The Brain in Cross-Cultural Adjustment: A Pilot Study of Japanese Expatriates Living in the United States
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. MRI Data Acquisition
2.3. MRI Data Analysis
2.4. Psychological Test
2.4.1. Cultural Adjustment
2.4.2. Lifestyle
2.4.3. Control Variables
2.5. Data Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spreitzer, G.M.; McCall, M.W., Jr.; Mahoney, J.D. Early Identification of International Executive Potential. J. Appl. Psychol. 1997, 82, 6–29. [Google Scholar] [CrossRef]
- Kolb, D.A. Experiential Learning: Experience as the Source of Learning and Development; Prentice Hall: Englewood Cliffs, NJ, USA, 1984. [Google Scholar]
- Argyris, D. On Organizational Learning, 2nd ed.; Blackwell: Malden, MA, USA, 1999. [Google Scholar]
- Senge, P.M. The Fifth Discipline: The Art and Practice of the Learning Organization; Currency Doubleday: New York, NY, USA, 1990. [Google Scholar]
- Black, J.S.; Mendenhall, M.; Oddou, G. Toward a comprehensive model of international adjustment: An integration of multiple theoretical perspectives. Acad. Manag. Rev. 1991, 16, 291–317. [Google Scholar] [CrossRef]
- Peltokorpi, V.; Zhang, L.E. Exploring expatriate adjustment through identity perspective. Int. Bus. Rev. 2020, 29, 101667. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Kayes, D.C. Expatriate learning: Exploring how Japanese managers adapt in the United States. Int. J. Hum. Resour. Manag. 2007, 18, 1373–1395. [Google Scholar] [CrossRef][Green Version]
- Yamazaki, Y.; Kayes, D.C. An Experiential Approach to Cross-cultural Learning: A Review and Integration of Success Factors in Expatriate Adaptation. Acad. Manag. Learn. Educ. 2004, 3, 354–379. [Google Scholar] [CrossRef]
- Berry, J.W. Immigration, acculturation, and adaptation. Appl. Psychol. 1997, 46, 5–34. [Google Scholar] [CrossRef]
- Haslberger, A. The complexities of expatriate adaptation. Hum. Resour. Manag. Rev. 2005, 15, 160–180. [Google Scholar] [CrossRef]
- Schenck, J.; Cruickshank, J. Evolving Kolb: Experiential education in the age of neuroscience. J. Exp. Educ. 2015, 38, 73–95. [Google Scholar] [CrossRef]
- Immordino-Yang, M.H. Me, my “self” and you: Neuropsychological relations between social emotion, self-awareness, and morality. Emot. Rev. 2011, 3, 313–315. [Google Scholar] [CrossRef]
- Piaget, J. The Psychology of Intelligence; Routledge: New York, NY, USA, 2001. [Google Scholar]
- Hebb, D. The Organization of Behavior: A Neuropsychological Theory; John Wiley: New York, NY, USA, 1949. [Google Scholar]
- Craik, F.I.; Tulving, E. Depth of processing and the retention of words in episodic memory. J. Exp. Psychol. Gen. 1975, 104, 268–294. [Google Scholar] [CrossRef]
