Is CT Still the Gold Standard in Semicircular Canal Dehiscence? Diagnostic Value of MRI in Poschl and Stenver Planes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Sample
2.2. Study Procedures
2.3. Intervention Protocol or Study Groups
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COM | Chronic otitis media |
| CT | Computed tomography |
| MRI | Magnetic resonance imaging |
| PSC | Posterior semicircular canal |
| PSCD | Posterior semicircular canal dehiscence |
| SC | Semicircular canal |
| SCs | Semicircular canals |
| SCD | Semicircular canal dehiscence |
| SSC | Superior semicircular canal |
| SSCD | Superior semicircular canal dehiscence |
References
- Bi, W.L.; Brewster, R.; Poe, D.; Vernick, D.; Lee, D.J.; Corrales, C.E.; Dunn, I.F. Superior semicircular canal dehiscence syndrome. J. Neurosurg. 2017, 127, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Castellucci, A.; Dumas, G.; Abuzaid, S.M.; Armato, E.; Martellucci, S.; Malara, P.; Alfarghal, M.; Ruberto, R.R.; Brizzi, P.; Ghidini, A.; et al. Posterior semicircular canal dehiscence with vestibulo-ocular reflex reduction for the affected canal at the video-head impulse test: Considerations to pathomechanisms. Audiol. Res. 2024, 14, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Ward, B.K.; van de Berg, R.; van Rompaey, V.; Bisdorff, A.; Hullar, T.E.; Welgampola, M.S.; Carey, J.P. Superior semicircular canal dehiscence syndrome: Diagnostic criteria consensus document of the committee for the classification of vestibular disorders of the Bárány Society. J. Vestib. Res. 2021, 31, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Cozart, A.C.; Kennedy, J.T., 3rd; Seidman, M.D. A basis for standardizing superior semicircular canal dehiscence management: Survey results from the American Neurotology Society and American Otologic Society. Ear Nose Throat J. 2021, 100, NP444–NP453. [Google Scholar] [CrossRef]
