Transcranial Direct Current Stimulation (tDCS) for Borderline Personality Disorder (BPD): Why and How?
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. tDCS
3.1.1. Description
3.1.2. Mechanisms of Action
3.2. Rationale for Using tDCS
3.2.1. Safety Profile of tDCS in Comparison to Pharmacotherapy
3.2.2. Affordability and Accessibility Compared to Conventional Treatments
3.2.3. Neurobiological Basis for tDCS in BPD
3.3. Possible Outcomes
3.3.1. Targeting BPD Symptoms
| Author | Participants | Anode | Cathode | Anodal Electrode Size (cm2) | Current Intensity (mA) | Number of Session | Session Duration (mn) | Main Effects |
|---|---|---|---|---|---|---|---|---|
| Schulze et al., 2019 [49] | 48 BPD: 25 sham; 23 active | Right DLPFC | Left deltoid | 35 | 1 | 1 | 20 | No improvement of cognitive control over negative stimuli |
| Molavi et al., 2020 [42] | 32 BPD: 16 sham; 16 active | Right DLPFC | Left DLPFC | 25 | 2 | 10 | 20 | Improvement of executive functions and cognitive emotion regulation, although emotional expression was unaffected |
| Lisoni et al., 2020 [44] | 30 BPD: 15 sham; 15 active | Left DLPFC | Right DLPFC | 35 | 2 | 15 | 20 | Improvement of impulsivity, aggression, and craving, while also marginally improving decision making |
| Wolkenstein et al., 2021 [43] | 40: 20 healthy; 20 BPD | Left DLPFC | Right Mastoïd | 35 | 1 | 1 | 20 | No reduction in emotional interference at the group level, but selectively improved cognitive control in participants—particularly BPD patients. |
| Lisco et al., 2025 [47] | 40 BPD: 20 sham; 20 active | Right VLPFC | Left supraorbital region | 25 | 1.5 | 20 | Reduction in rejection-related emotions |
3.3.2. Enhancing Psychosocial Functioning Through Executive Function
3.3.3. Efficacy of tDCS in Comorbid Conditions
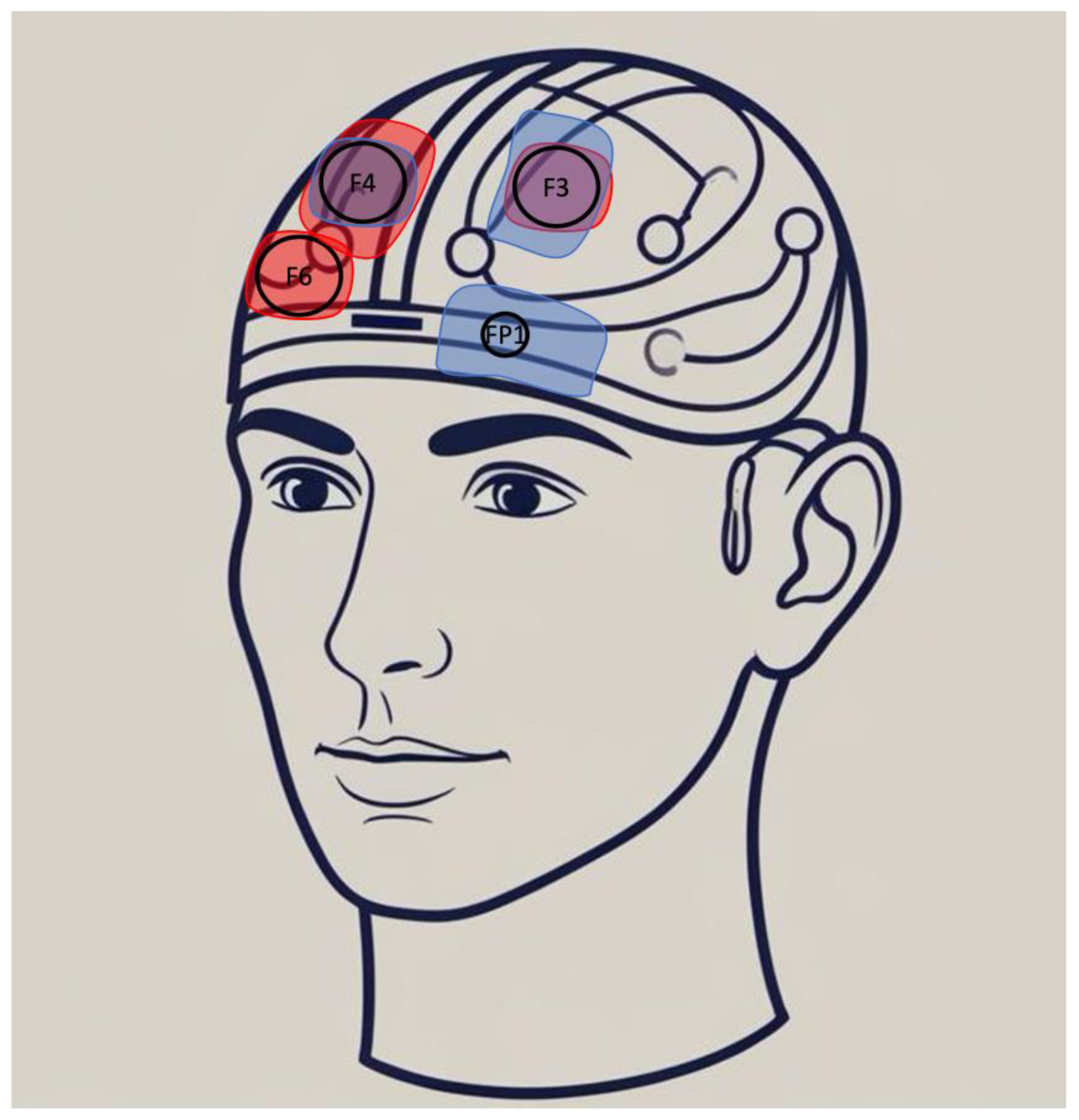
3.4. What Is the Optimal tDCS Protocol for BPD?
3.4.1. Stimulation Parameters
3.4.2. Combination with Psychotherapy
3.5. Future Research Directions
3.5.1. Caution Is Needed
3.5.2. Clinical Trials Needed
3.5.3. Long-Term Effects and Sustainability
3.5.4. Personalized Neuromodulation
3.5.5. Psychotherapeutic Combination
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BPD | Borderline Personality Disorder |
| DA | Dopamine |
| DBT | Dialectical Behavior Therapy |
| DLPFC | Dorso-Lateral Pre-Frontal Cortex |
| MDD | Major Depressive Disorder |
| MBT | Mentalization-Based Therapy |
| RCT | Randomized Controlled Trial |
| rVLPFC | Right Ventrolateral Prefrontal Cortex |
| rTMS | Repetitive Transcranial Magnetic Stimulation |
| SANRA | Scale for the Assessment of Narrative Review Articles |
| tDCS | Transcranial Direct Current Stimulation |
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2022. [Google Scholar]
- Volkert, J.; Gablonski, T.C.; Rabung, S. Prevalence of personality disorders in the general adult population in Western countries: Systematic review and meta-analysis. Br. J. Psychiatry 2018, 213, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Zanarini, M.C. Comorbidity of Borderline Personality Disorder: Current Status and Future Directions. Psychiatr. Clin. N. Am. 2018, 41, 583–593. [Google Scholar] [CrossRef]
