Rapid Eye Movements in Sleep Furnish a Unique Probe into the Ontogenetic and Phylogenetic Development of the Visual Brain: Implications for Autism Research
Abstract
1. Introduction
2. fMRI Study of the Neural Correlates of REMs in Sleep
2.1. Summary of the Findings
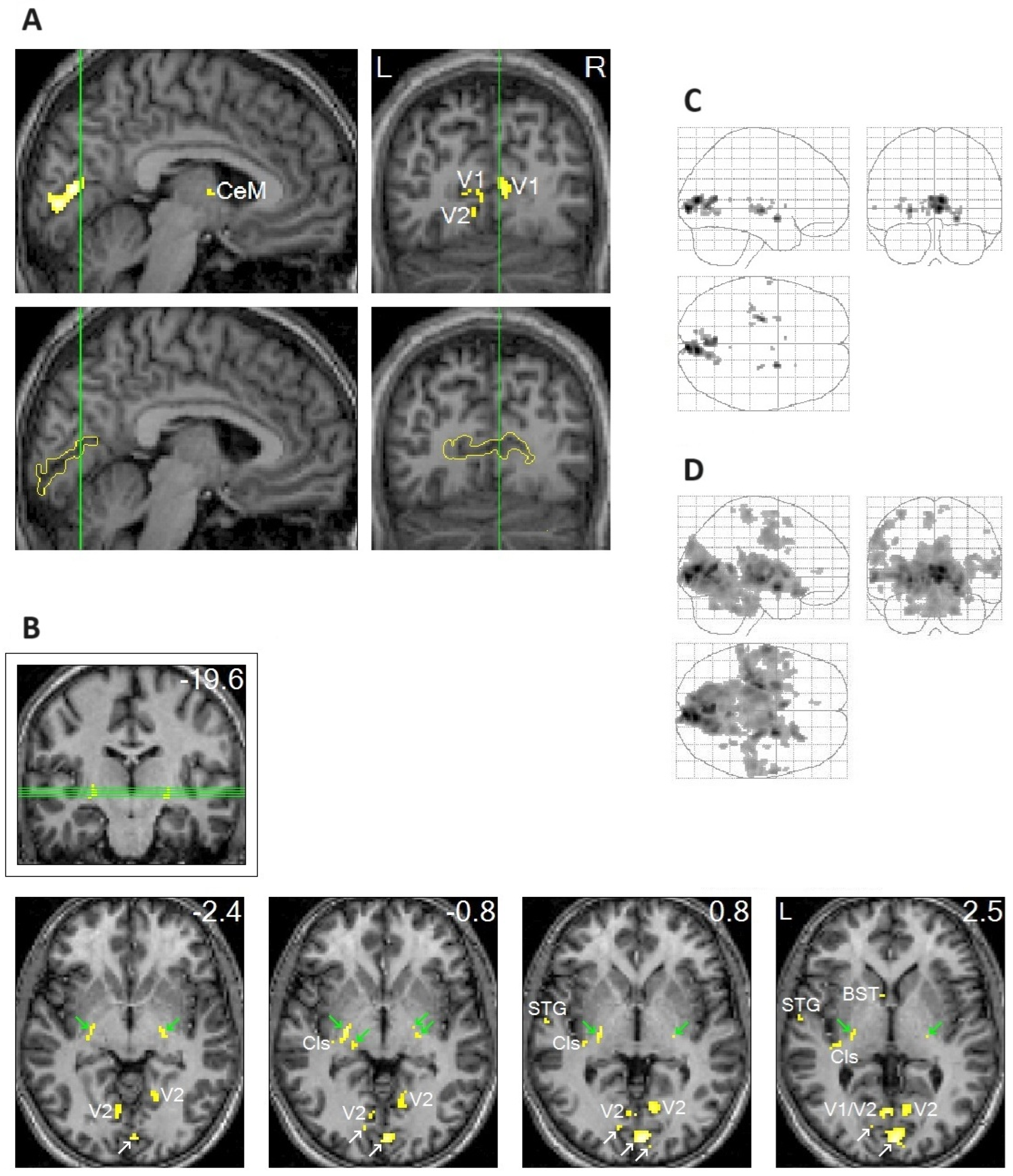

2.2. REM-Locked Peak Activation in the BNST
2.3. The Role of the RSC in Spatial Processing
2.4. Striking Contrast Between the RSC-Rt and the RSC-Lt
2.5. REM-Locked Activation Was Characteristically Widespread
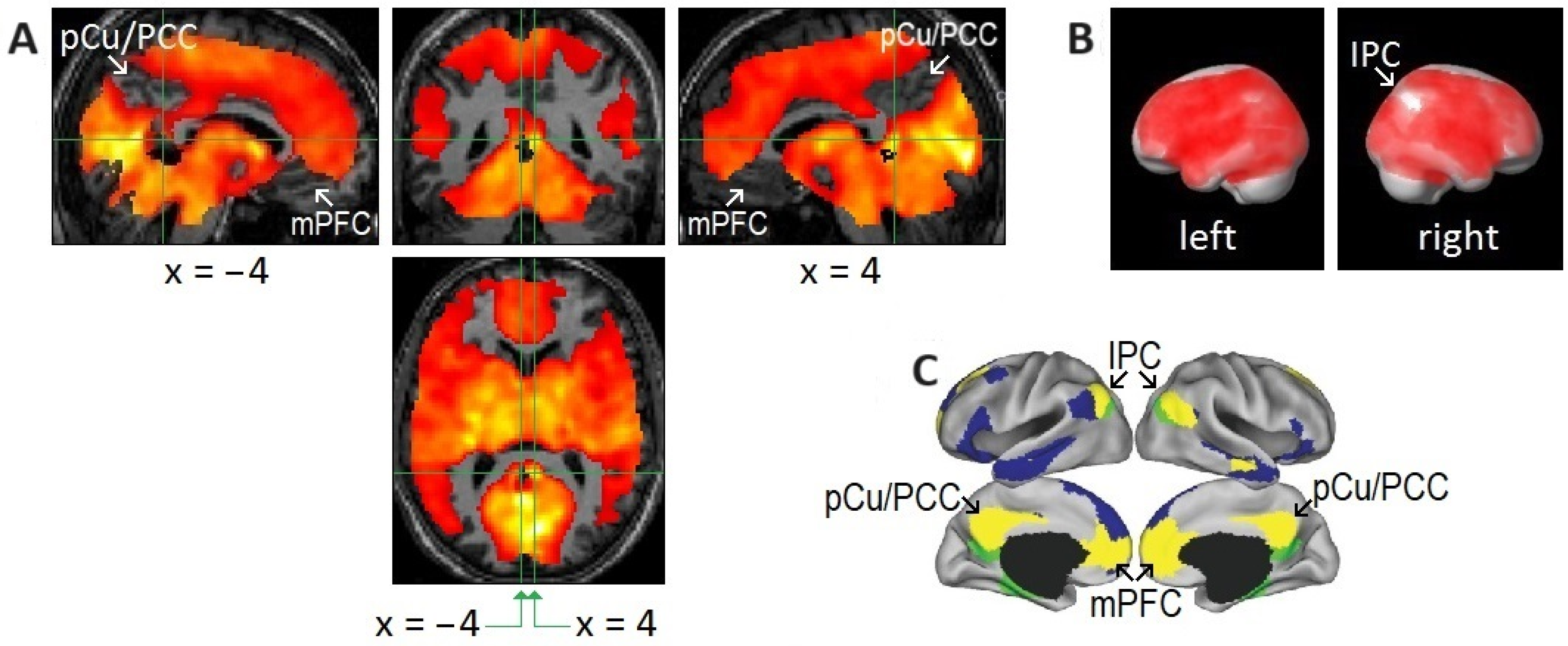
2.6. Relatively Small Islands of Attenuated REM-Locked Cortical Activation Were Restricted to the DMN
3. REM-Locked Peak Activation Sites and Neural Processing Overlap with Atypicality in Autism
3.1. REMs in Sleep Are Saccades
3.2. REMs Index Multisensory–Motor Integration
3.3. REM-Locked Peak Activation in the Cholinergic Basal Nuclei
3.4. REM-Locked Peak Activation in the BNST
3.5. REM-Locked RSC-Rt Peak | RSC-Lt Deactivation
3.6. REM-Associated DMN Deactivation
4. Strengths of REM-Probe Studies
4.1. REMs Are a Task-Free Probe
4.2. The Level of Attention Is Controlled
4.3. The Brain Is Relatively Isolated from the Environment
4.4. Sufficient Statistical Power for Longitudinal, Within-Individual Analysis
4.5. The Preponderance of REM Sleep in Late Pregnancy and in Infancy
4.6. Advantages over Traditional rs-fMRI Studies
5. Functional Ultrasound (fUS) Study of Animals and Human Infants
6. Practical Considerations in Conducting an REM-Probe Study
7. Limitations of REM Probe Approach
- In 6 of 11 adult participants in our study, a sufficient number of REMs occurred only on the second night. Presumably, head restraint, which is required for MRI studies, suppresses REM sleep, and REM sleep deprivation during the first night likely builds “REM pressure” for the second night [20]. This is a limitation of the REM-probe approach; two consecutive overnight studies are labor intensive, and it is costly to use an MRI scanner for two nights.
- Timing REMs by visual inspection is also labor-intensive. However, video recordings of closed eyes can be coupled with computerized analysis to time and quantify REMs automatically, as was done with REMs of Australian dragons [139].
- Timing REMs by visual inspection may be difficult for some people. We had difficulty timing REMs using video recording in 1 of the 14 participants [20].
- Out of the fourteen adult participants, one could not fall asleep and withdrew. Another participant had unusually large and frequent, jerky head movements and was not included in the analysis [20].
- We have limited experience in timing REMs in infants and no experience in timing REMs in animals. As the REM probe approach will lead researchers into uncharted territory, and alongside many surprising findings, we expect some challenges.
8. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rylaarsdam, L.; Guemez-Gamboa, A. Genetic causes and modifiers of autism spectrum disorder. Front. Cell. Neurosci. 2019, 13, 470832. [Google Scholar] [CrossRef] [PubMed]
- Maenner, M.J.; Warren, Z.; Williams, A.R.; Amoakohene, E.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Fitzgerald, R.T.; Furnier, S.M.; Hughes, M.M. Prevalence and characteristics of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2020. MMWR. Surveill. Summ. 2023, 72, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ozonoff, S.; Young, G.S.; Carter, A.; Messinger, D.; Yirmiya, N.; Zwaigenbaum, L.; Bryson, S.; Carver, L.J.; Constantino, J.N.; Dobkins, K. Recurrence risk for autism spectrum disorders: A Baby Siblings Research Consortium study. Pediatrics 2011, 128, e488–e495. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.D.; Piven, J. Brain and behavior development in autism from birth through infancy. Dialogues Clin. Neurosci. 2017, 19, 325–333. [Google Scholar] [CrossRef]
- Hazlett, H.C.; Gu, H.; Munsell, B.C.; Kim, S.H.; Styner, M.; Wolff, J.J.; Elison, J.T.; Swanson, M.R.; Zhu, H.; Botteron, K.N. Early brain development in infants at high risk for autism spectrum disorder. Nature 2017, 542, 348–351. [Google Scholar] [CrossRef]
- Girault, J.B.; Donovan, K.; Hawks, Z.; Talovic, M.; Forsen, E.; Elison, J.T.; Shen, M.D.; Swanson, M.R.; Wolff, J.J.; Kim, S.H. Infant visual brain development and inherited genetic liability in autism. Am. J. Psychiatry 2022, 179, 573–585. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5; American Psychiatric Association: Washington, DC, USA, 2013; Volume 10. [Google Scholar]
- Robertson, C.E.; Baron-Cohen, S. Sensory perception in autism. Nat. Rev. Neurosci. 2017, 18, 671–684. [Google Scholar] [CrossRef]
- Schaaf, R.C.; Puts, N.A.; Williams, Z.J.; Woynaroski, T. Forwarding the science of sensory features in autism and related conditions. J. Autism Dev. Disord. 2024, 54, 2663–2667. [Google Scholar] [CrossRef]
