Coping Styles and Defense Mechanisms in Healthy Young Adults—Correlations with tPA-BDNF Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Defense Mechanisms and Coping Strategies
2.3. Plasma BDNF, ProBDNF, t-Plasminogen Activator/tPA, Total Serpin E1/PAI-1, Serpin F2/Alpha 2-Antiplasmin, MMP-9 Quantification
2.4. Statistical Analyses
3. Results
3.1. Analysis of Plasma Levels of BDNF, ProBDNF, t-Plasminogen Activator/tPA, Total Serpin E1/PAI-1, Serpin F2/Alpha 2-Antiplasmin, and MMP-9 with Regard to Gender
3.2. Analysis of Defense Style Questionnaire (DSQ-40) and Coping Orientation to Problems Experienced Inventory (COPE) Scores with Regard to Gender
3.3. Correlation Analysis of Plasma Concentrations of tPA-BDNF Pathway
3.4. Correlations of tPA-BDNF Pathway Plasma Protein Levels with Coping Orientation to Problems Experienced Inventory (COPE) and Defense Style Questionnaire (DSQ-40) Scores
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wiszniewski, B.; Liberska, H. Styles of Coping with Stress among Healthy People and People with Diagnosis of Schizophrenia and Selected Personality Dimensions. Int. J. Environ. Res. Public Health 2022, 19, 5129. [Google Scholar] [CrossRef] [PubMed]
- Palego, L.; Giannaccini, G.; Betti, L. Neuroendocrine Response to Psychosocial Stressors, Inflammation Mediators and Brain-Periphery Pathways of Adaptation. Cent. Nerv. Syst. Agents Med. Chem. 2021, 21, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Folkman, S. Stress: Appraisal and Coping. In Encyclopedia of Behavioral Medicine; Gellman, M.D., Turner, J.R., Eds.; Springer: New York, NY, USA, 2013; pp. 1913–1915. ISBN 978-1-4419-1005-9. [Google Scholar]
- Stanisławski, K. The Coping Circumplex Model: An Integrative Model of the Structure of Coping With Stress. Front. Psychol. 2019, 10, 694. [Google Scholar] [CrossRef]
- Skinner, E.A.; Edge, K.; Altman, J.; Sherwood, H. Searching for the Structure of Coping: A Review and Critique of Category Systems for Classifying Ways of Coping. Psychol. Bull. 2003, 129, 216–269. [Google Scholar] [CrossRef] [PubMed]
- Bianca, C.-S.D.; Ramona, P.L.; Ioana, M.V. The Relationship between Coping Strategies and Life Quality in Major Depressed Patients. Egypt. J. Neurol. Psychiatry Neurosurg. 2022, 58, 110. [Google Scholar] [CrossRef]
- Ered, A.; Gibson, L.E.; Maxwell, S.D.; Cooper, S.; Ellman, L.M. Coping as a Mediator of Stress and Psychotic-like Experiences. Eur. Psychiatry 2017, 43, 9–13. [Google Scholar] [CrossRef]
