Pathway to Regulatory Approval of Digital Health Technologies in Progressive Supranuclear Palsy: A Scoping Review
Abstract
1. Introduction
1.1. Assessing Validity of Digital Health Technologies
1.2. Process to Obtain Regulatory Approval for DHTs in Other Neurologic Diseases
1.3. Objective of This Scoping Review
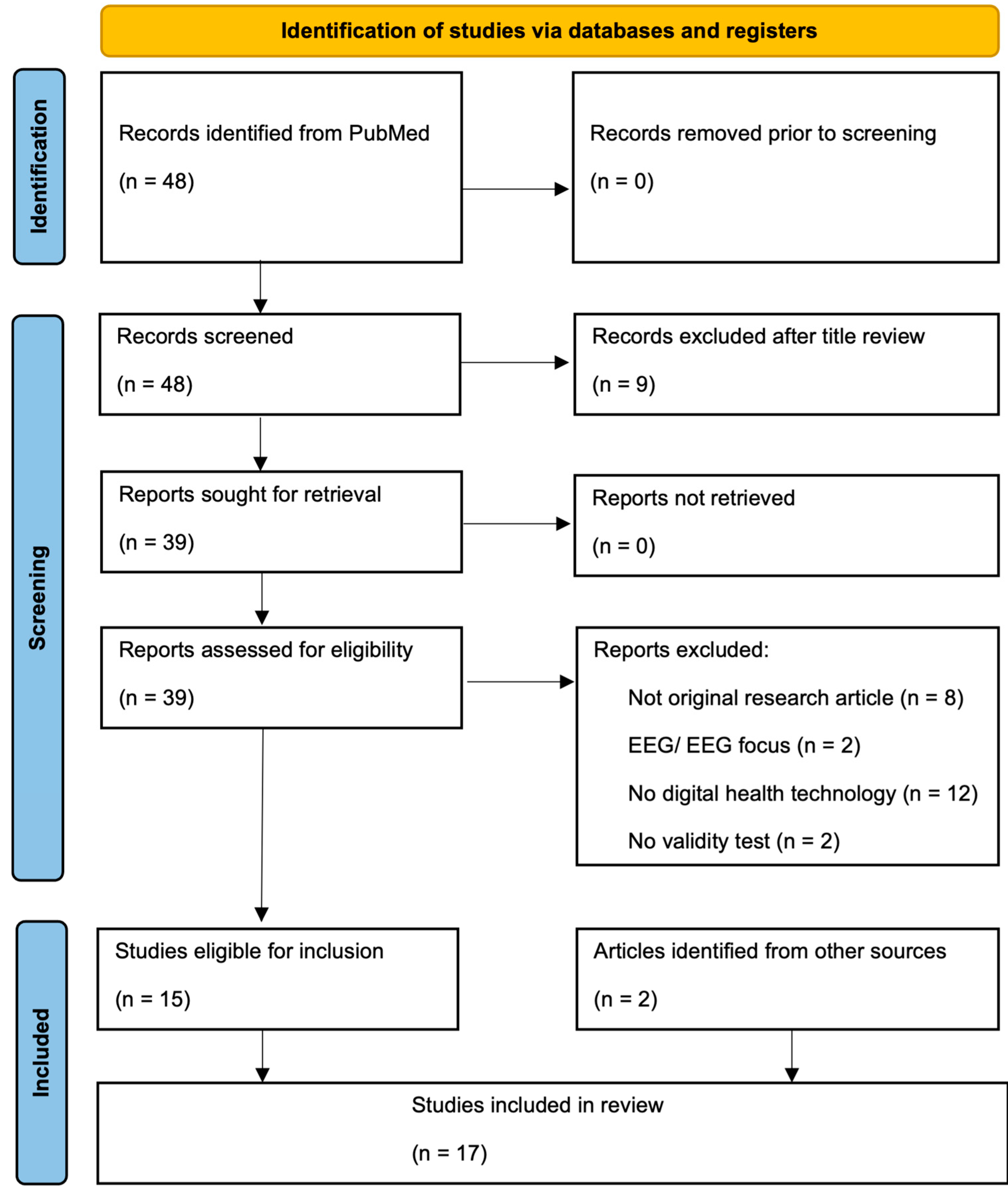
2. Materials and Methods
3. Results
| Study/ Year | Sensor | Country | Type of Participants (n) | Measurement Property Assessed | Outcomes |
|---|---|---|---|---|---|
| Lower Extremity Function/Gait | |||||
| Klenk et al., 2016 [31] | activPAL3 accelerometers (PAL Technologies Ltd., Glasgow, UK) | Germany | DA (34), PSP (15), PD (16), HC (38) | Discriminant validity | Significant decrease in average daily number of walking bouts and number of sit-to-stand transfers per day in PSP group compared to HC [31]. |
| Raccagni et al., 2018 [33] | SHIMMER 2 Sensors (Shimmer, Dublin, Ireland) | Austria, Germany | PD (25), MSA (13), PSP (12) | Construct validity | Significant correlation between total PSPRS score and sensor-measured stride length (SCC 0.682, p-value 0.021) [33]. |
| Gassner et al., 2019 [32] | SHIMMER 2 Sensors (Shimmer, Dublin, Ireland) | Austria, Germany | PD (40), APD 20 (MSA-p (11), PSP (9) | Discriminant validity | Statistically significant difference between PD and APD groups in sensor-based calculation of stride length, gait velocity, and toe-off angle [32]. |
| De Vos et al., 2020 [24] | Opal IMU Sensors (APDM, Portland, OR, USA) | UK | PSP (21) PD (20), HC (39) | Discriminant validity | Comparison of data from two-minute walk, static sway test, and timed up-and-go task using the Random Forest machine learning algorithm resulted in discrimination of PSP from PD with 86% sensitivity and 90% specificity, and PSP from HC with 90% sensitivity and 97% specificity [24]. |
| Sotirakis et al., 2022 [25] | Opal IMU Sensors (APDM, Portland, OR, USA) | UK | PSP (17) | Responsiveness | Significant longitudinal differences in a linear regression model incorporating sensor-measured mean turn velocity, standard deviation of stride length, and mean toe-off angle with ability to detect statistically significant progression 3 months earlier than clinical scores [25]. |
| Ricciardi et al., 2023 [26] | Opal IMU Sensors (APDM, Portland, OR, USA) | Italy | PSP (15) | Content validity | Sensor compared against optoelectronic measurement of gait showing concordance in gait speed (slope of Passing–Bablok regression line of 1.02 and intercept of 0.05), but systematic error in measurement of cadence and cycle duration [26]. |