- Li, M.; Jiang, J.; Qi, M. The mediating role of cultural intelligence to learning flexibility, cultural difference and expatriate effectiveness. J. Glob. Mob. 2024, 12, 715–737. [Google Scholar] [CrossRef]
- Wu, J.; Yang, J.; McIntyre, J.R.; Zhang, X. Revisiting the influence of cultural novelty and emotional stability on general adjustment of expatriates hosted in emerging economies. Cross Cult. Strateg. Manag. 2022, 29, 870–898. [Google Scholar] [CrossRef]
- Matsuo, M. Supporting experiential learning for expanding successes: Extending Kolb’s model. Hum. Resour. Dev. Int. 2024, 28, 1–23. [Google Scholar] [CrossRef]
- Bilderback, S.; Farrell, M. Integrating social media platforms into expatriate training and development programs: An experiential learning perspective. J. Glob. Mobil. 2025, 13, 59–76. [Google Scholar] [CrossRef]
- Owen, A.M.; McMillan, K.M.; Laird, A.R.; Bullmore, E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 2005, 25, 46–59. [Google Scholar] [CrossRef]
- Smith, E.E.; Jonides, J. Neuroimaging analyses of human working memory. Proc. Natl. Acad. Sci. USA 1998, 95, 12061–12068. [Google Scholar] [CrossRef]
- Wager, T.D.; Smith, E.E. Neuroimaging studies of working memory: A meta-analysis. Cogn. Affect. Behav. Neurosci. 2003, 3, 255–274. [Google Scholar] [CrossRef]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Goulden, N.; Khusnulina, A.; Davis, N.J.; Bracewell, R.M.; Bokde, A.L.; McNulty, J.P.; Mullins, P.G. The salience network is responsible for switching between the default mode network and the central executive network: Replication from DCM. Neuroimage 2014, 99, 180–190. [Google Scholar] [CrossRef]
- Sridharan, D.; Levitin, D.J.; Menon, V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. USA 2008, 105, 12569–12574. [Google Scholar] [CrossRef]
- Uddin, L.Q. Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 2015, 16, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Andrews-Hanna, J.R.; Reidler, J.S.; Sepulcre, J.; Poulin, R.; Buckner, R.L. Functional-anatomic fractionation of the brain’s default network. Neuron 2010, 65, 550–562. [Google Scholar] [CrossRef]
- Li, W.; Mai, X.; Liu, C. The default mode network and social understanding of others: What do brain connectivity studies tell us. Front. Hum. Neurosci. 2014, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Krönke, K.M.; Wolff, M.; Shi, Y.; Kräplin, A.; Smolka, M.N.; Bühringer, G.; Goschke, T. Functional connectivity in a triple-network saliency model is associated with real-life self-control. Neuropsychologia 2020, 149, 107667. [Google Scholar] [CrossRef]