- Josepho, S.A.; Plutchik, R. Stress, Coping, and Suicide Risk in Psychiatric Inpatients. Suicide Life-Threat. Behav. 1994, 24, 48–57. [Google Scholar] [CrossRef]
- Caredda, M.; Vescera, L.; Picardi, A.; Tarolla, E.; Pancheri, C.; Biondi, M.; Tondo, L. Positive Psychological Functioning, Resilience and Styles of Coping as Buffers against Suicidal Behaviours. A Case-Control Study. J. Affect. Disord. 2024, 367, 408–415. [Google Scholar] [CrossRef]
- Horesh, N.; Rolnick, T.; Iancu, I.; Dannon, P.; Lepkifker, E.; Apter, A.; Kotler, M. Coping Styles and Suicide Risk. Acta Psychiatr. Scand. 1996, 93, 489–493. [Google Scholar] [CrossRef]
- Orzechowska, A.; Bliźniewska-Kowalska, K.; Gałecki, P.; Szulc, A.; Płaza, O.; Su, K.-P.; Georgescu, D.; Gałecka, M. Ways of Coping with Stress among Patients with Depressive Disorders. J. Clin. Med. 2022, 11, 6500. [Google Scholar] [CrossRef]
- Aksoy Poyraz, C.; Özdemir, A.; Çakir Şen, C.; Usta Sağlam, N.G.; Enginkaya, S.; Tomruk, N. The Impact of Coping Strategies on Suicide Attempts and Suicidal Ideation in Bipolar Disorder. J. Nerv. Ment. Dis. 2021, 209, 564–570. [Google Scholar] [CrossRef]
- Lew, B.; Huen, J.; Yu, P.; Yuan, L.; Wang, D.-F.; Ping, F.; Abu Talib, M.; Lester, D.; Jia, C.-X. Associations between Depression, Anxiety, Stress, Hopelessness, Subjective Well-Being, Coping Styles and Suicide in Chinese University Students. PLoS ONE 2019, 14, e0217372. [Google Scholar] [CrossRef] [PubMed]
- Khazem, L.R.; Law, K.C.; Green, B.A.; Anestis, M.D. Examining the Relationship between Coping Strategies and Suicidal Desire in a Sample of United States Military Personnel. Compr. Psychiatry 2015, 57, 2–9. [Google Scholar] [CrossRef]
- Ambrus, L.; Sunnqvist, C.; Asp, M.; Westling, S.; Westrin, Å. Coping and Suicide Risk in High Risk Psychiatric Patients. J. Ment. Health 2020, 29, 27–32. [Google Scholar] [CrossRef]
- Kato, T. Frequently Used Coping Scales: A Meta-Analysis. Stress Health 2015, 31, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Bathina, S.; Das, U.N. Brain-Derived Neurotrophic Factor and Its Clinical Implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef]
- Autry, A.E.; Monteggia, L.M. Brain-Derived Neurotrophic Factor and Neuropsychiatric Disorders. Pharmacol. Rev. 2012, 64, 238–258. [Google Scholar] [CrossRef]