| Abate et al., 2023 [21] | Opal IMU Sensors (APDM, Portland, OR, USA) | Italy | PSP (35) | Construct validity | Inverse correlation between PSPRS total score and sensor-measured gait speed (r = −0.434; p < 0.001) and turning velocity (r = −0.579; p < 0.001) in 2 min walk test. Positive correlation between PSPRS total score and sensor-measured turn duration (r = 0.411; p < 0.001) in 2 min walk test [21]. |
| Responsiveness | Significant change in sensor measured gait cadence and cycle duration during 2 min walk test over 3 month follow-up [21]. | ||||
| Sharma et al., 2023 [27] | LEGSys+ Sensors BioSensics LLC, Newton, MA, USA) | USA | PSP (11), PD (12) | Construct validity | Correlation between virtually administered PSPRS score and sensor-measured Sit-to-Stand Transition time in Timed Up and Go test (SCC 0.84, uncorrected p-value 0.005) [27]. |
| Balance/Falls | |||||
| Baston et al., 2014 [38] | Opal IMU Sensors (APDM, Portland, OR, USA) | USA | PD (5), PSP (7) | Discriminant validity | PD and PSP subjects showed a predominant ankle strategy, unlike the HC group, but PSP subjects were not able to reduce sway area resulting in several falls for PSP group [38]. |
| Dale et al., 2017 [34] | Balance Master Clinical Research System (NeuroCom International, Clarckamas, OR, USA), Opal IMU Sensors (APDM, Portland, OR, USA) | USA | PSP (12), PD (12), HC (12) | Discriminant validity | Individuals with PSP were less able than PD or HC counterparts to perceive toes-up platform tilts and exhibited fewer corrective motor responses in reaction to forward platform translations and toes-up surface tilts [34]. |
| Srulijes et al., 2019 [35] | activPAL accelerometers (PAL Technologies Ltd., Glasgow, UK) | Germany | DA (31), PSP (12), PD (14), HC (31) | Discriminant validity | Physical activity measured via accelerometer was compared to recorded fall incidence. The PSP group with high walking bout length showed a significantly higher fall incidence of 45.3 falls/person years compared to the low-activity group 12.5 falls/person years [35]. |
| Upper Extremity Function | |||||
| Djurić-Jovičić et al., 2016 [36] | IMSUs from the University of Belgrade, Serbia (STMicroelectronics, Geneva, Switzerland) | Serbia | PD (13), PSP (15), MSA (14), HC (14) | Construct validity | Significant correlation between sensor-measured amplitude (ρ = −0.73; p = 0.007) and speed slope (ρ = −0.69; p = 0.012) of finger tapping as compared to the FAB total score [36]. |
| Bobić et al., 2019 [39] | IMSUs from the University of Belgrade, Serbia (STMicroelectronics, Geneva, Switzerland) | Serbia | PD (13), MSA (17), PSP (14), HC (12) | Content validity | Sensor-generated measure of bradykinesia (overall score computed by algorithm) was directly compared to movement disorder physicians’ rating of bradykinesia with an overall accuracy of 83.76 ± 7.86% in individuals with PSP [39]. |