- Bilevicius, E.; Kolesar, T.A.; Smith, S.D.; Trapnell, P.D.; Kornelsen, J. Trait emotional empathy and resting state functional connectivity in default mode, salience, and central executive networks. Brain Sci. 2018, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, S.E.; Kim, H.E.; Han, K.; Jeong, B.; Kim, J.J.; Namkoong, K.; Kim, J.W. Altered functional connectivity of the default mode network in low-empathy subjects. Yonsei Med. J. 2017, 58, 1061–1065. [Google Scholar] [CrossRef]
- Nummenmaa, L.; Hirvonen, J.; Parkkola, R.; Hietanen, J.K. Is emotional contagion special? An fMRI study on neural systems for affective and cognitive empathy. NeuroImage 2008, 43, 571–580. [Google Scholar] [CrossRef]
- Shamay-Tsoory, S.G.; Aharon-Peretz, J.; Perry, D. Two systems for empathy: A double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain 2009, 132, 617–627. [Google Scholar] [CrossRef]
- Frith, C.D.; Frith, U. Interacting minds--a biological basis. Science 1999, 286, 1692–1695. [Google Scholar] [CrossRef]
- Krueger, F.; Barbey, A.K.; Grafman, J. The medial prefrontal cortex mediates social event knowledge. Trends Cogn. Sci. 2009, 13, 103–109. [Google Scholar] [CrossRef]
- Luo, Y.; Eickhoff, S.B.; Hétu, S.; Feng, C. Social comparison in the brain: A coordinate-based meta-analysis of functional brain imaging studies on the downward and upward comparisons. Hum. Brain Mapp. 2018, 39, 440–458. [Google Scholar] [CrossRef] [PubMed]
- Montague, P.R.; Lohrenz, T. To detect and correct: Norm violations and their enforcement. Neuron 2007, 56, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Xiang, T.; Lohrenz, T.; Montague, P.R. Computational substrates of norms and their violations during social exchange. J. Neurosci. 2013, 33, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Buckholtz, J.W.; Marois, R. The roots of modern justice: Cognitive and neural foundations of social norms and their enforcement. Nat. Neurosci. 2012, 15, 655–661. [Google Scholar] [CrossRef]
- Krueger, F.; Hoffman, M. The emerging neuroscience of third-party punishment. Trends Neurosci. 2016, 39, 499–501. [Google Scholar] [CrossRef]
- Feng, C.; Eickhoff, S.B.; Li, T.; Wang, L.; Becker, B.; Camilleri, J.A.; Hetu, S.; Luo, Y. Common brain networks underlying human social interactions: Evidence from large-scale neuroimaging meta-analysis. Neurosci. Biobehav. Rev. 2021, 126, 289–303. [Google Scholar] [CrossRef]
- Bickart, K.C.; Brickhouse, M.; Negreira, A.; Sapolsky, D.; Barrett, L.F.; Dickerson, B.C. Atrophy in distinct corticolimbic networks in frontotemporal dementia relates to social impairments measured using the Social Impairment Rating Scale. J. Neurol. Neurosurg. Psychiatry 2014, 85, 438–448. [Google Scholar] [CrossRef]