- Mizui, T.; Ishikawa, Y.; Kumanogoh, H.; Lume, M.; Matsumoto, T.; Hara, T.; Yamawaki, S.; Takahashi, M.; Shiosaka, S.; Itami, C.; et al. BDNF Pro-Peptide Actions Facilitate Hippocampal LTD and Are Altered by the Common BDNF Polymorphism Val66Met. Proc. Natl. Acad. Sci. USA 2015, 112, E3067–E3074. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.L.; Grover, L.M.; Schwartzkroin, P.A.; Bothwell, M. Neurotrophin Expression in Rat Hippocampal Slices: A Stimulus Paradigm Inducing LTP in CA1 Evokes Increases in BDNF and NT-3 mRNAs. Neuron 1992, 9, 1081–1088. [Google Scholar] [CrossRef]
- Fan, Y.; Luan, X.; Wang, X.; Li, H.; Zhao, H.; Li, S.; Li, X.; Qiu, Z. Exploring the Association between BDNF Related Signaling Pathways and Depression: A Literature Review. Brain Res. Bull. 2025, 220, 111143. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, S.; Mehan, S.; Khan, Z.; Das Gupta, G.; Narula, A.S. Exploring the Connection Between BDNF/TrkB and AC/cAMP/PKA/CREB Signaling Pathways: Potential for Neuroprotection and Therapeutic Targets for Neurological Disorders. Mol. Neurobiol. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Hayat, M.R.; Umair, M.; Ikhtiar, H.; Wazir, S.; Palwasha, A.; Shah, M. The Relationship Between Brain-Derived Neurotrophic Factor and Serotonin in Major Depressive and Bipolar Disorders: A Cross-Sectional Analysis. Cureus 2024, 16, e70728. [Google Scholar] [CrossRef] [PubMed]
- Arévalo, J.C.; Deogracias, R. Mechanisms Controlling the Expression and Secretion of BDNF. Biomolecules 2023, 13, 789. [Google Scholar] [CrossRef]
- De Vincenti, A.P.; Ríos, A.S.; Paratcha, G.; Ledda, F. Mechanisms That Modulate and Diversify BDNF Functions: Implications for Hippocampal Synaptic Plasticity. Front. Cell. Neurosci. 2019, 13, 135. [Google Scholar] [CrossRef] [PubMed]
- Pang, P.T.; Teng, H.K.; Zaitsev, E.; Woo, N.T.; Sakata, K.; Zhen, S.; Teng, K.K.; Yung, W.-H.; Hempstead, B.L.; Lu, B. Cleavage of proBDNF by tPA/Plasmin Is Essential for Long-Term Hippocampal Plasticity. Science 2004, 306, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Kermani, P.; Teng, K.K.; Hempstead, B.L. Regulation of Cell Survival by Secreted Proneurotrophins. Science 2001, 294, 1945–1948. [Google Scholar] [CrossRef]
- Cesarman-Maus, G.; Hajjar, K.A. Molecular Mechanisms of Fibrinolysis. Br. J. Haematol. 2005, 129, 307–321. [Google Scholar] [CrossRef]
- Sprengers, E.D.; Kluft, C. Plasminogen Activator Inhibitors. Blood 1987, 69, 381–387. [Google Scholar] [CrossRef]
