Predictive Factors of Successful Spinal Cord Stimulation in Patients with Chronic Pain: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Data Collection
Statistical Analysis
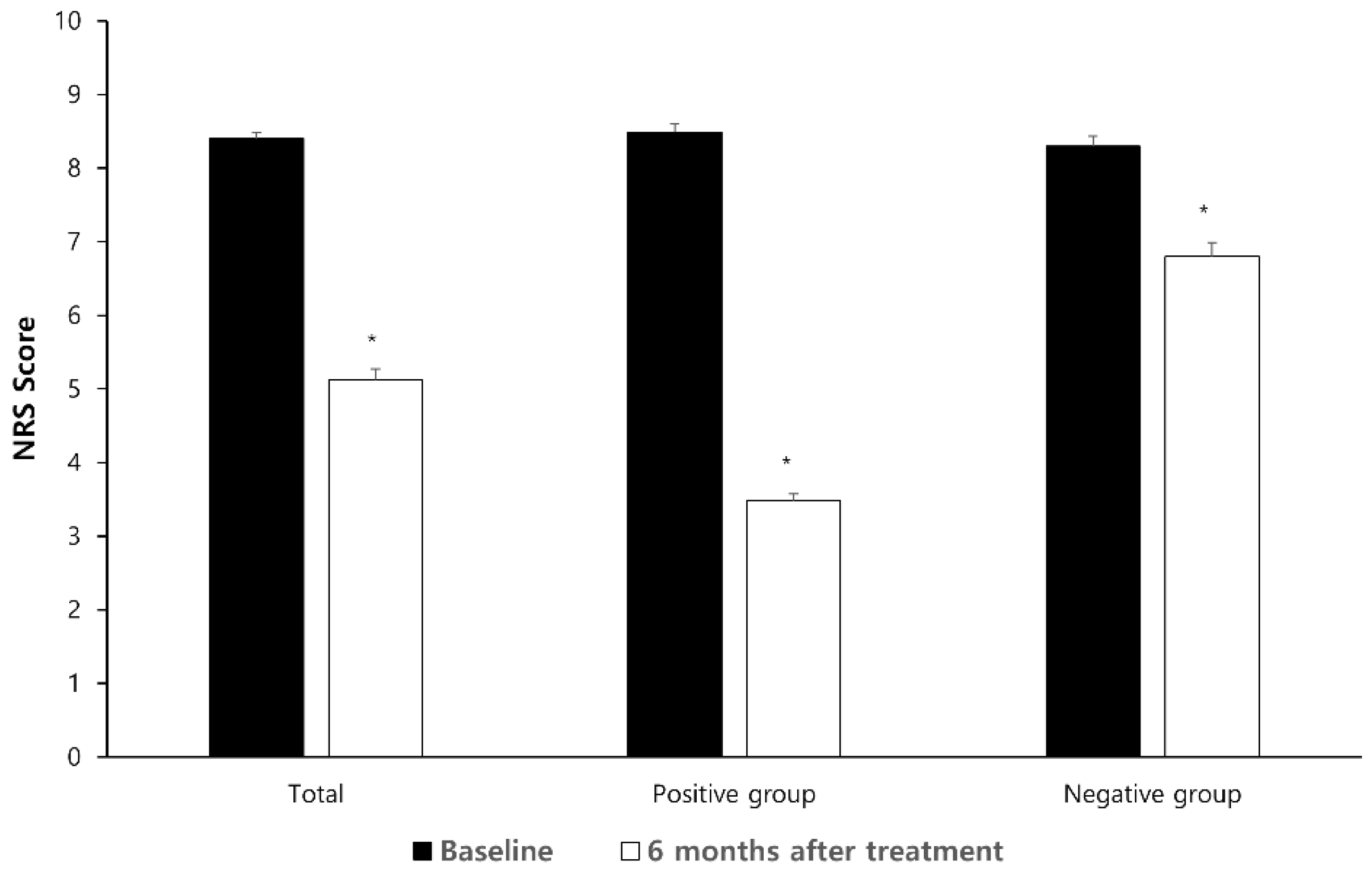
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SCS | Spinal cord stimulation |
| CRPS | Complex regional pain syndrome |
| PSPS | Persistent spinal pain syndrome |
| NRS | Numeric rating scale |
| MEDD | Morphine equivalent daily dosage |
References
- Sivanesan, E.; Maher, D.P.; Raja, S.N.; Linderoth, B.; Guan, Y. Supraspinal Mechanisms of Spinal Cord Stimulation for Modulation of Pain: Five Decades of Research and Prospects for the Future. Anesthesiology 2019, 130, 651–665. [Google Scholar] [CrossRef] [PubMed]
- De Groote, S.; De Jaeger, M.; Van Schuerbeek, P.; Sunaert, S.; Peeters, R.; Loeckx, D.; Goudman, L.; Forget, P.; De Smedt, A.; Moens, M. Functional magnetic resonance imaging: Cerebral function alterations in subthreshold and suprathreshold spinal cord stimulation. J. Pain Res. 2018, 11, 2517–2526. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.A.; Schatman, M.E.; Sayed, D.; Deer, T. Persistent Spinal Pain Syndrome: New Terminology for a New Era. J. Pain Res. 2021, 14, 1627–1630. [Google Scholar] [CrossRef] [PubMed]
- Früh, A.; Sargut, T.A.; Brüßeler, M.; Hallek, L.; Kuckuck, A.; Vajkoczy, P.; Bayerl, S. Spinal Cord Stimulation with Implantation of Surgical Leads is a Sufficient Salvage Therapy for Patients Suffering from Persistent Spinal Pain Syndrome-A Retrospective Single-center Experience. World Neurosurg. 2024, 192, e474–e479. [Google Scholar] [CrossRef]
- Oliveira, M.J.; Matis, G.K. Spinal cord stimulation as a treatment option for complex regional pain syndrome: A narrative review. Br. J. Neurosurg. 2024, 38, 1289–1293. [Google Scholar] [CrossRef]
- Kumar, K.; Taylor, R.S.; Jacques, L.; Eldabe, S.; Meglio, M.; Molet, J.; Thomson, S.; O’Callaghan, J.; Eisenberg, E.; Milbouw, G.; et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: A multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain 2007, 132, 179–188. [Google Scholar] [CrossRef]
