Digital Biomarkers and AI for Remote Monitoring of Fatigue Progression in Neurological Disorders: Bridging Mechanisms to Clinical Applications
Abstract
1. Introduction
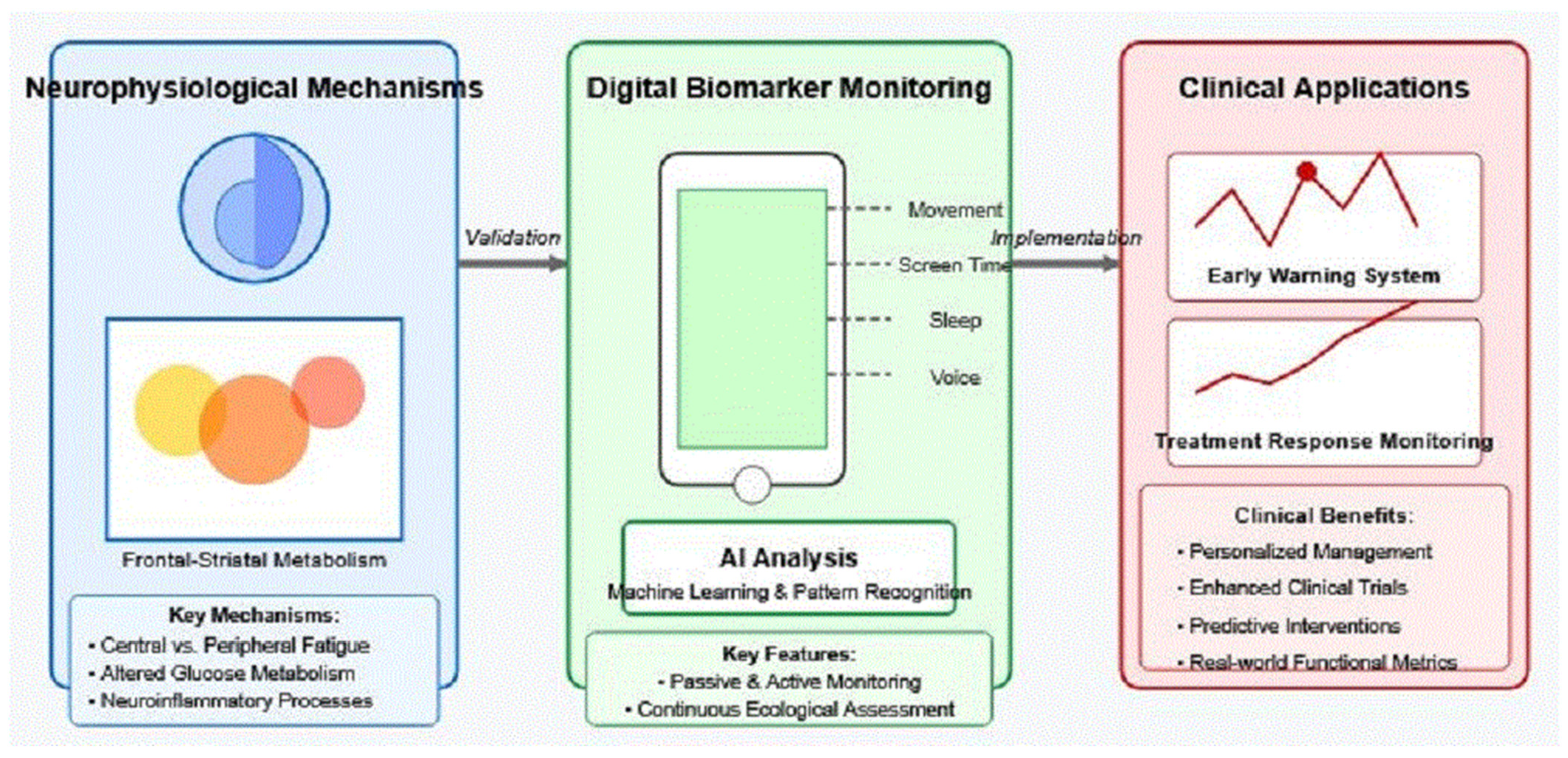
2. Neurophysiological Basis of Fatigue in Neurological Disorders
3. Smartphone-Based Digital Biomarkers for Fatigue
3.1. Passive Monitoring Approaches
3.2. Active Assessment Approaches
4. Artificial Intelligence Methods for Digital Phenotyping
4.1. Machine Learning Approaches for Multimodal Data Integration
4.1.1. Synthesis of Composite Fatigue Metrics
4.1.2. Weighting and Integration
4.1.3. Normalization and Adaptation
4.2. Personalized Modeling of Individual Fatigue Patterns