- Urano, T.; Suzuki, Y.; Iwaki, T.; Sano, H.; Honkura, N.; Castellino, F.J. Recognition of Plasminogen Activator Inhibitor Type 1 as the Primary Regulator of Fibrinolysis. Curr. Drug Targets 2019, 20, 1695–1701. [Google Scholar] [CrossRef]
- Mou, X.; Peterson, C.B.; Prosser, R.A. Tissue-Type Plasminogen Activator-Plasmin-BDNF Modulate Glutamate-Induced Phase-Shifts of the Mouse Suprachiasmatic Circadian Clock in Vitro. Eur. J. Neurosci. 2009, 30, 1451–1460. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Kuzniewska, B.; Rejmak, E.; Malik, A.R.; Jaworski, J.; Kaczmarek, L.; Kalita, K. Brain-Derived Neurotrophic Factor Induces Matrix Metalloproteinase 9 Expression in Neurons via the Serum Response Factor/c-Fos Pathway. Mol. Cell. Biol. 2013, 33, 2149–2162. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Y.; Sun, C.-Y.; Huang, J.; Chu, Z.-B. Brain-derived neurotrophic factor promotes the secretion of MMP-9 in human myeloma cell through modulation of nucleus factor-kappaB. Zhonghua Xue Ye Xue Za Zhi 2008, 29, 243–246. [Google Scholar] [PubMed]
- Nagase, H. Activation Mechanisms of Matrix Metalloproteinases. Biol. Chem. 1997, 378, 151–160. [Google Scholar]
- Yong, V.W.; Power, C.; Forsyth, P.; Edwards, D.R. Metalloproteinases in Biology and Pathology of the Nervous System. Nat. Rev. Neurosci. 2001, 2, 502–511. [Google Scholar] [CrossRef]
- Dziembowska, M.; Wlodarczyk, J. MMP9: A Novel Function in Synaptic Plasticity. Int. J. Biochem. Cell Biol. 2012, 44, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Rybakowski, J.K.; Remlinger-Molenda, A.; Czech-Kucharska, A.; Wojcicka, M.; Michalak, M.; Losy, J. Increased Serum Matrix Metalloproteinase-9 (MMP-9) Levels in Young Patients during Bipolar Depression. J. Affect. Disord. 2013, 146, 286–289. [Google Scholar] [CrossRef]
- Li, H.; Sheng, Z.; Khan, S.; Zhang, R.; Liu, Y.; Zhang, Y.; Yong, V.W.; Xue, M. Matrix Metalloproteinase-9 as an Important Contributor to the Pathophysiology of Depression. Front. Neurol. 2022, 13, 861843. [Google Scholar] [CrossRef] [PubMed]
- Bobińska, K.; Szemraj, J.; Czarny, P.; Gałecki, P. Expression and Activity of Metalloproteinases in Depression. Med. Sci. Monit. 2016, 22, 1334–1341. [Google Scholar] [CrossRef]
- Yang, J.; Harte-Hargrove, L.C.; Siao, C.-J.; Marinic, T.; Clarke, R.; Ma, Q.; Jing, D.; LaFrancois, J.J.; Bath, K.G.; Mark, W.; et al. proBDNF Negatively Regulates Neuronal Remodeling, Synaptic Transmission, and Synaptic Plasticity in Hippocampus. Cell Rep. 2014, 7, 796–806. [Google Scholar] [CrossRef]