- Nguyen, T.; Lagman, C.; Sheppard, J.P.; Romiyo, P.; Duong, C.; Prashant, G.N.; Gopen, Q.; Yang, I. Middle cranial fossa approach for the repair of superior semicircular canal dehiscence is associated with greater symptom resolution compared to transmastoid approach. Acta Neurochir. 2018, 160, 1219–1224. [Google Scholar] [CrossRef]
- Ward, B.K.; Carey, J.P.; Minor, L.B. Superior canal dehiscence syndrome: Lessons from the first 20 years. Front. Neurol. 2017, 8, 177. [Google Scholar] [CrossRef]
- Ionescu, E.C.; Reynard, P.; Damien, M.; Ltaief-Boudrigua, A.; Hermann, R.; Gianoli, G.J.; Thai-Van, H. Why should multiple dehiscences of the otic capsule be considered before surgically treating patients with superior semicircular canal dehiscence? A radiological monocentric review and a case series. Front. Neurol. 2023, 14, 1209567. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Lee, Y.; Choi, J.Y.; Bae, Y.J.; Kim, M.; Song, J.-J.; Choi, B.Y.; Jeong, W.-K.; Koo, J.-W. Quantitative three-dimensional image analysis of the superior canal after surgical plugging to treat superior semicircular canal dehiscence. Sci. Rep. 2021, 11, 16112. [Google Scholar] [CrossRef]
- Öztunali, Ç.; Şaylisoy, S.; İncesu, A. MDCT of dehiscent or thin bone coverage over the superior semicircular canal: Assessment of prevalence by age and contralateral canal bone thickness. Genel Tıp Dergisi 2023, 33, 255–260. [Google Scholar] [CrossRef]
- Waldeck, S.; Lanfermann, H.; von Falck, C.; Froelich, M.F.; Chapot, R.; Brockmann, M.; Overhoff, D. New classification of superior semicircular canal dehiscence in HRCT. PLoS ONE 2022, 17, e0262758. [Google Scholar] [CrossRef]