4.3. Temporal Dynamics and Progression Modeling
4.4. Explainable AI for Clinical Interpretation
5. Validation Framework: Connecting Digital Signals to Neural Mechanisms
5.1. Correlating Digital Biomarkers with Neuroimaging Findings
Mechanistic Pathways Linking Digital Biomarkers to Frontal–Striatal Metabolism
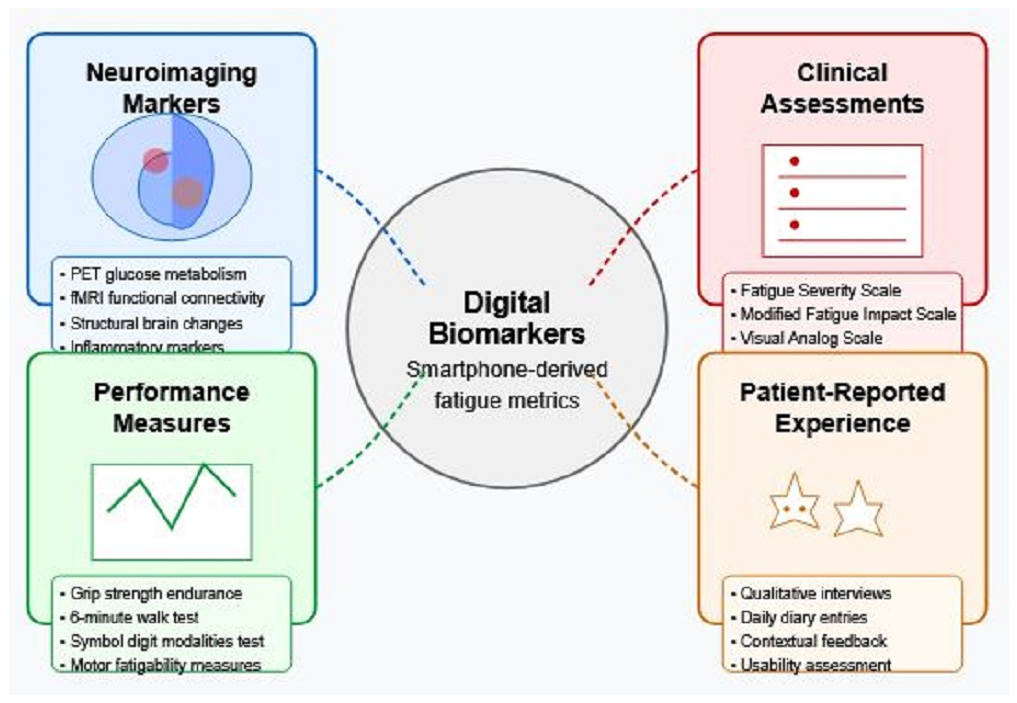
5.2. Establishing Ground Truth Through Multimodal Validation
- Neuroimaging markers: Beyond correlation analysis, machine learning approaches can identify which digital features best predict neuroimaging patterns, with recursive feature elimination techniques determining minimal feature sets needed for robust predictions [9].
