Chronic Stress Modulates Microglial Activation Dynamics, Shaping Priming Responses to Subsequent Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Stress Exposure Procedures
2.2.1. Stable Chronic Unpredictable Mild Stress Exposure Paradigm
2.2.2. Acute Stress (Re-)Exposure Before Sacrifice
2.3. Behavioral Testing
2.3.1. Open Field Test (OFT)
2.3.2. Tail Suspension Test (TST)
2.3.3. Forced Swimming Test (FST)
2.4. Immunohistochemical Assay
2.4.1. Diaminobenzidine Staining with Iba-1 Antibodies
2.4.2. Immunofluorescence Staining with Iba-1 Antibodies
2.5. Statistical Analysis
3. Results
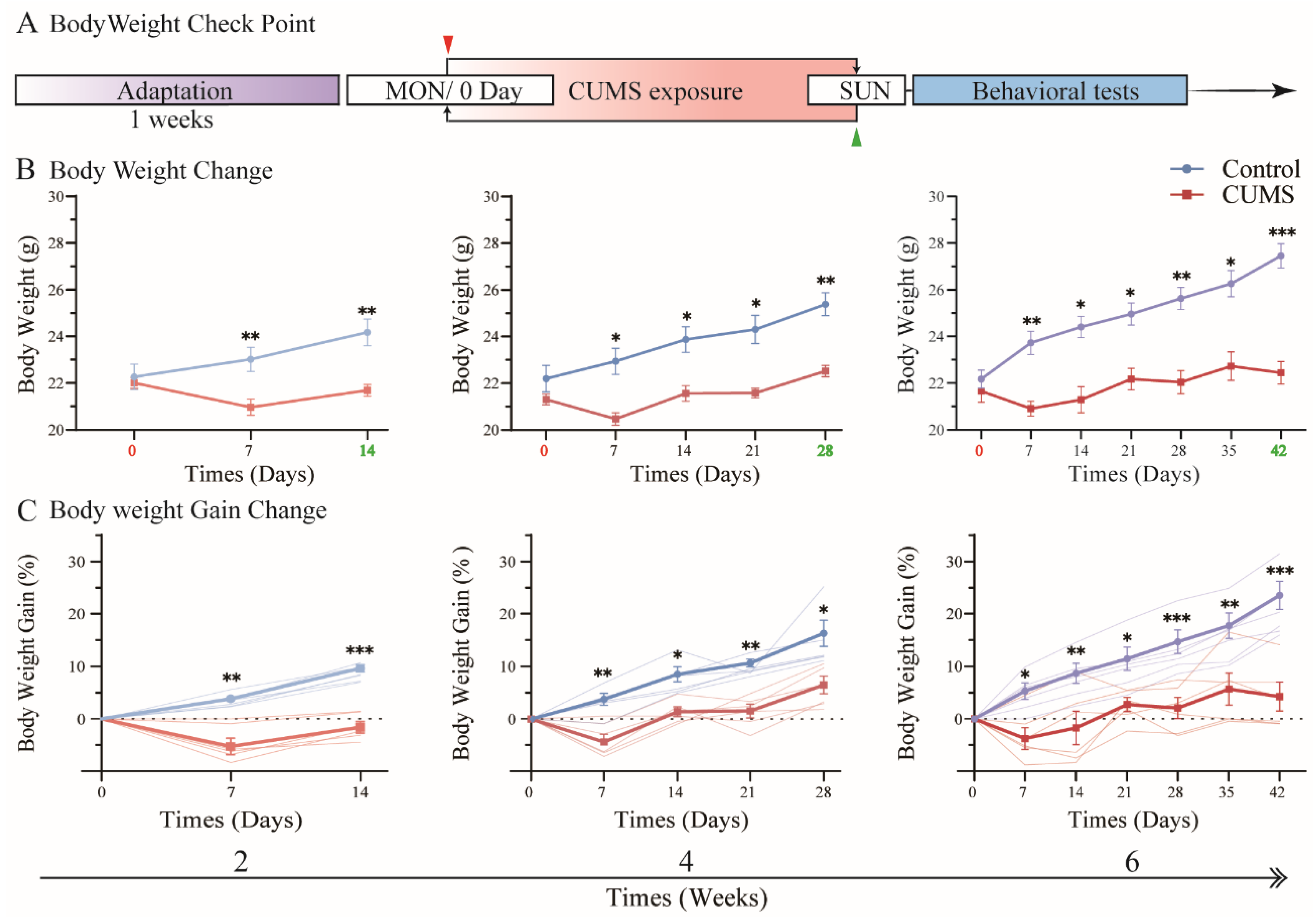
3.1. Body Weight Gain Was Suppressed During CUMS Exposure
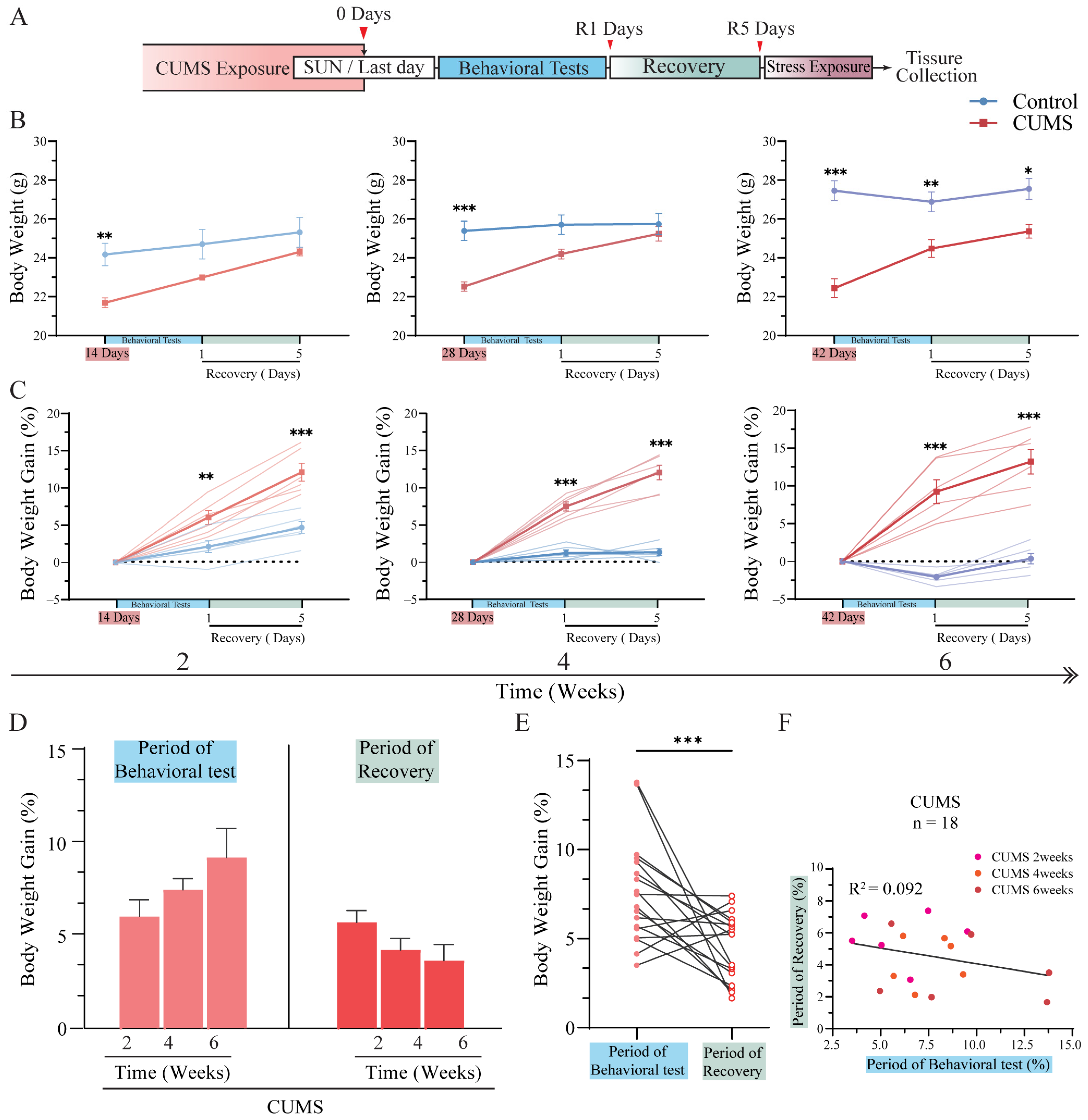
3.2. Body Weight Gain Increased Tremendously After CUMS Exposure
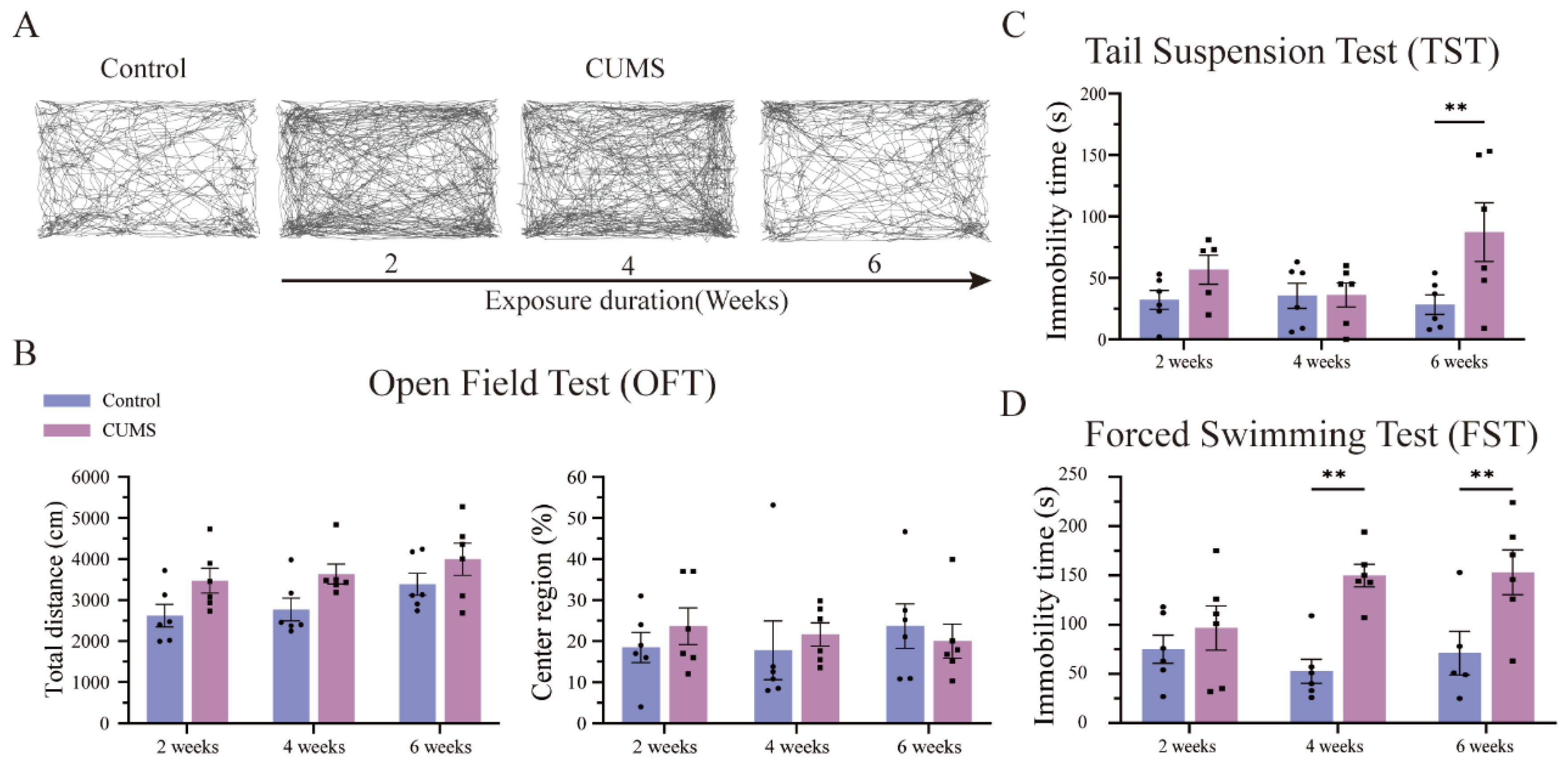
3.3. Long-Term CUMS Exposure Induced Depression-like Behavior in Mice, but Not in the Short Term
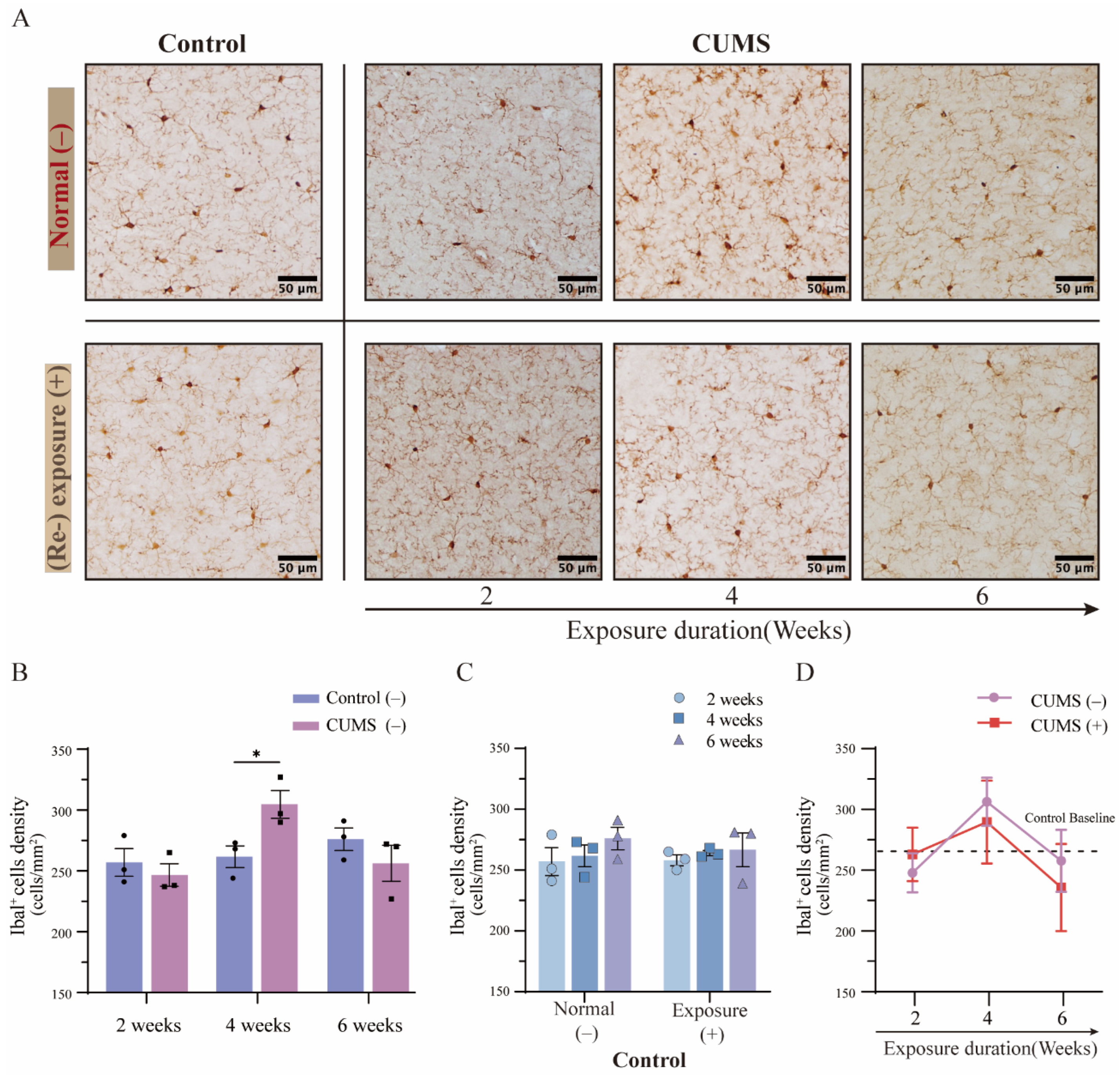
3.4. The Density of Microglia Showed Dynamic Variations in the Hippocampus After Different Durations of CUMS Exposure
3.5. The Phenomenon of Microglial Priming Showed a Converse Pattern Depending on the Duration of CUMS Exposure
3.6. The Density of Microglia Showed Relatively Stable Variations in the mPFC After Different Durations of CUMS Exposure
3.7. The Phenomenon of Microglial Priming in the mPFC Was Not Confirmed After Different Durations of CUMS Exposure
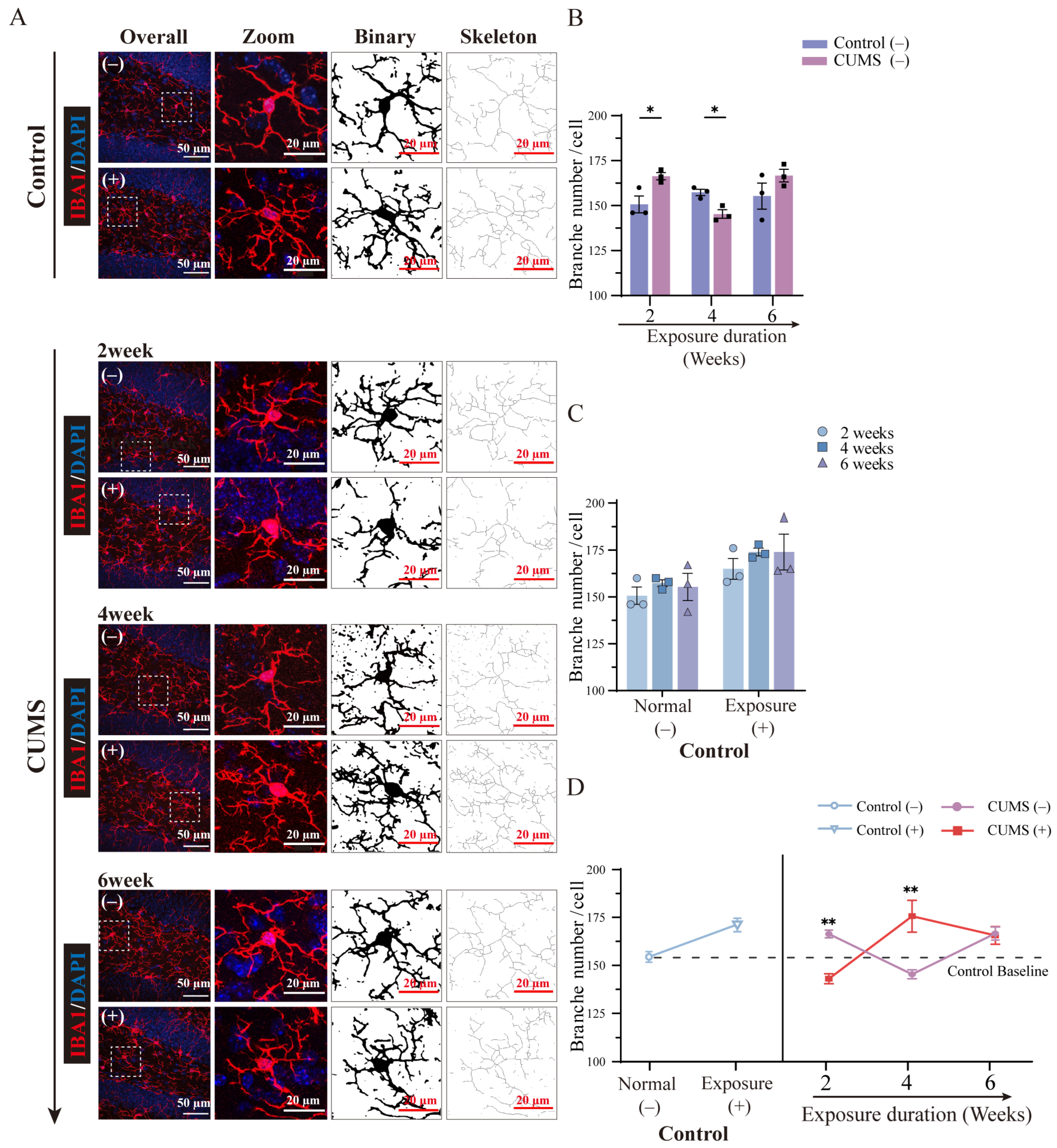
3.8. The Morphological Changes in Microglia Showed Diverse Fluctuations After Different Durations of CUMS Exposure
3.9. The Microglial Priming-Related Morphological Changes Showed a Converse Pattern Depending on the Duration of CUMS Exposure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- Szepesi, Z.; Manouchehrian, O.; Bachiller, S.; Deierborg, T. Bidirectional Microglia–Neuron Communication in Health and Disease. Front. Cell. Neurosci. 2018, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Wright-Jin, E.C.; Gutmann, D.H. Microglia as Dynamic Cellular Mediators of Brain Function. Trends Mol. Med. 2019, 25, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M.; Perry, V.H. Microglial Physiology: Unique Stimuli, Specialized Responses. Annu. Rev. Immunol. 2009, 27, 119–145. [Google Scholar] [CrossRef]
- Tynan, R.J.; Naicker, S.; Hinwood, M.; Nalivaiko, E.; Buller, K.M.; Pow, D.V.; Day, T.A.; Walker, F.R. Chronic Stress Alters the Density and Morphology of Microglia in a Subset of Stress-Responsive Brain Regions. Brain Behav. Immun. 2010, 24, 1058–1068. [Google Scholar] [CrossRef]