- Deer, T.R.; Mekhail, N.; Provenzano, D.; Pope, J.; Krames, E.; Leong, M.; Levy, R.M.; Abejon, D.; Buchser, E.; Burton, A.; et al. The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: The Neuromodulation Appropriateness Consensus Committee. Neuromodulation 2014, 17, 515–550; discussion 550. [Google Scholar] [CrossRef]
- Hoikkanen, T.; Nissen, M.; Ikäheimo, T.M.; Jyrkkänen, H.K.; Huttunen, J.; von Und Zu Fraunberg, M. Long-Term Outcome of Spinal Cord Stimulation in Complex Regional Pain Syndrome. Neurosurgery 2021, 89, 597–609. [Google Scholar] [CrossRef]
- Patel, S.K.; Gozal, Y.M.; Saleh, M.S.; Gibson, J.L.; Karsy, M.; Mandybur, G.T. Spinal cord stimulation failure: Evaluation of factors underlying hardware explantation. J. Neurosurg. Spine 2020, 32, 133–138. [Google Scholar] [CrossRef]
- Huygen, F.; Soulanis, K.; Rtveladze, K.; Kamra, S.; Schlueter, M. Spinal Cord Stimulation vs Medical Management for Chronic Back and Leg Pain: A Systematic Review and Network Meta-Analysis. JAMA Netw. Open 2024, 7, e2444608. [Google Scholar] [CrossRef]
- Caylor, J.; Reddy, R.; Yin, S.; Cui, C.; Huang, M.; Huang, C.; Ramesh, R.; Baker, D.G.; Simmons, A.; Souza, D.; et al. Spinal cord stimulation in chronic pain: Evidence and theory for mechanisms of action. Bioelectron. Med. 2019, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Bir, S.C.; Konar, S.; Maiti, T.; Nanda, A.; Guthikonda, B. Neuromodulation in intractable pain management: Outcomes and predictors of revisions of spinal cord stimulators. Neurosurg. Focus 2016, 40, E4. [Google Scholar] [CrossRef] [PubMed]
- Lad, S.P.; Petraglia, F.W., 3rd; Kent, A.R.; Cook, S.; Murphy, K.R.; Dalal, N.; Karst, E.; Staats, P.; Sharan, A. Longer Delay From Chronic Pain to Spinal Cord Stimulation Results in Higher Healthcare Resource Utilization. Neuromodulation 2016, 19, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Beletsky, A.; Liu, C.; Alexander, E.; Hassanin, S.W.; Vickery, K.; Loomba, M.; Winston, N.; Chen, J.; Gabriel, R.A. The Association of Psychiatric Comorbidities With Short-Term and Long-Term Outcomes Following Spinal Cord Stimulator Placement. Neuromodulation 2023, 26, 1081–1088. [Google Scholar] [CrossRef]
