Abstract
Background: Despite advances in glioblastoma (GBM) management, median overall survival (mOS) remains poor, and multi-modal disparities persist. We sought to evaluate trends in GBM treatment and survival outcomes from 2005–2020, with a focus on sociodemographic and geographic disparities. Methods: We conducted a retrospective US-based cohort study using the National Cancer Database (NCDB), stratifying study period into four intervals (2005–2008, 2009–2012, 2013–2016, and 2017–2020). Logistic regression was used to identified predictors of receipt of combination surgery, radiation, and chemotherapy (Sx+RT+Chemo). Kaplan–Meier and multivariable Cox proportional hazards approaches were used to assess mOS. Results: A total of 111,955 adults with GBM were included. From 2005–2008 to 2017–2020, mOS increased from 7.8 to 9.5 months, with geographically unequal gains in survival across the US. In multivariable logistic regression model adjusting for known confounders, combined Sx+RT+Chemo was less likely to be received by female patients (OR 0.90, 95% CI 0.88–0.92) vs. male, non-White patients (OR 0.90, 95% CI 0.86–0.94) vs. White, patients treated at community hospitals (OR: 0.78, 95% CI 0.76–0.80) vs. academic centers, publicly-insured patients (OR 0.74, 95% CI 0.71–0.76) or uninsured patients (OR 0.54, 95% CI 0.50–0.58) vs. privately-insured, and patients living in the South (OR 0.88, 95% CI 0.85–0.91), Midwest (OR 0.83, 95% CI 0.80–0.86), and West (OR 0.85, 95% CI 0.81–0.88) compared to the Northeast. In multivariable Cox regression, significantly poorer survival was seen amongst non-metropolitan patients, community-based hospital patients, and publicly-insured and uninsured patients (vs. privately-insured), despite adjusting for prognostic factors. Conclusions: Only modest improvement in mOS of GBM patients has occurred across 2005–2020, with persistent disparities linked to sociodemographic and structural factors, whose redressal warrants multi-pronged efforts.
1. Introduction
Glioblastoma (GBM) is the most common primary brain tumor in adults and accounts for 45.2% of all primary malignant brain and central nervous system (CNS) tumors [1,2]. As an aggressive and grade 4 astrocytic tumor, it is characterized by diffuse infiltration, necrosis and microvascular proliferation [3,4]. Therapeutic advances in GBM remain challenging given the rapid-growing, invasive nature of the disease and blood–brain barrier penetration limitations, which hinders effective drug delivery [5,6]. Conventional treatment modalities include maximal safe surgical resection followed by radiation and chemotherapy [7,8]. Recent approaches include tumor treating fields, targeted brachytherapy, and molecularly guided therapy, which are now being adopted in specialized settings [9,10]. Despite these advances, the prognosis of GBM remains poor, with a 5-year survival rate of 5.6% in adults aged ≥40 years [11,12]. These persistent sobering survival outcomes have been, accompanied by advances in our understanding of tumor biology and classification [13,14]. Molecular profiling with features such as O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation, observed in 35–40% of GBM cases, predicts improved responses to alkylating chemotherapy, associated with better survival outcomes [15,16,17]. However, despite advances in molecular understanding and treatment of GBM, there remains a paucity of large-scale, population-based data evaluating how sociodemographic disparities influence access to care and outcomes. This study aims to evaluate the trends in GBM management strategies and clinical outcomes in a large and diverse patient population across the United States (US), as well as to examine disparities in care and survival.
2. Materials and Methods
2.1. Study Design and Data Source
This was a retrospective cohort study performed using the National Cancer Database (NCDB), a cancer dataset jointly maintained by the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The work is reported following the STROBE reporting guidelines (STROBE Flow Diagram in Figure 1). The NCDB contains hospital registry data from over 1500 accredited facilities that represent nearly 70% of newly diagnosed cancers cases across the country [18]. Data are abstracted by certified tumor registrars at each participating institution and submitted following standardized coding protocols to ensure uniformity and quality across the dataset. National trends in GBM demographics, treatment patterns, and survival outcomes were analyzed. To assess temporal shifts, the 15-year study period was divided into four approximately equal intervals (2005–2008, 2009–2012, 2013–2016, and 2017–2020), allowing for evaluation of evolving treatment practices and disparities across consistent timeframes. The intervals were chosen to balance temporal resolution with sufficient sample sizes for robust statistical comparisons. The preliminary findings of this study were presented prior [19]. This project is registered in the Open Science Framework: DOI 10.17605/OSF.IO/VJ6FQ.
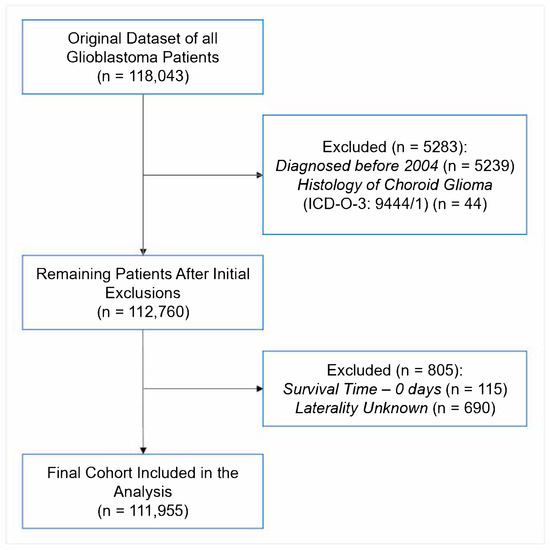
Figure 1.
STROBE Diagram of Patient Inclusion for Analysis.
2.2. Study Population
Patients were diagnosed with primary GBM prior to study enrollment. They were identified using the International Classification of Diseases for Oncology—Third Edition (ICD-O-3) morphology codes [20], which are further delineated in Supplementary Table S1. For consistency and to assess temporal trends, the data were stratified into four periods: 2005–2008, 2009–2012, 2013–2016, and 2017–2020. Patients with a survival time of 0 days or unknown laterality were excluded to ensure the completeness and reliability of the final analytic cohort (Figure 1).