- Gomez-Nicola, D.; Perry, V.H. Microglial Dynamics and Role in the Healthy and Diseased Brain: A Paradigm of Functional Plasticity. Neuroscientist 2015, 21, 169–184. [Google Scholar] [CrossRef]
- Charney, D.S. Psychobiological Mechanisms of Resilience and Vulnerability: Implications for Successful Adaptation to Extreme Stress. AJP 2004, 161, 195–216. [Google Scholar] [CrossRef]
- Slavich, G.M.; Irwin, M.R. From Stress to Inflammation and Major Depressive Disorder: A Social Signal Transduction Theory of Depression. Psychol. Bull. 2014, 140, 774–815. [Google Scholar] [CrossRef]
- Perry, V.H.; Holmes, C. Microglial Priming in Neurodegenerative Disease. Nat. Rev. Neurol. 2014, 10, 217–224. [Google Scholar] [CrossRef]
- Niraula, A.; Sheridan, J.F.; Godbout, J.P. Microglia Priming with Aging and Stress. Neuropsychopharmacology 2017, 42, 318–333. [Google Scholar] [CrossRef]
- Willner, P. The Chronic Mild Stress (CMS) Model of Depression: History, Evaluation and Usage. Neurobiol. Stress 2017, 6, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Sequeira-Cordero, A.; Salas-Bastos, A.; Fornaguera, J.; Brenes, J.C. Behavioural Characterisation of Chronic Unpredictable Stress Based on Ethologically Relevant Paradigms in Rats. Sci. Rep. 2019, 9, 17403. [Google Scholar] [CrossRef]
- Nollet, M. Models of Depression: Unpredictable Chronic Mild Stress in Mice. Curr. Protoc. 2021, 1, e208. [Google Scholar] [CrossRef]
- Burstein, O.; Doron, R. The Unpredictable Chronic Mild Stress Protocol for Inducing Anhedonia in Mice. JoVE 2018, 58184. [Google Scholar] [CrossRef]
- Morrison, H.W.; Filosa, J.A. A Quantitative Spatiotemporal Analysis of Microglia Morphology during Ischemic Stroke and Reperfusion. J. Neuroinflammation 2013, 10, 782. [Google Scholar] [CrossRef]
- Young, K.; Morrison, H. Quantifying Microglia Morphology from Photomicrographs of Immunohistochemistry Prepared Tissue Using ImageJ. JoVE 2018, 57648. [Google Scholar] [CrossRef]
- Alqurashi, G.K.; Hindi, E.A.; Zayed, M.A.; Abd El-Aziz, G.S.; Alturkistani, H.A.; Ibrahim, R.F.; Al-thepyani, M.A.; Bakhlgi, R.; Alzahrani, N.A.; Ashraf, G.M.; et al. The Impact of Chronic Unpredictable Mild Stress-Induced Depression on Spatial, Recognition and Reference Memory Tasks in Mice: Behavioral and Histological Study. Behav. Sci. 2022, 12, 166. [Google Scholar] [CrossRef]
- Iñiguez, S.D.; Aubry, A.; Riggs, L.M.; Alipio, J.B.; Zanca, R.M.; Flores-Ramirez, F.J.; Hernandez, M.A.; Nieto, S.J.; Musheyev, D.; Serrano, P.A. Social Defeat Stress Induces Depression-like Behavior and Alters Spine Morphology in the Hippocampus of Adolescent Male C57BL/6 Mice. Neurobiol. Stress 2016, 5, 54–64. [Google Scholar] [CrossRef]