- North, R.B.; Kidd, D.H.; Farrokhi, F.; Piantadosi, S.A. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: A randomized, controlled trial. Neurosurgery 2005, 56, 98–106; discussion 106–107. [Google Scholar] [CrossRef]
- Kapural, L.; Yu, C.; Doust, M.W.; Gliner, B.E.; Vallejo, R.; Sitzman, B.T.; Amirdelfan, K.; Morgan, D.M.; Brown, L.L.; Yearwood, T.L.; et al. Novel 10-kHz High-frequency Therapy (HF10 Therapy) Is Superior to Traditional Low-frequency Spinal Cord Stimulation for the Treatment of Chronic Back and Leg Pain: The SENZA-RCT Randomized Controlled Trial. Anesthesiology 2015, 123, 851–860. [Google Scholar] [CrossRef]
- De Ridder, D.; Plazier, M.; Kamerling, N.; Menovsky, T.; Vanneste, S. Burst spinal cord stimulation for limb and back pain. World Neurosurg. 2013, 80, 642–649.e641. [Google Scholar] [CrossRef]
- Mekhail, N.A.; Mathews, M.; Nageeb, F.; Guirguis, M.; Mekhail, M.N.; Cheng, J. Retrospective review of 707 cases of spinal cord stimulation: Indications and complications. Pain Pract. 2011, 11, 148–153. [Google Scholar] [CrossRef]
- Thomson, S.J.; Kruglov, D.; Duarte, R.V. A Spinal Cord Stimulation Service Review From a Single Centre Using a Single Manufacturer Over a 7.5 Year Follow-Up Period. Neuromodulation 2017, 20, 589–599. [Google Scholar] [CrossRef]
- Deer, T.R.; Stewart, C.D. Complications of Spinal Cord Stimulation: Identification, Treatment, and Prevention. Pain Med. 2008, 9, S93–S101. [Google Scholar] [CrossRef]
- Deer, T.R.; Mekhail, N.; Provenzano, D.; Pope, J.; Krames, E.; Thomson, S.; Raso, L.; Burton, A.; DeAndres, J.; Buchser, E.; et al. The appropriate use of neurostimulation: Avoidance and treatment of complications of neurostimulation therapies for the treatment of chronic pain. Neuromodulation Appropriateness Consensus Committee. Neuromodulation 2014, 17, 571–597; discussion 578–597. [Google Scholar] [CrossRef]