- Álvarez-Tomás, I.; Ruiz, J.; Guilera, G.; Bados, A. Long-term clinical and functional course of borderline personality disorder: A meta-analysis of prospective studies. Eur. Psychiatry 2019, 56, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Cailhol, L.; Pelletier, É.; Rochette, L.; Renaud, S.; Koch, M.; David, P.; Villeneuve, E.; Lunghi, C.; Lesage, A. Utilization of Health Care Services by Patients with Cluster B Personality Disorders or Schizophrenia. Psychiatr. Serv. 2021, 72, 1392–1399. [Google Scholar] [CrossRef]
- Kjær, J.N.R.; Biskin, R.; Vestergaard, C.; Munk-J Rgensen, P. All-Cause Mortality of Hospital-Treated Borderline Personality Disorder: A Nationwide Cohort Study. J. Pers. Disord. 2020, 34, 723–735. [Google Scholar] [CrossRef]
- Pompili, M.; Girardi, P.; Ruberto, A.; Tatarelli, R. Suicide in borderline personality disorder: A meta-analysis. Nord. J. Psychiatry 2005, 59, 319–324. [Google Scholar] [CrossRef]
- Cailhol, L.; Pelletier, É.; Rochette, L.; Laporte, L.; David, P.; Villeneuve, É.; Lesage, A. Prevalence, Mortality, and Health Care Use among Patients with Cluster B Personality Disorders Clinically Diagnosed in Quebec: A Provincial Cohort Study, 2001–2012. Can. J. Psychiatry 2017, 62, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Keepers, G.A.; Fochtmann, L.J.; Anzia, J.M.; Benjamin, S.; Lyness, J.M.; Mojtabai, R.; Servis, M.; Choi-Kain, L.; Nelson, K.J.; Oldham, J.M.; et al. The American Psychiatric Association Practice Guideline for the Treatment of Patients with Borderline Personality Disorder. Am. J. Psychiatry 2024, 181, 1024–1028. [Google Scholar] [CrossRef]
- Leichsenring, F.; Fonagy, P.; Heim, N.; Kernberg, O.F.; Leweke, F.; Luyten, P.; Salzer, S.; Spitzer, C.; Steinert, C. Borderline personality disorder: A comprehensive review of diagnosis and clinical presentation, etiology, treatment, and current controversies. World Psychiatry 2024, 23, 4–25. [Google Scholar] [CrossRef]
- Bohus, M.; Stoffers-Winterling, J.; Sharp, C.; Krause-Utz, A.; Schmahl, C.; Lieb, K. Borderline personality disorder. Lancet 2021, 398, 1528–1540. [Google Scholar] [CrossRef]
- Stoffers-Winterling, J.M.; Storebø, O.J.; Kongerslev, M.T.; Faltinsen, E.; Todorovac, A.; Sedoc Jørgensen, M.; Sales, C.P.; Edemann Callesen, H.; Pereira Ribeiro, J.; Völlm, B.A.; et al. Psychotherapies for borderline personality disorder: A focused systematic review and meta-analysis. Br. J. Psychiatry 2022, 221, 538–552. [Google Scholar] [CrossRef] [PubMed]
- Paris, J. Stepped care: An alternative to routine extended treatment for patients with borderline personality disorder. Psychiatr. Serv. 2013, 64, 1035–1037. [Google Scholar] [CrossRef] [PubMed]
- Arntz, A.; Mensink, K.; Cox, W.R.; Verhoef, R.E.J.; van Emmerik, A.A.P.; Rameckers, S.A.; Badenbach, T.; Grasman, R.P.P.P. Dropout from psychological treatment for borderline personality disorder: A multilevel survival meta-analysis. Psychol. Med. 2023, 53, 668–686. [Google Scholar] [CrossRef] [PubMed]