2.3. Variables and Measures
Patient demographics included age at diagnosis (categorized as 40–49, 50–64, 65–74, and 74–90 years), sex (male, female), race (White, non-White), ethnicity (Hispanic, non-Hispanic), geographic region (Northeast, South, Midwest, West), household income, and facility type (academic, community, or network). Median household income was estimated using the American Community Survey (ACS) 2016–2020 5-year estimates, as matched by patient ZIP code. Income was categorized as <$74,063 versus ≥$74,063, based on the NCDB 2022 Participant User File income quartiles. The income cutoff of $74,063 was selected to align closely with the national median household income reported by the U.S. Census Bureau for 2022. According to the 2023 Current Population Survey Annual Social and Economic Supplement (CPS ASEC), the real median household income in the United States was $74,580 in 2022 [21]. Given that the patient cohort spans diagnoses from 2005 to 2020, $74,063 represents a conservative threshold reflective of contemporary national medians while accounting for inflation adjustments and ensuring consistency across socioeconomic analyses. Educational attainment was defined by the percentage of adults without a high school diploma in the patient’s ZIP code, derived from the ACS 2016–2020 data, and categorized as ≥9.1% vs. <9.1%. Urbanicity was determined using the NCDB-provided urban/rural classification based on U.S. Department of Agriculture urban influence codes (metro vs. non-metro).
Clinical variables included primary tumor site, tumor laterality (unilateral vs. bilateral/midline), histologic subtype (glioblastoma NOS, gliosarcoma, giant cell glioblastoma, and IDH-mutant glioblastoma), and comorbidity burden assessed by the Charlson–Deyo Comorbidity Index (0, 1, 2, 3). Treatment characteristics included receipt of surgery, radiation therapy, chemotherapy (Sx+RT+Chemo), and immunotherapy, as well as facility type and time interval between diagnosis and initiation of radiation. Outcomes evaluated were overall survival (OS), 30-day mortality, 90-day mortality, and 30-day hospital readmission rates.
2.4. Statistical Analysis
Descriptive statistics were used to summarize patient demographic, clinical, and treatment characteristics. Continuous variables were reported as means with standard deviations (SD) or medians with interquartile ranges (IQRs), depending on distribution, and categorical variables were summarized as frequencies and percentages. Comparisons across the four study periods (2005–2008, 2009–2012, 2013–2016, and 2017–2020) were performed using Chi-square tests for categorical variables and one-way analysis of variance (ANOVA) or Kruskal–Wallis tests for continuous variables, based on normality assessments.
Overall survival (OS) was estimated using Kaplan–Meier methods, and survival differences between groups were evaluated using the log-rank test. Multivariable Cox proportional hazards regression models were fitted to identify independent predictors of OS, adjusting for demographic, clinical, and treatment-related covariates. The proportional hazards assumption was assessed using scaled Schoenfeld residuals. Minor deviations from proportionality were noted for selected covariates (e.g., insurance status, urbanicity, treatment type, diagnosis year, and age). Multicollinearity was assessed using variance inflation factors (VIFs), with all included variables demonstrating VIFs < 2. To address these deviations and validate findings, supplementary parametric survival analyses were conducted using accelerated failure time (AFT) models under Weibull, log-normal, and log-logistic distributions. The AFT framework was selected because it models survival times directly rather than hazard rates, offering a robust alternative when the proportional hazards assumption is not fully satisfied.
Multivariable logistic regression analyses were also performed to identify factors associated with the receipt of combined Sx+RT+Chemo.
Complete case analysis was applied for variables with missing data. All analyses were conducted using RStudio (version 4.1.2; The R Foundation for Statistical Computing). A two-sided p-value of ≤0.05 was considered statistically significant. No formal adjustment for multiple comparisons was performed; results from secondary and exploratory analyses should be interpreted with caution due to the potential for Type I error inflation.
3. Results
3.1. Cohort Characteristics
In total, 111,955 patients between 2005 and 2020 were included in this analysis (Table 1). Of these, 8.9% of patients were aged 40–49 years, 38.4% were 50–64 years, 30.1% were 65–74 years, and 22.7% were 74–90 years at diagnosis. Males comprised 57.5% of the cohort, and females accounted for 42.5%. The majority of patients were White (91.3%), while Non-White patients represented 8.7%. Regarding ethnicity, 94.7% of patients were non-Hispanic and 5.3% were Hispanic. Treatment facilities included academic centers (41.3%), community hospitals (39.0%), and network facilities (19.7%). A proportion of 59.8% of patients resided in areas with a median household income below $74,063, while 40.2% lived in areas with income at or above $74,063. Educational attainment, defined by the proportion of adults aged 25 years or older in the ZIP code without a high school diploma, showed that 44.8% of patients lived in areas where ≥9.1% of adults had not graduated, whereas 55.2% lived in areas with lower rates of non-completion. Most patients (84.1%) resided in metropolitan areas, while 15.9% lived in non-metropolitan regions. Regarding comorbidity, Charlson–Deyo scores were distributed as 69.6% with a score of 0, 17.5% with a score of 1, 8.0% with a score of 2, and 4.9% with a score of 3. Insurance status included private insurance (41.0%), government insurance (55.9%), and uninsured (3.1%). Geographically, 36.8% of patients lived in the Northeast, 28.5% in the South, 20.3% in the Midwest, and 14.4% in the West. Histologically, 96.8% of tumors were classified as GBM, 2.1% as gliosarcoma, 0.7% as giant cell GBM, and 0.3% as IDH-mutant GBM. Tumor laterality was unilateral in 74.0% of cases and bilateral or midline in 26.0%. Primary tumor sites included the frontal-temporal lobes (52.1%), parietal-occipital lobes (19.6%), overlapping lesions (14.2%), ventricles or cerebellum (0.9%), brainstem (0.3%), brain not otherwise specified (9.1%), and cerebrum (3.7%) (Table 1).

Table 1.
Demographic and Disease Characteristics.
3.2. Temporal Trends and Treatment Characteristics
Temporal trends in treatment delivery and early outcomes are summarized in Table 2. Use of chemotherapy modestly increased over the study period, from 62.8% in 2005–2008 to 68.3% in 2017–2020. Radiation therapy and surgical treatment also saw slight increases in use, from 69.7% to 72.2% and from 72.3% to 77.2%, respectively. Immunotherapy use remained limited overall but was more frequently recorded in recent years, increasing from 0.3% to 6.3% across the time periods.