- Kemler, M.A.; de Vet, H.C.; Barendse, G.A.; van den Wildenberg, F.A.; van Kleef, M. Effect of spinal cord stimulation for chronic complex regional pain syndrome Type I: Five-year final follow-up of patients in a randomized controlled trial. J. Neurosurg. 2008, 108, 292–298. [Google Scholar] [CrossRef]
- Negoita, S.; Duy, P.Q.; Mahajan, U.V.; Anderson, W.S. Timing and prevalence of revision and removal surgeries after spinal cord stimulator implantation. J. Clin. Neurosci. 2019, 62, 80–82. [Google Scholar] [CrossRef]
- Cameron, T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: A 20-year literature review. J. Neurosurg. 2004, 100, 254–267. [Google Scholar] [CrossRef]
- Kapural, L.; Yu, C.; Doust, M.W.; Gliner, B.E.; Vallejo, R.; Sitzman, B.T.; Amirdelfan, K.; Morgan, D.M.; Yearwood, T.L.; Bundschu, R.; et al. Comparison of 10-kHz High-Frequency and Traditional Low-Frequency Spinal Cord Stimulation for the Treatment of Chronic Back and Leg Pain: 24-Month Results From a Multicenter, Randomized, Controlled Pivotal Trial. Neurosurgery 2016, 79, 667–677. [Google Scholar] [CrossRef]
- Kemler, M.A.; de Vet, H.C.; Barendse, G.A.; van den Wildenberg, F.A.; van Kleef, M. Spinal cord stimulation for chronic reflex sympathetic dystrophy—Five-year follow-up. N. Engl. J. Med. 2006, 354, 2394–2396. [Google Scholar] [CrossRef]
- Shanthanna, H.; Eldabe, S.; Provenzano, D.A.; Bouche, B.; Buchser, E.; Chadwick, R.; Doshi, T.L.; Duarte, R.; Hunt, C.; Huygen, F.; et al. Evidence-based consensus guidelines on patient selection and trial stimulation for spinal cord stimulation therapy for chronic non-cancer pain. Reg. Anesth. Pain Med. 2023, 48, 273–287. [Google Scholar] [CrossRef]
- Williams, K.A.; Gonzalez-Fernandez, M.; Hamzehzadeh, S.; Wilkinson, I.; Erdek, M.A.; Plunkett, A.; Griffith, S.; Crooks, M.; Larkin, T.; Cohen, S.P. A multi-center analysis evaluating factors associated with spinal cord stimulation outcome in chronic pain patients. Pain Med. 2011, 12, 1142–1153. [Google Scholar] [CrossRef]