- Su, W.-J.; Zhang, Y.; Chen, Y.; Gong, H.; Lian, Y.-J.; Peng, W.; Liu, Y.-Z.; Wang, Y.-X.; You, Z.-L.; Feng, S.-J.; et al. NLRP3 Gene Knockout Blocks NF-κB and MAPK Signaling Pathway in CUMS-Induced Depression Mouse Model. Behav. Brain Res. 2017, 322, 1–8. [Google Scholar] [CrossRef]
- Surget, A.; Saxe, M.; Leman, S.; Ibarguen-Vargas, Y.; Chalon, S.; Griebel, G.; Hen, R.; Belzung, C. Drug-Dependent Requirement of Hippocampal Neurogenesis in a Model of Depression and of Antidepressant Reversal. Biol. Psychiatry 2008, 64, 293–301. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Liu, A.-H.; Xu, Y.; Tie, L.; Yu, H.-M.; Li, X.-J. Effect of Chronic Unpredictable Mild Stress on Brain–Pancreas Relative Protein in Rat Brain and Pancreas. Behav. Brain Res. 2005, 165, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Nollet, M.; Guisquet, A.L.; Belzung, C. Models of Depression: Unpredictable Chronic Mild Stress in Mice. CP Pharmacol. 2013, 61, 61:5.65.1–5.65.17. [Google Scholar] [CrossRef] [PubMed]
- Prut, L.; Belzung, C. The Open Field as a Paradigm to Measure the Effects of Drugs on Anxiety-like Behaviors: A Review. Eur. J. Pharmacol. 2003, 463, 3–33. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. Behavioral Despair in Mice: A Primary Screening Test for Antidepressants. Arch. Int. Pharmacodyn. Ther. 1977, 229, 327–336. [Google Scholar]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The Tail Suspension Test: A New Method for Screening Antidepressants in Mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef]
- Moser, M.-B.; Moser, E.I. Functional Differentiation in the Hippocampus. Hippocampus 1998, 8, 608–619. [Google Scholar] [CrossRef]
- Lurie, D.I. An Integrative Approach to Neuroinflammation in Psychiatric Disorders and Neuropathic Pain. J. Exp. Neurosci. 2018, 12, 1179069518793639. [Google Scholar] [CrossRef]
- Hori, H.; Nakamura, S.; Yoshida, F.; Teraishi, T.; Sasayama, D.; Ota, M.; Hattori, K.; Kim, Y.; Higuchi, T.; Kunugi, H. Integrated Profiling of Phenotype and Blood Transcriptome for Stress Vulnerability and Depression. J. Psychiatr. Res. 2018, 104, 202–210. [Google Scholar] [CrossRef]
- Zubin, J.; Spring, B. Vulnerability: A New View of Schizophrenia. J. Abnorm. Psychol. 1977, 86, 103–126. [Google Scholar] [CrossRef]
- Sugama, S.; Fujita, M.; Hashimoto, M.; Conti, B. Stress Induced Morphological Microglial Activation in the Rodent Brain: Involvement of Interleukin-18. Neuroscience 2007, 146, 1388–1399. [Google Scholar] [CrossRef]