- Cao, W.; Duan, J.; Wang, X.; Zhong, X.; Hu, Z.; Huang, F.; Wang, H.; Zhang, J.; Li, F.; Zhang, J.; et al. Early Enriched Environment Induces an Increased Conversion of proBDNF to BDNF in the Adult Rat’s Hippocampus. Behav. Brain Res. 2014, 265, 76–83. [Google Scholar] [CrossRef]
- Gawęda, Ł.; Prochwicz, K.; Adamczyk, P.; Frydecka, D.; Misiak, B.; Kotowicz, K.; Szczepanowski, R.; Florkowski, M.; Nelson, B. The Role of Self-Disturbances and Cognitive Biases in the Relationship between Traumatic Life Events and Psychosis Proneness in a Non-Clinical Sample. Schizophr. Res. 2018, 193, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Andrews, G.; Singh, M.; Bond, M. The Defense Style Questionnaire. J. Nerv. Ment. Dis. 1993, 181, 246–256. [Google Scholar] [CrossRef]
- Carver, C.; Scheier, M.; Weintraub, J. Assessing Coping Strategies: A Theoretically Based Approach. J. Personal. Soc. Psychol. 1989, 56, 267–283. [Google Scholar] [CrossRef]
- Skibinska, M.; Kapelski, P.; Dmitrzak-Weglarz, M.; Lepczynska, N.; Pawlak, J.; Twarowska-Hauser, J.; Szczepankiewicz, A.; Rajewska-Rager, A. Elevated Epidermal Growth Factor (EGF) as Candidate Biomarker of Mood Disorders—Longitudinal Study in Adolescent and Young Adult Patients. J. Clin. Med. 2021, 10, 4064. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, H.; Liu, Y.; Hou, Z.; Yue, Y.; Zhang, Y.; Zhao, F.; Xu, Z.; Li, Y.; Mou, X.; et al. Combined Serum Levels of Multiple Proteins in tPA-BDNF Pathway May Aid the Diagnosis of Five Mental Disorders. Sci. Rep. 2017, 7, 6871. [Google Scholar] [CrossRef]
- Carvalho, L.D.F.; Reis, A.M.; Pianowski, G. Investigating Correlations Between Defence Mechanisms and Pathological Personality Characteristics. Rev. Colomb. Psiquiatr. 2019, 48, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Rajewska-Rager, A.; Dmitrzak-Weglarz, M.; Lepczynska, N.; Kapelski, P.; Pawlak, J.; Skibinska, M. Clinical Assessment of Impulsiveness and Defence Mechanisms in Young Patients with Mood Disorders in a Two-Year Prospective Study. Early Interv. Psychiatry 2023, 17, 1001–1011. [Google Scholar] [CrossRef]
