Spreading New Light on Attention Restoration Theory: An Environmental Posner Paradigm
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Size and Power Analysis
2.2. Participants
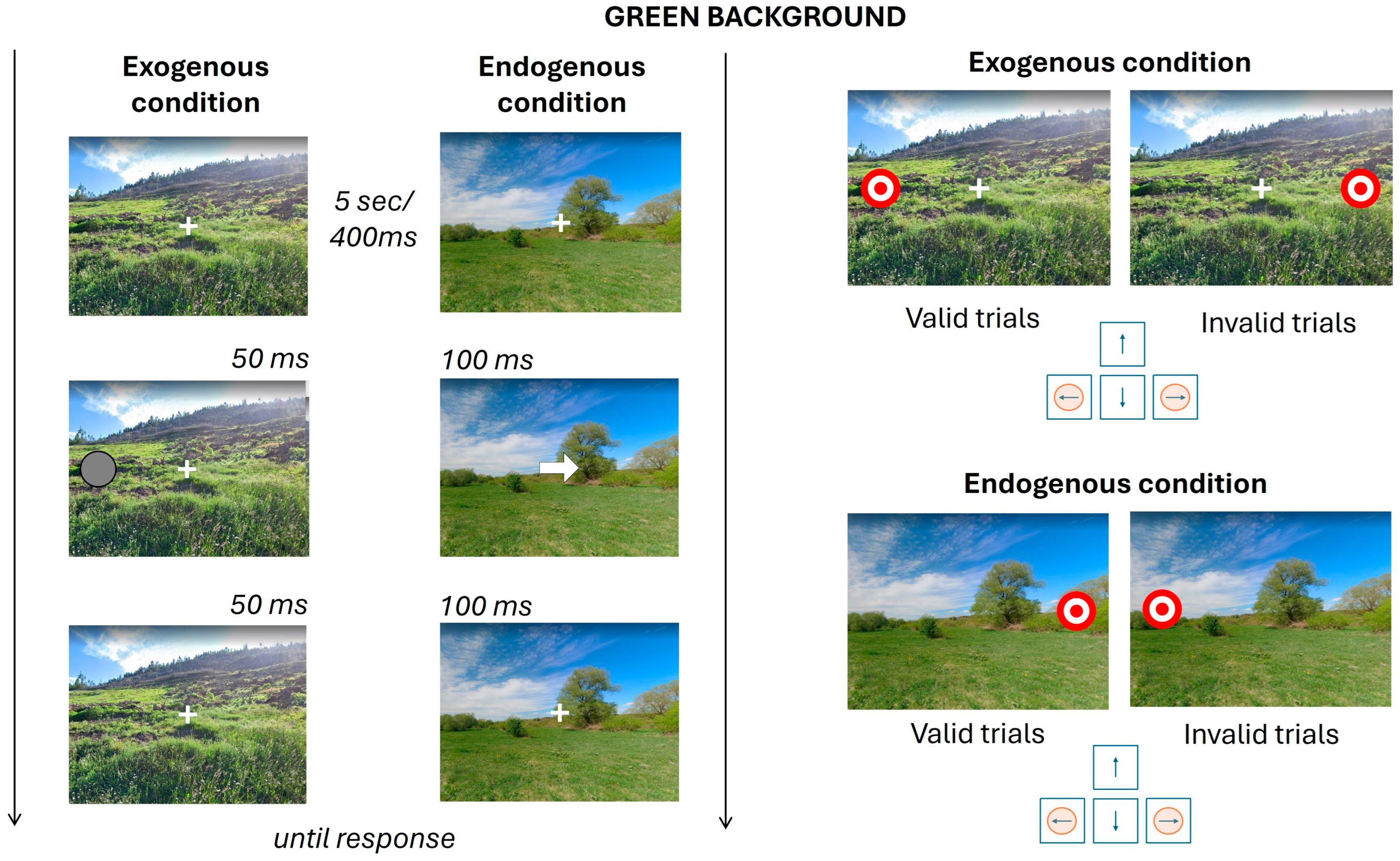
2.3. Experimental Procedure
2.3.1. Endogenous Condition
2.3.2. Exogenous Condition
2.4. Electroencephalography
2.5. Behavioral Data Analysis
2.6. Electrophysiological Analysis
3. Results
3.1. Behavioral Results
3.2. Electrophysiological Results
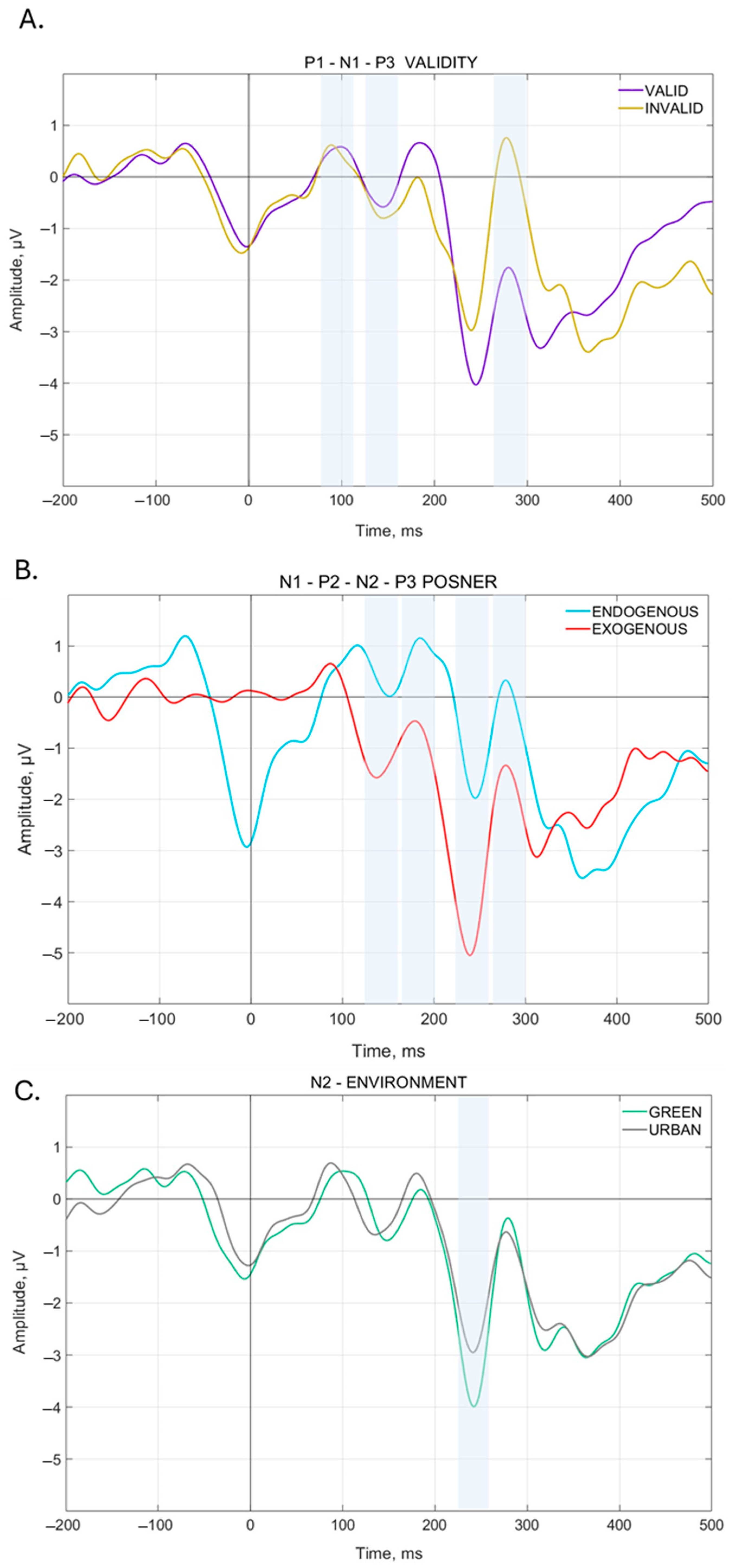
3.2.1. VEP Analysis for Validity, PCond, and Environment
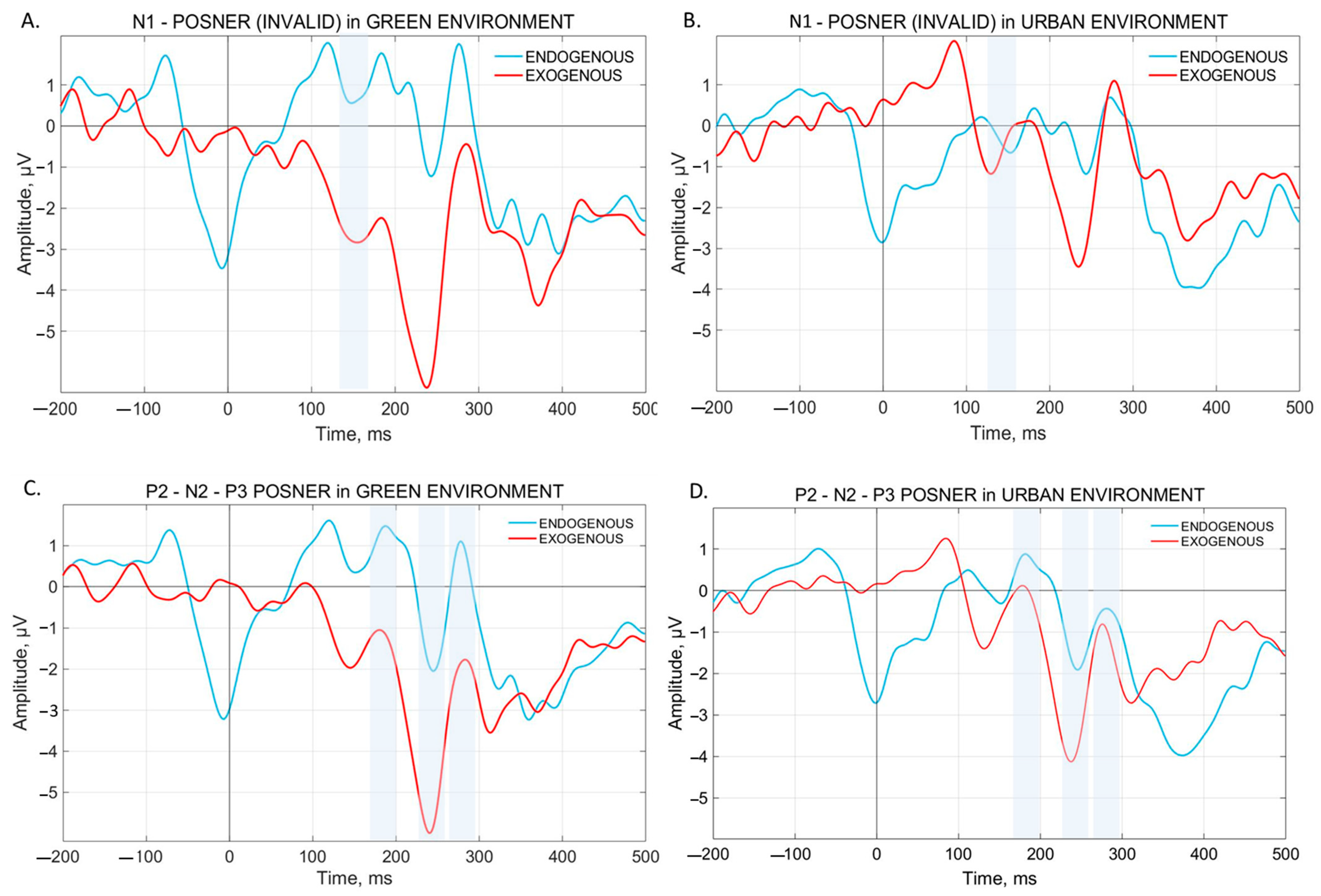
3.2.2. N1 Component
3.2.3. P1 Component
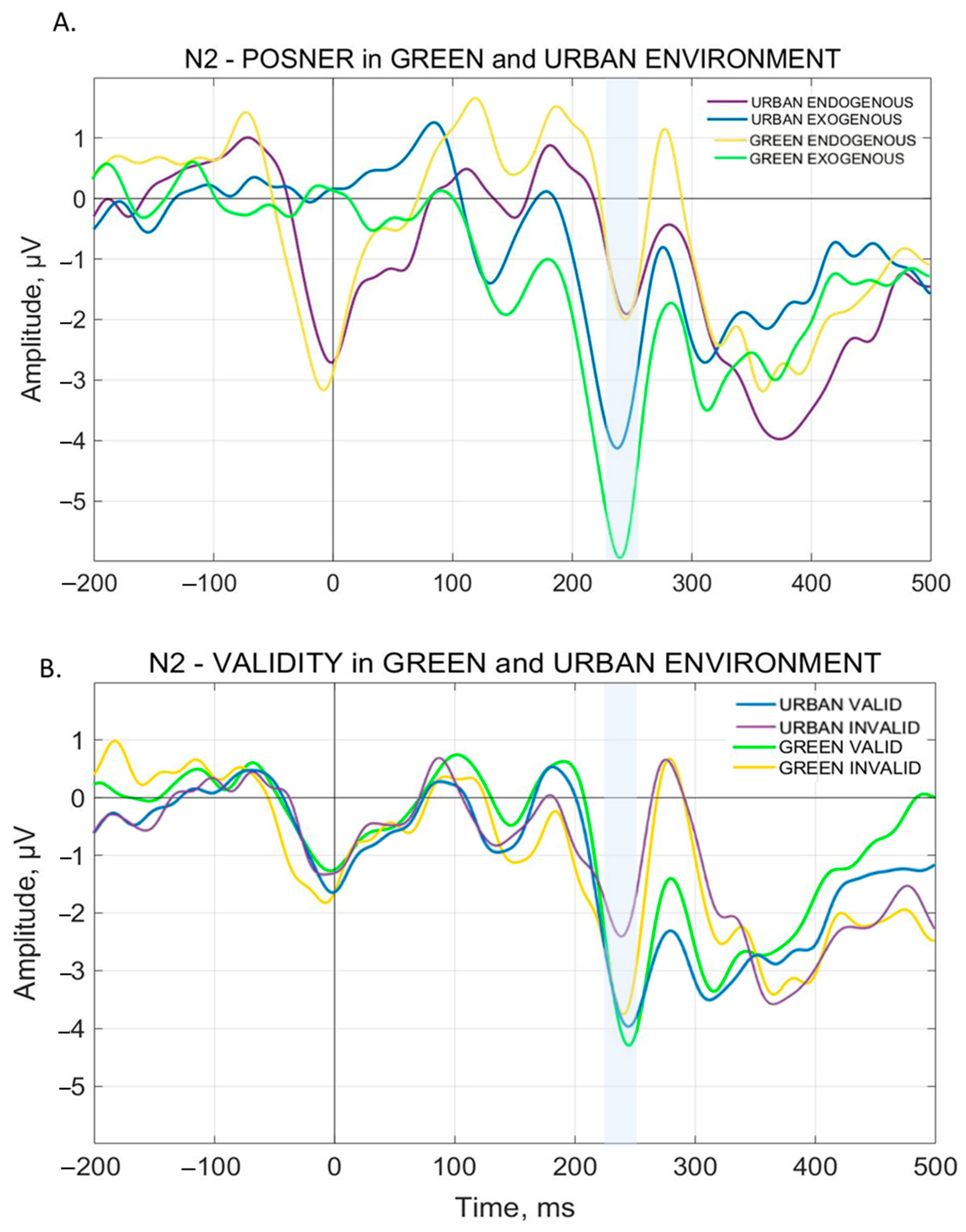
3.2.4. N2 Component
3.2.5. P2 Component
3.2.6. P3 Component
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berman, M.G.; Kardan, O.; Kotabe, H.P.; Nusbaum, H.C.; London, S.E. The promise of environmental neuroscience. Nat. Hum. Behav. 2019, 3, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, H.; Samborska, V.; Roser, M. Urbanization; Our World in Data: Oxford, UK, 2024. [Google Scholar]
- Unidas, O.D.N. Department of Economic and Social Affairs, Population Division (2019). In World Population Prospects 2019: Highlights; United Nations: New York, NY, USA, 2019; ST/ESA/SER.A/423. [Google Scholar]
- Kaplan, S.; Berman, M.G. Directed attention as a common resource for executive functioning and Self-Regulation. Perspect. Psychol. Sci. 2010, 5, 43–57. [Google Scholar] [CrossRef]
- Pol, E. Blueprints for a History of Environmental Psychology (II): From Architectural Psychology to the challenge of sustainability. Medio Ambiente Y Comport. Hum. 2007, 8, 1–28. [Google Scholar]
- Ulrich, R.S.; Simons, R.F.; Losito, B.D.; Fiorito, E.; Miles, M.A.; Zelson, M. Stress recovery during exposure to natural and urban environments. J. Env. Psychol. 1991, 11, 201–230. [Google Scholar] [CrossRef]
- Kaplan, R.; Kaplan, S. The experience of nature: A psychological perspective. In The Experience of Nature: A Psychological Perspective; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]
- Kaplan, S. The restorative benefits of nature: Toward an integrative framework. J. Env. Psychol. 1995, 15, 169–182. [Google Scholar] [CrossRef]
- Berman, M.G.; Jonides, J.; Kaplan, S. The cognitive benefits of interacting with nature. Psychol. Sci. 2008, 19, 1207–1212. [Google Scholar] [CrossRef]