- Gandal, M.J.; Haney, J.R.; Wamsley, B.; Yap, C.X.; Parhami, S.; Emani, P.S.; Chang, N.; Chen, G.T.; Hoftman, G.D.; de Alba, D. Broad transcriptomic dysregulation occurs across the cerebral cortex in ASD. Nature 2022, 611, 532–539. [Google Scholar] [CrossRef]
- Falck-Ytter, T.; Bussu, G. The sensory-first account of autism. Neurosci. Biobehav. Rev. 2023, 153, 105405. [Google Scholar] [CrossRef]
- Thye, M.D.; Bednarz, H.M.; Herringshaw, A.J.; Sartin, E.B.; Kana, R.K. The impact of atypical sensory processing on social impairments in autism spectrum disorder. Dev. Cogn. Neurosci. 2018, 29, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Siemann, J.K.; Veenstra-VanderWeele, J.; Wallace, M.T. Approaches to understanding multisensory dysfunction in autism spectrum disorder. Autism Res. 2020, 13, 1430–1449. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, K.; Ostrolenk, A.; Irion, C.; Bertone, A. Reduced multisensory facilitation exists at different periods of development in autism. Cortex 2021, 134, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Feldman, J.I.; Dunham, K.; Cassidy, M.; Wallace, M.T.; Liu, Y.; Woynaroski, T.G. Audiovisual multisensory integration in individuals with autism spectrum disorder: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2018, 95, 220–234. [Google Scholar] [CrossRef]
- Foss-Feig, J.H.; Kwakye, L.D.; Cascio, C.J.; Burnette, C.P.; Kadivar, H.; Stone, W.L.; Wallace, M.T. An extended multisensory temporal binding window in autism spectrum disorders. Exp. Brain Res. 2010, 203, 381–389. [Google Scholar] [CrossRef]
- Hill, E.L.; Crane, L.; Bremner, A.J. Developmental disorders and multisensory perception. In Multisensory Development; Bremner, A.J., Lewkowicz, D.J., Spence, C., Eds.; Oxford University Press: Oxford, UK, 2012; pp. 273–300. [Google Scholar]
- Stevenson, R.A.; Segers, M.; Ferber, S.; Barense, M.D.; Wallace, M.T. The impact of multisensory integration deficits on speech perception in children with autism spectrum disorders. Front. Psychol. 2014, 5, 379. [Google Scholar] [CrossRef]
- Wallace, M.T.; Woynaroski, T.G.; Stevenson, R.A. Multisensory integration as a window into orderly and disrupted cognition and communication. Annu. Rev. Psychol. 2020, 71, 193–219. [Google Scholar] [CrossRef]
- Hong, C.C.-H.; Harris, J.C.; Pearlson, G.D.; Kim, J.-S.; Calhoun, V.D.; Fallon, J.H.; Golay, X.; Gillen, J.S.; Simmonds, D.J.; van Zijl, P.C.M. fMRI evidence for multisensory recruitment associated with rapid eye movements during sleep. Hum. Brain Mapp. 2009, 30, 1705–1722. [Google Scholar] [CrossRef]
- Hong, C.C.-H.; Fallon, J.H.; Friston, K.J. fMRI evidence for default mode network deactivation associated with rapid eye movements in sleep. Brain Sci. 2021, 11, 1528. [Google Scholar] [CrossRef]
- Roffwarg, H.P.; Muzio, J.N.; Dement, W.C. Ontogenetic development of the human sleep-dream cycle. Science 1966, 152, 604–619. [Google Scholar] [CrossRef]
- Hobson, J.A. REM sleep and dreaming: Towards a theory of protoconsciousness. Nat. Rev. Neurosci. 2009, 10, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Yarbus, A.L. Eye Movements and Vision; Plenum Press: New York, NY, USA, 1967. [Google Scholar]
- Hong, C.C.-H.; Fallon, J.H.; Friston, K.J.; Harris, J.C. Rapid eye movements in sleep furnish a unique probe into consciousness. Front. Psychol. 2018, 9, 2087. [Google Scholar]
- Hong, C.C.-H.; Gillin, J.C.; Dow, B.M.; Wu, J.; Buchsbaum, M.S. Localized and lateralized cerebral glucose metabolism associated with eye movements during REM sleep and wakefulness: A positron emission tomography (PET) study. Sleep 1995, 18, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Leigh, R.J.; Zee, D.S. The saccadic system. In The Neurology of Eye Movements, 5th ed.; Oxford University Press: New York, NY, USA, 2015; pp. 169–288. [Google Scholar]
- Senzai, Y.; Scanziani, M. A cognitive process occurring during sleep is revealed by rapid eye movements. Science 2022, 377, 999–1004. [Google Scholar] [CrossRef]
- Apicella, F.; Costanzo, V.; Purpura, G. Are early visual behavior impairments involved in the onset of autism spectrum disorders? Insights for early diagnosis and intervention. Eur. J. Pediatr. 2020, 179, 225–234. [Google Scholar] [CrossRef]
- Jones, W.; Klaiman, C.; Richardson, S.; Aoki, C.; Smith, C.; Minjarez, M.; Bernier, R.; Pedapati, E.; Bishop, S.; Ence, W. Eye-tracking–based measurement of social visual engagement compared with expert clinical diagnosis of autism. JAMA 2023, 330, 854–865. [Google Scholar] [CrossRef]
- Clark, A. Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behav. Brain Sci. 2013, 36, 181–204. [Google Scholar] [CrossRef]