- Lommatzsch, M.; Zingler, D.; Schuhbaeck, K.; Schloetcke, K.; Zingler, C.; Schuff-Werner, P.; Virchow, J.C. The Impact of Age, Weight and Gender on BDNF Levels in Human Platelets and Plasma. Neurobiol. Aging 2005, 26, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Tsiknia, A.A.; Sundermann, E.E.; Reas, E.T.; Edland, S.D.; Brewer, J.B.; Galasko, D.; Banks, S.J. Alzheimer’s Disease Neuroimaging Initiative Sex Differences in Alzheimer’s Disease: Plasma MMP-9 and Markers of Disease Severity. Alzheimer’s Res. Ther. 2022, 14, 160. [Google Scholar] [CrossRef]
- Jonsson, A.; Hjalmarsson, C.; Falk, P.; Ivarsson, M.-L. Levels of Matrix Metalloproteinases Differ in Plasma and Serum—Aspects Regarding Analysis of Biological Markers in Cancer. Br. J. Cancer 2016, 115, 703–706. [Google Scholar] [CrossRef]
- Kelly, M.M.; Tyrka, A.R.; Price, L.H.; Carpenter, L.L. Sex Differences in the Use of Coping Strategies: Predictors of Anxiety and Depressive Symptoms. Depress. Anxiety 2008, 25, 839–846. [Google Scholar] [CrossRef]
- Matud, M.P. Gender Differences in Stress and Coping Styles. Personal. Individ. Differ. 2004, 37, 1401–1415. [Google Scholar] [CrossRef]
- Carver, C.S. Active Coping. In Encyclopedia of Quality of Life and Well-Being Research; Michalos, A.C., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 16–19. ISBN 978-94-007-0753-5. [Google Scholar]
- Uchino, B.N. Social Support and Health: A Review of Physiological Processes Potentially Underlying Links to Disease Outcomes. J. Behav. Med. 2006, 29, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Mennesson, M.; Revest, J.-M. Glucocorticoid-Responsive Tissue Plasminogen Activator (tPA) and Its Inhibitor Plasminogen Activator Inhibitor-1 (PAI-1): Relevance in Stress-Related Psychiatric Disorders. Int. J. Mol. Sci. 2023, 24, 4496. [Google Scholar] [CrossRef] [PubMed]
- Angelucci, F.; Veverova, K.; Katonová, A.; Vyhnalek, M.; Hort, J. Serum PAI-1/BDNF Ratio Is Increased in Alzheimer’s Disease and Correlates with Disease Severity. ACS Omega 2023, 8, 36025–36031. [Google Scholar] [CrossRef]
- Tanrikulu, A.M.; Ozdilek, B.; Agirbasli, M. Serum Levels of Plasminogen Activator Inhibitor-1 in Patients with Parkinson’s Disease. Med. Princ. Pract. 2024, 33, 562–568. [Google Scholar] [CrossRef]
- Stevenson, T.K.; Moore, S.J.; Murphy, G.G.; Lawrence, D.A. Tissue Plasminogen Activator in Central Nervous System Physiology and Pathology: From Synaptic Plasticity to Alzheimer’s Disease. Semin. Thromb. Hemost. 2022, 48, 288–300. [Google Scholar] [CrossRef]