- Bora, E.; Walterfang, M.; Velakoulis, D. Theory of mind in behavioural-variant frontotemporal dementia and Alzheimer’s disease: A meta-analysis. J. Neurol. Neurosurg. Psychiatry 2015, 86, 714–719. [Google Scholar] [CrossRef]
- Boublay, N.; Schott, A.M.; Krolak-Salmon, P. Neuroimaging correlates of neuropsychiatric symptoms in Alzheimer’s disease: A review of 20 years of research. Eur. J. Neurol. 2016, 23, 1500–1509. [Google Scholar] [CrossRef]
- Pasquini, L.; Nana, A.L.; Toller, G.; Brown, J.A.; Deng, J.; Staffaroni, A.; Kim, E.-J.; Hwang, J.-H.L.; Li, L.; Park, Y.; et al. Salience network atrophy links neuron type-specific pathobiology to loss of empathy in frontotemporal dementia. Cereb. Cortex. 2020, 30, 5387–5399. [Google Scholar] [CrossRef]
- Yuan, P.; Raz, N. Prefrontal cortex and executive functions in healthy adults: A meta-analysis of structural neuroimaging studies. Neurosci. Biobehav. Rev. 2014, 42, 180–192. [Google Scholar] [CrossRef]
- Truman, S.D.; Sharar, D.A.; Pompe, J.C. The mental health status of expatriate versus US domestic workers: A comparative study. Int. J. Ment. Health 2011, 40, 3–18. [Google Scholar] [CrossRef]
- Fujimoto, W.Y. 2015 Yutaka Seino distinguished leadership award lecture: The Japanese American community diabetes study and the ‘canary in the coal mine’. J. Diabetes Investig. 2016, 7, 664–673. [Google Scholar] [CrossRef]
- Naithani, D.P. Impact of health and recreation on work-life balance: A case study of expatriates. J. Soc. Sci. Bus. 2016, 1, 1–21. [Google Scholar] [CrossRef]
- Prestes, V.A.; Grisci, C.L.I.; Fraga, A.M. Lifestyles of workers in the expatriation context. RAM Rev. De Adm. Mackenzie 2016, 17, 39–59. [Google Scholar] [CrossRef][Green Version]
- Grant-Vallone, E.J.; Ensher, E.A. An examination of work and personal life conflict, organizational support, and employee health among international expatriates. Int. J. Intercult. Relat. 2001, 25, 261–278. [Google Scholar] [CrossRef]
- Kokubun, K.; Yamakawa, Y. Association between food patterns and gray matter volume. Front. Hum. Neurosci. 2019, 13, 384. [Google Scholar] [CrossRef] [PubMed]
- Kokubun, K.; Pineda, J.C.D.; Yamakawa, Y. Unhealthy lifestyles and brain condition: Examining the relations of BMI, living alone, alcohol intake, short sleep, smoking, and lack of exercise with gray matter volume. PLoS ONE 2021, 16, e0255285. [Google Scholar] [CrossRef]
- Binnewies, J.; Nawijn, L.; Brandmaier, A.M.; Baaré, W.F.; Boraxbekk, C.J.; Demnitz, N.; Drevon, C.A.; Fjell, A.M.; Lindenberger, U.; Madsen, K.S.; et al. Lifestyle-related risk factors and their cumulative associations with hippocampal and total grey matter volume across the adult lifespan: A pooled analysis in the European Lifebrain consortium. Brain Res. Bull. 2023, 200, 110692. [Google Scholar] [CrossRef]