| Belić et al., 2023 [28] | IMSUs from the University of Belgrade, Serbia (STMicroelectronics, Geneva, Switzerland) | Serbia | PD (14), PSP (16), MSA (13), HC (11) | Discriminant validity | Using sensors to evaluate finger tapping data resulted in correct identification of 11 of the 16 individuals with PSP (2 individuals with PSP incorrectly identified as MSA, and 3 as HC) [28]. |
| Speech | |||||
| Parjane et al., 2021 [23] | Speech Activity Detector (University of Pennsylvania Linguistic Data Consortium, Philadelphia, PA, USA) | USA | PSPS-CBS (87), naPPA (25), HC (41) | Construct validity | Longer pause segment duration and lower speech rate correlated with phonemic fluency score in PSPS-CBS; however, no correlation between sensor outcomes and other standard neuropsychological assessment [23]. |
| Discriminant validity | PSPS-CBS had statistically significant shorter speech segments, longer pause segments, higher pause rate, and reduced f0 range compared to HC [23]. | ||||
| Kang et al., 2023 [29] | BioDigit Speech Sensor (BioSensics LLC, Newton, MA, USA) | USA | PSP (11), PD (10) | Construct validity | Negative correlation between PSPRS dysphagia score and sensor-derived similarity dynamic time warping in rainbow passage reading (r 0.78, p 0.007). Positive correlation between PSPRS dysphagia score and sensor-derived ratio of extra words (r 0.82, p 0.004) and ratio of missing words (r 0.78, p 0.007) in rainbow passage reading. Positive correlation between PSPRS dysarthria and bulbar scores and sensor-derived articulation rate in reverse number count assessment [29]. |
| Tröger et al., 2024 [22] | ki: SB-M intelligibility score (ki:elements, Saarbrücken, Germany) | Czech Republic, Columbia, Germany | Czech: HD (39), PD (43), ALS (16), PSP (17), HC (46); Colombian: HC (50), PD (50); German PD (98) | Reliability | Comparison of two different automatic speech recognition systems as basis for SB-M intelligibility score resulted in ICC of 0.841 [22]. |
| Construct validity | Non-statistically significant correlation (suspected to be due to small sample size) in ki: SB-M intelligibility score and NNIPPS (r = −0.42, p < 0.10, d = 0.92) [22]. | ||||
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DHT | Digital health technologies |
| PSP | Progressive Supranuclear Palsy |
| PSPRS | Progressive Supranuclear Palsy Rating Scale |
| PD | Parkinson’s disease |
| DA | Degenerative ataxia |
| ALS | Amyotrophic Lateral Sclerosis |