- Trammell, J.P.; Aguilar, S.C. Natural Is Not Always Better: The Varied Effects of a Natural Environment and Exercise on Affect and Cognition. Front. Psychol. 2021, 11, 575245. [Google Scholar] [CrossRef]
- Theeuwes, J. Exogenous and endogenous control of attention: The effect of visual onsets and offsets. Percept. Psychophys. 1991, 49, 83–90. [Google Scholar] [CrossRef]
- Posner, M.I.; Snyder, C.R.; Davidson, B.J. Attention and the detection of signals. J. Exp. Psychol. Gen. 1980, 109, 160. [Google Scholar] [CrossRef]
- Bourrier, S.C.; Berman, M.G.; Enns, J.T. Cognitive strategies and natural environments interact in influencing executive function. Front. Psychol. 2018, 9, 1248. [Google Scholar] [CrossRef]
- Crossan, C.; Salmoni, A. A Simulated Walk in Nature: Testing Predictions From the Attention Restoration Theory. Environ. Behav. 2021, 53, 277–295. [Google Scholar] [CrossRef]
- Gamble, K.R.; Howard, J.H.; Howard, D.V. Not just scenery: Viewing nature pictures improves executive attention in older adults. Exp. Aging Res. 2014, 40, 513–530. [Google Scholar] [CrossRef]
- Gidlow, C.J.; Jones, M.V.; Hurst, G.; Masterson, D.; Clark-Carter, D.; Tarvainen, M.P.; Smith, G.; Nieuwenhuijsen, M. Where to put your best foot forward: Psycho-physiological responses to walking in natural and urban environments. J. Env. Psychol. 2016, 45, 22–29. [Google Scholar] [CrossRef]
- Raanaas, R.K.; Evensen, K.H.; Rich, D.; Sjøstrøm, G.; Patil, G. Benefits of indoor plants on attention capacity in an office setting. J. Environ. Psychol. 2011, 31, 99–105. [Google Scholar] [CrossRef]
- Stenfors, C.U.; Van Hedger, S.C.; Schertz, K.E.; Meyer, F.A.; Smith, K.E.; Norman, G.J.; Bourrier, S.C.; Enns, J.T.; Kardan, O.; Jonides, J.; et al. Positive effects of nature on cognitive performance across multiple experiments: Test order but not affect modulates the cognitive effects. Front. Psychol. 2019, 10, 438004. [Google Scholar] [CrossRef]
- Tennessen, C.M.; Cimprich, B. Views to nature: Effects on attention. J. Environ. Psychol. 1995, 15, 77–85. [Google Scholar] [CrossRef]
- Berto, R. Assessing the restorative value of the environment: A study on the elderly in comparison with young adults and adolescents. Int. J. Psychol. 2007, 42, 331–341. [Google Scholar] [CrossRef]
- Matsuoka, R.H. Student performance and high school landscapes: Examining the links. Landsc. Urban Plan. 2010, 97, 273–282. [Google Scholar] [CrossRef]
- McDonnell, A.S.; Strayer, D.L. Immersion in nature enhances neural indices of executive attention. Sci. Rep. 2024, 14, 1845. [Google Scholar] [CrossRef]