- Roth, M.K.; Bingham, B.; Shah, A.; Joshi, A.; Frazer, A.; Strong, R.; Morilak, D.A. Effects of Chronic plus Acute Prolonged Stress on Measures of Coping Style, Anxiety, and Evoked HPA-Axis Reactivity. Neuropharmacology 2012, 63, 1118–1126. [Google Scholar] [CrossRef]
- Radley, J.J.; Johnson, S.B. Anteroventral Bed Nuclei of the Stria Terminalis Neurocircuitry: Towards an Integration of HPA Axis Modulation with Coping Behaviors—Curt Richter Award Paper 2017. Psychoneuroendocrinology 2018, 89, 239–249. [Google Scholar] [CrossRef]
- Mobbs, D.; Greicius, M.D.; Abdel-Azim, E.; Menon, V.; Reiss, A.L. Humor Modulates the Mesolimbic Reward Centers. Neuron 2003, 40, 1041–1048. [Google Scholar] [CrossRef]
- Simione, L.; Gnagnarella, C. Humor Coping Reduces the Positive Relationship between Avoidance Coping Strategies and Perceived Stress: A Moderation Analysis. Behav. Sci. 2023, 13, 179. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.P.; Lengacher, C. Humor and Laughter May Influence Health IV. Humor and Immune Function. Evid.-Based Complement. Altern. Med. 2009, 6, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Bowins, B. Psychological Defense Mechanisms: A New Perspective. Am. J. Psychoanal. 2004, 64, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Hüfner, K.; Koudouovoh-Tripp, P.; Kandler, C.; Hochstrasser, T.; Malik, P.; Giesinger, J.; Semenitz, B.; Humpel, C.; Sperner-Unterweger, B. Differential Changes in Platelet Reactivity Induced by Acute Physical Compared to Persistent Mental Stress. Physiol. Behav. 2015, 151, 284–291. [Google Scholar] [CrossRef]
- Lutgendorf, S.K.; Lamkin, D.M.; Jennings, N.B.; Arevalo, J.M.G.; Penedo, F.; DeGeest, K.; Langley, R.R.; Lucci, J.A.; Cole, S.W.; Lubaroff, D.M.; et al. Biobehavioral Influences on Matrix Metalloproteinase Expression in Ovarian Carcinoma. Clin. Cancer Res. 2008, 14, 6839–6846. [Google Scholar] [CrossRef]
- van der Kooij, M.A.; Fantin, M.; Rejmak, E.; Grosse, J.; Zanoletti, O.; Fournier, C.; Ganguly, K.; Kalita, K.; Kaczmarek, L.; Sandi, C. Role for MMP-9 in Stress-Induced Downregulation of Nectin-3 in Hippocampal CA1 and Associated Behavioural Alterations. Nat. Commun. 2014, 5, 4995. [Google Scholar] [CrossRef]
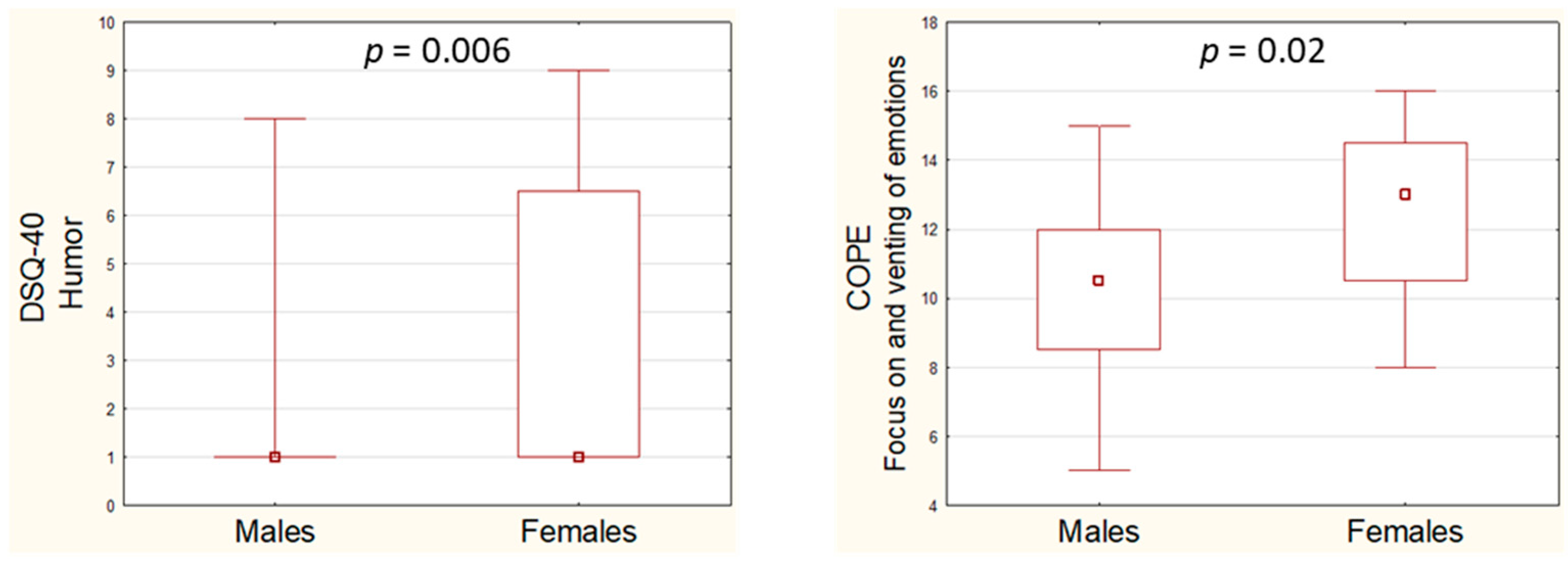
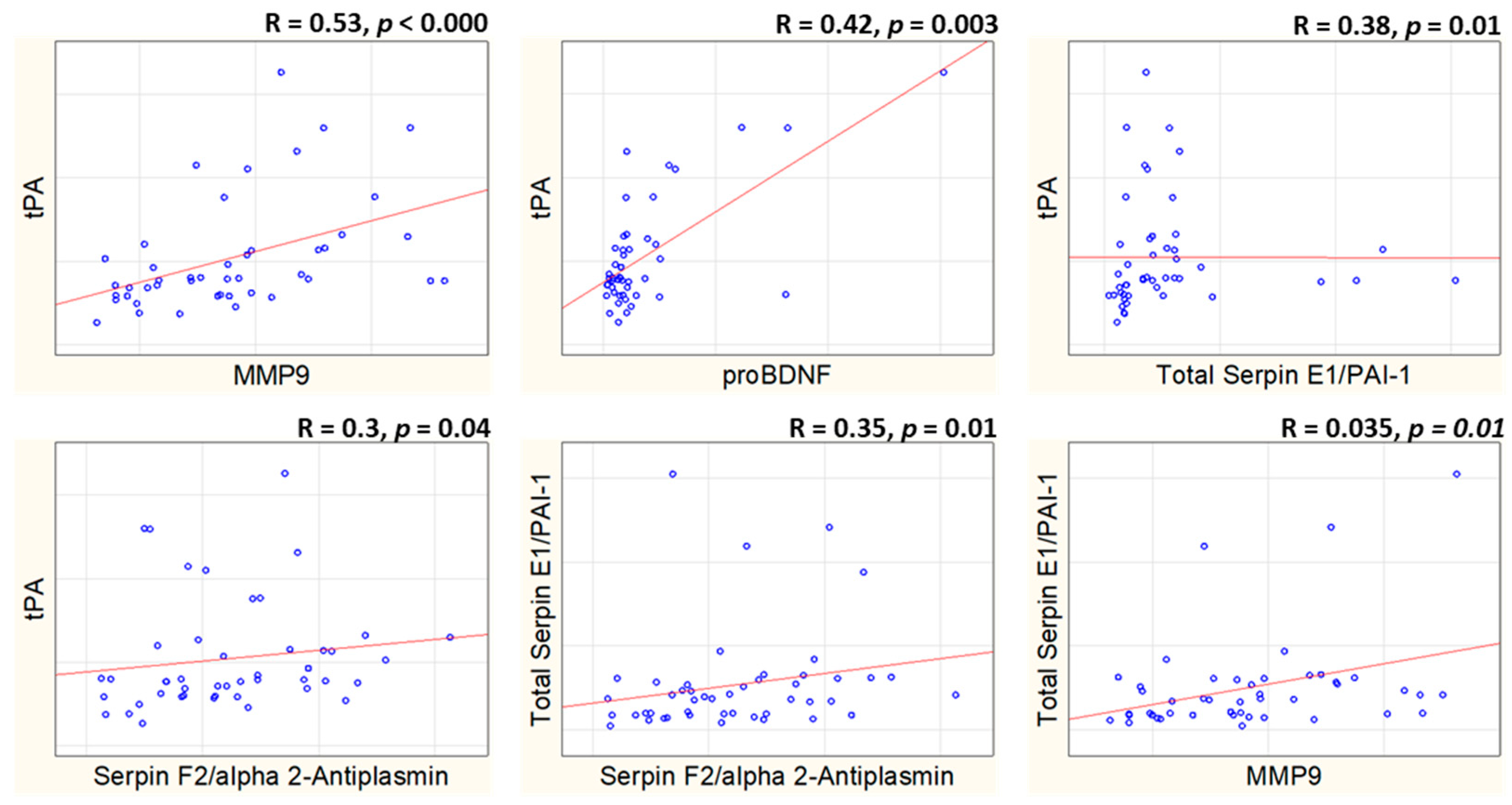
| Whole Group | Males | Females | |||
|---|---|---|---|---|---|
| n | Mean (SD) | Mean (SD) | Mean (SD) | p 1 | |
| Age | 48 | 24 (3) | 25 (3) | 24 (3) | ns |