- Hall, E.T. Beyond Culture; Anchor Press/Doubleday: Garden City, NY, USA, 1976. [Google Scholar]
- Hofstede, G.H. Culture and Organization: Software of Mind; McGraw Hill: New York, NY, USA, 1997. [Google Scholar]
- Triandis, H.C. Individualism and Collectivism; Westview Press: Boulder, CO, USA, 1995. [Google Scholar]
- Furnham, A.; Bochner, S. Culture Shock: Psychological Reactions to Unfamiliar Environments; Methuen: London, UK, 1982. [Google Scholar]
- Linowes, R.G. The Japanese manager’s traumatic entry into the United States: Understanding the American-Japanese cultural divide. Acad. Manag. Exec. 1993, 7, 21–40. [Google Scholar] [CrossRef]
- Hayashi, K. Ibunka Intafeisu Keiei, 3rd ed.; Nihon Keizai Shinbunsha: Tokyo, Japan, 1999. [Google Scholar]
- Nemoto, K.; Oka, H.; Fukuda, H.; Yamakawa, Y. MRI based Brain Healthcare Quotients: A bridge between neural and behavioral analyses for keeping the brain healthy. PLoS ONE 2017, 12, e0187137. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, T.; Kokubun, K.; Okamoto, M.; Yamakawa, Y. The brain that understands diversity: A pilot study focusing on the triple network. Brain Sci. 2025, 15, 233. [Google Scholar] [CrossRef]
- Tarrant, M.A.; Cordell, H.K. The effect of respondent characteristics on general environmental attitude-behavior correspondence. Environ. Behav. 1997, 29, 618–637. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Ashburner, J. A fast diffeomorphic image registration algorithm. Neuroimage 2007, 38, 95–113. [Google Scholar] [CrossRef]
- Tzourio-Mazoyer, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Tzourio-Mazoyer, B.; Joliot, M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002, 15, 273–289. [Google Scholar] [CrossRef]
- Logothetis, N.K. What we can do and what we cannot do with fMRI. Nature 2008, 453, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Winkler, A.M.; Kochunov, P.; Blangero, J.; Almasy, L.; Zilles, K.; Fox, P.T.; Duggirala, R.; Glahn, D.C. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage 2010, 53, 1135–1146. [Google Scholar] [CrossRef]
- Black, J.S.; Stephens, G.K. The influence of the spouse on American expatriate adjustment and intent to stay in Pacific Rim overseas assignments. J. Manag. 1989, 15, 529–544. [Google Scholar] [CrossRef]
- Kokubun, K.; Nemoto, K.; Yamakawa, Y. Cultural and emotional intelligence correlates with healthy lifestyles. Acta Psychol. 2025, 255, 104854. [Google Scholar] [CrossRef]
- Zhang, Z.; Castelló, A. Principal components analysis in clinical studies. Ann. Transl. Med. 2017, 5, 351. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.C.; Whitehead, A.L.; Jacques, R.M.; Julious, S.A. The statistical interpretation of pilot trials: Should significance thresholds be reconsidered? BMC Med. Res. Methodol. 2014, 14, 1–8. [Google Scholar] [CrossRef]