- Menardo, E.; Brondino, M.; Hall, R.; Pasini, M. Restorativeness in Natural and Urban Environments: A Meta-Analysis; National Institutes of Health: Rockville, MA, USA, 2021. [CrossRef]
- Evensen, K.H.; Raanaas, R.K.; Hagerhall, C.M.; Johansson, M.; Patil, G.G. Restorative Elements at the Computer Workstation: A Comparison of Live Plants and Inanimate Objects With and Without Window View. Environ. Behav. 2015, 47, 288–303. [Google Scholar] [CrossRef]
- Chung, K.; Lee, D.; Park, J.Y. Involuntary attention restoration during exposure to mobile-based 360° virtual nature in healthy adults with different levels of restorative experience: Event-related potential study. J. Med. Internet Res. 2018, 20, e11152. [Google Scholar] [CrossRef] [PubMed]
- Grassini, S.; Revonsuo, A.; Castellotti, S.; Petrizzo, I.; Benedetti, V.; Koivisto, M. Processing of natural scenery is associated with lower attentional and cognitive load compared with urban ones. J. Environ. Psychol. 2019, 62, 1–11. [Google Scholar] [CrossRef]
- Mahamane, S.; Wan, N.; Porter, A.; Hancock, A.S.; Campbell, J.; Lyon, T.E.; Jordan, K.E. Natural Categorization: Electrophysiological Responses to Viewing Natural Versus Built Environments. Front. Psychol. 2020, 11, 990. [Google Scholar] [CrossRef]
- Grassini, S. EEG for the Study of Environmental Neuroscience. In Environmental Neuroscience; Springer Nature: Cham, Switzerland, 2024; pp. 547–561. [Google Scholar] [CrossRef]
- Mangun, G.R.; Hillyard, S.A. Modulations of Sensory-Evoked Brain Potentials Indicate Changes in Perceptual Processing During Visual-Spatial Priming. J. Exp. Psychol. Hum. Percept. Perform. 1991, 17, 1057. [Google Scholar] [CrossRef] [PubMed]
- Perchet, C.; Revol, O.; Fourneret, P.; Mauguière, F.; Garcia-Larrea, L. Attention shifts and anticipatory mechanisms in hyperactive children: An ERP study using the Posner paradigm. Biol. Psychiatry. 2001, 50, 44–57. [Google Scholar] [CrossRef]
- Perchet, C.; García-Larrea, L. Visuospatial attention and motor reaction in children: An electrophysiological study of the “Posner” paradigm. Psychophysiology 2000, 37, 231–241. [Google Scholar] [CrossRef]
- Folstein, J.R.; Van Petten, C. Influence of Cognitive Control and Mismatch on the N2 Component of the ERP: A Review; National Institutes of Health: Rockville, MA, USA, 2008. [CrossRef]
- Berto, R. Exposure to restorative environments helps restore attentional capacity. J. Environ. Psychol. 2005, 25, 249–259. [Google Scholar] [CrossRef]