- Gupta, M.; Abd-Elsayed, A.; Hughes, M.; Rotte, A. A Retrospective Review of Lead Migration Rate in Patients Permanently Implanted with Percutaneous Leads and a 10 kHz SCS Device. Pain Res. Manag. 2021, 2021, 6639801. [Google Scholar] [CrossRef]
- Oakley, J.C.; Krames, E.S.; Stamatos, J.; Foster, A.M. Successful long-term outcomes of spinal cord stimulation despite limited pain relief during temporary trialing. Neuromodulation 2008, 11, 66–73. [Google Scholar] [CrossRef]
- Eldabe, S.; Duarte, R.V.; Gulve, A.; Thomson, S.; Baranidharan, G.; Houten, R.; Jowett, S.; Sandhu, H.; Chadwick, R.; Brookes, M.; et al. Does a screening trial for spinal cord stimulation in patients with chronic pain of neuropathic origin have clinical utility and cost-effectiveness (TRIAL-STIM)? A randomised controlled trial. Pain 2020, 161, 2820–2829. [Google Scholar] [CrossRef]
- Graham, D.T.; Lambert, M.; Mirzadeh, Z.; Ponce, F.A. Factors Contributing to Spinal Cord Stimulation Outcomes for Chronic Pain. Neuromodulation 2022, 25, 145–154. [Google Scholar] [CrossRef]
- Remacle, T.; Mauviel, S.; Renwart, H.J.; Ghassempour, K.; Belle, F.; Lückers, O.; Bex, V.; Remacle, J.M.; Bonhomme, V. Long-Term Multicolumn-Lead Spinal Cord Stimulation Efficacy in Patients with Failed Back Surgery Syndrome: A Six-Year Prospective Follow-up Study. World Neurosurg. 2020, 142, e245–e252. [Google Scholar] [CrossRef]
- Song, I.A.; Lee, J.H.; Han, W.K.; Nahm, F.S. The actual duration of spinal cord stimulator use in patients with complex regional pain syndrome: A Korean nationwide cohort study. Korean J. Pain 2025, 38, 51–57. [Google Scholar] [CrossRef]
- Jensen, M.P.; Brownstone, R.M. Mechanisms of spinal cord stimulation for the treatment of pain: Still in the dark after 50 years. Eur. J. Pain 2019, 23, 652–659. [Google Scholar] [CrossRef]
- Sdrulla, A.D.; Guan, Y.; Raja, S.N. Spinal Cord Stimulation: Clinical Efficacy and Potential Mechanisms. Pain Pract. 2018, 18, 1048–1067. [Google Scholar] [CrossRef]
- De La Rosa, J.S.; Brady, B.R.; Ibrahim, M.M.; Herder, K.E.; Wallace, J.S.; Padilla, A.R.; Vanderah, T.W. Co-occurrence of chronic pain and anxiety/depression symptoms in U.S. adults: Prevalence, functional impacts, and opportunities. Pain 2024, 165, 666–673. [Google Scholar] [CrossRef]