- Hobson, J.A.; Friston, K.J. Waking and dreaming consciousness: Neurobiological and functional considerations. Prog. Neurobiol. 2012, 98, 82–98. [Google Scholar] [CrossRef]
- Friston, K.J.; Stephan, K.E.; Montague, R.; Dolan, R.J. Computational psychiatry: The brain as a phantastic organ. Lancet Psychiatry 2014, 1, 148–158. [Google Scholar] [CrossRef]
- Hobson, J.A.; Hong, C.C.-H.; Friston, K. Virtual reality and consciousness inference in dreaming. Front. Psychol. 2014, 5, 1133. [Google Scholar] [CrossRef]
- von Helmholtz, H. Helmholtz’s Treatise on Physiological Optics (Translated from the Third German Edition); The Optical Society of America: Menasha, WI, USA, 1925; Volume 3. [Google Scholar]
- Andrillon, T.; Nir, Y.; Cirelli, C.; Tononi, G.; Fried, I. Single-neuron activity and eye movements during human REM sleep and awake vision. Nat. Commun. 2015, 6, 7884. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, T.; Tamaki, M.; Miyawaki, Y.; Kamitani, Y. Neural decoding of visual imagery during sleep. Science 2013, 340, 639–642. [Google Scholar] [CrossRef]
- Siclari, F.; Baird, B.; Perogamvros, L.; Bernardi, G.; LaRocque, J.J.; Riedner, B.; Boly, M.; Postle, B.R.; Tononi, G. The neural correlates of dreaming. Nat. Neurosci. 2017, 20, 872. [Google Scholar] [CrossRef] [PubMed]
- Seth, A.K. Being You: A New Science of Consciousnes; Penguin Publishing Group: London, UK, 2021. [Google Scholar]
- Metzinger, T. Being No One: The Self-Model Theory of Subjectivity; MIT Press: Cambridge, MA, USA, 2003. [Google Scholar]
- Metzinger, T. Empirical perspectives from the self-model theory of subjectivity: A brief summary with examples. In Progress in Brain Research; Rahul, B., Bikas, K.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 215–278. [Google Scholar]
- Blanke, O. Multisensory brain mechanisms of bodily self-consciousness. Nat. Rev. Neurosci. 2012, 13, 556–571. [Google Scholar] [CrossRef] [PubMed]
- Ehrsson, H.H. The concept of body ownership and its relation to multisensory integration. In The New Handbook of Multisensory Processes; Stein, B.E., Ed.; MIT Press: Cambridge, UK, 2012; pp. 775–792. [Google Scholar]
- Staunton, H. The function of dreaming. Rev. Neurosci. 2001, 12, 365–371. [Google Scholar] [CrossRef]
- Metzinger, T. The Ego Tunnel: The Science of the Mind and the Myth of the Self; Basic Books: New York, NY, USA, 2009. [Google Scholar]
- Windt, J.M. Predictive brains, dreaming selves, sleeping bodies: How the analysis of dream movement can inform a theory of self- and world-simulation in dreams. Synthese 2018, 195, 2577–2625. [Google Scholar] [CrossRef]
- Hobson, A. Conscious States: The AIM Model of Waking, Sleeping, and Dreaming; Tranquillo, N., Shin, T., Eds.; Create Space: Scotts Valley, CA, USA, 2017. [Google Scholar]
- Bayne, T.; Frohlich, J.; Cusack, R.; Moser, J.; Naci, L. Consciousness in the cradle: On the emergence of infant experience. Trends Cogn. Sci. 2023, 27, 1135–1149. [Google Scholar] [CrossRef]
- Courchesne, E.; Gazestani, V.H.; Lewis, N.E. Prenatal origins of ASD: The when, what, and how of ASD development. Trends Neurosci. 2020, 43, 326–342. [Google Scholar] [CrossRef]
- Friston, K.; Adams, R.A.; Perrinet, L.; Breakspear, M. Perceptions as hypotheses: Saccades as experiments. Front. Psychol. 2012, 3, 151. [Google Scholar] [CrossRef]
- Crick, F. Function of the thalamic reticular complex: The searchlight hypothesis. Proc. Natl. Acad. Sci. USA 1984, 81, 4586–4590. [Google Scholar] [CrossRef]
- Crick, F.C.; Koch, C. What is the function of the claustrum? Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Koubeissi, M.Z.; Bartolomei, F.; Beltagy, A.; Picard, F. Electrical stimulation of a small brain area reversibly disrupts consciousness. Epilepsy Behav. 2014, 37, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Remedios, R.; Logothetis, N.K.; Kayser, C. Unimodal responses prevail within the multisensory claustrum. J. Neurosci. 2010, 30, 12902–12907. [Google Scholar] [CrossRef] [PubMed]
- Reale, R.A.; Calvert, G.A.; Thesen, T.; Jenison, R.L.; Kawasaki, H.; Oya, H.; Howard, M.A.; Brugge, J.F. Auditory-visual processing represented in the human superior temporal gyrus. Neuroscience 2007, 145, 162–184. [Google Scholar] [CrossRef]
- Gao, C.; Green, J.J.; Yang, X.; Oh, S.; Kim, J.; Shinkareva, S.V. Audiovisual integration in the human brain: A coordinate-based meta-analysis. Cereb. Cortex 2023, 33, 5574–5584. [Google Scholar] [CrossRef]
- Szymusiak, R. Magnocellular nuclei of the basal forebrain: Substrates of sleep and arousal regulation. Sleep 1995, 18, 478–500. [Google Scholar] [CrossRef]
- Solari, N.; Hangya, B. Cholinergic modulation of spatial learning, memory and navigation. Eur. J. Neurosci. 2018, 48, 2199–2230. [Google Scholar] [CrossRef]