- Noorani, M.; Bolognone, R.K.; Graville, D.J.; Palmer, A.D. The association between dysphagia symptoms, DIGEST scores, and severity ratings in individuals with Parkinson’s disease. Dysphagia 2023, 38, 1295–1307. [Google Scholar] [CrossRef]
- Nykänen, M.; Kurki, A.L.; Airila, A. Promoting workplace guidance and workplace–school collaboration in vocational training: A mixed-methods pilot study. Vocat. Learn. 2022, 15, 317–339. [Google Scholar] [CrossRef]
- Lincoln, T.M.; Schlier, B.; Müller, R.; Hayward, M.; Fladung, A.K.; Bergmann, N.; Böge, K.; Gallinat, J.; Mahlke, C.; Gonther, U.; et al. Reducing Distress from Auditory Verbal Hallucinations: A Multicenter, Parallel, Single-Blind, Randomized Controlled Feasibility Trial of Relating Therapy. Psychother. Psychosom. 2024, 93, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Kakeda, S.; Nemoto, K.; Onoda, K.; Yamaguchi, S.; Kobayashi, S.; Yamakawa, Y. Grey-matter brain healthcare quotient and cognitive function: A large cohort study of an MRI brain screening system in Japan. Cortex 2021, 145, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Kokubun, K.; Yamakawa, Y.; Nemoto, K. The link between the brain volume derived index and the determinants of social performance. Curr. Psychol. 2023, 42, 12309–12321. [Google Scholar] [CrossRef]
- Kokubun, K.; Nemoto, K.; Yamakawa, Y. Whole-brain gray matter volume mediates the relationship between psychological distress and job satisfaction. Acta Psychol. 2025, 256, 105059. [Google Scholar] [CrossRef]
- de Andrade, A.P.M.; Amaro, E., Jr.; Farhat, S.C.L.; Schvartsman, C. Higher burnout scores in paediatric residents are associated with increased brain activity during attentional functional magnetic resonance imaging task. Acta Paediatr. 2016, 105, 705–713. [Google Scholar] [CrossRef]
- Muraven, M.; Baumeister, R.F. Self-regulation and depletion of limited resources: Does self-control resemble a muscle? Psychol. Bull. 2000, 126, 247–259. [Google Scholar] [CrossRef]
- Nakahara, J. Experiential Learning: Theoretical Genealogies and Research Trends. Japan. J. Labor Stud. 2013, 55, 4–14. [Google Scholar]
- Takeuchi, R.; Yun, S.; Tesluk, P.E. An examination of crossover and spillover effects of spousal and expatriate cross-cultural adjustment on expatriate outcomes. J. Appl. Psychol. 2002, 87, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.P.; Harrison, D.A. Using intra-national diversity for international assignments: A model of bicultural competence and expatriate adjustment. Hum. Resour. Manag. Rev. 1996, 6, 47–74. [Google Scholar] [CrossRef]