- Trout, A.T.; Abu-El-Haija, M.; Anupindi, S.A.; Marine, M.B.; Murati, M.; Phelps, A.S.; Rees, M.A.; Squires, J.H.; Ellery, K.M.; Gariepy, C.E.; et al. Interobserver Agreement for CT and MRI Findings of Chronic Pancreatitis in Children: A Multicenter Ancillary Study Under the INSPPIRE Consortium. AJR Am. J. Roentgenol. 2022, 219, 303–313. [Google Scholar] [CrossRef]
- Bolgen, C. MRI-CT correlation for semicircular canal dehiscence. Radiology Master’s Thesis, Kirikkale University, Kirikkale, Türkiye, 2012. [Google Scholar]
- İnal, M.; Muluk, N.B.; Şahan, M.H.; Asal, N.; Şimşek, G.; Arıkan, O.K. A comparison of diagnostic accuracy of superior semicircular canal dehiscence in MDCT and MRI, and coexistence with tegmen tympani dehiscence. J. Int. Adv. Otol. 2020, 16, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, K.E.; Chari, D.A.; Nakajima, H.H.; Klokker, M.; Caye-Thomasen, P.; Lee, D.J. Current trends, controversies, and future directions in the evaluation and management of superior canal dehiscence syndrome. Front. Neurol. 2021, 12, 636574. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, E.; Reynard, P.; Coudert, A.; Roiban, L.; Boudrigua, A.L.; Thai-Van, H.; Lyon, I.-L.U. Superior semicircular canal dehiscence by superior petrosal sinus: Proposal for classification. J. Int. Adv. Otol. 2021, 17, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Fritz, C.G.; Casale, G.G.; Kana, I.A.; Hong, R.S. An evidenced-based diagnostic tool for superior semicircular canal dehiscence syndrome. J. Otol. 2023, 18, 230–234. [Google Scholar] [CrossRef]
- Gurbuz, D.; Tunç, M.K.; Karaketir, S.; Berkiten, G.; Akan, O. Investigation of the relationship between idiopathic intracranial hypertension and superior semicircular canal dehiscence. Pamukkale Med. J. 2022, 15, 133–139. [Google Scholar] [CrossRef]