- Lebow, M.A.; Chen, A. Overshadowed by the amygdala: The bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol. Psychiatry 2016, 21, 450–463. [Google Scholar] [CrossRef]
- Mobbs, D.; Yu, R.; Rowe, J.B.; Eich, H.; FeldmanHall, O.; Dalgleish, T. Neural activity associated with monitoring the oscillating threat value of a tarantula. Proc. Natl. Acad. Sci. USA 2010, 107, 20582–20586. [Google Scholar] [CrossRef]
- Beauchamp, M.S.; Nath, A.R.; Pasalar, S. fMRI-Guided transcranial magnetic stimulation reveals that the superior temporal sulcus is a cortical locus of the McGurk effect. J. Neurosci. 2010, 30, 2414–2417. [Google Scholar] [CrossRef]
- Guterstam, A.; Björnsdotter, M.; Gentile, G.; Ehrsson, H.H. Posterior cingulate cortex integrates the senses of self-location and body ownership. Curr. Biol. 2015, 25, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.S.; Place, R.; Starrett, M.J.; Chrastil, E.R.; Nitz, D.A. Rethinking retrosplenial cortex: Perspectives and predictions. Neuron 2023, 111, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Maguire, E.A. The retrosplenial contribution to human navigation: A review of lesion and neuroimaging findings. Scand. J. Psychol. 2001, 42, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Harker, K.T.; Whishaw, I.Q. A reaffirmation of the retrosplenial contribution to rodent navigation: Reviewing the influences of lesion, strain, and task. Neurosci. Biobehav. Rev. 2004, 28, 485–496. [Google Scholar] [CrossRef]
- Vann, S.D.; Aggleton, J.P.; Maguire, E.A. What does the retrosplenial cortex do? Nat. Rev. Neurosci. 2009, 10, 792–802. [Google Scholar] [CrossRef]
- Agron, A.M.; Martin, A.; Gilmore, A.W. Scene construction and autobiographical memory retrieval in autism spectrum disorder. Autism Res. 2024, 17, 204–214. [Google Scholar] [CrossRef]
- Hassabis, D.; Maguire, E.A. Deconstructing episodic memory with construction. Trends Cogn. Sci. 2007, 11, 299–306. [Google Scholar] [CrossRef]
- Spreng, R.N.; Mar, R.A.; Kim, A.S. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta-analysis. J. Cogn. Neurosci. 2009, 21, 489–510. [Google Scholar] [CrossRef]
- Auger, S.D.; Mullally, S.L.; Maguire, E.A. Retrosplenial cortex codes for permanent landmarks. PLoS ONE 2012, 7, e43620. [Google Scholar] [CrossRef]
- Auger, S.D.; Maguire, E.A. Assessing the mechanism of response in the retrosplenial cortex of good and poor navigators. Cortex 2013, 49, 2903–2913. [Google Scholar] [CrossRef]
- Auger, S.D.; Zeidman, P.; Maguire, E.A. A central role for the retrosplenial cortex in de novo environmental learning. eLife 2015, 4, e09031. [Google Scholar] [CrossRef] [PubMed]
- Minoshima, S.; Giordani, B.; Berent, S.; Frey, K.A.; Foster, N.L.; Kuhl, D.E. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann. Neurol. 1997, 42, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Nestor, P.J.; Fryer, T.D.; lkeda, M.; Hodges, J.R. Retrosplenial cortex (BA 29/30) hypometabolism in mild cognitive impairment (prodromal Alzheimer’s disease). Eur. J. Neurosci. 2003, 18, 2663–2667. [Google Scholar] [CrossRef] [PubMed]
- Pengas, G.; Hodges, J.R.; Watson, P.; Nestor, P.J. Focal posterior cingulate atrophy in incipient Alzheimer’s disease. Neurobiol. Aging 2010, 31, 25–33. [Google Scholar] [CrossRef]
- Lind, S.E.; Bowler, D.M.; Raber, J. Spatial navigation, episodic memory, episodic future thinking, and theory of mind in children with autism spectrum disorder: Evidence for impairments in mental simulation? Front. Psychol. 2014, 5, 1411. [Google Scholar] [CrossRef]
- Pellicano, E.; Smith, A.D.; Cristino, F.; Hood, B.M.; Briscoe, J.; Gilchrist, I.D. Children with autism are neither systematic nor optimal foragers. Proc. Natl. Acad. Sci. USA 2011, 108, 421–426. [Google Scholar] [CrossRef]
- Smith, M.; Cameron, L.; Ferguson, H.J. Scene construction ability in neurotypical and autistic adults. Autism 2024, 28, 1919–1933. [Google Scholar] [CrossRef]
- Darby, R.R.; Laganiere, S.; Pascual-Leone, A.; Prasad, S.; Fox, M.D. Finding the imposter: Brain connectivity of lesions causing delusional misidentifications. Brain 2017, 140, 497–507. [Google Scholar] [CrossRef]
- Anderson, D.N. The delusion of inanimate doubles: Implications for understanding the Capgras phenomenon. Br. J. Psychiatry 1988, 153, 694–699. [Google Scholar] [CrossRef]
- Dehaene, S.; Changeux, J.P. Experimental and theoretical approaches to conscious processing. Neuron 2011, 70, 200–227. [Google Scholar] [CrossRef]
- Hohwy, J. The Predictive Mind; Oxford University Press: New York, NY, USA, 2013. [Google Scholar]
- Matei, M.; Bergel, A.; Pezet, S.; Tanter, M. Global dissociation of the posterior amygdala from the rest of the brain during REM sleep. Commun. Biol. 2022, 5, 1306. [Google Scholar] [CrossRef] [PubMed]