- Bhaskar-Shrinivas, P.; Harrison, D.A.; Shaffer, M.A.; Luk, D.M. Input-based and time-based models of international adjustment: Meta-analytic evidence and theoretical extensions. Acad. Manag. J. 2005, 48, 257–281. [Google Scholar] [CrossRef]



| N | % | |
|---|---|---|
| Sex | ||
| Male | 9 | 90 |
| Female | 1 | 10 |
| Occupation | ||
| Managerial | 5 | 50 |
| Professional and technical | 4 | 40 |
| Sales | 1 | 10 |
| Accompanying family | ||
| Accompanied | 8 | 80 |
| Unaccompanied | 1 | 10 |
| Single | 1 | 10 |
| Pre-departure cross-cultural training | ||
| Yes | 6 | 60 |
| No | 4 | 40 |
| N | Min | Max | Mean | SD | |
|---|---|---|---|---|---|
| GMV | |||||
| Whole-brain | 10 | 84.9 | 105.8 | 93.9 | 7.4 |
| DMN | 10 | 86.7 | 108.6 | 95.7 | 6.5 |
| CEN | 10 | 82.0 | 107.4 | 94.0 | 7.4 |
| SN | 10 | 86.6 | 123.8 | 101.5 | 10.7 |
| Cross-cultural adjustment | |||||
| General adjustment | 10 | 4.7 | 6.1 | 5.3 | 0.5 |
| Interaction adjustment | 10 | 2.8 | 6.0 | 4.2 | 0.8 |
| Work adjustment | 10 | 3.0 | 7.0 | 5.2 | 1.2 |
| Lifestyle | 10 | 2.9 | 4.1 | 3.4 | 0.5 |
| Age (years) | 10 | 32.0 | 56.0 | 44.9 | 7.3 |
| BMI (kg/m2) | 10 | 17.9 | 27.8 | 22.5 | 3.1 |
| Length of education (months) | 10 | 16.0 | 21.0 | 17.3 | 1.6 |
| Length of service (months) | 10 | 1.0 | 6.0 | 3.7 | 1.8 |
| Period of stay in the US (months) | 10 | 5.0 | 54.0 | 26.6 | 18.7 |
| Period overseas for work (months) | 10 | 5.0 | 130.0 | 43.6 | 37.5 |
| Period overseas for study (months) | 10 | 0.0 | 24.0 | 5.2 | 7.7 |
| Period overseas for others (months) | 10 | 0.0 | 74.0 | 17.2 | 28.7 |
| Network | AAL Code | Region |
|---|---|---|
| DMN | AAL023 | Superior medial frontal gyrus (Left) |
| AAL024 | Superior medial frontal gyrus (Right) | |
| AAL035 | Posterior cingulate gyrus (Left) | |
| AAL036 | Posterior cingulate gyrus (Right) | |
| AAL061 | Inferior parietal lobule (Left) | |
| AAL062 | Inferior parietal lobule (Right) | |
| AAL067 | Precuneus (Left) | |
| AAL068 | Precuneus (Right) | |
| CEN | AAL003 | Superior frontal gyrus (Left) |
| AAL004 | Superior frontal gyrus (Right) | |
| AAL059 | Superior parietal lobule (Left) | |
| AAL060 | Superior parietal lobule (Right) | |
| SN | AAL029 | Insula (Left) |
| AAL030 | Insula (Right) | |
| AAL031 | Anterior cingulate gyrus (Left) | |
| AAL032 | Anterior cingulate gyrus (Right) |
| Factor 1 | |
|---|---|
| Sex (male 1; female 0) | 0.770 |
| Age (years) | 0.908 |
| BMI (kg/m2) | 0.714 |
| Length of education (months) | 0.595 |
| Period of stay in the US (months) | 0.304 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
|---|---|---|---|---|---|---|---|---|---|
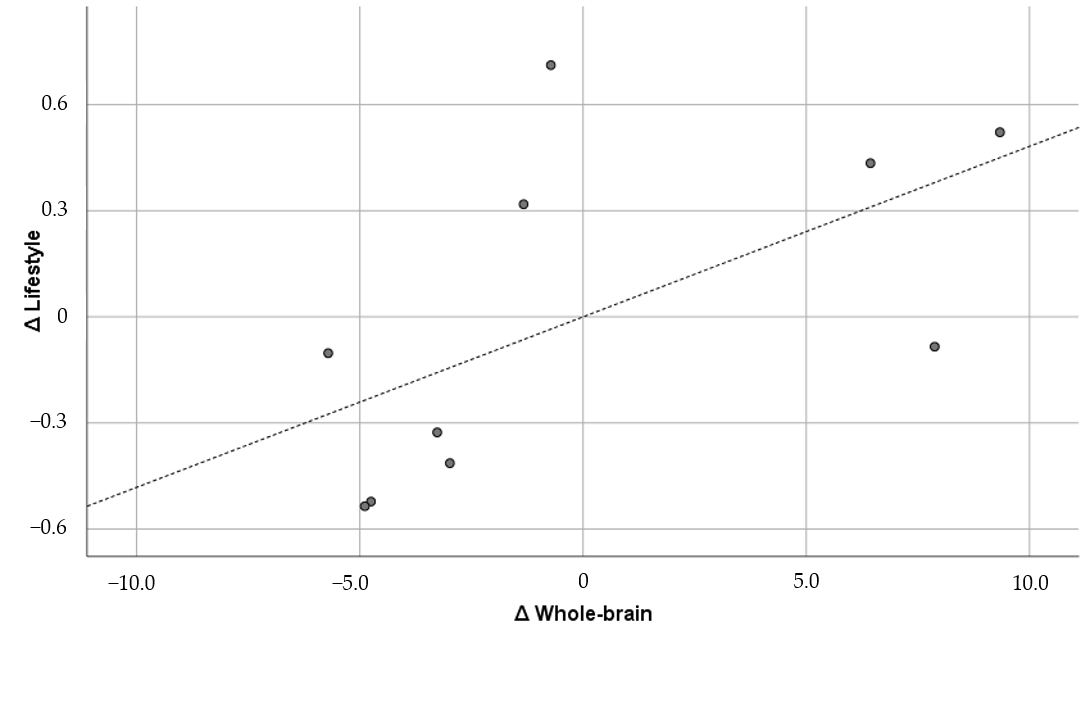
| 1 | Whole-brain | 0.865 ** | 0.854 ** | 0.892 ** | 0.733 * | 0.554 | 0.342 | 0.593 † | |