| HD | Huntington’s disease |
| MSA | Multiple system atrophy |
| naPPA | Non-fluent/agrammatic primary progressive aphasia |
| CBS | Corticobasal syndrome |
| HC | Healthy controls |
| DMD | Duchenne muscular dystrophy |
| EMA | European Medicines Agency |
| FDA | Food and Drug Administration |
| FAB | Frontal Assessment Battery (FAB) |
| IMU | Inertial Measurement Unit |
| IMSUs | Inertial Measurement Sensor Units |
| LCC | Lin’s concordance correlation coefficient |
| PCC | Pearson correlation coefficient |
| SCC | Spearman’s correlation coefficient |
| ICC | Intraclass correlation coefficient |
| SB-M | Speech biomarker score for motor speech disorders (SB-M) |
| NNIPPS | Natural History and Neuroprotection in Parkinson Plus Syndromes—Parkinson Plus Scale |
References
- Boxer, A.L.; Yu, J.-T.; Golbe, L.I.; Litvan, I.; Lang, A.E.; Höglinger, G.U. New Diagnostics and Therapeutics for Progressive Supranuclear Palsy. Lancet Neurol. 2017, 16, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Giagkou, N.; Höglinger, G.U.; Stamelou, M. Chapter Three—Progressive Supranuclear Palsy. In International Review of Neurobiology; Stamelou, M., Höglinger, G.U., Eds.; Parkinsonism Beyond Parkinson’s Disease; Academic Press: Cambridge, MA, USA, 2019; Volume 149, pp. 49–86. [Google Scholar]
- Roemer, S.F.; Grinberg, L.T.; Crary, J.F.; Seeley, W.W.; McKee, A.C.; Kovacs, G.G.; Beach, T.G.; Duyckaerts, C.; Ferrer, I.A.; Gelpi, E.; et al. Rainwater Charitable Foundation Criteria for the Neuropathologic Diagnosis of Progressive Supranuclear Palsy. Acta Neuropathol. 2022, 144, 603. [Google Scholar] [CrossRef]
- Golbe, L.I.; Ohman-Strickland, P.A. A Clinical Rating Scale for Progressive Supranuclear Palsy. Brain 2007, 130, 1552–1565. [Google Scholar] [CrossRef]
- Wills, A.-M.; Pantelyat, A.; Espay, A.; Chan, J.; Litvan, I.; Xie, T.; Dale, M.L.; Gunzler, S.A.; Tartaglia, M.C.; Fox, S.H.; et al. A Modified Progressive Supranuclear Palsy Rating Scale for Virtual Assessments. Mov. Disord. 2022, 37, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Wills, A.; Golbe, L.I.; Lang, A.E.; Xie, T.; Dale, M.L.; Espay, A.; Tartaglia, M.C.; Fox, S.H.; Parashos, S.A.; McFarland, N.R.; et al. Recommendations for Virtual Administration of the PSP Rating Scale. Mov. Disord. 2022, 37, 1960–1961. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services: Food and Drug Administration. Digital Health Technologies for Remote Data Acquisition in Clinical Investigations: Guidance for Industry, Investigators, and Other Stakeholders. 2023. Available online: https://www.fda.gov/media/155022/download (accessed on 17 February 2025).