- Stevenson, M.P.; Schilhab, T.; Bentsen, P. Attention Restoration Theory II: A Systematic Review to Clarify Attention Processes Affected by Exposure to Natural Environments; National Institutes of Health: Rockville, MA, USA, 2018. [CrossRef]
- Posner, M.I.; Cohen, Y. Components of visual orienting. Atten. Perform. 1984, 32, 531–556. [Google Scholar] [CrossRef]
- Wen, W.; Yamashita, A.; Asama, H. Measurement of the perception of control during continuous movement using electroencephalography. Front. Hum. Neurosci. 2017, 11, 392. [Google Scholar] [CrossRef]
- Odom, J.V.; Bach, M.; Brigell, M.; Holder, G.E.; McCulloch, D.L.; Tormene, A.P.; Vaegan. ISCEV Standard for Clinical Visual Evoked Potentials (2009 Update); Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Hopfinger, J.B.; West, V.M. Interactions between endogenous and exogenous attention on cortical visual processing. Neuroimage 2006, 31, 774–789. [Google Scholar] [CrossRef]
- Meyer, K.N.; Du, F.; Parks, E.; Hopfinger, J.B. Exogenous vs. endogenous attention: Shifting the balance of fronto-parietal activity. Neuropsychologia 2018, 111, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Peirce, J.; Gray, J.R.; Simpson, S.; MacAskill, M.; Höchenberger, R.; Sogo, H.; Kastman, E.; Lindeløv, J.K. PsychoPy2: Experiments in behavior made easy. Behav. Res. Methods 2019, 51, 195–203. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Klug, M.; Gramann, K. Identifying key factors for improving ICA-based decomposition of EEG data in mobile and stationary experiments. Eur. J. Neurosci. 2021, 54, 8406–8420. [Google Scholar] [CrossRef]
- Pion-Tonachini, L.; Kreutz-Delgado, K.; Makeig, S. ICLabel: An automated electroencephalographic independent component classifier, dataset, and website. Neuroimage 2019, 198, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Xu, G.; Zhang, K.; Liang, R.; Yan, W.; Tian, P.; Jia, Y.; Zhang, S.; Du, C. Assessment of Human Visual Acuity Using Visual Evoked Potential: A Review. Sensors 2020, 20, 5542. [Google Scholar] [CrossRef]
- Park, J.; Chiang, C.; Brannon, E.M.; Woldorff, M.G. Experience-dependent hemispheric specialization of letters and numbers is revealed in early visual processing. J. Cogn. Neurosci. 2014, 26, 2239–2249. [Google Scholar] [CrossRef] [PubMed]
- Hemptinne, C.; Liu-Shuang, J.; Yuksel, D.; Rossion, B. Rapid objective assessment of contrast sensitivity and visual acuity with sweep visual evoked potentials and an extended electrode array. Invest. Ophthalmol. Vis. Sci. 2018, 59, 1144–1157. [Google Scholar] [CrossRef]