- Stoffers-Winterling, J.M.; Storebø, O.J.; Pereira Ribeiro, J.; Kongerslev, M.T.; Völlm, B.A.; Mattivi, J.T.; Faltinsen, E.; Todorovac, A.; Jørgensen, M.S.; Callesen, H.E.; et al. Pharmacological interventions for people with borderline personality disorder. Cochrane Database Syst Rev. 2022, 11, CD012956. [Google Scholar]
- Lisoni, J.; Nibbio, G.; Baldacci, G.; Cicale, A.; Zucchetti, A.; Bertoni, L.; Calzavara Pinton, I.; Necchini, N.; Deste, G.; Barlati, S.; et al. What impact can brain stimulation interventions have on borderline personality disorder? Expert Rev. Neurother. 2024, 24, 343–360. [Google Scholar] [CrossRef]
- Lisoni, J.; Barlati, S.; Deste, G.; Ceraso, A.; Nibbio, G.; Baldacci, G.; Vita, A. Efficacy and tolerability of Brain Stimulation interventions in Borderline Personality Disorder: State of the art and future perspectives—A systematic review. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 116, 110537. [Google Scholar] [CrossRef]
- Woodham, R.D.; Selvaraj, S.; Lajmi, N.; Hobday, H.; Sheehan, G.; Ghazi-Noori, A.R.; Largerberg, P.J.; Rizvi, M.; Kwon, S.S.; Orhii, P.; et al. Home-based transcranial direct current stimulation treatment for major depressive disorder: A fully remote phase 2 randomized sham-controlled trial. Nat. Med. 2025, 31, 87–95. [Google Scholar] [CrossRef]
- Ruocco, A.C.; Marceau, E.M. Update on the Neurobiology of Borderline Personality Disorder: A Review of Structural, Resting-State and Task-Based Brain Imaging Studies. Curr. Psychiatry Rep. 2024, 26, 807–815. [Google Scholar] [CrossRef]
- van Zutphen, L.; Siep, N.; Jacob, G.A.; Goebel, R.; Arntz, A. Emotional sensitivity, emotion regulation and impulsivity in borderline personality disorder: A critical review of fMRI studies. Neurosci. Biobehav. Rev. 2015, 51, 64–76. [Google Scholar] [CrossRef]
- Sicorello, M.; Schmahl, C. Emotion dysregulation in borderline personality disorder: A fronto-limbic imbalance? Curr. Opin. Psychol. 2021, 37, 114–120. [Google Scholar] [CrossRef]
- Amad, A.; Radua, J. Resting-state meta-analysis in Borderline Personality Disorder: Is the fronto-limbic hypothesis still valid? J. Affect. Disord. 2017, 212, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA-a scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Cao, L.; Wang, Q.; Sheng, Y.; Yu, J.; Liang, Z. The Physiological Mechanisms of Transcranial Direct Current Stimulation to Enhance Motor Performance: A Narrative Review. Biology 2024, 13, 790. [Google Scholar] [CrossRef] [PubMed]
- Keeser, D.; Meindl, T.; Bor, J.; Palm, U.; Pogarell, O.; Mulert, C.; Brunelin, J.; Möller, H.J.; Reiser, M.; Padberg, F.; et al. Prefrontal transcranial direct current stimulation changes connectivity of resting-state networks during fMRI. J. Neurosci. 2011, 31, 15284–15293. [Google Scholar] [CrossRef]
- Keeser, D.; Padberg, F.; Reisinger, E.; Pogarell, O.; Kirsch, V.; Palm, U.; Karsh, S.; Möller, H.J.; Nitsche, M.A.; Mulert, C. Prefrontal direct current stimulation modulates resting EEG and event-related potentials in healthy subjects: A standardized low resolution tomography (sLORETA) study. Neuroimage 2011, 55, 644–657. [Google Scholar] [CrossRef]
- Fonteneau, C.; Redoute, J.; Haesebaert, F.; Le Bars, D.; Costes, N.; Suaud-Chagny, M.F.; Brunelin, J. Frontal Transcranial Direct Current Stimulation Induces Dopamine Release in the Ventral Striatum in Human. Cereb. Cortex. 2018, 28, 2636–2646. [Google Scholar] [CrossRef] [PubMed]