- Marra, C.; Chen, J.L.; Coravos, A.; Stern, A.D. Quantifying the Use of Connected Digital Products in Clinical Research. NPJ Digit. Med. 2020, 3, 50. [Google Scholar] [CrossRef] [PubMed]
- Espay, A.J.; Hausdorff, J.M.; Sánchez-Ferro, Á.; Klucken, J.; Merola, A.; Bonato, P.; Paul, S.S.; Horak, F.B.; Vizcarra, J.A.; Mestre, T.A.; et al. A Roadmap for Implementation of Patient-Centered Digital Outcome Measures in Parkinson’s Disease Obtained Using Mobile Health Technologies. Mov. Disord. 2019, 34, 657–663. [Google Scholar] [CrossRef]
- Toosizadeh, N.; Mohler, J.; Lei, H.; Parvaneh, S.; Sherman, S.; Najafi, B. Motor Performance Assessment in Parkinson’s Disease: Association between Objective In-Clinic, Objective In-Home, and Subjective/Semi-Objective Measures. PLoS ONE 2015, 10, e0124763. [Google Scholar] [CrossRef]
- Espay, A.J.; Bonato, P.; Nahab, F.B.; Maetzler, W.; Dean, J.M.; Klucken, J.; Eskofier, B.M.; Merola, A.; Horak, F.; Lang, A.E.; et al. Technology in Parkinson’s Disease: Challenges and Opportunities. Mov. Disord. 2016, 31, 1272–1282. [Google Scholar] [CrossRef]
- Awad, A.; Trenfield, S.J.; Pollard, T.D.; Ong, J.J.; Elbadawi, M.; McCoubrey, L.E.; Goyanes, A.; Gaisford, S.; Basit, A.W. Connected Healthcare: Improving Patient Care Using Digital Health Technologies. Adv. Drug Deliv. Rev. 2021, 178, 113958. [Google Scholar] [CrossRef]
- COSMIN Taxonomy of Measurement Properties. Available online: https://www.cosmin.nl/tools/cosmin-taxonomy-measurement-properties/ (accessed on 1 March 2025).
- Del Din, S.; Kirk, C.; Yarnall, A.J.; Rochester, L.; Hausdorff, J.M. Body-Worn Sensors for Remote Monitoring of Parkinson’s Disease Motor Symptoms: Vision, State of the Art, and Challenges Ahead. J. Park. Dis. 2021, 11, S35–S47. [Google Scholar] [CrossRef]
- Ilg, W.; Milne, S.; Schmitz-Hübsch, T.; Alcock, L.; Beichert, L.; Bertini, E.; Mohamed Ibrahim, N.; Dawes, H.; Gomez, C.M.; Hanagasi, H.; et al. Quantitative Gait and Balance Outcomes for Ataxia Trials: Consensus Recommendations by the Ataxia Global Initiative Working Group on Digital-Motor Biomarkers. Cerebellum 2023, 23, 1566–1592. [Google Scholar] [CrossRef] [PubMed]
- Mitsi, G.; Mendoza, E.U.; Wissel, B.D.; Barbopoulou, E.; Dwivedi, A.K.; Tsoulos, I.; Stavrakoudis, A.; Espay, A.J.; Papapetropoulos, S. Biometric Digital Health Technology for Measuring Motor Function in Parkinson’s Disease: Results from a Feasibility and Patient Satisfaction Study. Front. Neurol. 2017, 8, 273. [Google Scholar] [CrossRef] [PubMed]
- Servais, L.; Camino, E.; Clement, A.; McDonald, C.M.; Lukawy, J.; Lowes, L.P.; Eggenspieler, D.; Cerreta, F.; Strijbos, P. First Regulatory Qualification of a Novel Digital Endpoint in Duchenne Muscular Dystrophy: A Multi-Stakeholder Perspective on the Impact for Patients and for Drug Development in Neuromuscular Diseases. Digit. Biomark. 2021, 5, 183–190. [Google Scholar] [CrossRef]
- European Medicines Agency Committee for Medicinal Products for Human Use. Qualification Opinion for Stride Velocity 95th Centile as Primary Endpoint in Studies in Ambulatory Duchenne Muscular Dystrophy Studies. 2023. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/qualification-opinion-stride-velocity-95th-centile-primary-endpoint-studies-ambulatory-duchenne-muscular-dystrophy-studies_en.pdf (accessed on 2 February 2025).