- Anllo-Vento, L. Shifting attention in visual space: The effects of peripheral cueing on brain cortical potentials. Int. J. Neurosci. 1995, 80, 353–370. [Google Scholar] [CrossRef]
- Coull, J.T. Neural correlates of attention and arousal: Insights from electrophysiology, functional neuroimaging and psychopharmacology. Prog. Neurobiol. 1998, 55, 343–361. [Google Scholar] [CrossRef]
- Eason, R.G. Visual evoked potential correlates of early neural filtering during selective attention. Bull. Psychon. Soc. 1981, 18, 203–206. [Google Scholar] [CrossRef]
- Harter, M.R.; Aine, C.; Schroeder, C. Hemispheric differences in the neural processing of stimulus location and type: Effects of selective attention on visual evoked potentials. Neuropsychologia 1982, 20, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Hillyard, S.A.; Mangun, G.R. Sensory gating as a physiological mechanism for visual selective attention. Electroencephalogr. Clin. Neurophysiol. 1987, 40, 61–67. [Google Scholar]
- Hillyard, S.A.; Münte, T.F. Selective attention to color and location: An analysis with event-related brain potentials. Percept. Psychophys. 1984, 36, 185–198. [Google Scholar] [CrossRef]
- Schomaker, J. The Relationship between Response Time and the Strength of Top-Down Attentional Control: An ERP Study. J. Eur. Psychol. Stud. 2009, 1, Art.2. [Google Scholar] [CrossRef][Green Version]
- Finnigan, S.; O’Connell, R.G.; Cummins, T.D.R.; Broughton, M.; Robertson, I.H. ERP measures indicate both attention and working memory encoding decrements in aging. Psychophysiology 2011, 48, 601–611. [Google Scholar] [CrossRef]
- Dien, J.; Spencer, K.M.; Donchin, E. Localization of the event-related potential novelty response as defined by principal components analysis. Cogn. Brain Res. 2003, 17, 637–650. [Google Scholar] [CrossRef]
- Donchin, E.; Coles, M.G.H. Is the P300 component a manifestation of context updating? Behav. Brain Sci. 1988, 11, 357–374. [Google Scholar] [CrossRef]
- Friedman, D.; Cycowicz, Y.M.; Gaeta, H. The Novelty P3: An Event-Related Brain Potential (ERP) Sign of the Brain’s Evaluation of Novelty; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar] [CrossRef]
- Eimer, M. Spatial cueing, sensory gating and selective response preparation: An ERP study on visuo-spatial orienting. In Electroencephalography and Clinical Neurophysiology/Evoked Potentials; Elsevier: Amsterdam, The Netherlands, 1993; Volume 88. [Google Scholar] [CrossRef]