Table 2.
Treatment Patterns and Outcomes Across Four Time Periods.
The median time from diagnosis to the start of radiation increased from 29.0 to 36.0 days. Meanwhile, the median number of elapsed days for radiation therapy declined from 42.0 to 37.0 days. Median length of hospital stay following surgical treatment decreased from 4.0 days to 3.0 days.
Early outcomes remained generally stable or showed modest changes. Thirty-day mortality decreased from 6.2% to 4.3%, and ninety-day mortality from 18.0% to 14.5% over the study period. Thirty-day hospital readmission rates remained between 5.6% and 5.2%. Median overall survival increased slightly from 7.8 months in 2005–2008 to 9.5 months in 2017–2020.
3.3. Predictors of Receipt of Combined Surgery + Radiation + Chemotherapy
In multivariable logistic regression, several demographic, clinical, and socioeconomic factors were associated with a higher likelihood of receiving combined surgery, radiation, and chemotherapy (Sx+RT+Chemo) (Figure 2). Patients aged ≥65 years (OR: 0.52, 95% CI: 0.50–0.53), female patients (OR: 0.90, 95% CI: 0.88–0.92), and non-White patients (OR: 0.90, 95% CI: 0.86–0.94) had lower odds of receiving combined Sx+RT+Chemo. Treatment at community (OR: 0.78, 95% CI: 0.76–0.80) or network facilities (OR: 0.87, 95% CI: 0.84–0.90) was also associated with lower odds compared to academic centers. Patients residing in areas with higher median income (≥$74,063) (OR: 1.15, 95% CI: 1.12–1.19) and lower proportions of adults without high school education (<9.1%) (OR: 1.14, 95% CI: 1.11–1.17) were more likely to receive combined Sx+RT+Chemo.
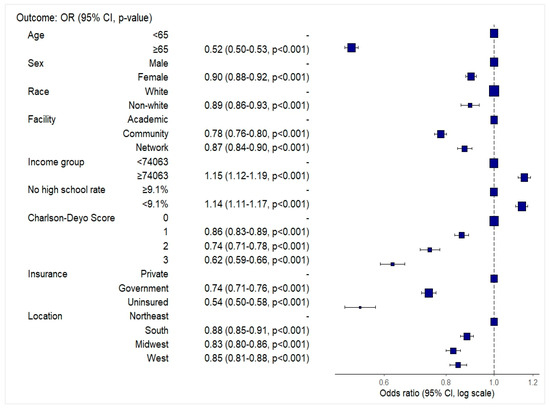
Figure 2.
Forest plot depicting odds ratios and 95% confidence intervals for receipt of combined surgery, radiation, and chemotherapy among glioblastoma patients, based on multivariable logistic regression.
Higher comorbidity burden was associated with reduced odds of treatment, with a stepwise decline from Charlson–Deyo scores of 1 (OR: 0.86, 95% CI: 0.83–0.89) to 3 (OR: 0.62, 95% CI: 0.59–0.66). Compared to patients with private insurance, those with government insurance (OR: 0.74, 95% CI: 0.71–0.76) and uninsured patients (OR: 0.54, 95% CI: 0.50–0.58) had significantly lower odds of receiving combined Sx+RT+Chemo. Regional variation was also observed, with lower odds of combination therapy receipt in the South (OR: 0.88, 95% CI: 0.85–0.91), the Midwest (OR: 0.83, 95% CI: 0.80–0.86), and the West (OR: 0.85, 95% CI: 0.81–0.88) compared to the Northeast US.
A visual summary of the adjusted odds ratios is presented in Figure 2. Full model and reduced coefficients are provided in Supplementary Tables S2 and S3, respectively.
3.4. Overall Survival by Treatment Category
For the entire cohort, the median OS was 9.30 months (95% CI: 9.20–9.40). Patients receiving combined Sx+RT+Chemo had a median OS of 14.62 months (95% CI: 14.52–14.75), with 1-year and 3-year survival rates of 59.9% and 15.2%, respectively. Patients receiving RT+Chemo or Sx+RT had median OS of 7.00 months (95% CI: 6.80–7.20) and 7.16 months (95% CI: 6.90–7.39), respectively. Median OS among patients receiving surgery alone or other therapies was 3.09 months (95% CI: 3.00–3.15) and 4.57 months (95% CI: 4.44–4.73), respectively. Patients who did not receive surgery, radiation, or chemotherapy had a median OS of 1.64 months (95% CI: 1.61–1.68) (Table 3, Figure 3).

Table 3.
Median OS and Survival Rates by Treatment Category.
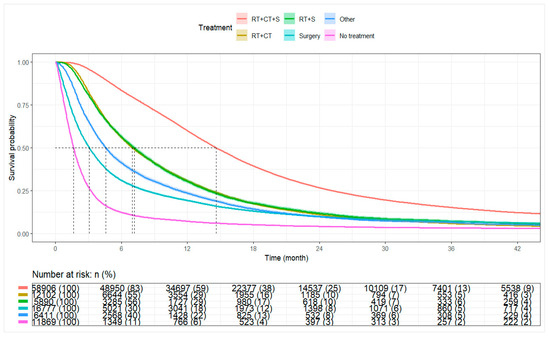
Figure 3.
Kaplan–Meier curve for overall survival by treatment categories. Abbreviations: RT+CT+S: Radiation, chemotherapy, and surgery; RT+CT: Radiation and chemotherapy; RT+S: Radiation and surgery; Surgery: Surgery alone; No Treatment: No radiation, chemotherapy, or surgery.
3.5. Overall Survival by Receipt of Combined Surgery, Radiation, and Chemotherapy
Among all patients, the median OS was 9.30 months (95% CI: 9.20–9.40). Patients receiving combined Sx+RT+Chemo had a median OS of 14.62 months (95% CI: 14.52–14.75), whereas patients not receiving combination therapy had a median OS of 3.84 months (95% CI: 3.81–3.91). At 1 year, the survival rate was 59.9% among combination therapy recipients compared to 21.0% among non-recipients. The 3-year survival rates were 15.2% and 5.8%, respectively (Table 4, Figure 4).

Table 4.
Median Overall Survival by Combined RT+CT+S vs. Other.