- FDA Center for Devices and Radiological Health (CDRH). Medical Device Safety and the 510(k) Clearance Process. 2024. Available online: https://www.fda.gov/medical-devices/510k-clearances/medical-device-safety-and-510k-clearance-process (accessed on 2 February 2025).
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Abate, F.; Russo, M.; Ricciardi, C.; Tepedino, M.F.; Romano, M.; Erro, R.; Pellecchia, M.T.; Amboni, M.; Barone, P.; Picillo, M. Wearable Sensors for Assessing Disease Severity and Progression in Progressive Supranuclear Palsy. Park. Relat. Disord. 2023, 109, 105345. [Google Scholar] [CrossRef]
- Tröger, J.; Dörr, F.; Schwed, L.; Linz, N.; König, A.; Thies, T.; Barbe, M.T.; Orozco-Arroyave, J.R.; Rusz, J. An Automatic Measure for Speech Intelligibility in Dysarthrias—Validation across Multiple Languages and Neurological Disorders. Front. Digit. Health 2024, 6, 1440986. [Google Scholar] [CrossRef]
- Parjane, N.; Cho, S.; Ash, S.; Cousins, K.A.Q.; Shellikeri, S.; Liberman, M.; Shaw, L.M.; Irwin, D.J.; Grossman, M.; Nevler, N. Digital Speech Analysis in Progressive Supranuclear Palsy and Corticobasal Syndromes. J. Alzheimers Dis. 2021, 82, 33–45. [Google Scholar] [CrossRef]
- De Vos, M.; Prince, J.; Buchanan, T.; FitzGerald, J.J.; Antoniades, C.A. Discriminating Progressive Supranuclear Palsy from Parkinson’s Disease Using Wearable Technology and Machine Learning. Gait Posture 2020, 77, 257–263. [Google Scholar] [CrossRef]
- Sotirakis, C.; Conway, N.; Su, Z.; Villarroel, M.; Tarassenko, L.; FitzGerald, J.J.; Antoniades, C.A. Longitudinal Monitoring of Progressive Supranuclear Palsy Using Body-Worn Movement Sensors. Mov. Disord. 2022, 37, 2263–2271. [Google Scholar] [CrossRef]
- Ricciardi, C.; Pisani, N.; Donisi, L.; Abate, F.; Amboni, M.; Barone, P.; Picillo, M.; Cesarelli, M.; Amato, F. Agreement between Optoelectronic System and Wearable Sensors for the Evaluation of Gait Spatiotemporal Parameters in Progressive Supranuclear Palsy. Sensors 2023, 23, 9859. [Google Scholar] [CrossRef]
- Sharma, M.; Mishra, R.K.; Hall, A.J.; Casado, J.; Cole, R.; Nunes, A.S.; Barchard, G.; Vaziri, A.; Pantelyat, A.; Wills, A.-M. Remote At-Home Wearable-Based Gait Assessments in Progressive Supranuclear Palsy Compared to Parkinson’s Disease. BMC Neurol. 2023, 23, 434. [Google Scholar] [CrossRef] [PubMed]
- Belić, M.; Radivojević, Z.; Bobić, V.; Kostić, V.; Đurić-Jovičić, M. Quick Computer Aided Differential Diagnostics Based on Repetitive Finger Tapping in Parkinson’s Disease and Atypical Parkinsonisms. Heliyon 2023, 9, e14824. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Nunes, A.S.; Sharma, M.; Hall, A.J.; Mishra, R.K.; Casado, J.; Cole, R.; Derhammer, M.; Barchard, G.; Najafi, B.; et al. Utilizing Speech Analysis to Differentiate Progressive Supranuclear Palsy from Parkinson’s Disease. Park. Relat. Disord. 2023, 115, 105835. [Google Scholar] [CrossRef]
- Hoglinger, G.U.; Respondek, G.; Stamelou, M.; Kurz, C.; Josephs, K.A.; Lang, A.E.; Mollenhauer, B.; Muller, U.; Nilsson, C.; Whitwell, J.L.; et al. Clinical Diagnosis of Progressive Supranuclear Palsy: The Movement Disorder Society Criteria. Mov. Disord. 2017, 32, 853–864. [Google Scholar] [CrossRef]