- Mangun, G.R.; Hillyard, S.A. Mechanisms and models of selective attention. In Electrophysiology of Mind; Oxford University Press: Oxford, UK, 2008. [Google Scholar] [CrossRef]
- Luck, S.J.; Hillyard, S.A. Electrophysiological correlates of feature analysis during visual search. Psychophysiology 1994, 31, 291–308. [Google Scholar] [CrossRef]
- Doro, M.; Bellini, F.; Brigadoi, S.; Eimer, M.; Dell’Acqua, R. A bilateral N2pc (N2pcb) component is elicited by search targets displayed on the vertical midline. Psychophysiology 2020, 57, e13512. [Google Scholar] [CrossRef]
- González-Espinar, J.; Ortells, J.J.; Sánchez-García, L.; Montoro, P.R.; Hutchison, K. Exposure to natural environments consistently improves visuospatial working memory performance. J. Environ. Psychol. 2023, 91, 102138. [Google Scholar] [CrossRef]
- Weintraub, J.; Nolan, K.P.; Sachdev, A.R. The Cognitive Control Model of Work-related Flow. Front. Psychol. 2023, 14, 1174152. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, P.; Sarasso, P.; Lodico, F.; Aliverti, A.; Murayama, K.; Sacco, K.; Ronga, I. The aesthetic valve: How aesthetic appreciation may switch emotional states from anxiety to curiosity. Philos. Trans. R. Soc. B Biol. Sci. 2024, 379, 20220413. [Google Scholar] [CrossRef] [PubMed]
- Wessel, J.R.; Aron, A.R. On the Globality of Motor Suppression: Unexpected Events and Their Influence on Behavior and Cognition; National Institutes of Health: Rockville, MA, USA, 2017. [CrossRef]
- Grassini, S.; Segurini, G.V.; Koivisto, M. Watching Nature Videos Promotes Physiological Restoration: Evidence From the Modulation of Alpha Waves in Electroencephalography. Front. Psychol. 2022, 13, 871143. [Google Scholar] [CrossRef] [PubMed]
- Franěk, M.; Petružálek, J.; Šefara, D. Eye movements in viewing urban images and natural images in diverse vegetation periods. Urban For. Urban Green. 2019, 46, 126477. [Google Scholar] [CrossRef]
- Nguyen, P.Y.; Astell-Burt, T.; Rahimi-Ardabili, H.; Feng, X. Green space quality and health: A systematic review. Int. J. Environ. Res. Public Health 2021, 18, 11028. [Google Scholar] [CrossRef]
- Markevych, I.; Schoierer, J.; Hartig, T.; Chudnovsky, A.; Hystad, P.; Dzhambov, A.M.; De Vries, S.; Triguero-Mas, M.; Brauer, M.; Nieuwenhuijsen, M.J.; et al. Exploring pathways linking greenspace to health: Theoretical and methodological guidance. Environ. Res. 2017, 158, 301–317. [Google Scholar] [CrossRef]