- Brunelin, J.; Fecteau, S. Impact of bifrontal transcranial Direct Current Stimulation on decision-making and stress reactivity. A pilot study. J. Psychiatr. Res. 2021, 135, 15–19. [Google Scholar] [CrossRef]
- Antal, A.; Alekseichuk, I.; Bikson, M.; Brockmöller, J.; Brunoni, A.R.; Chen, R.; Cohen, L.G.; Dowthwaite, G.; Ellrich, J.; Flöel, A.; et al. Low intensity transcranial electric stimulation: Safety, ethical, legal regulatory and application guidelines. Clin. Neurophysiol. 2017, 128, 1774–1809. [Google Scholar] [CrossRef]
- Bikson, M.; Grossman, P.; Thomas, C.; Zannou, A.L.; Jiang, J.; Adnan, T.; Mourdoukoutas, A.P.; Kronberg, G.; Truong, D.; Boggio, P.; et al. Safety of Transcranial Direct Current Stimulation: Evidence Based Update 2016. Brain Stimul. 2016, 9, 641–661. [Google Scholar] [CrossRef]
- Lunghi, C.; Cailhol, L.; Massamba, V.; Renaud, S.; David, P.; Laouan Sidi, E.A.; Biskin, R.; Koch, M.; Martineau, C.; Rahme, E.; et al. Cluster B personality disorders and psychotropic medications: A focused analysis of trends and patterns across sex and age groups. Soc. Psychiatry Psychiatr. Epidemiol. 2024, 1–11. [Google Scholar] [CrossRef]
- Temes, C.M.; Frankenburg, F.R.; Fitzmaurice, G.M.; Zanarini, M.C. Deaths by Suicide and Other Causes Among Patients with Borderline Personality Disorder and Personality-Disordered Comparison Subjects Over 24 Years of Prospective Follow-Up. J. Clin. Psychiatry 2019, 80, 18m12436. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.L.; Cowdry, R.W. Alprazolam-induced dyscontrol in borderline personality disorder. Am. J. Psychiatry 1985, 142, 98–100. [Google Scholar]
- Broadbear, J.H.; Dwyer, J.; Bugeja, L.; Rao, S. Coroners’ investigations of suicide in Australia: The hidden toll of borderline personality disorder. J. Psychiatr. Res. 2020, 129, 241–249. [Google Scholar] [CrossRef]
- Hastrup, L.H.; Jennum, P.; Ibsen, R.; Kjellberg, J.; Simonsen, E. Societal costs of Borderline Personality Disorders: A matched-controlled nationwide study of patients and spouses. Acta Psychiatr. Scand. 2019, 140, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Sauvaget, A.; Lagalice, L.; Schirr-Bonnans, S.; Volteau, C.; Péré, M.; Dert, C.; Rivalland, A.; Tessier, F.; Le page, A.; Tostivint, A.; et al. Cost-utility analysis of transcranial direct current stimulation (tDCS) in non-treatment-resistant depression: The DISCO randomised controlled study protocol. BMJ Open 2020, 10, e033376. [Google Scholar] [CrossRef]
- Sauvaget, A.; Tostivint, A.; Etcheverrigaray, F.; Pichot, A.; Dert, C.; Schirr-Bonnais, S.; Clouet, J.; Sellal, O.; Mauduit, N.; Leux, C.; et al. Hospital production cost of transcranial direct current stimulation (tDCS) in the treatment of depression. Neurophysiol. Clin. 2019, 49, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Koutsomitros, T.; Schwarz, S.A.; van der Zee, K.T.; Schuhmann, T.; Sack, A.T. Home-administered transcranial direct current stimulation with asynchronous remote supervision in the treatment of depression: Feasibility, tolerability, and clinical effectiveness. Front. Psychiatry 2023, 14, 1206805. [Google Scholar] [CrossRef]