- Klenk, J.; Srulijes, K.; Schatton, C.; Schwickert, L.; Maetzler, W.; Becker, C.; Synofzik, M. Ambulatory Activity Components Deteriorate Differently across Neurodegenerative Diseases: A Cross-Sectional Sensor-Based Study. Neurodegener. Dis. 2016, 16, 317–323. [Google Scholar] [CrossRef]
- Gassner, H.; Raccagni, C.; Eskofier, B.M.; Klucken, J.; Wenning, G.K. The Diagnostic Scope of Sensor-Based Gait Analysis in Atypical Parkinsonism: Further Observations. Front. Neurol. 2019, 10, 5. [Google Scholar] [CrossRef]
- Raccagni, C.; Gaßner, H.; Eschlboeck, S.; Boesch, S.; Krismer, F.; Seppi, K.; Poewe, W.; Eskofier, B.M.; Winkler, J.; Wenning, G.; et al. Sensor-Based Gait Analysis in Atypical Parkinsonian Disorders. Brain Behav. 2018, 8, e00977. [Google Scholar] [CrossRef]
- Dale, M.L.; Horak, F.B.; Wright, W.G.; Schoneburg, B.M.; Nutt, J.G.; Mancini, M. Impaired Perception of Surface Tilt in Progressive Supranuclear Palsy. PLoS ONE 2017, 12, e0173351. [Google Scholar] [CrossRef]
- Srulijes, K.; Klenk, J.; Schwenk, M.; Schatton, C.; Schwickert, L.; Teubner-Liepert, K.; Meyer, M.; Srijana, K.C.; Maetzler, W.; Becker, C.; et al. Fall Risk in Relation to Individual Physical Activity Exposure in Patients with Different Neurodegenerative Diseases: A Pilot Study. Cerebellum 2019, 18, 340–348. [Google Scholar] [CrossRef]
- Djurić-Jovičić, M.; Petrović, I.; Ječmenica-Lukić, M.; Radovanović, S.; Dragašević-Mišković, N.; Belić, M.; Miler-Jerković, V.; Popović, M.B.; Kostić, V.S. Finger Tapping Analysis in Patients with Parkinson’s Disease and Atypical Parkinsonism. J. Clin. Neurosci. 2016, 30, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Litvan, I.; Agid, Y.; Calne, D.; Campbell, G.; Dubois, B.; Duvoisin, R.C.; Goetz, C.; Golbe, L.I.; Grafman, J.; Growdon, J.H.; et al. Clinical Research Criteria for the Diagnosis of Progressive Supranuclear Palsy (Steele-Richardson-Olszewski Syndrome): Report of the NINDS-SPSP International Workshop. Neurology 1996, 47, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Baston, C.; Mancini, M.; Schoneburg, B.; Horak, F.; Rocchi, L. Postural Strategies Assessed with Inertial Sensors in Healthy and Parkinsonian Subjects. Gait Posture 2014, 40, 70–75. [Google Scholar] [CrossRef]
- Bobić, V.; Djurić-Jovičić, M.; Dragašević, N.; Popović, M.B.; Kostić, V.S.; Kvaščev, G. An Expert System for Quantification of Bradykinesia Based on Wearable Inertial Sensors. Sensors 2019, 19, 2644. [Google Scholar] [CrossRef]
- Kluge, F.; Gaßner, H.; Hannink, J.; Pasluosta, C.; Klucken, J.; Eskofier, B.M. Towards Mobile Gait Analysis: Concurrent Validity and Test-Retest Reliability of an Inertial Measurement System for the Assessment of Spatio-Temporal Gait Parameters. Sensors 2017, 17, 1522. [Google Scholar] [CrossRef]
- Homes, R.; Clark, D.; Moridzadeh, S.; Tosovic, D.; Van den Hoorn, W.; Tucker, K.; Midwinter, M. Comparison of a Wearable Accelerometer/Gyroscopic, Portable Gait Analysis System (LEGSYS+TM) to the Laboratory Standard of Static Motion Capture Camera Analysis. Sensors 2023, 23, 537. [Google Scholar] [CrossRef]