- Picton, T.W.; Stuss, D.T.; Alexander, M.P.; Shallice, T.; Binns, M.A.; Gillingham, S. Effects of focal frontal lesions on response inhibition. Cereb. Cortex 2007, 17, 826–838. [Google Scholar] [CrossRef]
- Aron, A.R.; Robbins, T.W.; Poldrack, R.A. Inhibition and the right inferior frontal cortex. Trends Cogn. Sci. 2004, 8, 170–177. [Google Scholar] [CrossRef]
- Dinu Roman Szabo, M.; Dumitras, A.; Mircea, D.M.; Doroftei, D.; Sestras, P.; Boscaiu, M.; Brzuszek, R.F.; Sestras, A.F. Touch, feel, heal. The use of hospital green spaces and landscape as sensory-therapeutic gardens: A case study in a university clinic. Front. Psychol. 2023, 14, 1201030. [Google Scholar] [CrossRef]


| Subject | Green | |||||
| Exogenous | Endogenous | |||||
| Mean RT | Mean Valid RT | Mean Invalid RT | Mean RT | Mean Valid RT | Mean Invalid RT | |
| 1 | 0.249137024 | 0.240151741 | 0.285078154 | 0.257391674 | 0.241511387 | 0.320912823 |
| 2 | 0.334997077 | 0.324027356 | 0.378875963 | 0.306402855 | 0.29073568 | 0.369071553 |
| 3 | 0.314887324 | 0.302259423 | 0.365398927 | 0.270287685 | 0.25885686 | 0.316010983 |
| 4 | 0.246914843 | 0.242665717 | 0.263911347 | 0.284231804 | 0.275132427 | 0.320629315 |
| 5 | 0.245307665 | 0.237926057 | 0.274834098 | 0.262065705 | 0.247940076 | 0.318568218 |
| 6 | 0.208870532 | 0.20893939 | 0.208595102 | 0.162348879 | 0.157734365 | 0.180806933 |
| 7 | 0.308951705 | 0.283142809 | 0.412187291 | 0.326095302 | 0.319530306 | 0.352355286 |
| 8 | 0.300592355 | 0.280657166 | 0.380333109 | 0.284099017 | 0.269768323 | 0.34142179 |
| 9 | 0.328078283 | 0.307884736 | 0.408852468 | 0.330228997 | 0.31762009 | 0.380664627 |
| 10 | 0.286731258 | 0.277493841 | 0.323680927 | 0.270894826 | 0.259204828 | 0.317654821 |
| 11 | 0.277554035 | 0.263471209 | 0.333885339 | 0.283397209 | 0.270130053 | 0.336465833 |
| 12 | 0.304645934 | 0.280679632 | 0.400511143 | 0.295713726 | 0.288500067 | 0.324568361 |
| 13 | 0.229191206 | 0.220708353 | 0.263122622 | 0.222430795 | 0.214108593 | 0.255719607 |
| 14 | 0.396158187 | 0.38003358 | 0.460656614 | 0.325574109 | 0.311220268 | 0.382989475 |
| 15 | 0.192131213 | 0.191224892 | 0.195756499 | 0.173118983 | 0.172210673 | 0.176752226 |
| Means | 0.281609909 | 0.269417727 | 0.33037864 | 0.270285438 | 0.2596136 | 0.31297279 |
| Subject | Urban | |||||
| Exogenous | Endogenous | |||||
| Mean RT | Mean Valid RT | Mean Invalid RT | Mean RT | Mean Valid RT | Mean Invalid RT | |