- Matic, J.; Winklhofer, S.; Pfiffner, F.; Roosli, C.; Veraguth, D.; Huber, A.; Dalbert, A. Influence of semicircular canal dehiscence on cochlear implant outcome. Audiol. Neurotol. 2021, 26, 135–139. [Google Scholar] [CrossRef]
- Connor, S.E.J.; Dudau, C.; Pai, I.; Gaganasiou, M. Is CT or MRI the optimal imaging investigation for the diagnosis of large vestibular aqueduct syndrome and large endolymphatic sac anomaly? Eur. Arch. Otorhinolaryngol. 2019, 276, 693–702. [Google Scholar] [CrossRef]
- Johanis, M.; De Jong, R.; Miao, T.; Hwang, L.; Lum, M.; Kaur, T.; Willis, S.; Arsenault, J.J.; Duong, C.; Yang, I.; et al. Concurrent superior semicircular canal dehiscence and endolymphatic hydrops: A novel case series. Int. J. Surg. Case. Rep. 2021, 78, 382–386. [Google Scholar] [CrossRef]
- Nada, A.; Agunbiade, S.A.; Whitehead, M.T.; Cousins, J.P.; Ahsan, H.; Mahdi, E. Cross-sectional imaging evaluation of congenital temporal bone anomalies: What each radiologist should know. Curr. Probl. Diagn. Radiol. 2021, 50, 716–724. [Google Scholar] [CrossRef]
- Whyte, J.; Cisneros, A.I.; Garcia-Barrios, A.; Fraile, J.; Whyte, A.; Crovetto, R.; Lahoz, M. Association between superior semicircular canal dehiscence and other dehiscences in temporal bone. Folia Morphol. 2020, 79, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Chemtob, R.A.; Epprecht, L.; Reinshagen, K.L.; Huber, A.; Caye-Thomasen, P.; Nakajima, H.H.; Lee, D.J. Utility of postoperative magnetic resonance imaging in patients who fail superior canal dehiscence surgery. Otol. Neurotol. 2019, 40, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Davvaz, B.; Hasani, M.; Haghnegahdar, A. Evaluation of superior semicircular canal morphology and its relationship with glenoid fossa roof thickness using cone beam computed tomography. Radiol. Res. Pract. 2022, 2022, 1565038. [Google Scholar] [CrossRef] [PubMed]