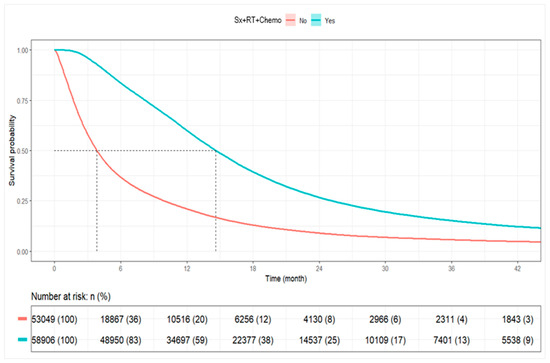
Figure 4.
Kaplan–Meier Plot for Overall Survival by Combined RT+CT+S vs. Other. Abbreviations: Sx+RT+Chemo: Surgery, radiation, and chemotherapy.
3.6. Predictors of Overall Survival (Cox Models)
Multivariable Cox proportional hazards regression findings are summarized in Table 5.

Table 5.
Cox Proportional Hazard Models for Overall Survival.
Older age (≥65 years) was associated with poorer survival (aHR 1.43, 95% CI 1.41–1.46), while female sex was modestly associated with improved survival (aHR 0.95, 95% CI 0.94–0.96). Non-White race (aHR 0.78, 95% CI 0.76–0.80) and Hispanic ethnicity (aHR 0.75, 95% CI 0.73–0.77) were associated with better survival compared with White and non-Hispanic patients, respectively.
Treatment at community and network facilities was associated with poorer survival compared with academic centers (aHRs 1.09 and 1.12, respectively). Additional factors associated with inferior survival included lower household income, higher Charlson–Deyo comorbidity scores (≥1), residence in non-metropolitan areas, and bilateral or midline tumor laterality.
Scaled Schoenfeld residuals were used to evaluate the proportional hazards assumption (Supplementary Figures S1–S3). Residuals for race, laterality, and geographic region were generally flat, supporting proportionality. Minor deviations were observed for insurance status, urbanicity, treatment type, age, and diagnosis year, but these were modest and not deemed clinically meaningful given the large cohort. Overall, the Cox proportional hazards model was considered appropriate.
Sensitivity analyses using stratified Cox models by histology, facility type, age, insurance, comorbidity burden, and geographic region demonstrated consistent findings and are presented in Supplementary Tables S4–S10.
3.7. Sensitivity Analyses Using Parametric Survival Models
Given minor deviations from proportional hazards, particularly for insurance, urbanicity, treatment, age, and year, we supplemented our analysis with parametric AFT models. AFT models offer a robust alternative when proportionality is imperfect, and findings were broadly consistent with Cox results, with time ratios (TRs) < 1 indicating shorter survival and TRs >1 indicating longer survival.
Predictors of interest of shorter survival included older age (≥65 years; TR 0.67, 95% CI 0.66–0.68), treatment at community or network facilities compared to academic centers, lower income, higher comorbidity burden, residence in non-metropolitan areas, and bilateral or midline tumor laterality. In contrast, female sex (TR 1.07, 95% CI 1.06–1.08), Non-White race (TR 1.33, 95% CI 1.30–1.36), and Hispanic ethnicity (TR 1.40, 95% CI 1.34–1.46) were associated with longer survival.
Results from the Weibull AFT model are presented in Table 6. Supplementary Tables S11 and S12 present findings from log-normal and log-logistic AFT models, respectively, which yielded comparable results.

Table 6.
Multivariable Weibull Accelerated Failure Time Model for Overall Survival.
4. Discussion
This study evaluated trends, progress, and ongoing challenges in GBM management across a 15-year period. In patients receiving combined Sx+RT+Chemo the median OS was 14.6 months. These findings are consistent with prior reports, such as that of Delgado-López et al., who documented a median OS of 14 months following maximum safe resection and chemoradiotherapy [22]. The use of triple-modality therapy increased by approximately 8% from 2005 to 2020, reflecting improved adoption of evidence-based standards of care. Despite these gains, demographic, socioeconomic, and geographic disparities continued to impact treatment receipt and survival outcomes. These results align with broader national trends toward optimizing GBM care [23,24,25].
Thomas-Joulié and colleagues reported that median OS improved between the 2005–2012 and 2013–2018 periods, with a HR of 0.64 (p < 0.001) favoring the more recent cohort [26]. These findings were attributed to advancements in supportive care, earlier GBM detection, and optimization of treatment delivery. Similarly, Touat et al. highlighted that modest survival gains may also reflect greater adoption of molecular markers, such as MGMT promoter methylation, for treatment stratification [26,27,28]. Despite these developments, median survival for GBM remains limited at 14–20 months, and the 5-year survival rate remains approximately 5%, with prognosis heavily influenced by factors, such as patient age, performance status, molecular characteristics, and extent of surgical resection [29,30]. The aggressive, infiltrative nature of GBM further compounds the challenges related to systemic therapy penetration across the blood–brain barrier [31].
While this study observed increasing receipt of combined Sx+RT+Chemo over time, improvements in perioperative and postoperative care also contributed to significantly reduced early mortality. The decline in 30- and 90-day mortality rates across the study period suggests improved surgical techniques, better management of comorbidities, and reductions in treatment-related toxicities, all of which are pivotal components of modern GBM care [32,33]. Supportive care interventions remain critical in minimizing side effects and optimizing outcomes in glioblastoma management [26]. Nonetheless, these advances remain modest. Despite improvements in supportive care and multimodal treatment delivery, the “Stupp protocol”, introduced in the mid-2000s, continues to define the standard of care for GBM [33,34]. Emerging targeted therapies and immunotherapies have yet to demonstrate consistent benefit in clinical trials, largely due to the immunosuppressive tumor microenvironment and challenges in delivering brain-penetrant agents, as highlighted by Touat et al. [28]. The persistent lack of effective systemic therapies highlights the substantial challenges that remain in achieving meaningful therapeutic breakthroughs for GBM.