- Ohara, M.; Hattori, T.; Chen, Q.; Shimano, K.; Hirata, K.; Matsui, M.; Yokota, T. Is There a Spinal Tap Responder in Progressive Supranuclear Palsy? The First Prospective Study. J. Neurol. 2024, 271, 4473–4484. [Google Scholar] [CrossRef]
- Krzosek, P.; Madetko, N.; Migda, A.; Migda, B.; Jaguś, D.; Alster, P. Differential Diagnosis of Rare Subtypes of Progressive Supranuclear Palsy and PSP-Like Syndromes—Infrequent Manifestations of the Most Common Form of Atypical Parkinsonism. Front. Aging Neurosci. 2022, 14, 804385. [Google Scholar] [CrossRef]
- Inan, O.T.; Tenaerts, P.; Prindiville, S.A.; Reynolds, H.R.; Dizon, D.S.; Cooper-Arnold, K.; Turakhia, M.; Pletcher, M.J.; Preston, K.L.; Krumholz, H.M.; et al. Digitizing Clinical Trials. NPJ Digit. Med. 2020, 3, 101. [Google Scholar] [CrossRef]
| Limitations | Opportunities |
|---|---|
| DHTs often capture a single disease metric, not necessarily a global picture of an individual’s disease [9]. | DHTs provide more continuous, objective and reliable measures in comparison with ordinal rating scales, as has been shown in PD [10,11]. |
| DHTs characterize motor dysfunction but often neglect non-motor aspects of an individual’s disease [9]. | DHTs allow for remote data collection and expand access to individuals who may be geographically or economically separated from academic medical centers [12]. |
| DHTs produce excessive data requiring the user to sort through acquired data [9]. | DHTs may provide more ecologically valid assessments by measuring function in a low-stress, home environment as compared to hospital-based evaluations [12]. |
| Use and adoption of DHTs may be limited by technological literacy. | Improved healthcare accessibility via DHT use leads to inclusivity and enrollment of cohorts more representative of the general population [12]. |
| Term 1 | Term 2 | |
|---|---|---|
| progressive supranuclear palsy | AND | digital health technology |
| digital technology | ||
| digital health | ||
| DHT | ||
| sensor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isroff, C.; Kang, K.; Espay, A.J.; Dale, M.L.; Pantelyat, A.; Wills, A.-M.; Antoniades, C.A. Pathway to Regulatory Approval of Digital Health Technologies in Progressive Supranuclear Palsy: A Scoping Review. Brain Sci. 2025, 15, 587. https://doi.org/10.3390/brainsci15060587
Isroff C, Kang K, Espay AJ, Dale ML, Pantelyat A, Wills A-M, Antoniades CA. Pathway to Regulatory Approval of Digital Health Technologies in Progressive Supranuclear Palsy: A Scoping Review. Brain Sciences. 2025; 15(6):587. https://doi.org/10.3390/brainsci15060587
Chicago/Turabian StyleIsroff, Catherine, Kyurim Kang, Alberto J. Espay, Marian L. Dale, Alexander Pantelyat, Anne-Marie Wills, and Chrystalina A. Antoniades. 2025. "Pathway to Regulatory Approval of Digital Health Technologies in Progressive Supranuclear Palsy: A Scoping Review" Brain Sciences 15, no. 6: 587. https://doi.org/10.3390/brainsci15060587
APA StyleIsroff, C., Kang, K., Espay, A. J., Dale, M. L., Pantelyat, A., Wills, A.-M., & Antoniades, C. A. (2025). Pathway to Regulatory Approval of Digital Health Technologies in Progressive Supranuclear Palsy: A Scoping Review. Brain Sciences, 15(6), 587. https://doi.org/10.3390/brainsci15060587