- Kraus, M.; Hassannia, F.; Dabiri, S.; Vergara Olmos, G.; Rutka, J.A. Central Vestibular Dysfunction in Head Injury. J. Otolaryngol. Head Neck Surg. 2024, 53. [Google Scholar] [CrossRef]
- Stefanescu, E.H.; Balica, N.C.; Motoi, S.B.; Grigorita, L.; Georgescu, M.; Iovanescu, G. Evaluation of temporal bone cholesteatoma and the correlation between high resolution computed tomography and surgical finding. Medicina 2023, 59, 1712. [Google Scholar] [CrossRef]
- Rodrigues, M.; Nunes, J.; Figueiredo, S.; Martins de Campos, A.; Geraldo, A.F. Neuroimaging assessment in Down syndrome: A pictorial review. Insights Imaging 2019, 10, 52. [Google Scholar] [CrossRef]
- Berning, A.W.; Arani, K.; Branstetter, B.F., IV. Prevalence of superior semicircular canal dehiscence on high-resolution CT imaging in patients without vestibular or auditory abnormalities. AJNR Am. J. Neuroradiol. 2019, 40, e1–e6. [Google Scholar] [CrossRef]
- Cocca, S.; Mignacco, G.; Mandalà, M.; Giannitto, C.; Esposito, A.A.; Porcino, S. A “Double” Third Window Syndrome: The Case of Semicircular Canal Dehiscence in Twin Sisters. Rep. Med. Imaging 2022, 15, 1–7. [Google Scholar] [CrossRef]
- Romiyo, P.; Duong, C.; Ng, E.; Wung, V.; Udawatta, M.; Nguyen, T.; Sheppard, J.P.; Preet, K.; Alemnew, M.; Seo, D.; et al. Superior semicircular canal dehiscence postoperative outcomes: A case series of 156 repairs. J. Clin. Neurosci. 2019, 68, 69–72. [Google Scholar] [CrossRef]
- Kurihara, Y.Y.; Fujikawa, A.; Tachizawa, N.; Takaya, M.; Ikeda, H.; Starkey, J. Temporal bone trauma: Typical CT and MRI appearances and important points for evaluation. Radiographics 2020, 40, 1148–1162. [Google Scholar] [CrossRef]