- Le Bars, T.; Bulteau, S.; Bonnot, O.; Gollier-Briant, F.; Prevotel, A.; Choneau, D.; Grymazewski, C.; Riche, V.P.; Rothärmel, M.; Poulet, E.; et al. Home-based transcranial direct current stimulation in schizophrenia: Systematic literature review, a teenager case report with cost-utility analysis. Schizophr. Res. 2024, 267, 441–443. [Google Scholar] [CrossRef]
- Sosic-Vasic, Z.; Schaitz, C.; Mayer, B.; Maier, A.; Connemann, B.; Kroener, J. Treating emotion dysregulation in patients with borderline personality disorder using imagery rescripting: A two-session randomized controlled trial. Behav. Res. Ther. 2024, 173, 104454. [Google Scholar] [CrossRef]
- Chen, S.Y.; Cheng, Y.; Zhao, W.W.; Zhang, Y.H. Effects of dialectical behaviour therapy on reducing self-harming behaviours and negative emotions in patients with borderline personality disorder: A meta-analysis. J. Psychiatr. Ment. Health Nurs. 2021, 28, 1128–1139. [Google Scholar] [CrossRef]
- Molavi, P.; Aziziaram, S.; Basharpoor, S.; Atadokht, A.; Nitsche, M.A.; Salehinejad, M.A. Repeated transcranial direct current stimulation of dorsolateral-prefrontal cortex improves executive functions, cognitive reappraisal emotion regulation, and control over emotional processing in borderline personality disorder: A randomized, sham-controlled, parallel-group study. J. Affect. Disord. 2020, 274, 93–102. [Google Scholar] [PubMed]
- Wolkenstein, L.; Rombold-Bruehl, F.; Bingmann, T.; Sommer, A.; Kanske, P.; Plewnia, C. Challenging control over emotions in borderline personality disorder—A tDCS study. Neuropsychologia 2021, 156, 107850. [Google Scholar] [CrossRef]
- Lisoni, J.; Miotto, P.; Barlati, S.; Calza, S.; Crescini, A.; Deste, G.; Sacchetti, E.; Vita, A. Change in core symptoms of borderline personality disorder by tDCS: A pilot study. Psychiatry Res. 2020, 291, 113261. [Google Scholar] [CrossRef]
- Teti Mayer, J.; Chopard, G.; Nicolier, M.; Gabriel, D.; Masse, C.; Giustiniani, J.; Vandel, P.; Haffen, E.; Bennabi, D. Can transcranial direct current stimulation (tDCS) improve impulsivity in healthy and psychiatric adult populations? A systematic review. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 98, 109814. [Google Scholar] [CrossRef] [PubMed]
- Brevet-Aeby, C.; Brunelin, J.; Iceta, S.; Padovan, C.; Poulet, E. Prefrontal cortex and impulsivity: Interest of noninvasive brain stimulation. Neurosci. Biobehav. Rev. 2016, 71, 112–134. [Google Scholar] [CrossRef]
- Lisco, A.; Gallucci, A.; Fabietti, C.; Fornaroli, A.; Marchesi, C.; Preti, E.; Riva, P.; De Panfilis, C.; Romero Lauro, R.J. Reduction of rejection-related emotions by transcranial direct current stimulation over right ventrolateral prefrontal cortex in borderline personality disorder: A double-blind randomized pilot study. Psychiatry Clin. Neurosci. 2025, 79, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Tolomeo, S.; Yang, C.; Wang, Y.; Yu, R. tDCS effect on prosocial behavior: A meta-analytic review. Soc. Cogn. Affect. Neurosci. 2022, 17, 26–42. [Google Scholar] [CrossRef]