- Bergel, A.; Deffieux, T.; Demené, C.; Tanter, M.; Cohen, I. Local hippocampal fast gamma rhythms precede brain-wide hyperemic patterns during spontaneous rodent REM sleep. Nat. Commun. 2018, 9, 5364. [Google Scholar] [CrossRef] [PubMed]
- Andrews-Hanna, J.R.; Smallwood, J.; Spreng, R.N. The default network and self-generated thought: Component processes, dynamic control, and clinical relevance. Ann. N. Y. Acad. Sci. 2014, 1316, 29–52. [Google Scholar] [CrossRef] [PubMed]
- Yarkoni, T.; Poldrack, R.A.; Nichols, T.E.; Van Essen, D.C.; Wager, T.D. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods 2011, 8, 665–670. [Google Scholar] [CrossRef]
- Yeo, B.T.T.; Krienen, F.M.; Sepulcre, J.; Sabuncu, M.R.; Lashkari, D.; Hollinshead, M.; Roffman, J.L.; Smoller, J.W.; Zöllei, L.; Pollimeni, J.R. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011, 106, 1125–1165. [Google Scholar]
- Raichle, M.E.; MacLeod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. A default mode of brain function. Proc. Natl. Acad. Sci. USA 2001, 98, 676–682. [Google Scholar] [CrossRef]
- Margulies, D.S.; Ghosh, S.S.; Goulas, A.; Falkiewicz, M.; Huntenburg, J.M.; Langs, G.; Bezgin, G.; Eickhoff, S.B.; Castellanos, F.X.; Petrides, M. Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc. Natl. Acad. Sci. USA 2016, 113, 12574–12579. [Google Scholar] [CrossRef]
- Sormaz, M.; Murphy, C.; Wang, H.-t.; Hymers, M.; Karapanagiotidis, T.; Poerio, G.; Margulies, D.S.; Jefferies, E.; Smallwood, J. Default mode network can support the level of detail in experience during active task states. Proc. Natl. Acad. Sci. USA 2018, 115, 9318–9323. [Google Scholar] [CrossRef]
- Smallwood, J.; Bernhardt, B.C.; Leech, R.; Bzdok, D.; Jefferies, E.; Margulies, D.S. The default mode network in cognition: A topographical perspective. Nat. Rev. Neurosci. 2021, 22, 503–513. [Google Scholar] [CrossRef]
- Raichle, M.E. Two views of brain function. Trends Cogn. Sci. 2010, 14, 180–190. [Google Scholar] [CrossRef]
- Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Corbetta, M.; Van Essen, D.C.; Raichle, M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. USA 2005, 102, 9673–9678. [Google Scholar] [CrossRef] [PubMed]
- Chow, H.M.; Horovitz, S.G.; Carr, W.S.; Picchioni, D.; Coddington, N.; Fukunaga, M.; Yisheng, X.; Balkin, T.J.; Duyn, J.H.; Braun, A.R. Rhythmic alternating patterns of brain activity distinguish rapid eye movement sleep from other states of consciousness. Proc. Natl. Acad. Sci. USA 2013, 110, 10300–10305. [Google Scholar] [CrossRef] [PubMed]
- Vatansever, D.; Menon, D.K.; Manktelow, A.E.; Sahakian, B.J.; Stamatakis, E.A. Default mode network connectivity during task execution. NeuroImage 2015, 122, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Elison, J.T.; Paterson, S.J.; Wolff, J.J.; Reznick, J.S.; Sasson, N.J.; Gu, H.; Botteron, K.N.; Dager, S.R.; Estes, A.M.; Evans, A.C. White matter microstructure and atypical visual orienting in 7-month-olds at risk for autism. Am. J. Psychiatry 2013, 170, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Yeshurun, Y.; Nguyen, M.; Hasson, U. The default mode network: Where the idiosyncratic self meets the shared social world. Nat. Rev. Neurosci. 2021, 22, 181–192. [Google Scholar] [CrossRef]
- Braga, R.M.; Sharp, D.J.; Leeson, C.; Wise, R.J.S.; Leech, R. Echoes of the brain within default mode, association, and heteromodal cortices. J. Neurosci. 2013, 33, 14031–14039. [Google Scholar] [CrossRef]
- Leech, R.; Smallwood, J. The posterior cingulate cortex: Insights from structure and function. Handb. Clin. Neurol. 2019, 166, 73–85. [Google Scholar]
- Johnson, B.P.; Lum, J.A.; Rinehart, N.J.; Fielding, J. Ocular motor disturbances in autism spectrum disorders: Systematic review and comprehensive meta-analysis. Neurosci. Biobehav. Rev. 2016, 69, 260–279. [Google Scholar] [CrossRef]
- Bast, N.; Mason, L.; Freitag, C.M.; Smith, T.; Portugal, A.M.; Poustka, L.; Banaschewski, T.; Johnson, M.; The EU-AIMS LEAP Group. Saccade dysmetria indicates attenuated visual exploration in autism spectrum disorder. J. Child Psychol. Psychiatry 2021, 62, 149–159. [Google Scholar] [CrossRef]
- Caldani, S.; Steg, S.; Lefebvre, A.; Atzori, P.; Peyre, H.; Delorme, R.; Bucci, M.P. Oculomotor behavior in children with autism spectrum disorders. Autism 2020, 24, 670–679. [Google Scholar] [CrossRef]
- Bedford, R.; Gliga, T.; Shephard, E.; Elsabbagh, M.; Pickles, A.; Charman, T.; Johnson, M.H. Neurocognitive and observational markers: Prediction of autism spectrum disorder from infancy to mid-childhood. Mol. Autism 2017, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Wass, S.V.; Jones, E.J.; Gliga, T.; Smith, T.J.; Charman, T.; Johnson, M.H.; BASIS Team. Shorter spontaneous fixation durations in infants with later emerging autism. Sci. Rep. 2015, 5, 8284. [Google Scholar] [CrossRef] [PubMed]