| (0.599, 0.959) | (0.571, 0.956) | (0.670, 0.968) | (0.304, 0.914) | (0.002, 0.847) | (−0.259, 0.752) | (0.061, 0.863) | |||
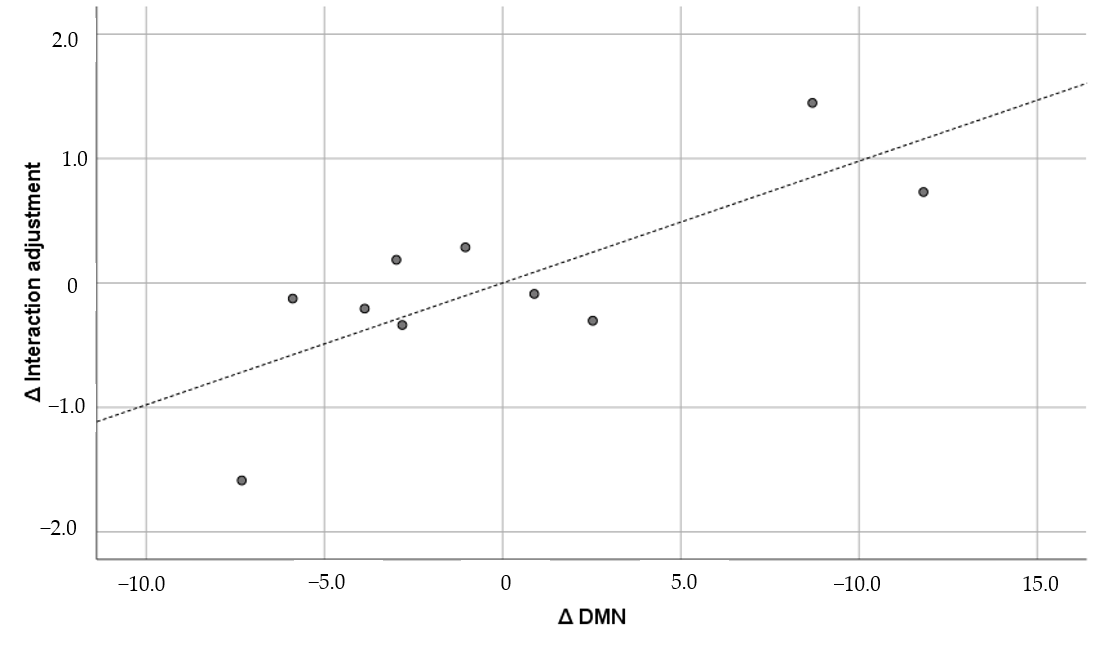
| 2 | DMN | 0.830 ** | 0.965 *** | 0.950 *** | 0.630 † | 0.771 * | 0.614 † | 0.508 | |
| (0.513, 0.948) | (0.884, 0.990) | (0.837, 0.985) | (0.119, 0.877) | (0.381, 0.928) | (0.093, 0.871) | (−0.062, 0.828) | |||
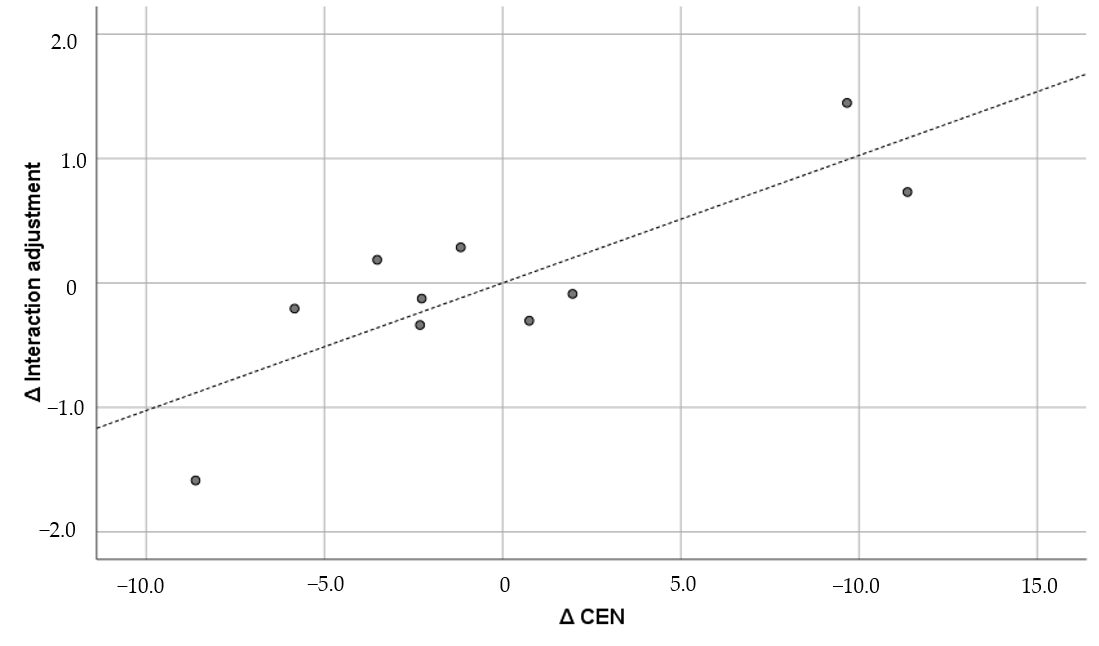
| 3 | CEN | 0.894 *** | 0.944 *** | 0.938 *** | 0.691 * | 0.826 ** | 0.551 | 0.660 † | |
| (0.675, 0.968) | (0.818, 0.984) | (0.800, 0.982) | (0.224, 0.900) | (0.503, 0.947) | (−0.002, 0.846) | (0.169, 0.888) | |||
| 4 | SN | 0.937 *** | 0.884 ** | 0.946 *** | 0.682 * | 0.663 † | 0.572 | 0.593 † | |
| (0.797, 0.981) | (0.648, 0.965) | (0.824, 0.984) | (0.208, 0.897) | (0.175, 0.890) | (0.029, 0.854) | (0.061, 0.863) | |||
| 5 | General adjustment | 0.443 | 0.536 | 0.490 | 0.392 | 0.408 | 0.052 | 0.810 ** | |
| (−0.145, 0.800) | (−0.023, 0.840) | (−0.085, 0.820) | (−0.204, 0.776) | (−0.186, 0.784) | (−0.515, 0.587) | (0.466, 0.941) | |||
| 6 | Interaction adjustment | 0.227 | 0.615 | 0.527 | 0.293 | 0.434 | 0.730* | 0.516 | |
| (−0.372, 0.692) | (0.095, 0.871) | (−0.036, 0.836) | (−0.309, 0.728) | (−0.156, 0.796) | (0.298, 0.914) | (−0.051, 0.831) | |||
| 7 | Work adjustment | 0.298 | 0.600 † | 0.498 | 0.467 | 0.041 | 0.684 * | 0.065 | |
| (−0.304, 0.730) | (0.071, 0.865) | (−0.075, 0.824) | (−0.115, 0.813) | (−0.523, 0.580) | (0.212, 0.897) | (−0.505, 0.596) | |||
| 8 | Lifestyle | 0.395 | 0.452 | 0.513 | 0.385 | 0.810 ** | 0.518 | 0.059 | |
| (−0.201, 0.778) | (−0.134, 0.804) | (−0.055, 0.830) | (−0.212, 0.773) | (0.466, 0.941) | (−0.048, 0.832) | (−0.510, 0.592) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokubun, K.; Nemoto, K.; Yamakawa, Y. The Brain in Cross-Cultural Adjustment: A Pilot Study of Japanese Expatriates Living in the United States. Brain Sci. 2025, 15, 617. https://doi.org/10.3390/brainsci15060617
Kokubun K, Nemoto K, Yamakawa Y. The Brain in Cross-Cultural Adjustment: A Pilot Study of Japanese Expatriates Living in the United States. Brain Sciences. 2025; 15(6):617. https://doi.org/10.3390/brainsci15060617
Chicago/Turabian StyleKokubun, Keisuke, Kiyotaka Nemoto, and Yoshinori Yamakawa. 2025. "The Brain in Cross-Cultural Adjustment: A Pilot Study of Japanese Expatriates Living in the United States" Brain Sciences 15, no. 6: 617. https://doi.org/10.3390/brainsci15060617
APA StyleKokubun, K., Nemoto, K., & Yamakawa, Y. (2025). The Brain in Cross-Cultural Adjustment: A Pilot Study of Japanese Expatriates Living in the United States. Brain Sciences, 15(6), 617. https://doi.org/10.3390/brainsci15060617