- Etkin, A.; Egner, T.; Kalisch, R. Emotional Processing in Anterior Cingulate and Medial Prefrontal Cortex. Trends Cogn. Sci. 2011, 15, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Diorio, D.; Viau, V.; Meaney, M. The Role of the Medial Prefrontal Cortex (Cingulate Gyrus) in the Regulation of Hypothalamic-Pituitary-Adrenal Responses to Stress. J. Neurosci. 1993, 13, 3839–3847. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.B.; Moura, C.F.H.; Gomes-Filho, E.; Marco, C.A.; Urban, L.; Miranda, M.R.A. The Impact of Organic Farming on Quality of Tomatoes Is Associated to Increased Oxidative Stress during Fruit Development. PLoS ONE 2013, 8, e56354. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Barres, B.A. Microglia and Macrophages in Brain Homeostasis and Disease. Nat. Rev. Immunol. 2018, 18, 225–242. [Google Scholar] [CrossRef]
- Vidal-Itriago, A.; Radford, R.A.W.; Aramideh, J.A.; Maurel, C.; Scherer, N.M.; Don, E.K.; Lee, A.; Chung, R.S.; Graeber, M.B.; Morsch, M. Microglia Morphophysiological Diversity and Its Implications for the CNS. Front. Immunol. 2022, 13, 997786. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, H.; Ning, W.; Yang, Z.; Wang, Y.; Zhang, T. Knockdown of FSTL1 Inhibits Microglia Activation and Alleviates Depressive-like Symptoms through Modulating TLR4/MyD88/NF-κB Pathway in CUMS Mice. Exp. Neurol. 2022, 353, 114060. [Google Scholar] [CrossRef]
- Giulian, D.; Baker, T. Characterization of Ameboid Microglia Isolated from Developing Mammalian Brain. J. Neurosci. 1986, 6, 2163–2178. [Google Scholar] [CrossRef]
- Hinwood, M.; Morandini, J.; Day, T.A.; Walker, F.R. Evidence That Microglia Mediate the Neurobiological Effects of Chronic Psychological Stress on the Medial Prefrontal Cortex. Cereb. Cortex 2012, 22, 1442–1454. [Google Scholar] [CrossRef]
- Hu, C.; Luo, Y.; Wang, H.; Kuang, S.; Liang, G.; Yang, Y.; Mai, S.; Yang, J. Re-Evaluation of the Interrelationships among the Behavioral Tests in Rats Exposed to Chronic Unpredictable Mild Stress. PLoS ONE 2017, 12, e0185129. [Google Scholar] [CrossRef]
- Willner, P.; Moreau, J.-L.; Kurre Nielsen, C.; Papp, M.; Sluzewska, A. Decreased Hedonic Responsiveness Following Chronic Mild Stress Is Not Secondary to Loss of Body Weight. Physiol. Behav. 1996, 60, 129–134. [Google Scholar] [CrossRef]
- Wu, J.; Li, J.; Gaurav, C.; Muhammad, U.; Chen, Y.; Li, X.; Chen, J.; Wang, Z. CUMS and Dexamethasone Induce Depression-like Phenotypes in Mice by Differentially Altering Gut Microbiota and Triggering Macroglia Activation. Gen. Psych 2021, 34, e100529. [Google Scholar] [CrossRef]
- Van Den Bosch, M.; Meyer-Lindenberg, A. Environmental Exposures and Depression: Biological Mechanisms and Epidemiological Evidence. Annu. Rev. Public Health 2019, 40, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Grassini, S. A Systematic Review and Meta-Analysis of Nature Walk as an Intervention for Anxiety and Depression. J. Clin. Med. 2022, 11, 1731. [Google Scholar] [CrossRef]