| 1 | 0.245175936 | 0.241321198 | 0.260594888 | 0.254439064 | 0.24780198 | 0.280987399 |
| 2 | 0.332952607 | 0.323645963 | 0.370179181 | 0.278007826 | 0.262958739 | 0.338204173 |
| 3 | 0.287662048 | 0.278070822 | 0.326026954 | 0.292104551 | 0.280773914 | 0.337427101 |
| 4 | 0.258678978 | 0.253121243 | 0.280909916 | 0.268210498 | 0.265306041 | 0.279828326 |
| 5 | 0.268228474 | 0.263271148 | 0.288057774 | 0.276304924 | 0.274288303 | 0.284371408 |
| 6 | 0.183940172 | 0.181863903 | 0.192245249 | 0.202987578 | 0.197332076 | 0.225609583 |
| 7 | 0.30550634 | 0.294627381 | 0.349022175 | 0.314165882 | 0.305839286 | 0.347472265 |
| 8 | 0.287193544 | 0.264362553 | 0.378517512 | 0.315462459 | 0.299863006 | 0.37786027 |
| 9 | 0.328048234 | 0.313167996 | 0.387569183 | 0.317294426 | 0.306452926 | 0.360660425 |
| 10 | 0.286416865 | 0.286912626 | 0.284433822 | 0.257804996 | 0.250971788 | 0.285137825 |
| 11 | 0.284355723 | 0.272209128 | 0.332942103 | 0.25881389 | 0.247810467 | 0.302827583 |
| 12 | 0.313099547 | 0.300254705 | 0.364478916 | 0.275954405 | 0.266226896 | 0.314864444 |
| 13 | 0.246286015 | 0.238789332 | 0.276272744 | 0.238854801 | 0.235539774 | 0.252114909 |
| 14 | 0.337127176 | 0.320183819 | 0.404900604 | 0.357536685 | 0.335300835 | 0.446480088 |
| 15 | 0.217737504 | 0.216865175 | 0.221226818 | 0.157133369 | 0.162206261 | 0.136841799 |
| Means | 0.278827277 | 0.269911133 | 0.314491856 | 0.271005024 | 0.262578153 | 0.304712507 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piedimonte, A.; Lanzo, G.; Campaci, F.; Volpino, V.; Carlino, E. Spreading New Light on Attention Restoration Theory: An Environmental Posner Paradigm. Brain Sci. 2025, 15, 578. https://doi.org/10.3390/brainsci15060578
Piedimonte A, Lanzo G, Campaci F, Volpino V, Carlino E. Spreading New Light on Attention Restoration Theory: An Environmental Posner Paradigm. Brain Sciences. 2025; 15(6):578. https://doi.org/10.3390/brainsci15060578
Chicago/Turabian StylePiedimonte, Alessandro, Gianluca Lanzo, Francesco Campaci, Valeria Volpino, and Elisa Carlino. 2025. "Spreading New Light on Attention Restoration Theory: An Environmental Posner Paradigm" Brain Sciences 15, no. 6: 578. https://doi.org/10.3390/brainsci15060578
APA StylePiedimonte, A., Lanzo, G., Campaci, F., Volpino, V., & Carlino, E. (2025). Spreading New Light on Attention Restoration Theory: An Environmental Posner Paradigm. Brain Sciences, 15(6), 578. https://doi.org/10.3390/brainsci15060578