- Brenner, L.A.; Turner, K.C.; Müller, R.-A. Eye movement and visual search: Are there elementary abnormalities in autism? J. Autism Dev. Disord. 2007, 37, 1289–1309. [Google Scholar] [CrossRef] [PubMed]
- Chawarska, K.; Macari, S.; Shic, F. Decreased spontaneous attention to social scenes in 6-month-old infants later diagnosed with autism spectrum disorders. Biol. Psychiatry 2013, 74, 195–203. [Google Scholar] [CrossRef]
- Cheung, C.H.M.; Bedford, R.; Johnson, M.H.; Charman, T.; Gliga, T. Visual search performance in infants associates with later ASD diagnosis. Dev. Cogn. Neurosci. 2018, 29, 4–10. [Google Scholar] [CrossRef]
- Palmer, C.J.; Lawson, R.P.; Hohwy, J. Bayesian approaches to autism: Towards volatility, action, and behavior. Psychol. Bull. 2017, 143, 521. [Google Scholar] [CrossRef]
- Gliga, T.; Bedford, R.; Charman, T.; Johnson, M.H.; The BASIS Team. Enhanced visual search in infancy predicts emerging autism symptoms. Curr. Biol. 2015, 25, 1727–1730. [Google Scholar] [CrossRef]
- Chita-Tegmark, M. Social attention in ASD: A review and meta-analysis of eye-tracking studies. Res. Dev. Disabil. 2016, 48, 79–93. [Google Scholar] [CrossRef]
- Wen, T.H.; Cheng, A.; Andreason, C.; Zahiri, J.; Xiao, Y.; Xu, R.; Bao, B.; Courchesne, E.; Barnes, C.C.; Arias, S.J. Large scale validation of an early-age eye-tracking biomarker of an autism spectrum disorder subtype. Sci. Rep. 2022, 12, 4253. [Google Scholar] [CrossRef]
- Constantino, J.N.; Kennon-McGill, S.; Weichselbaum, C.; Marrus, N.; Haider, A.; Glowinski, A.L.; Gillespie, S.; Klakman, C.; Klin, A.; Jones, W. Infant viewing of social scenes is under genetic control and is atypical in autism. Nature 2017, 547, 340–344. [Google Scholar] [CrossRef]
- Jones, W.; Klin, A. Attention to eyes is present but in decline in 2–6-month-old infants later diagnosed with autism. Nature 2013, 504, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Meng, Y.; He, J.; Xiang, Y.; Wu, C.; Wang, S.; Yuan, Z. McGurk effect by individuals with autism spectrum disorder and typically developing controls: A systematic review and meta-analysis. J. Autism Dev. Disord. 2019, 49, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Jertberg, R.M.; Wienicke, F.J.; Andruszkiewicz, K.; Begeer, S.; Chakrabarti, B.; Geurts, H.M.; de Vries, R.; Van der Burg, E. Differences between autistic and non-autistic individuals in audiovisual speech integration: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2024, 164, 105787. [Google Scholar] [CrossRef] [PubMed]
- McGurk, H.; MacDonald, J. Hearing lips and seeing voices. Nature 1976, 264, 746–748. [Google Scholar] [CrossRef]
- Redcay, E. The superior temporal sulcus performs a common function for social and speech perception: Implications for the emergence of autism. Neurosci. Biobehav. Rev. 2008, 32, 123–142. [Google Scholar] [CrossRef]
- Boddaert, N.; Chabane, N.; Gervais, H.; Good, C.D.; Bourgeois, M.; Plumet, M.; Barthélémy, C.; Mouren, M.-C.; Artiges, E.; Sampson, Y. Superior temporal sulcus anatomical abnormalities in childhood autism: A voxel-based morphometry MRI study. Neuroimage 2004, 23, 364–369. [Google Scholar] [CrossRef]
- Perry, E.; Lee, M. The cholinergic system in autism. In The Neurobiology of Autism; Bauman, M.L., Kemper, T.L., Eds.; JHU Press: Baltimore, MD, USA, 2005; pp. 331–348. [Google Scholar]
- Riva, D.; Bulgheroni, S.; Aquino, D.; Di Salle, F.; Savoiardo, M.; Erbetta, A. Basal forebrain involvement in low-functioning autistic children: A voxel-based morphometry study. Am. J. Neuroradiol. 2011, 32, 1430–1435. [Google Scholar] [CrossRef]
- Coria-Avila, G.A.; Manzo, J.; Garcia, L.I.; Carrillo, P.; Miquel, M.; Pfaus, J.G. Neurobiology of social attachments. Neurosci. Biobehav. Rev. 2014, 43, 173–182. [Google Scholar] [CrossRef]
- Luo, P.X.; Zakharenkov, H.C.; Torres, L.Y.; Rios, R.A.; Gegenhuber, B.; Black, A.M.; Xu, C.K.; Minie, A.; Tran, A.M.; Tollkuhn, J. Oxytocin receptor behavioral effects and cell types in the bed nucleus of the stria terminalis. Horm. Behav. 2022, 143, 105203. [Google Scholar] [CrossRef]
- Kremarik, P.; Freund-Mercier, M.; Stoeckel, M. Autoradiographic detection of oxytocin-and vasopressin-binding sites in various subnuclei of the bed nucleus of the stria terminalis in the rat. Effects of functional and experimental sexual steroid variations. J. Neuroendocrinol. 1991, 3, 689–698. [Google Scholar] [CrossRef]
- Quattrocki, E.; Friston, K. Autism, oxytocin and interoception. Neurosci. Biobehav. Rev. 2014, 47, 410–430. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, Y.; Tachigori, S.-I.; Arakawa, H. Faded neural projection from the posterior bed nucleus of the stria terminalis to the lateral habenula contributes to social signaling deficit in male BTBR mice as a mouse model of autism. Psychoneuroendocrinology 2023, 149, 106004. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Shi, Y.; Du, X.; Wang, J.; Zhang, Y.; Shan, S.; Yuan, Y.; Wang, R.; Zhou, C.; Liu, Y. SENP1 in the retrosplenial agranular cortex regulates core autistic-like symptoms in mice. Cell Rep. 2021, 37, 109939. [Google Scholar] [CrossRef] [PubMed]