- Schulze, L.; Grove, M.; Tamm, S.; Renneberg, B.; Roepke, S. Effects of transcranial direct current stimulation on the cognitive control of negative stimuli in borderline personality disorder. Sci. Rep. 2019, 9, 332. [Google Scholar] [CrossRef] [PubMed]
- D’Iorio, A.; Benedetto, G.L.D.; Santangelo, G. A meta-analysis on the neuropsychological correlates of Borderline Personality Disorder: An update. Neurosci. Biobehav. Rev. 2024, 165, 105860. [Google Scholar] [CrossRef]
- Imburgio, M.J.; Orr, J.M. Effects of prefrontal tDCS on executive function: Methodological considerations revealed by meta-analysis. Neuropsychologia 2018, 117, 156–166. [Google Scholar] [CrossRef]
- Frankenburg, F.R.; Zanarini, M.C. The association between borderline personality disorder and chronic medical illnesses, poor health-related lifestyle choices, and costly forms of health care utilization. J. Clin. Psychiatry 2004, 65, 1660–1665. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Luo, H.; Schülke, R.; Geng, X.; Sahakian, B.J.; Wang, S. Is transcranial direct current stimulation, alone or in combination with antidepressant medications or psychotherapies, effective in treating major depressive disorder? A systematic review and meta-analysis. BMC Med. 2021, 19, 319. [Google Scholar] [CrossRef] [PubMed]
- Sabé, M.; Hyde, J.; Cramer, C.; Eberhard, A.; Crippa, A.; Brunoni, A.R.; Aleman, A.; Kaiser, S.; Baldwin, D.S.; Garner, M. Transcranial Magnetic Stimulation and Transcranial Direct Current Stimulation Across Mental Disorders: A Systematic Review and Dose-Response Meta-Analysis. JAMA Netw. Open 2024, 7, e2412616. [Google Scholar] [CrossRef]
- Razza, L.B.; De Smet, S.; Moffa, A.; Sudbrack-Oliveira, P.; Vanderhasselt, M.A.; Brunoni, A.R. Follow-up effects of transcranial direct current stimulation (tDCS) for the major depressive episode: A systematic review and meta-analysis. Psychiatry Res. 2021, 302, 114024. [Google Scholar] [CrossRef]
- Dondé, C.; Amad, A.; Nieto, I.; Brunoni, A.R.; Neufeld, N.H.; Bellivier, F.; Poulet, E.; Geoffroy, P.A. Transcranial direct-current stimulation (tDCS) for bipolar depression: A systematic review and meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 78, 123–131. [Google Scholar] [CrossRef]
- Moshfeghinia, R.; Shekouh, D.; Mostafavi, S.; Hosseinzadeh, M.; Bahadori, A.R.; Abdollahifard, S.; Razmkon, A. The effects of transcranial direct-current stimulation (tDCS) on pain intensity of patients with fibromyalgia: A systematic review and meta-analysis. BMC Neurol. 2023, 23, 395. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.L.; Cai, D.B.; Sun, C.H.; Yin, F.; Goerigk, S.; Brunoni, A.R.; Zhao, X.W.; Mayes, T.L.; Zheng, W.; Xiang, Y.T. Adjunctive tDCS for treatment-refractory auditory hallucinations in schizophrenia: A meta-analysis of randomized, double-blinded, sham-controlled studies. Asian J. Psychiatr. 2022, 73, 103100. [Google Scholar] [CrossRef]
- Yang, F.; Fang, X.; Tang, W.; Hui, L.; Chen, Y.; Zhang, C.; Tian, X. Effects and potential mechanisms of transcranial direct current stimulation (tDCS) on auditory hallucinations: A meta-analysis. Psychiatry Res. 2019, 273, 343–349. [Google Scholar] [CrossRef]