- Norrbrink Budh, C.; Lund, I.; Hultling, C.; Levi, R.; Werhagen, L.; Ertzgaard, P.; Lundeberg, T. Gender related differences in pain in spinal cord injured individuals. Spinal Cord 2003, 41, 122–128. [Google Scholar] [CrossRef]
- Zuidema, X.; van Daal, E.; van Geel, I.; de Geus, T.J.; van Kuijk, S.M.J.; de Galan, B.E.; de Meij, N.; Van Zundert, J. Long-Term Evaluation of Spinal Cord Stimulation in Patients With Painful Diabetic Polyneuropathy: An Eight-to-Ten-Year Prospective Cohort Study. Neuromodulation 2023, 26, 1074–1080. [Google Scholar] [CrossRef]
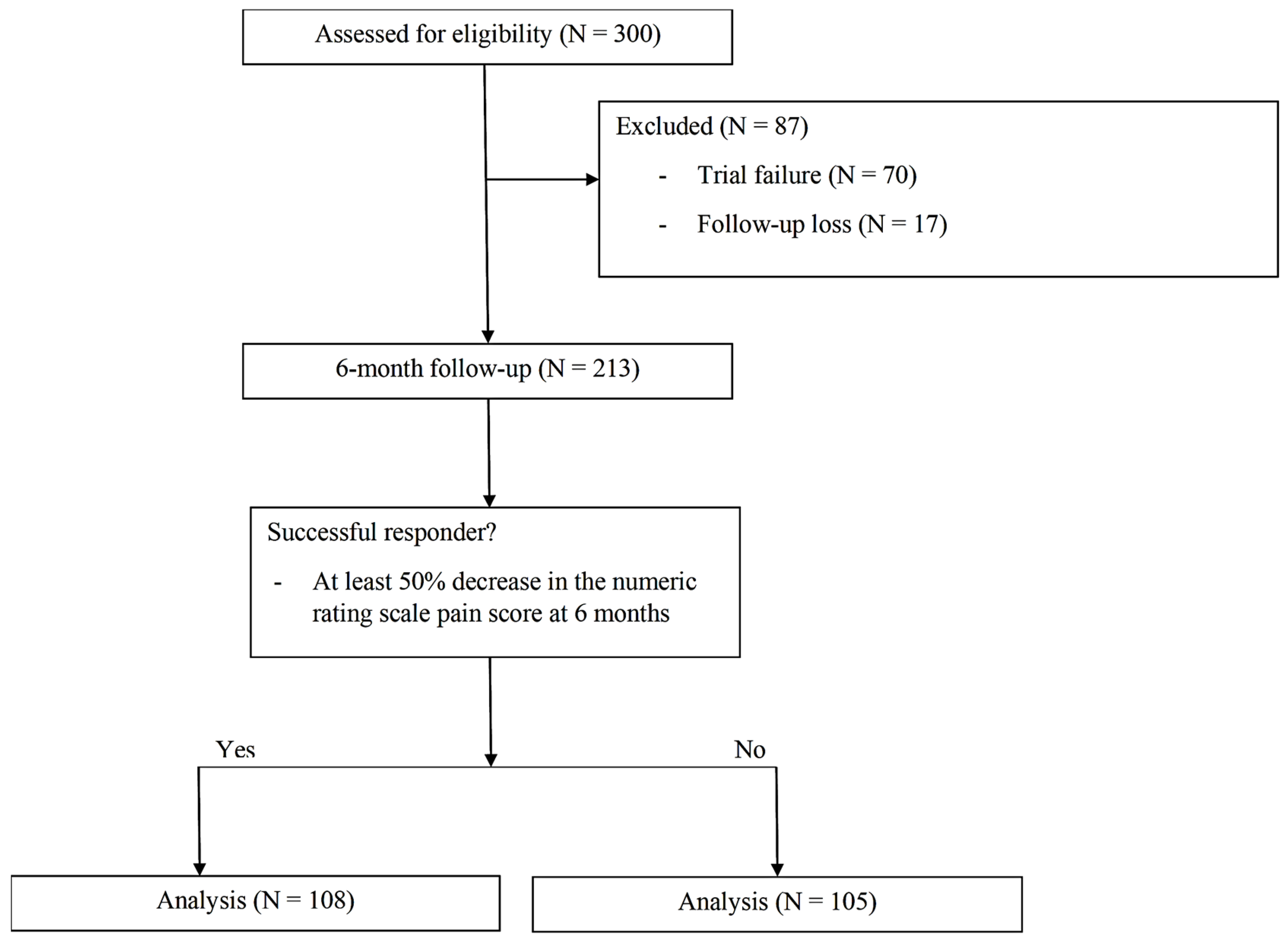
| Variables | Total (N = 213) | Positive Outcome (N = 108) | Negative Outcome (N = 103) | p Value |
|---|---|---|---|---|
| Age (yrs) | 53.00 ± 17.59 | 53.23 ± 17.79 | 52.76 ± 17.47 | 0.846 |
| Sex (M/F) | 120/93 | 68/40 | 52/53 | 0.048 |
| BMI (Kg/m2) | 24.99 ± 4.31 | 24.87 ± 3.97 | 25.11 ± 4.65 | 0.676 |
| Comorbid psychiatric disorder | 89 (41.8) | 47 (43.5) | 42 (40.0) | 0.677 |
| Litigation status | 83 (38.0) | 41 (38.0) | 42 (40.0) | 0.780 |
| Disease duration (months) | 25.0 (14.5–36.0) | 25.0 (20.5–27.0) | 26.0 (12.00–46.0) | 0.174 |
| Diagnosis | ||||
| CRPS type 1 | 114 (53.5) | 56 (51.9) | 58 (55.2) | 0.681 |
| CRPS type 2 | 27 (12.7) | 16 (14.8) | 11 (10.5) | 0.412 |
| PSPS | 39 (18.3) | 19 (17.6) | 20 (19.0) | 0.860 |
| Others | 33 (15.5) | 17 (15.7) | 16 (15.2) | >0.999 |
| Smoking | 46 (21.6) | 22 (20.4) | 24 (22.9) | 0.740 |
| Employment | 47 (22.1) | 19 (17.6) | 28 (26.7) | 0.137 |
| Lead position | 0.876 | |||
| Cervical | 55 (25.8) | 27 (25.0) | 28 (26.7) | |
| Thoracolumbar | 158 (74.2) | 81 (75.0) | 77 (73.3) | |
| Number of leads | 0.463 | |||
| 1 | 147 (69.0) | 72 (66.7) | 75 (71.4) | |
| 2 | 66 (31.0) | 36 (33.3) | 30 (28.6) | |
| MEDD (mg/day) at baseline | 38.80 ± 55.61 | 34.45 ± 54.66 | 43.28 ± 56.47 | 0.248 |
| Preoperative NRS | 8.00 (8.00–10.00) | 8.00 (8.00–10.00) | 8.00 (7.00–10.00) | 0.384 |
| Patient satisfaction | 122 (57.8) | 85 (80.2) | 37 (35.2) | <0.001 |
| Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age | 1.002 (0.986, 1.017) | 0.845 | 1.943 (1.096, 3.444) | 0.023 |
| Male | 1.733 (1.003, 2.993) | 0.049 | ||
| BMI | 0.987 (0.926, 1.051) | 0.675 | ||
| Litigation | 0.918 (0.529, 1.592) | 0.761 | ||
| Pain duration | 0.984 (0.972, 0.996) | 0.010 | 0.983 (0.971, 0.996) | 0.011 |
| Employment | 0.587 (0.304, 1.133) | 0.112 | 0.507 (0.254, 1.013) | 0.054 |
| Diagnosis | ||||
| CRPS type 1 | 0.873 (0.509, 1.496) | 0.620 | ||
| CRPS type 2 | 1.486 (0.655, 3.373) | 0.343 | ||
| PSPS | 0.907 (0.453, 1.817) | 0.784 | ||
| Others | 1.039 (0.495, 2.184) | 0.919 | ||
| Lead position | ||||
| Cervical | 0.917 (0.496, 1.694) | 0.781 | ||
| Thoracolumbar | 1.091 (0.590, 2.015) | 0.781 | ||
| Psychiatric comorbidity | 1.156 (0.670, 1.993) | 0.603 | ||
| Opioid use | 0.698 (0.388, 1.256) | 0.230 | ||
| Baseline NRS pain score (0–10) | 1.129 (0.908, 1.405) | 0.275 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, Y.; Roh, H.; Moon, J.Y.; Choi, E.J.; Nahm, F.S.; Lee, P.B. Predictive Factors of Successful Spinal Cord Stimulation in Patients with Chronic Pain: A Retrospective Cohort Study. Brain Sci. 2025, 15, 614. https://doi.org/10.3390/brainsci15060614
Yoo Y, Roh H, Moon JY, Choi EJ, Nahm FS, Lee PB. Predictive Factors of Successful Spinal Cord Stimulation in Patients with Chronic Pain: A Retrospective Cohort Study. Brain Sciences. 2025; 15(6):614. https://doi.org/10.3390/brainsci15060614
Chicago/Turabian StyleYoo, Yongjae, Hyungsang Roh, Jee Youn Moon, Eun Joo Choi, Francis Sahngun Nahm, and Pyung Bok Lee. 2025. "Predictive Factors of Successful Spinal Cord Stimulation in Patients with Chronic Pain: A Retrospective Cohort Study" Brain Sciences 15, no. 6: 614. https://doi.org/10.3390/brainsci15060614
APA StyleYoo, Y., Roh, H., Moon, J. Y., Choi, E. J., Nahm, F. S., & Lee, P. B. (2025). Predictive Factors of Successful Spinal Cord Stimulation in Patients with Chronic Pain: A Retrospective Cohort Study. Brain Sciences, 15(6), 614. https://doi.org/10.3390/brainsci15060614