- Churuangsuk, C.; Hall, J.; Reynolds, A.; Griffin, S.J.; Combet, E.; Lean, M.E.J. Diets for Weight Management in Adults with Type 2 Diabetes: An Umbrella Review of Published Meta-Analyses and Systematic Review of Trials of Diets for Diabetes Remission. Diabetologia 2022, 65, 14–36. [Google Scholar] [CrossRef]
- Setiawan, T.; Sari, I.N.; Wijaya, Y.T.; Julianto, N.M.; Muhammad, J.A.; Lee, H.; Chae, J.H.; Kwon, H.Y. Cancer Cachexia: Molecular Mechanisms and Treatment Strategies. J. Hematol. Oncol. 2023, 16, 54. [Google Scholar] [CrossRef]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting Microglial Cells Are Highly Dynamic Surveillants of Brain Parenchyma in Vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef]
- Wake, H.; Moorhouse, A.J.; Jinno, S.; Kohsaka, S.; Nabekura, J. Resting Microglia Directly Monitor the Functional State of Synapses In Vivo and Determine the Fate of Ischemic Terminals. J. Neurosci. 2009, 29, 3974–3980. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; et al. Synaptic Pruning by Microglia Is Necessary for Normal Brain Development. Science 2011, 333, 1456–1458. [Google Scholar] [CrossRef]
- Parkhurst, C.N.; Yang, G.; Ninan, I.; Savas, J.N.; Yates, J.R.; Lafaille, J.J.; Hempstead, B.L.; Littman, D.R.; Gan, W.-B. Microglia Promote Learning-Dependent Synapse Formation through Brain-Derived Neurotrophic Factor. Cell 2013, 155, 1596–1609. [Google Scholar] [CrossRef]
- Morizawa, Y.M.; Matsumoto, M.; Nakashima, Y.; Endo, N.; Aida, T.; Ishikane, H.; Beppu, K.; Moritoh, S.; Inada, H.; Osumi, N.; et al. Synaptic Pruning through Glial Synapse Engulfment upon Motor Learning. Nat. Neurosci. 2022, 25, 1458–1469. [Google Scholar] [CrossRef]
- Yirmiya, R.; Rimmerman, N.; Reshef, R. Depression as a Microglial Disease. Trends Neurosci. 2015, 38, 637–658. [Google Scholar] [CrossRef] [PubMed]
- Gunner, G.; Cheadle, L.; Johnson, K.M.; Ayata, P.; Badimon, A.; Mondo, E.; Nagy, M.A.; Liu, L.; Bemiller, S.M.; Kim, K.-W.; et al. Sensory Lesioning Induces Microglial Synapse Elimination via ADAM10 and Fractalkine Signaling. Nat. Neurosci. 2019, 22, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Lee, D.; You, H.; Lee, M.; Kim, H.; Cheong, E.; Um, J.W. LPS Induces Microglial Activation and GABAergic Synaptic Deficits in the Hippocampus Accompanied by Prolonged Cognitive Impairment. Sci. Rep. 2023, 13, 6547. [Google Scholar] [CrossRef]