While increased uptake of combined Sx+RT+Chemo reflects overall progress in the real-world treatment of GBM, persistent inequities in access remain due to demographic, socioeconomic, and systemic factors. In this study, multivariable analyses revealed that females had 10% lower odds of receiving combined Sx+RT+Chemo compared to males (OR: 0.90, 95% CI: 0.88–0.92, p < 0.001). Non-White patients also had lower odds of receiving combination therapy (OR: 0.90, 95% CI: 0.86–0.94, p < 0.001). Despite disparities in treatment access, Non-White and Hispanic patients demonstrated significantly better adjusted survival compared with White and non-Hispanic patients, respectively. These findings align with those observed by Reihanian et al., where women exhibited a higher median survival than men (16.14 months vs. 10.75 months, p = 0.023) [35]. Patients who did not receive Sx+RT+Chemo were likely to have been influenced by factors such as poor performance status or high comorbidity burden, limiting eligibility for aggressive multimodal therapy [36]. Geographic disparities were also evident, with patients in rural or underserved regions facing greater barriers to combination therapy receipt. Prior studies have noted that counties with higher Black populations and counties located in the Midwest (OR: 3.042) or West (OR: 3.175) were more likely to experience surgical delays [36]. These findings highlight opportunities for expanding telemedicine infrastructure, strengthening regional referral networks, and increasing investment in the neurosurgical workforce to enhance equitable access to standard-of-care therapies [37].
While these disparities are well-documented in the U.S. healthcare system, recent studies suggest that inequities in cancer treatment are also present in countries with universal healthcare models. For instance, Coppini et al. reported that patients in Central and Eastern Europe encounter barriers related to health misinformation and structural inefficiencies, despite broad coverage frameworks [38]. Similarly, Ferraris et al. highlighted persistent socioeconomic disparities in access and treatment navigation among Italian cancer patients [39]. Berchet et al. further emphasized that pan-European gaps in prevention and care contribute to unequal outcomes even within predominantly Caucasian populations [40]. Disparities in cancer treatment are not solely products of insurance status or racial demographics but can persist due to cultural, structural, and geographic challenges across healthcare systems.
Socioeconomic status was a significant determinant of treatment access. Patients residing in ZIP codes with median household incomes < $74,063 had lower odds of receiving combined Sx+RT+Chemo compared with patients from higher-income areas (≥$74,063) (OR: 1.15, 95% CI: 1.12–1.19, p < 0.001). This finding is supported by prior studies, where patients treated at safety-net hospitals were less likely to complete radiation or systemic therapy compared to those obtaining private care [41,42]. Insurance status further contributed to disparities: patients with government insurance (Medicare/Medicaid) and uninsured patients had significantly lower odds of receiving triple-modality therapy compared to privately insured patients [43,44]. Brown et al. similarly reported reduced odds of receiving surgery among Medicaid and uninsured patients (ORs: 0.72 and 0.77, respectively), with associated gaps in access to chemotherapy and radiation therapy [45].
Beyond standard oncologic management, anticoagulation in GBM remains an area of clinical complexity due to the competing risks of thromboembolic events and intracerebral hemorrhage. As highlighted by Bianconi et al., patients with high-grade gliomas face increased thrombotic risks, although prophylactic anticoagulation strategies must carefully weigh bleeding risks [46]. Future real-world studies should integrate data on anticoagulant management to further characterize its impact on GBM outcomes.
Survival analyses demonstrated a paradoxical pattern: despite facing barriers to treatment, Non-White and Hispanic patients exhibited significantly better adjusted survival compared to White and non-Hispanic patients, respectively. Survival outcomes varied by tumor histology. Patients with giant cell GBM exhibited improved survival relative to those with conventional GBM (aHR: 0.81, p < 0.001), reflecting the less infiltrative biology of this variant. Gliosarcoma, however, did not demonstrate a significant difference in survival compared to GBM NOS.
The impact of comorbidity burden on survival was notable. A stepwise increase in mortality risk was observed with higher Charlson–Deyo comorbidity scores. Patients with a CCI of 3 had a 44% higher risk of death compared to patients with no comorbidities (HR: 1.44, 95% CI: 1.40–1.48, p < 0.001). These results partly explain why survival gains observed in real-world populations were more modest compared to clinical trial populations. For instance, while Stupp et al. reported median OS of 14–16 months with standard chemoradiation, median OS in this cohort increased only from 8.2 to 9.8 months between 2005 and 2020. This discrepancy highlights the role of patient heterogeneity, access disparities, and real-world treatment limitations often underrepresented in trial populations.
4.1. Limitations
This study has several important limitations. First, the retrospective nature of the NCDB introduces inherent selection bias and restricts the ability to infer causality. The reliance on hospital-reported data from Commission on Cancer-accredited facilities limits generalizability, as non-hospital settings and international populations are not depicted [47]. Additionally, granular treatment-level details, such as specific chemotherapy agents (e.g., temozolomide), radiation dosages, or number of therapy cycles, are unavailable, precluding definitive assessment of adherence to full Stupp protocol standards.
Second, patient-reported outcomes, quality-of-life measures, and functional performance status are absent from the NCDB. As a result, the relationship between treatment receipt and broader patient well-being could not be evaluated. Third, while socioeconomic data are available at the ZIP code level, individual-level socioeconomic status and healthcare access measures (e.g., transportation barriers, specialist availability) are lacking. Differences in healthcare infrastructure, referral patterns, and hospital resources could not be fully delineated.
Additionally, during the 15-year study period, administrative coding transitioned from ICD-9 to ICD-10 classifications in 2016 for comorbidity and administrative variables, although tumor morphology coding (ICD-O-3) remained consistent. Due to the NCDB data structure, only aggregated Charlson–Deyo scores were available; specific comorbid conditions contributing to higher scores (e.g., CCI = 3) could not be individually assessed. While comorbidity burden was associated with worse survival outcomes, the impact of individual comorbid conditions could not be separately analyzed due to limitations in NCDB data granularity. Observed survival differences across facility types may partly reflect differences in access to specialized therapies, clinical trials, and supportive care resources, although these variables were not directly tabulated in the NCDB.
Finally, the increasing number of GBM cases reported over time is likely to reflect expansion of NCDB coverage through hospital accreditation rather than a true rise in disease incidence, a point that must be interpreted cautiously.
4.2. Implications and Future Research Directions
The findings of this study highlight persistent disparities in GBM treatment access and survival outcomes despite overall improvements in multimodal therapy delivery. Efforts to bridge these gaps must prioritize expanding telemedicine services, strengthening regional neuro-oncology networks, and enhancing access to specialized care, particularly in underserved rural and low-income areas.