- Garrido, D.; Beretta, S.; Grabrucker, S.; Bauer, H.F.; Bayer, D.; Sala, C.; Verpelli, C.; Roselli, F.R.; Bockmann, J.; Proepper, C. Shank2/3 double knockout-based screening of cortical subregions links the retrosplenial area to the loss of social memory in autism spectrum disorders. Mol. Psychiatry 2022, 27, 4994–5006. [Google Scholar] [CrossRef]
- Kennedy, D.P.; Redcay, E.; Courchesne, E. Failing to deactivate: Resting functional abnormalities in autism. Proc. Natl. Acad. Sci. USA 2006, 103, 8275–8280. [Google Scholar] [CrossRef]
- Murdaugh, D.L.; Shinkareva, S.V.; Deshpande, H.R.; Wang, J.; Pennick, M.R.; Kana, R.K. Differential deactivation during mentalizing and classification of autism based on default mode network connectivity. PLoS ONE 2012, 7, e50064. [Google Scholar] [CrossRef]
- Spencer, M.D.; Chura, L.R.; Holt, R.J.; Suckling, J.; Calder, A.J.; Bullmore, E.T.; Baron-Cohen, S. Failure to deactivate the default mode network indicates a possible endophenotype of autism. Mol. Autism 2012, 3, 15. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, X.; Gu, H.; Yang, Y. Resting-state glutamate and GABA concentrations predict task-induced deactivation in the default mode network. J. Neurosci. 2013, 33, 18566–18573. [Google Scholar] [CrossRef]
- Padmanabhan, A.; Lynch, C.J.; Schaer, M.; Menon, V. The default mode network in autism. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2017, 2, 476–486. [Google Scholar] [CrossRef]
- Lombardo, M.V.; Eyler, L.; Moore, A.; Datko, M.; Carter Barnes, C.; Cha, D.; Courchesne, E.; Pierce, K. Default mode-visual network hypoconnectivity in an autism subtype with pronounced social visual engagement difficulties. eLife 2019, 8, e47427. [Google Scholar] [CrossRef]
- McKinnon, C.J.; Eggebrecht, A.T.; Todorov, A.; Wolff, J.J.; Elison, J.T.; Adams, C.M.; Snyder, A.Z.; Estes, A.M.; Zwaigenbaum, Z.; Botteron, K.N. Restricted and repetitive behavior and brain functional connectivity in infants at risk for developing autism spectrum disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2019, 4, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-J.; Vos de Wael, R.; Bethlehem, R.A.I.; Lariviere, S.; Paquola, C.; Valk, S.L.; Milham, M.P.; Di Martino, A.; Margulies, D.S.; Smallwood, J.S. Atypical functional connectome hierarchy in autism. Nat. Commun. 2019, 10, 1022. [Google Scholar] [CrossRef] [PubMed]
- Rolison, M.; Lacadie, C.; Chawarska, K.; Spann, M.; Scheinost, D. Atypical intrinsic hemispheric interaction associated with autism spectrum disorder is present within the first year of life. Cereb. Cortex 2021, 32, 1212–1222. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.M.; Honjoh, S.; Rodriguez, A.V.; Cirelli, C.; Tononi, G. Local slow waves in superficial layers of primary cortical areas during REM sleep. Curr. Biol. 2016, 26, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Seth, A.K.; Bayne, T. Theories of consciousness. Nat. Rev. Neurosci. 2022, 23, 439–452. [Google Scholar] [CrossRef]
- Shein-Idelson, M.; Ondracek, J.M.; Liaw, H.-P.; Reiter, S.; Laurent, G. Slow waves, sharp waves, ripples, and REM in sleeping dragons. Science 2016, 352, 590–595. [Google Scholar] [CrossRef]
- Deiber, M.P.; Ibañez, V.; Bastuji, H.; Fischer, C.; Mauguière, F. Changes of middle latency auditory evoked potentials during natural sleep in humans. Neurology 1989, 39, 806–813. [Google Scholar] [CrossRef]
- Issa, E.B.; Wang, X. Sensory Responses during Sleep in Primate Primary and Secondary Auditory Cortex. J. Neurosci. 2008, 28, 14467–14480. [Google Scholar] [CrossRef]
- Blume, C.; del Giudice, R.; Wislowska, M.; Heib, D.P.J.; Schabus, M. Standing sentinel during human sleep: Continued evaluation of environmental stimuli in the absence of consciousness. NeuroImage 2018, 178, 638–648. [Google Scholar] [CrossRef]
- Hong, C.C.-H.; Harris, J.C. Study of neural correlates of rapid eye movements in dreaming sleep using video camera for timing of REMs and functional MRI: Its implications [editorial]. Sleep Hypn. 2009, 11, 1–4. [Google Scholar]
- Thomason, M.E. Development of brain networks in utero: Relevance for common neural disorders. Biol. Psychiatry 2020, 88, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Schöpf, V.; Schlegl, T.; Jakab, A.; Kasprian, G.; Woitek, R.; Prayer, D.; Langs, G. The relationship between eye movement and vision develops before birth. Front. Hum. Neurosci. 2014, 8, 775. [Google Scholar]