- Aladham, Y.; Ahmed, O.; Hassan, S.A.S.; Francis-Khoury, E. Traumatic superior semicircular canal dehiscence syndrome: Case report and literature review. J. Surg. Case Rep. 2021, 2021, rjaa592. [Google Scholar] [CrossRef] [PubMed]
- Campion, T.; Taranath, A.; Pinelli, L.; Ugga, L.; Nash, R.; Talenti, G.; Dahmoush, H.; D’arco, F. Imaging of temporal bone inflammations in children: A pictorial review. Neuroradiology 2019, 61, 965–980. [Google Scholar] [CrossRef] [PubMed]
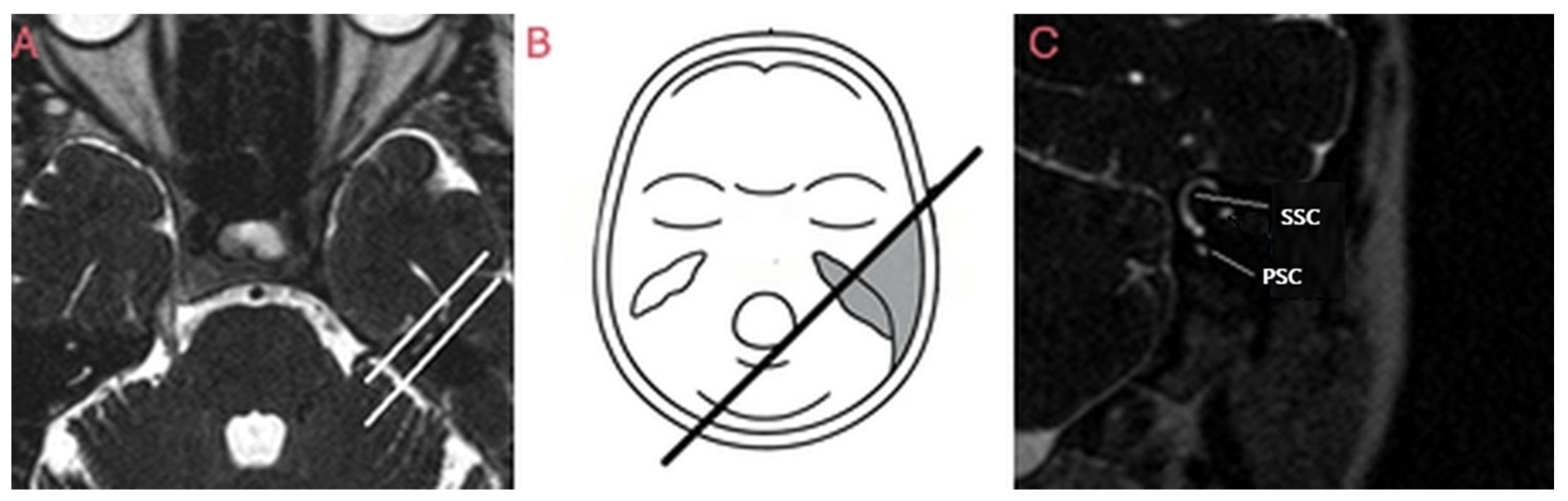
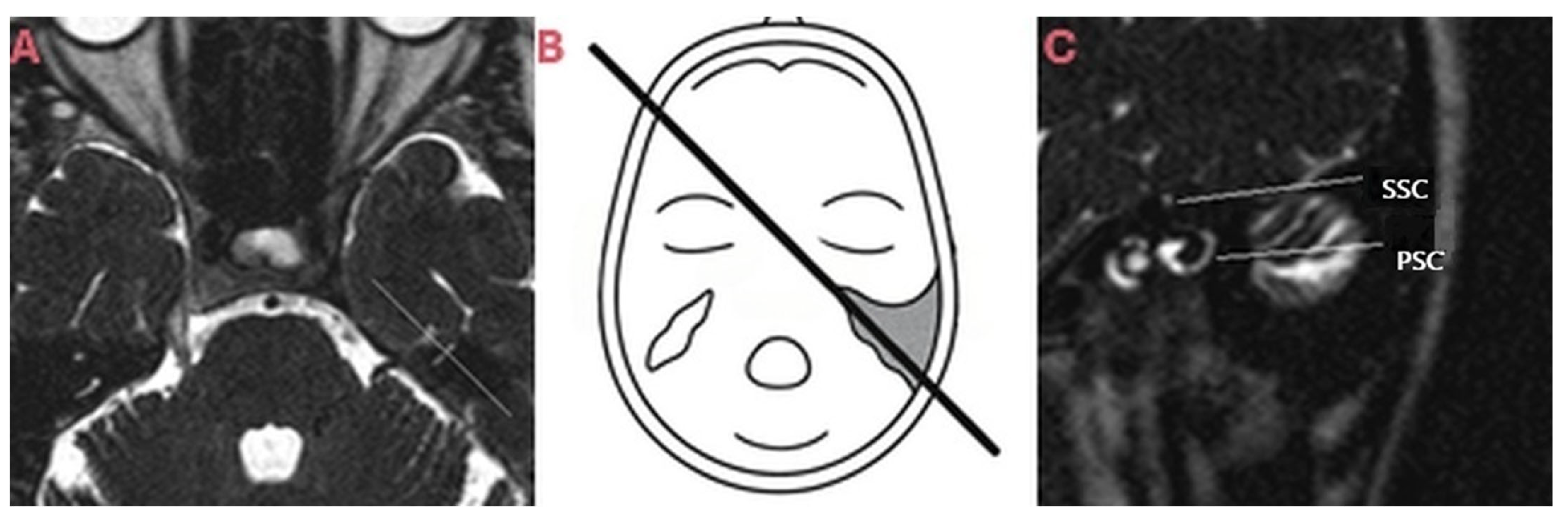
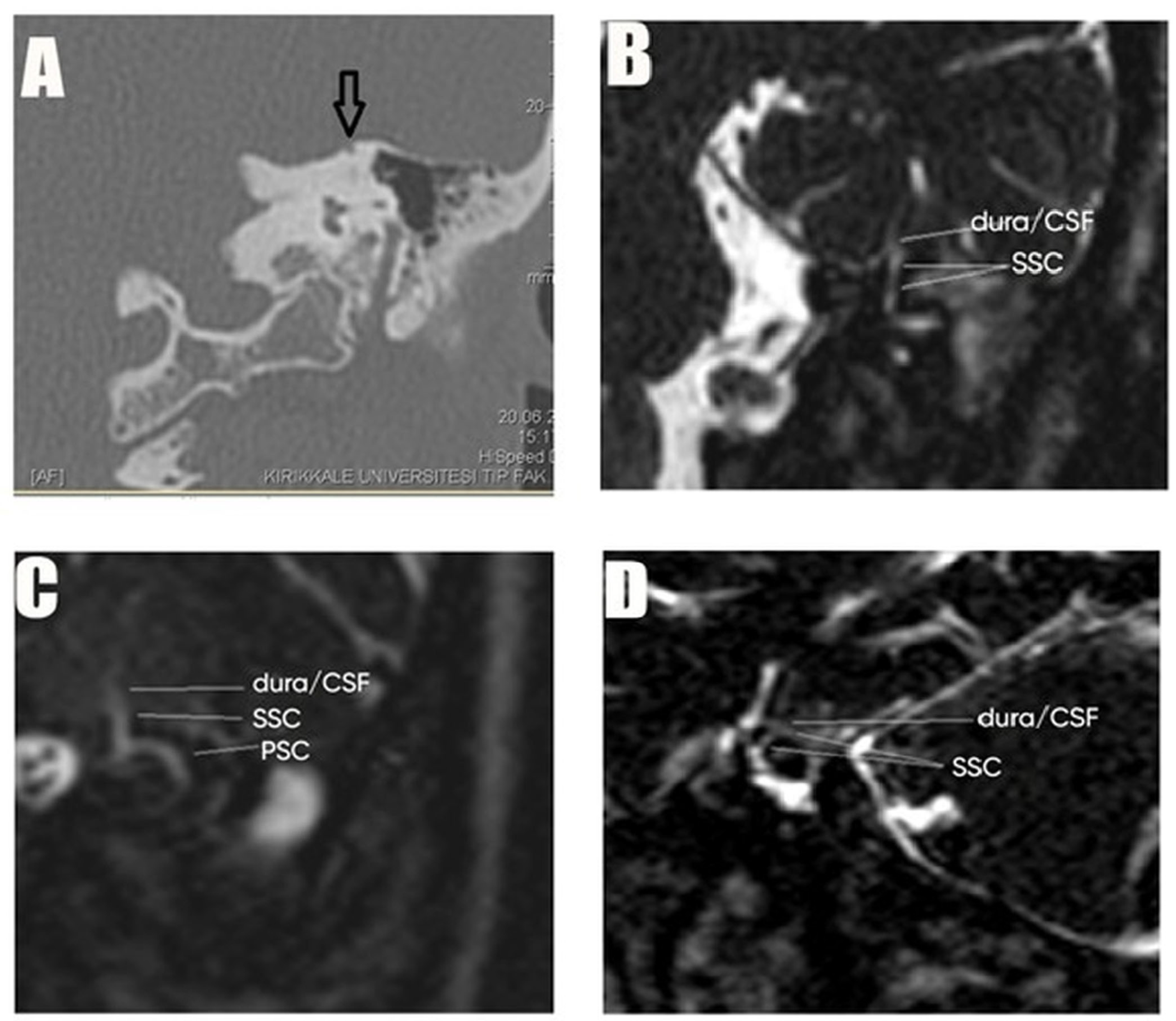
| CT Request Reasons | n = Patients (%) | CT Findings | n = Patients (%) |
|---|---|---|---|
| Vertigo—balance loss | 15 (14.0%) | Normal | 29 (14.0%) |
| Hearing loss | 50 (48.5%) | COM | 42 (40.0%) |
| Tinnitus | 19 (18.4%) | Mastoid inflammation | 21 (20.4%) |
| COM | 47 (45.6%) | Ossicular chain defect | 12 (11.7%) |
| Otalgia | 15 (14.6%) | Surgical defect | 11 (10.7%) |
| Hyperacusis | 3 (2.9%) | High jugular bulb | 5 (4.8%) |
| Tympanic membrane perforation | 12 (11.7%) | Ossicular chain sclerosis | 4 (3.9%) |
| Ear fullness | 2 (1.9%) | Cholesteatoma | 3 (2.9%) |
| Cranial dysmorphism | 1 (1.0%) | Cranial dysmorphism | 1 (1.0%) |
| Eagle syndrome | 2 (1.9%) | Eagle syndrome | 2 (1.9%) |
| Trauma | 3 (2.9%) | Skull base fracture | 2 (1.9%) |
| Findings | SSC | PSC |
|---|---|---|
| Group 1: Dehiscence/suspected dehiscence | 43 | 19 |
| -Definite dehiscence | 21 | 7 |
| -Suspected dehiscence | 22 | 12 |
| Group 2: No dehiscence | 163 | 187 |
| -Thin bone roof | 19 | 13 |
| -Normal | 144 | 174 |
| Total | 206 | 206 |
| CT Findings | MRI Results | MRI Axial (n = 53) | MRI Coronal (n = 53) | MRI Poschl (n = 53) | MRI Stenver (n = 53) | MRI Final Decision (n = 53) |
|---|---|---|---|---|---|---|
| Normal (n = 20) | Total | 20 | 20 | 20 | 20 | 20 |
| Normal | 17 | 16 | 18 | 14 | 17 | |