Future research should focus on integrating molecular and genomic profiling into large datasets to enable precision medicine approaches for GBM. Brain-penetrant therapies, targeted agents, and novel delivery methods should be incorporated into combination regimens to address the challenges of the blood–brain barrier and tumor invasiveness. Prospective studies incorporating patient-reported outcomes and quality-of-life measures are essential to better understand the full impact of treatment beyond survival alone.
In addition, future analyses should explore disparities in clinical trial participation and therapeutic innovations across different healthcare systems. Comparative studies between U.S. and European populations, where universal healthcare systems may influence disparity patterns, could offer valuable insights into system-level drivers of inequity. Finally, designing inclusive, representative clinical trials with diverse socioeconomic and racial/ethnic enrollment will be critical to ensuring equitable therapeutic advancements for GBM patients worldwide.
5. Conclusions
This study highlights a significant increase in the adoption of combination therapy with Sx+RT+Chemo for GBM over a 15-year period, accompanied by modest improvements in survival. Despite these gains, median overall survival for combination therapy recipients remained limited at 14.6 months, reflecting the inherent aggressiveness of GBM. Persistent disparities were evident, with women, racial and ethnic minorities, and patients from lower-income regions less likely to receive triple-modality treatment. These inequities are likely to stem from differences in healthcare infrastructure, resource allocation, and systemic biases. The underutilization of molecular profiling and its capture in national datasets further constrains efforts to personalize therapy and optimize outcomes in real-world settings. Future efforts must prioritize the integration of emerging therapies with a commitment to equitable access. Reducing structural disparities and advancing precision medicine approaches will be essential to achieving meaningful, transformative improvements in GBM care.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/brainsci15060556/s1, Table S1: ICD-O-3 Coding; Table S2: Full Multivariable Model Assessing Sociodemographic and Clinical Predictors of Combined Sx+RT+Chemo; Table S3: Multivariable Logistic Regression: Reduced Model Assessing Sociodemographic Predictors of Receiving Combined Sx+RT+Chemo; Table S4: Cox Model Stratified by Histology, Facility, Age, Insurance, Charlson–Deyo Score, and Location; Table S5: Cox Model Stratified by Histology; Table S6: Cox Model Stratified by Facility Type; Table S7: Cox Model Stratified by Age Group; Table S8: Cox Model Stratified by Insurance Statusl; Table S9: Cox Model Stratified by Charlson–Deyo Comorbidity Score; Table S10: Cox Model Stratified by Geographic Location; Table S11: Multivariable Log-Normal Accelerated Failure Time Model for Overall Survival; Table S12: Multivariable Log-Logistic Accelerated Failure Time Model for Overall Survival; Figure S1: Scaled Schoenfeld Residual Plots for Race, Insurance, and Laterality; Figure S2: Scaled Schoenfeld Residual Plots for Urbanicity (Metro/Non-Metro) and Location (Region); Figure S3: Scaled Schoenfeld Residual Plots for Age, Treatment, and Diagnosis Year.
Author Contributions
Conceptualization, Z.S., D.J., S.B. and A.O.; methodology, Z.S., D.J. and A.O.; software, L.H. formal analysis, D.J. and L.H.; investigation, Z.S. and D.J.; data curation, D.J. and L.H.; writing—original draft preparation, Z.S., D.J., A.O. and L.H.; writing—review and editing, Z.S., D.J., S.B., A.M. (Arun Maharaj), L.H., A.O., A.M. (Alireza Mansouri) and M.S.A.; visualization, Z.S. and D.J.; supervision, M.S.A.; project administration, M.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. Ethical review and approval were waived for this study by the Institutional Review Board at Miami Cancer Institute because only de-identified data was used, containing no private or personally identifiable information.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the findings of this study are available from the National Cancer Database.
Acknowledgments
We extend our thanks to Shahzaib Ahmad, Samuel Sommer, Numa Frias, Vivek Podder, and Mohammed Arfat Ganiyani for their invaluable early contributions to this study. The authors acknowledge data access provided by the National Cancer Database (NCDB). The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Conflicts of Interest
Manmeet Singh Ahluwalia (M.S.A.) has received consulting fees from Bayer, Xoft, Apollomics, Viewray, Cairn Therapeutics, Anheart Therapeutics, Theraguix, Menarini Ricerche, Sumitomo Pharma Oncology, Autem Therapeutics, GT Medical Technologies, Allovir, EquilliumBio, QV Bioelectronics, Servier Pharmaceuticals, Incyte, and Recordati. He has served on a Data Safety Monitoring Committee (DSMC) for VBI Vaccines and on the Scientific Advisory Board for Modifibiosciences and Bugworks. M.S.A. is a shareholder in Mimivax, CytoDyn, MedInnovate Advisors LLC, and Trisalus Life Sciences. His research has been supported by grants 1R01CA277728-01A1 and 1R01CA264017-01A1. All other authors declare no conflicts of interest. The funders had no role in the study design, data collection, analysis, interpretation, manuscript writing, or the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| CCI | Charlson Comorbidity Index |