- Leffa, D.T.; Grevet, E.H.; Bau, C.H.D.; Schneider, M.; Ferrazza, C.P.; da Silva, R.F.; Miranda, M.S.; Picon, F.; Teche, S.P.; Sanches, P. Transcranial Direct Current Stimulation vs Sham for the Treatment of Inattention in Adults with Attention-Deficit/Hyperactivity Disorder: The TUNED Randomized Clinical Trial. JAMA Psychiatry 2022, 79, 847–856. [Google Scholar] [CrossRef]
- Yang, X.; Ma, L.; Fan, C.; Wang, H.; Zhang, M.; Du, H.; Zhou, T.; Li, X. Efficacy and acceptability of brain stimulation for anxiety disorders, OCD, and PTSD: A systematic review and network meta-analysis of randomized controlled trials. J. Affect. Disord. 2025, 370, 62–75. [Google Scholar] [CrossRef]
- Mehta, D.D.; Praecht, A.; Ward, H.B.; Sanches, M.; Sorkhou, M.; Tang, V.M.; Steele, V.R.; Hanlon, C.A.; George, T.P. A systematic review and meta-analysis of neuromodulation therapies for substance use disorders. Neuropsychopharmacology 2024, 49, 649–680. [Google Scholar] [CrossRef] [PubMed]
- Aust, S.; Brakemeier, E.L.; Spies, J.; Herrera-Melendez, A.L.; Kaiser, T.; Fallgatter, A.; Plewnia, C.; Mayer, S.V.; Dechantsreiter, E.; Burkhardt, G.; et al. Efficacy of Augmentation of Cognitive Behavioral Therapy with Transcranial Direct Current Stimulation for Depression: A Randomized Clinical Trial. JAMA Psychiatry 2022, 79, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Kujovic, M.; Bahr, C.; Riesbeck, M.; Benz, D.; Wingerter, L.; Deiß, M.; Margittai, Z.; Reinermann, D.; Plewnia, C.; Meisenzahl, E. Effects of intermittent theta burst stimulation add-on to dialectical behavioral therapy in borderline personality disorder: Results of a randomized, sham-controlled pilot trial. Eur. Arch. Psychiatry Clin. Neurosci. 2024, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Krause-Utz, A.; Walther, J.C.; Schweizer, S.; Lis, S.; Hampshire, A.; Schmahl, C.; Bohus, M. Effectiveness of an Emotional Working Memory Training in Borderline Personality Disorder: A Proof-of-Principle Study. Psychother. Psychosom. 2020, 89, 122–124. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cailhol, L.; Soltani, K.; Neige, C.; Mondino, M.; Brunelin, J.; Blay, M. Transcranial Direct Current Stimulation (tDCS) for Borderline Personality Disorder (BPD): Why and How? Brain Sci. 2025, 15, 547. https://doi.org/10.3390/brainsci15060547
Cailhol L, Soltani K, Neige C, Mondino M, Brunelin J, Blay M. Transcranial Direct Current Stimulation (tDCS) for Borderline Personality Disorder (BPD): Why and How? Brain Sciences. 2025; 15(6):547. https://doi.org/10.3390/brainsci15060547
Chicago/Turabian StyleCailhol, Lionel, Kamilia Soltani, Cécilia Neige, Marine Mondino, Jérôme Brunelin, and Martin Blay. 2025. "Transcranial Direct Current Stimulation (tDCS) for Borderline Personality Disorder (BPD): Why and How?" Brain Sciences 15, no. 6: 547. https://doi.org/10.3390/brainsci15060547
APA StyleCailhol, L., Soltani, K., Neige, C., Mondino, M., Brunelin, J., & Blay, M. (2025). Transcranial Direct Current Stimulation (tDCS) for Borderline Personality Disorder (BPD): Why and How? Brain Sciences, 15(6), 547. https://doi.org/10.3390/brainsci15060547