- Pagani, M.; Gutierrez-Barragan, D.; de Guzman, A.E.; Xu, T.; Gozzi, A. Mapping and comparing fMRI connectivity networks across species. Commun. Biol. 2023, 6, 1238. [Google Scholar] [CrossRef] [PubMed]
- Deffieux, T.; Demené, C.; Tanter, M. Functional ultrasound imaging: A new imaging modality for neuroscience. Neuroscience 2021, 474, 110–121. [Google Scholar] [CrossRef]
- Macé, E.; Montaldo, G.; Cohen, I.; Baulac, M.; Fink, M.; Tanter, M. Functional ultrasound imaging of the brain. Nat. Methods 2011, 8, 662–664. [Google Scholar] [CrossRef]
- Ferrier, J.; Tiran, E.; Deffieux, T.; Tanter, M.; Lenkei, Z. Functional imaging evidence for task-induced deactivation and disconnection of a major default mode network hub in the mouse brain. Proc. Natl. Acad. Sci. USA 2020, 117, 15270–15280. [Google Scholar] [CrossRef]
- Bianciardi, M.; Fukunaga, M.; Van Gelderen, P.; De Zwart, J.A.; Duyn, J.H. Negative BOLD-fMRI signals in large cerebral veins. J. Cereb. Blood Flow Metab. 2011, 31, 401–412. [Google Scholar] [CrossRef]
- Dizeux, A.; Gesnik, M.; Ahnine, H.; Blaize, K.; Arcizet, F.; Picaud, S.; Sahel, J.-A.; Deffieux, T.; Pouget, P.; Tanter, M. Functional ultrasound imaging of the brain reveals propagation of task-related brain activity in behaving primates. Nat. Commun. 2019, 10, 1400. [Google Scholar] [CrossRef]
- Demene, C.; Baranger, J.; Bernal, M.; Delanoe, C.; Auvin, S.; Biran, V.; Alison, M.; Mairesse, J.; Harribaud, E.; Pernot, M. Functional ultrasound imaging of brain activity in human newborns. Sci. Transl. Med. 2017, 9, eaah6756. [Google Scholar] [CrossRef]
- Baranger, J.; Demene, C.; Frerot, A.; Faure, F.; Delanoë, C.; Serroune, H.; Houdouin, A.; Mairesse, J.; Biran, V.; Baud, O. Bedside functional monitoring of the dynamic brain connectivity in human neonates. Nat. Commun. 2021, 12, 1080. [Google Scholar] [CrossRef]
- Wehrle, R.; Czisch, M.; Kaufmann, C.; Wetter, T.C.; Holsboer, F.; Auer, D.P.; Pollmächer, T. Rapid eye movement-related brain activation in human sleep: A functional magnetic resonance imaging study. NeuroReport 2005, 16, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, S.; Misaki, M.; Kan, S.; Fukunaga, T.; Koike, T. Human brain activity time-locked to rapid eye movements during REM sleep. Exp. Brain Res. 2009, 192, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Kimmig, H.; Greenlee, M.W.; Gondan, M.; Schira, M.; Kassubek, J.; Mergner, T. Relationship between saccadic eye movements and cortical activity as measured by fMRI, quantitative and qualitative aspects. Exp. Brain Res. 2001, 141, 184–194. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tanguay, P.E.; Ornitz, E.M.; Forsythe, A.B.; Ritvo, E.R. Rapid eye movement (REM) activity in normal and autistic children during REM sleep. J. Autism Child. Schizophr. 1976, 6, 275–288. [Google Scholar] [CrossRef]
- Mason, T.B., II; Teoh, L.; Calabro, K.; Traylor, J.; Karamessinis, L.; Schultz, B.; Samuel, J.; Gallagher, P.R.; Marcus, C.L. Rapid eye movement latency in children and adolescents. Pediatr. Neurol. 2008, 39, 162–169. [Google Scholar] [CrossRef]
- Ohayon, M.M.; Carskadon, M.A.; Guilleminault, C.; Vitiello, M.V. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: Developing normative sleep values across the human lifespan. Sleep 2004, 27, 1255–1273. [Google Scholar] [CrossRef]
- Born, P.; Leth, H.; Miranda, M.J.; Rostrup, E.; Stensgaard, A.; Peitersen, B.; Larsson, H.B.W.; Lou, H.C. Visual activation in infants and young children studied by functional magnetic resonance imaging. Pediatr. Res. 1998, 44, 578–583. [Google Scholar] [CrossRef]
- Grigg-Damberger, M.M. The visual scoring of sleep in infants 0 to 2 months of age. J. Clin. Sleep Med. 2016, 12, 429–445. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, C.C.-H. Rapid Eye Movements in Sleep Furnish a Unique Probe into the Ontogenetic and Phylogenetic Development of the Visual Brain: Implications for Autism Research. Brain Sci. 2025, 15, 574. https://doi.org/10.3390/brainsci15060574
Hong CC-H. Rapid Eye Movements in Sleep Furnish a Unique Probe into the Ontogenetic and Phylogenetic Development of the Visual Brain: Implications for Autism Research. Brain Sciences. 2025; 15(6):574. https://doi.org/10.3390/brainsci15060574
Chicago/Turabian StyleHong, Charles Chong-Hwa. 2025. "Rapid Eye Movements in Sleep Furnish a Unique Probe into the Ontogenetic and Phylogenetic Development of the Visual Brain: Implications for Autism Research" Brain Sciences 15, no. 6: 574. https://doi.org/10.3390/brainsci15060574
APA StyleHong, C. C.-H. (2025). Rapid Eye Movements in Sleep Furnish a Unique Probe into the Ontogenetic and Phylogenetic Development of the Visual Brain: Implications for Autism Research. Brain Sciences, 15(6), 574. https://doi.org/10.3390/brainsci15060574