| CI | Confidence Interval |
| CNS | Central Nervous System |
| EGFR | Epidermal Growth Factor Receptor |
| GBM | Glioblastoma |
| HR | Hazard Ratio |
| ICD-O-3 | International Classification of Diseases for Oncology—Third Edition |
| IQR | Interquartile Range |
| MGMT | O6-Methylguanine-DNA Methyltransferase |
| NCDB | National Cancer Database |
| OS | Overall Survival |
| SD | Standard Deviation |
| TERT | Telomerase Reverse Transcriptase |
| US | United States |
References
- Agarwal, A.; Edgar, M.A.; Desai, A.; Gupta, V.; Soni, N.; Bathla, G. Molecular GBM versus histopathological GBM: Radiology-pathology-genetic correlation and the new WHO 2021 definition of glioblastoma. Am. J. Neuroradiol. 2024, 45, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef]
- Motomura, K.; Kibe, Y.; Ohka, F.; Aoki, K.; Yamaguchi, J.; Saito, R. Clinical characteristics and radiological features of glioblastoma, IDH-wildtype, grade 4 with histologically lower-grade gliomas. Brain Tumor Pathol. 2023, 40, 48–55. [Google Scholar] [CrossRef] [PubMed]
- D’Alessio, A.; Proietti, G.; Sica, G.; Scicchitano, B.M. Pathological and molecular features of glioblastoma and its peritumoral tissue. Cancers 2019, 11, 469. [Google Scholar] [CrossRef]
- Ganipineni, L.P.; Danhier, F.; Préat, V. Drug delivery challenges and future of chemotherapeutic nanomedicine for glioblastoma treatment. J. Control. Release 2018, 281, 42–57. [Google Scholar] [CrossRef]
- Janjua, T.I.; Rewatkar, P.; Ahmed-Cox, A.; Saeed, I.; Mansfeld, F.M.; Kulshreshtha, R.; Kumeria, T.; Ziegler, D.S.; Kavallaris, M.; Mazzieri, R. Frontiers in the treatment of glioblastoma: Past, present and emerging. Adv. Drug Deliv. Rev. 2021, 171, 108–138. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Costa, A.; Osório, L.; Lago, R.C.; Linhares, P.; Carvalho, B.; Caeiro, C. Current standards of care in glioblastoma therapy. In Glioblastoma; Exon Publications: Brisbane, Australia, 2017; pp. 197–241. [Google Scholar]
- Sulman, E.P.; Ismaila, N.; Armstrong, T.S.; Tsien, C.; Batchelor, T.T.; Cloughesy, T.; Galanis, E.; Gilbert, M.; Gondi, V.; Lovely, M. Radiation therapy for glioblastoma: American Society of Clinical Oncology clinical practice guideline endorsement of the American Society for Radiation Oncology guideline. J. Clin. Oncol. 2017, 35, 361–369. [Google Scholar] [CrossRef]
- Angom, R.S.; Nakka, N.M.R.; Bhattacharya, S. Advances in glioblastoma therapy: An update on current approaches. Brain Sci. 2023, 13, 1536. [Google Scholar] [CrossRef]
- Cruz, N.; Herculano-Carvalho, M.; Roque, D.; Faria, C.C.; Cascão, R.; Ferreira, H.A.; Reis, C.P.; Matela, N. Highlighted advances in therapies for difficult-to-treat brain tumours such as glioblastoma. Pharmaceutics 2023, 15, 928. [Google Scholar] [CrossRef]
- ABTA. Glioblastoma (GBM). Available online: https://www.abta.org/tumor_types/glioblastoma-gbm/ (accessed on 4 December 2024).
- Keegan, T.H.; Abrahão, R.; Alvarez, E.M. Survival trends among adolescents and young adults diagnosed with cancer in the United States: Comparisons with children and older adults. J. Clin. Oncol. 2024, 42, 630–641. [Google Scholar] [CrossRef]
- Johnson, D.R.; Giannini, C.; Vaubel, R.A.; Morris, J.M.; Eckel, L.J.; Kaufmann, T.J.; Guerin, J.B. A radiologist’s guide to the 2021 WHO Central Nervous System Tumor Classification: Part I—Key concepts and the spectrum of diffuse gliomas. Radiology 2022, 304, 494–508. [Google Scholar] [CrossRef] [PubMed]
- Osborn, A.; Louis, D.; Poussaint, T.; Linscott, L.; Salzman, K. The 2021 World Health Organization classification of tumors of the central nervous system: What neuroradiologists need to know. Am. J. Neuroradiol. 2022, 43, 928–937. [Google Scholar] [CrossRef]
- Reuss, D.E. Updates on the WHO diagnosis of IDH-mutant glioma. J. Neuro-Oncol. 2023, 162, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Pandith, A.A.; Qasim, I.; Zahoor, W.; Shah, P.; Bhat, A.R.; Sanadhya, D.; Shah, Z.A.; Naikoo, N.A. Concordant association validates MGMT methylation and protein expression as favorable prognostic factors in glioma patients on alkylating chemotherapy (Temozolomide). Sci. Rep. 2018, 8, 6704. [Google Scholar] [CrossRef]
- Weller, M.; Stupp, R.; Reifenberger, G.; Brandes, A.A.; Van Den Bent, M.J.; Wick, W.; Hegi, M.E. MGMT promoter methylation in malignant gliomas: Ready for personalized medicine? Nat. Rev. Neurol. 2010, 6, 39–51. [Google Scholar] [CrossRef]
- Merkow, R.P.; Rademaker, A.W.; Bilimoria, K.Y. Practical guide to surgical data sets: National Cancer Database (NCDB). JAMA Surg. 2018, 153, 850–851. [Google Scholar] [CrossRef]
- Jayram, D.; Bellur, S.; Ozair, A.; Ahmad, S.; Hodgson, L.; Perez, N.F.; Podder, V.; Ganiyani, M.; Ahluwalia, M. 458P Patterns of care and clinical outcomes of patients with glioblastoma in the United States from 2005–2020: A real-world analysis. In Proceedings of the European Society for Medical Oncology (ESMO) Congress 2024, Barcelona, Spain, 13–17 September 2024. [Google Scholar]
- Edition, T. International Classification of Diseases for Oncology; World Health Organization: Geneva, Swiss, 2020. [Google Scholar]
- Bureau, U.S.C. Income in the United States: 2022. Available online: https://www.census.gov/library/publications/2023/demo/p60-279.html?utm_source=chatgpt.com (accessed on 18 April 2025).