- Kreisel, T.; Frank, M.G.; Licht, T.; Reshef, R.; Ben-Menachem-Zidon, O.; Baratta, M.V.; Maier, S.F.; Yirmiya, R. Dynamic Microglial Alterations Underlie Stress-Induced Depressive-like Behavior and Suppressed Neurogenesis. Mol. Psychiatry 2014, 19, 699–709. [Google Scholar] [CrossRef]
- Rimmerman, N.; Schottlender, N.; Reshef, R.; Dan-Goor, N.; Yirmiya, R. The Hippocampal Transcriptomic Signature of Stress Resilience in Mice with Microglial Fractalkine Receptor (CX3CR1) Deficiency. Brain Behav. Immun. 2017, 61, 184–196. [Google Scholar] [CrossRef]
- Clarke, D.; Crombag, H.S.; Hall, C.N. An Open-Source Pipeline for Analysing Changes in Microglial Morphology. Open Biol. 2021, 11, 210045. [Google Scholar] [CrossRef]
- Neumann, J.; Gunzer, M.; Gutzeit, H.O.; Ullrich, O.; Reymann, K.G.; Dinkel, K. Microglia Provide Neuroprotection after Ischemia. FASEB J. 2006, 20, 714–716. [Google Scholar] [CrossRef]
- Batchelor, P.E.; Liberatore, G.T.; Wong, J.Y.F.; Porritt, M.J.; Frerichs, F.; Donnan, G.A.; Howells, D.W. Activated Macrophages and Microglia Induce Dopaminergic Sprouting in the Injured Striatum and Express Brain-Derived Neurotrophic Factor and Glial Cell Line-Derived Neurotrophic Factor. J. Neurosci. 1999, 19, 1708–1716. [Google Scholar] [CrossRef]
- Stein, D.J.; Vasconcelos, M.F.; Albrechet-Souza, L.; Ceresér, K.M.M.; De Almeida, R.M.M. Microglial Over-Activation by Social Defeat Stress Contributes to Anxiety- and Depressive-Like Behaviors. Front. Behav. Neurosci. 2017, 11, 207. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, Y.; Ye, W.; Chen, Z.; Yuan, Z. Cynaroside Improved Depressive-like Behavior in CUMS Mice by Suppressing Microglial Inflammation and Ferroptosis. Biomed. Pharmacother. 2024, 173, 116425. [Google Scholar] [CrossRef]
- Tong, L.; Gong, Y.; Wang, P.; Hu, W.; Wang, J.; Chen, Z.; Zhang, W.; Huang, C. Microglia Loss Contributes to the Development of Major Depression Induced by Different Types of Chronic Stresses. Neurochem. Res. 2017, 42, 2698–2711. [Google Scholar] [CrossRef] [PubMed]
- Filipović, D.; Gavrilović, L.; Dronjak, S.; Radojčić, M.B. Brain Glucocorticoid Receptor and Heat Shock Protein 70 Levels in Rats Exposed to Acute, Chronic or Combined Stress. Neuropsychobiology 2005, 51, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Polazzi, E.; Contestabile, A. Reciprocal Interactions Between Microglia and Neurons: From Survival to Neuropathology. Rev. Neurosci. 2002, 13, 221–242. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Akgul, G.; Wollmuth, L.P.; Tsirka, S.E. Microglia Actively Regulate the Number of Functional Synapses. PLoS ONE 2013, 8, e56293. [Google Scholar] [CrossRef]