| Definite dehiscence | 0 | 0 | 1 | 1 | 0 | |
| Suspected dehiscence | 1 | 2 | 0 | 1 | 1 | |
| Thin bone roof | 2 | 2 | 1 | 4 | 2 | |
| Definite dehiscence (n = 11) | Total | 11 | 11 | 11 | 11 | 11 |
| Normal | 1 | 1 | 2 | 1 | 1 | |
| Definite dehiscence | 3 | 7 | 7 | 5 | 9 | |
| Suspected dehiscence | 5 | 3 | 2 | 3 | 1 | |
| Thin bone roof | 2 | 0 | 0 | 2 | 0 | |
| Suspected dehiscence (n = 11) | Total | 11 | 11 | 11 | 11 | 11 |
| [MRI Results’ Distribution...] | ||||||
| Thin bone roof (n = 11) | Total | 11 | 11 | 11 | 11 | 11 |
| [MRI Results’ Distribution...] |
| CT Findings | MRI Results | MRI Axial (n = 54) | MRI Coronal (n = 54) | MRI Poschl (n = 54) | MRI Stenver (n = 54) | MRI Final Decision (n = 54) |
|---|---|---|---|---|---|---|
| Normal (n = 35) | Total | 35 | 35 | 35 | 35 | 35 |
| Normal | 33 | 31 | 31 | 33 | 33 | |
| Definite dehiscence | 0 | 2 | 2 | 0 | 0 | |
| Suspected dehiscence | 0 | 0 | 1 | 0 | 0 | |
| Thin bone roof | 2 | 2 | 1 | 2 | 2 | |
| Definite dehiscence (n = 3) | Total | 3 | 3 | 3 | 3 | 3 |
| Normal | 1 | 1 | 1 | 1 | 1 | |
| Definite dehiscence | 1 | 1 | 2 | 2 | 2 | |
| Suspected dehiscence | 1 | 1 | 0 | 0 | 0 | |
| Thin bone roof | 0 | 0 | 0 | 0 | 0 | |
| Suspected dehiscence (n = 8) | Total | 8 | 8 | 8 | 8 | 8 |
| Normal | 2 | 3 | 2 | 2 | 2 | |
| Definite dehiscence | 3 | 3 | 4 | 4 | 4 | |
| Suspected dehiscence | 1 | 2 | 0 | 1 | 1 | |
| Thin bone roof | 2 | 0 | 2 | 1 | 1 | |
| Thin bone roof (n = 8) | Total | 8 | 8 | 8 | 8 | 8 |
| Normal | 1 | 4 | 2 | 2 | 2 | |
| Definite dehiscence | 2 | 3 | 1 | 1 | 1 | |
| Suspected dehiscence | 0 | 1 | 0 | 0 | 0 | |
| Thin bone roof | 5 | 0 | 5 | 5 | 5 |
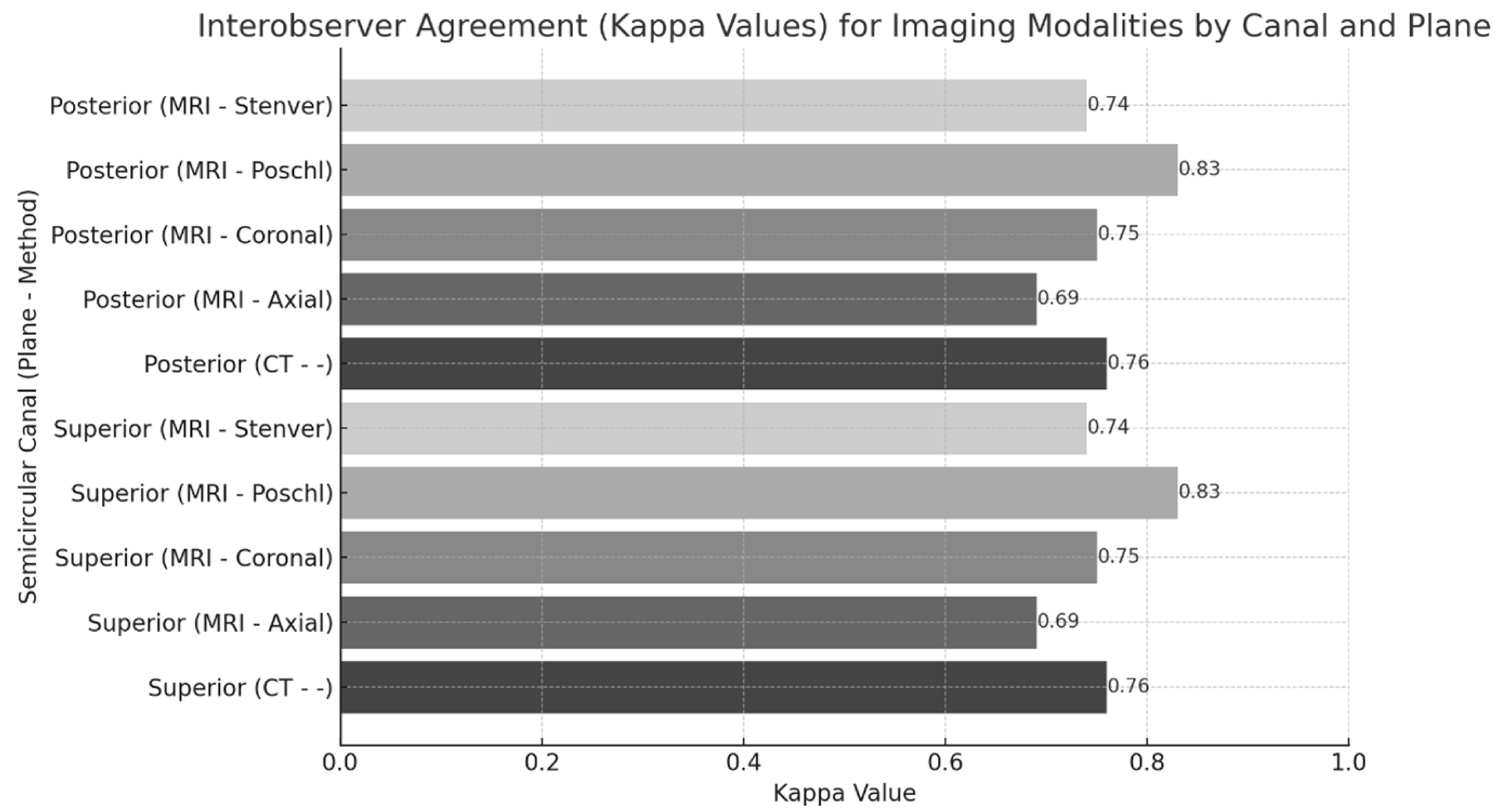
| Canal | MRI Planes | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | PPV (%) | NPV (%) | Agreement (κ) | p-Value |