| BDNF (ng/mL) | 48 | 4.55 (8.91) | 2.61 (2.57) | 6.5 (12.15) | 0.04 |
| ProBDNF (ng/mL) | 48 | 1.99 (2.91) | 1.96 (2.13) | 2.01 (3.57) | ns |
| tPA (pg/mL) | 47 | 832 (528) | 918 (510) | 750 (543) | ns |
| Total serpin E1/PAI-1 (ng/mL) | 48 | 51.07 (59.1) | 59.74 (61.21) | 42.4 (56.86) | ns |
| Serpin F2/alpha 2-antiplasmin (mg/mL) | 47 | 9.32 (5.38) | 8.23 (4.77) | 10.36 (5.80) | ns |
| MMP-9 (ng/mL) | 46 | 201.36 (89.87) | 224.34 (66.83) | 180.3 (103.71) | 0.04 |
| R | p 1 | |
|---|---|---|
| COPE | ||
| Total serpin E1/PAI-1 and denial | 0.43 | 0.002 |
| Total serpin E1/PAI-1 and denial | 0.30 | 0.03 |
| Total serpin E1/PAI-1 and use of emotional social support | −0.44 | 0.002 |
| Total serpin E1/PAI-1 and focus on and venting of emotions | −0.32 | 0.03 |
| Total serpin E1/PAI-1 and use of instrumental social support | −0.3 | 0.04 |
| MMP-9 and focus on and venting of emotions | −0.35 | 0.02 |
| DSQ-40 | ||
| BDNF and humor | 0.31 | 0.03 |
| proBDNF and neurotic | 0.29 | 0.05 |
| proBDNF and acting out | −0.29 | 0.04 |
| Total serpin E1/PAI-1 and devaluation | −0.29 | 0.04 |
| MMP-9 and rationalization | 0.32 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilecka, J.; Wojciechowski, J.; Bargiel, W.; Terczynska, M.; Zakowicz, P.; Bojarski, D.; Wasicka-Przewozna, K.; Skibinska, M. Coping Styles and Defense Mechanisms in Healthy Young Adults—Correlations with tPA-BDNF Pathway. Brain Sci. 2025, 15, 575. https://doi.org/10.3390/brainsci15060575
Pilecka J, Wojciechowski J, Bargiel W, Terczynska M, Zakowicz P, Bojarski D, Wasicka-Przewozna K, Skibinska M. Coping Styles and Defense Mechanisms in Healthy Young Adults—Correlations with tPA-BDNF Pathway. Brain Sciences. 2025; 15(6):575. https://doi.org/10.3390/brainsci15060575
Chicago/Turabian StylePilecka, Julia, Jedrzej Wojciechowski, Weronika Bargiel, Maria Terczynska, Przemyslaw Zakowicz, Dawid Bojarski, Karolina Wasicka-Przewozna, and Maria Skibinska. 2025. "Coping Styles and Defense Mechanisms in Healthy Young Adults—Correlations with tPA-BDNF Pathway" Brain Sciences 15, no. 6: 575. https://doi.org/10.3390/brainsci15060575
APA StylePilecka, J., Wojciechowski, J., Bargiel, W., Terczynska, M., Zakowicz, P., Bojarski, D., Wasicka-Przewozna, K., & Skibinska, M. (2025). Coping Styles and Defense Mechanisms in Healthy Young Adults—Correlations with tPA-BDNF Pathway. Brain Sciences, 15(6), 575. https://doi.org/10.3390/brainsci15060575