- Liu, M.; Li, J.; Dai, P.; Zhao, F.; Zheng, G.; Jing, J.; Wang, J.; Luo, W.; Chen, J. Microglia Activation Regulates GluR1 Phosphorylation in Chronic Unpredictable Stress-Induced Cognitive Dysfunction. Stress 2015, 18, 96–106. [Google Scholar] [CrossRef]
- Geng, M.; Shao, Q.; Fu, J.; Gu, J.; Feng, L.; Zhao, L.; Liu, C.; Mu, J.; Zhang, X.; Zhao, M.; et al. Down-Regulation of MKP-1 in Hippocampus Protects against Stress-Induced Depression-like Behaviors and Neuroinflammation. Transl. Psychiatry 2024, 14, 130. [Google Scholar] [CrossRef]
- Sasaki, Y.; Ohsawa, K.; Kanazawa, H.; Kohsaka, S.; Imai, Y. Iba1 Is an Actin-Cross-Linking Protein in Macrophages/Microglia. Biochem. Biophys. Res. Commun. 2001, 286, 292–297. [Google Scholar] [CrossRef]
- Kierdorf, K.; Masuda, T.; Jordão, M.J.C.; Prinz, M. Macrophages at CNS Interfaces: Ontogeny and Function in Health and Disease. Nat. Rev. Neurosci. 2019, 20, 547–562. [Google Scholar] [CrossRef]
- Utz, S.G.; See, P.; Mildenberger, W.; Thion, M.S.; Silvin, A.; Lutz, M.; Ingelfinger, F.; Rayan, N.A.; Lelios, I.; Buttgereit, A.; et al. Early Fate Defines Microglia and Non-Parenchymal Brain Macrophage Development. Cell 2020, 181, 557–573.e18. [Google Scholar] [CrossRef]
- Bennett, M.L.; Bennett, F.C.; Liddelow, S.A.; Ajami, B.; Zamanian, J.L.; Fernhoff, N.B.; Mulinyawe, S.B.; Bohlen, C.J.; Adil, A.; Tucker, A.; et al. New Tools for Studying Microglia in the Mouse and Human CNS. Proc. Natl. Acad. Sci. USA 2016, 113, E1738–E1746. [Google Scholar] [CrossRef]
- Satoh, J.; Kino, Y.; Asahina, N.; Takitani, M.; Miyoshi, J.; Ishida, T.; Saito, Y. TMEM119 Marks a Subset of Microglia in the Human Brain. Neuropathology 2016, 36, 39–49. [Google Scholar] [CrossRef]
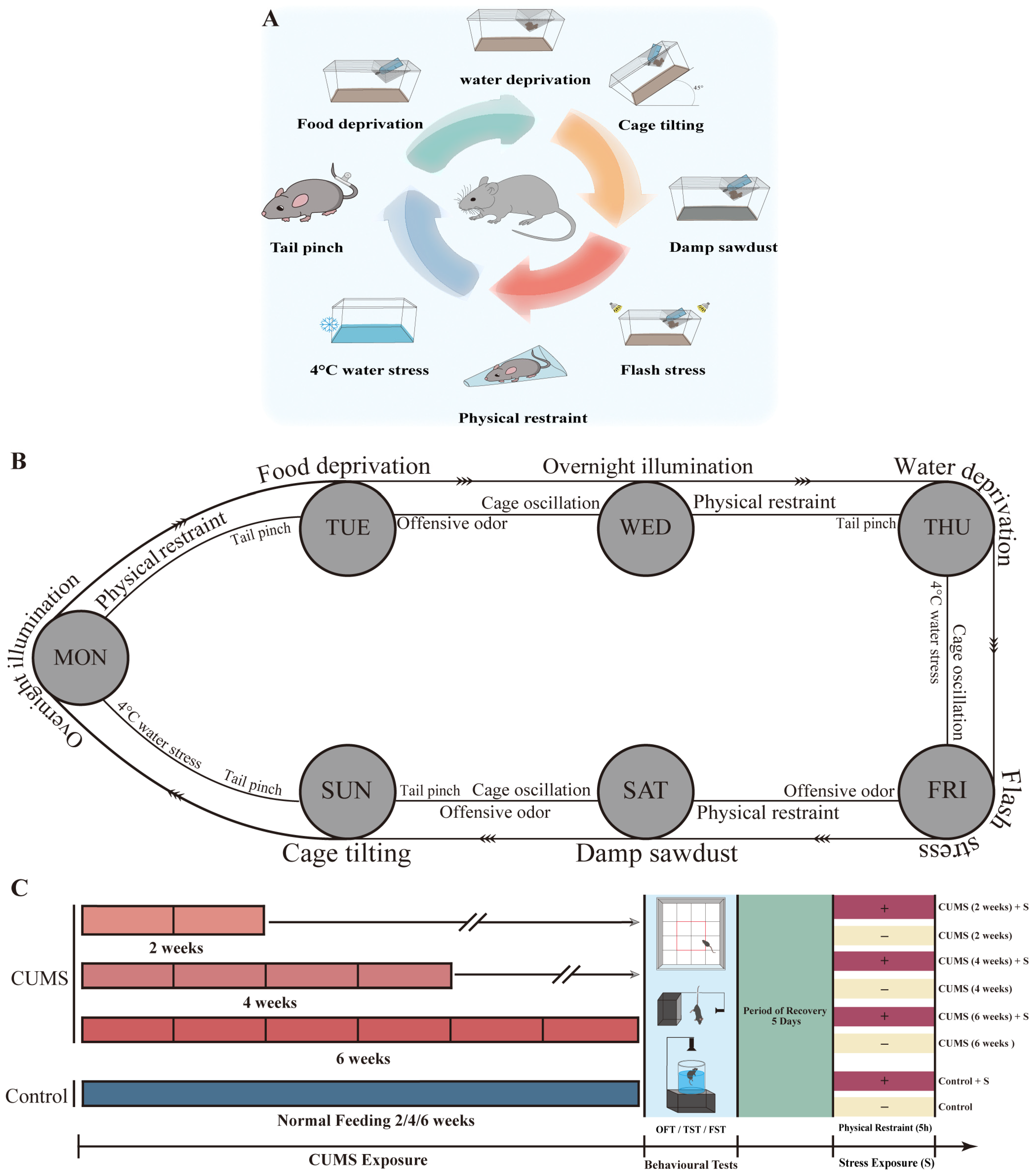

| Stressor | Description |
|---|---|
| Tail pinch | A steel binder clip (20 mm opening width, KOKUYO, Osaka, Japan) was placed 4 cm from the tip of the tail for 2 min (after each clip was removed, the steel binder clip was disinfected with 70% alcohol). |
| 4 °C water stress | About 1 L of 4 °C tap water was poured into the cage (27.0 cm length, 16.0 cm width, 11.5 cm height) and maintained at a water depth of approximately 3 cm. The CUMS group mice were placed in this cage for 2 min. |
| Cage oscillation | The CUMS group mice were placed in the cage, which was fixed on top of the shaker for 30 min (each oscillation speed was kept the same, Double-Action Lab Shaker SRR-2, AS ONE Corp., Osaka, Japan). |
| Offensive odor | 2,4,5-Trimethylthiazole (TMT) is a secretion oozed by a natural rodent predator. Four µL TMT was dropped onto the filter paper, which was placed in the center of the cage, and the CUMS group mice were placed in this cage for 30 min. |