|---|---|---|---|---|---|---|---|
| SSCD | Axial | 68 (49.7–86.3) | 93 (82.9–100.0) | 88 | 80 | 0.63 * | <0.001 |
| Coronal | 68 (49.7–86.3) | 83 (69.6–96.4) | 75 | 78 | 0.51 | 0.118 | |
| Poschl | 63 (44.1–81.9) | 80 (65.7–94.3) | 70 | 75 | 0.43 * | 0.002 | |
| Stenver | 63 (44.1–81.9) | 87 (75.1–98.9) | 77 | 77 | 0.51 * | <0.001 | |
| MRI Final Decision | 78 (62.0–94.0) | 92 (81.4–100.0) | 83 | 77 | 0.71 * | <0.001 | |
| PSCD | Axial | 60 (35.2–84.8) | 95 (88.3–100.0) | 75 | 91 | 0.60 * | <0.001 |
| Coronal | 70 (47.1–92.9) | 86 (75.7–96.3) | 53 | 92 | 0.48 * | <0.001 | |
| Poschl | 60 (35.2–84.8) | 88 (78.5–97.5) | 54 | 90 | 0.45 * | <0.001 | |
| Stenver | 60 (35.2–84.8) | 88 (78.5–97.5) | 54 | 90 | 0.46 * | <0.001 | |
| MRI Final Decision | 70 (47.1–92.9) | 97 (91.6–100.0) | 87 | 93 | 0.73 * | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolgen, C.; Unal Daphan, B. Is CT Still the Gold Standard in Semicircular Canal Dehiscence? Diagnostic Value of MRI in Poschl and Stenver Planes. Brain Sci. 2025, 15, 555. https://doi.org/10.3390/brainsci15060555
Bolgen C, Unal Daphan B. Is CT Still the Gold Standard in Semicircular Canal Dehiscence? Diagnostic Value of MRI in Poschl and Stenver Planes. Brain Sciences. 2025; 15(6):555. https://doi.org/10.3390/brainsci15060555
Chicago/Turabian StyleBolgen, Cagatay, and Birsen Unal Daphan. 2025. "Is CT Still the Gold Standard in Semicircular Canal Dehiscence? Diagnostic Value of MRI in Poschl and Stenver Planes" Brain Sciences 15, no. 6: 555. https://doi.org/10.3390/brainsci15060555
APA StyleBolgen, C., & Unal Daphan, B. (2025). Is CT Still the Gold Standard in Semicircular Canal Dehiscence? Diagnostic Value of MRI in Poschl and Stenver Planes. Brain Sciences, 15(6), 555. https://doi.org/10.3390/brainsci15060555