- Delgado-López, P.; Corrales-García, E. Survival in glioblastoma: A review on the impact of treatment modalities. Clin. Transl. Oncol. 2016, 18, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Poon, M.T.; Sudlow, C.L.; Figueroa, J.D.; Brennan, P.M. Longer-term (≥2 years) survival in patients with glioblastoma in population-based studies pre-and post-2005: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 11622. [Google Scholar] [CrossRef]
- Grech, N.; Dalli, T.; Mizzi, S.; Meilak, L.; Calleja, N.; Zrinzo, A. Rising incidence of glioblastoma multiforme in a well-defined population. Cureus 2020, 12, e8195. [Google Scholar] [CrossRef]
- Chen, B.; Chen, C.; Zhang, Y.; Xu, J. Recent incidence trend of elderly patients with glioblastoma in the United States, 2000–2017. BMC Cancer 2021, 21, 54. [Google Scholar] [CrossRef]
- Thomas, T.; Khader, S.A.; Kumar, K.S. Survival Analysis and Factors Affecting Treatment Outcome in Glioblastoma Multiforme: A Cohort Study. J. Clin. Diagn. Res. 2023, 17, 1026–1036. [Google Scholar] [CrossRef]
- Furtak, J.; Kwiatkowski, A.; Śledzińska, P.; Bebyn, M.; Krajewski, S.; Szylberg, T.; Birski, M.; Druszcz, A.; Krystkiewicz, K.; Gasiński, P. Survival after reoperation for recurrent glioblastoma multiforme: A prospective study. Surg. Oncol. 2022, 42, 101771. [Google Scholar] [CrossRef] [PubMed]
- Touat, M.; Idbaih, A.; Sanson, M.; Ligon, K. Glioblastoma targeted therapy: Updated approaches from recent biological insights. Ann. Oncol. 2017, 28, 1457–1472. [Google Scholar] [CrossRef]
- Verdugo, E.; Puerto, I.; Medina, M.Á. An update on the molecular biology of glioblastoma, with clinical implications and progress in its treatment. Cancer Commun. 2022, 42, 1083–1111. [Google Scholar] [CrossRef] [PubMed]
- Cahill, D.P. Extent of resection of glioblastoma: A critical evaluation in the molecular era. Neurosurg. Clin. 2021, 32, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Xia, Y.; Bettegowda, C.; Weller, M. Current state of immunotherapy for glioblastoma. Nat. Rev. Clin. Oncol. 2018, 15, 422–442. [Google Scholar] [CrossRef]
- Tan, A.C.; Ashley, D.M.; López, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of glioblastoma: State of the art and future directions. CA A Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef]
- Dencker, E.E.; Bonde, A.; Troelsen, A.; Varadarajan, K.M.; Sillesen, M. Postoperative complications: An observational study of trends in the United States from 2012 to 2018. BMC Surg. 2021, 21, 393. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; Van Den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Reihanian, Z.; Abbaspour, E.; Zaresharifi, N.; Karimzadhagh, S.; Mahmoudalinejad, M.; Sourati, A.; Farzin, M.; EslamiKenarsari, H. Impact of Age and Gender on Survival of Glioblastoma Multiforme Patients: A Multicenter Retrospective Study. Cancer Rep. 2024, 7, e70050. [Google Scholar] [CrossRef]
- Ramapriyan, R.; Ramesh, T.; Yu, H.; Richardson, L.G.; Nahed, B.V.; Carter, B.S.; Barker, F.G.; Curry, W.T.; Choi, B.D. County-level disparities in care for patients with glioblastoma. Neurosurg. Focus 2023, 55, E12. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Pham, A.; Yoo, S.; Attenello, F.J.; Jennelle, R.; Wagle, N.; Chang, E.L.; Zada, G. Identifying disparities in care in treating glioblastoma: A retrospective cohort study of patients treated at a safety-net versus private hospital setting. World Neurosurg. 2020, 137, e213–e220. [Google Scholar] [CrossRef]
- Coppini, V.; Ferraris, G.; Ferrari, M.V.; Dahò, M.; Kirac, I.; Renko, I.; Monzani, D.; Grasso, R.; Pravettoni, G. Patients’ perspectives on cancer care disparities in Central and Eastern European countries: Experiencing taboos, misinformation and barriers in the healthcare system. Front. Oncol. 2024, 14, 1420178. [Google Scholar] [CrossRef]
- Ferraris, G.; Coppini, V.; Ferrari, M.V.; Monzani, D.; Grasso, R.; Pravettoni, G. Understanding reasons for cancer disparities in Italy: A qualitative study of barriers and needs of cancer patients and healthcare providers. Cancer Control 2024, 31, 10732748241258589. [Google Scholar] [CrossRef]
- Berchet, C.; Dedet, G.; Klazinga, N.; Colombo, F. Inequalities in cancer prevention and care across Europe. Lancet Oncol. 2023, 24, 10–11. [Google Scholar] [CrossRef]
- Nabi, J.; Tully, K.H.; Cole, A.P.; Marchese, M.; Cone, E.B.; Melnitchouk, N.; Kibel, A.S.; Trinh, Q.D. Access denied: The relationship between patient insurance status and access to high-volume hospitals. Cancer 2021, 127, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Seo, V.; Baggett, T.P.; Thorndike, A.N.; Hull, P.; Hsu, J.; Newhouse, J.P.; Fung, V. Access to care among Medicaid and uninsured patients in community health centers after the Affordable Care Act. BMC Health Serv. Res. 2019, 19, 291. [Google Scholar] [CrossRef] [PubMed]
- Estevez-Ordonez, D.; Abdelrashid, M.; Coffee, E.; Laskay, N.M.; Atchley, T.J.; Chkheidze, R.; Fiveash, J.B.; Markert, J.M.; Lobbous, M.; Maveal, B.M. Racial and socioeconomic disparities in glioblastoma outcomes: A single-center, retrospective cohort study. Cancer 2023, 129, 3010–3022. [Google Scholar] [CrossRef]
- Hodges, T.R.; Labak, C.M.; Mahajan, U.V.; Wright, C.H.; Wright, J.; Cioffi, G.; Gittleman, H.; Herring, E.Z.; Zhou, X.; Duncan, K. Impact of race on care, readmissions, and survival for patients with glioblastoma: An analysis of the National Cancer Database. Neuro-Oncol. Adv. 2021, 3, vdab040. [Google Scholar] [CrossRef]
- Brown, D.A.; Himes, B.T.; Kerezoudis, P.; Chilinda-Salter, Y.M.; Grewal, S.S.; Spear, J.A.; Bydon, M.; Burns, T.C.; Parney, I.F. Insurance correlates with improved access to care and outcome among glioblastoma patients. Neuro-Oncol. 2018, 20, 1374–1382. [Google Scholar] [CrossRef]
- Bianconi, A.; Prior, A.; Zona, G.; Fiaschi, P. Anticoagulant therapy in high grade gliomas: A systematic review on state of the art and future perspectives. J. Neurosurg. Sci. 2021, 67, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.-D.; Cole, A.P.; Trinh, Q.-D. Limitations of using the National Cancer Database to examine the effect of policy change on stage at presentation at the population level. J. Am. Acad. Dermatol. 2021, 85, e195–e196. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).