| Physical restraint | Each mouse in the CUMS group was stuffed into the polyethylene disposable mouse restrainer for 3 h (15.5 cm length, the width of topside opening is 1 cm, the radius of Bottom opening is 13 cm, MDC-20, Nihon Bioresearch Inc., Gifu, Japan). |
| Overnight illumination | The light/dark cycle was disrupted (e.g., during the dark-cycle period, illumination for 12 h). |
| Water deprivation | The bottle of drinking water was removed for 15 h. However, food was provided as usual. |
| Food deprivation | The food was removed for 15 h. Drinking water was provided as usual. |
| Flash stress | The CUMS group mice were subjected to flash stress for 24 h (the two flash LED devices were placed on the left and right of the home cage). The strobe speed was set as the fastest (S81 LED II, ADJ Products, LLC, Los Angeles, CA, USA). Food and water were provided during this period. |
| Damp sawdust | A total of 400 mL tap water was poured into 20 g sawdust in the cage, and the CUMS group mice were placed in the cage for 24 h (tap water was at room temperature, food and water were provided during this period). |
| Cage tilting | The cage was tilted forward by 45 degrees for 24 h (food and water were provided during this period). |
| Stressor | Period of Exposure | Times of Exposure |
|---|---|---|
| Tail pinch | 2 min | 4 |
| 4 °C water stress | 2 min | 2 |
| Cage oscillation | 30 min | 3 |
| Offensive odor | 30 min | 3 |
| Physical restraint | 3 h | 3 |
| Overnight illumination | 12 h | 2 |
| Water deprivation | 15 h | 1 |
| Food deprivation | 15 h | 1 |
| Flash stress | 24 h | 1 |
| Damp sawdust | 24 h | 1 |
| Cage tilting | 24 h | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Huang, J.; Han, T.; Kojima, N. Chronic Stress Modulates Microglial Activation Dynamics, Shaping Priming Responses to Subsequent Stress. Brain Sci. 2025, 15, 534. https://doi.org/10.3390/brainsci15050534
Chen J, Huang J, Han T, Kojima N. Chronic Stress Modulates Microglial Activation Dynamics, Shaping Priming Responses to Subsequent Stress. Brain Sciences. 2025; 15(5):534. https://doi.org/10.3390/brainsci15050534
Chicago/Turabian StyleChen, Junyu, Jiacheng Huang, Taolei Han, and Nobuhiko Kojima. 2025. "Chronic Stress Modulates Microglial Activation Dynamics, Shaping Priming Responses to Subsequent Stress" Brain Sciences 15, no. 5: 534. https://doi.org/10.3390/brainsci15050534
APA StyleChen, J., Huang, J., Han, T., & Kojima, N. (2025). Chronic Stress Modulates Microglial Activation Dynamics, Shaping Priming Responses to Subsequent Stress. Brain Sciences, 15(5), 534. https://doi.org/10.3390/brainsci15050534