- Clinical assessments: Digital metrics should be validated against established clinical measures, including the Modified Fatigue Impact Scale and Fatigue Severity Scale, with statistical approaches accounting for the ordinal nature of these scales [63]. Preliminary validation studies show moderate-to-strong correlations (r = 0.68) between digital metrics and clinical scales [8].
- Performance measures: Objective performance-based measures of fatigability provide complementary validation targets. Digital metrics should predict performance decrements in standardized cognitive and motor tasks, with validation studies demonstrating correlations of r = 0.65 between smartphone-derived features and laboratory measures of motor fatigability [57].
- Patient-reported experience: Ecological momentary assessments provide critical ground truths for algorithm development, with correlations between passive sensor data and momentary fatigue ratings (r = 0.59) establishing ecological validity [20].
5.3. Methodological Considerations and Validation Study Designs
- Discovery phase: Cross-sectional studies (n = 100+) correlating digital features with established measures to identify promising biomarkers.
- Validation phase: Longitudinal studies (6–12 months) assessing stability, sensitivity to change, and predictive value of digital biomarkers identified in phase 1.
- Implementation phase: Pragmatic trials evaluating the utility of digital biomarkers in clinical decision-making and patient self-management.
Addressing Attrition Bias
5.4. Challenges in Bridging Subjective Experience, Digital Signals, and Neural Substrate
6. Clinical Applications and Future Directions
6.1. Early Warning Systems for Fatigue Episodes
6.2. Objective Measurement of Treatment Response
6.3. Personalized Fatigue Management Strategies
6.4. Optimized Clinical Trial Design Using Digital Endpoints
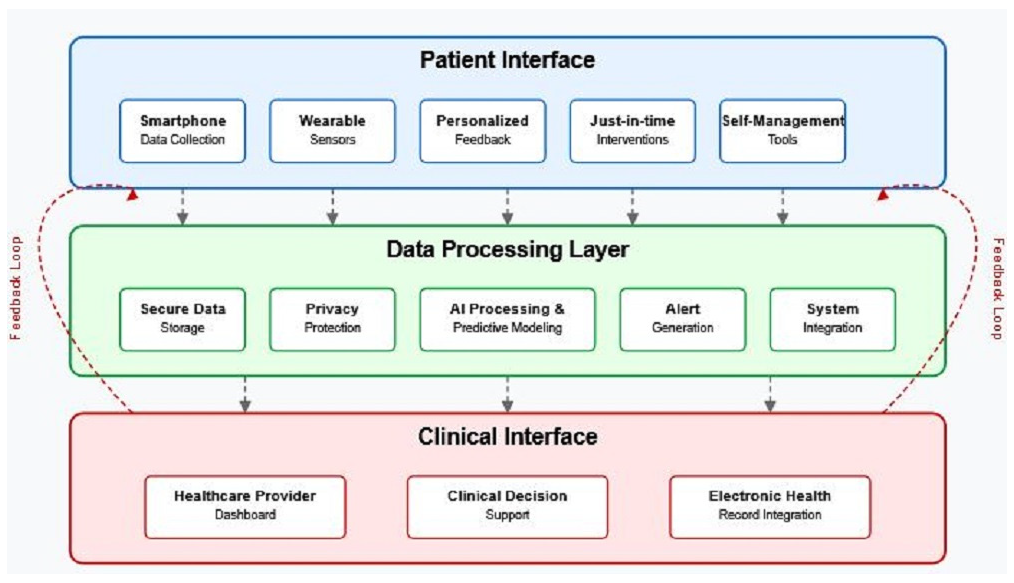
7. Ethical and Implementation Considerations
7.1. Privacy and Data Security
7.2. Digital Equity and Accessibility
7.3. Regulatory Pathways and Clinical Adoption Barriers
7.4. Patient Perspectives and Engagement
7.5. Clinical Feasibility and Implementation Challenges
- Individual variability: Despite high group-level correlations, individual patient trajectories show substantial variability. The Floodlight study revealed that while digital biomarkers could distinguish between disability levels, predicting individual disease progression remained challenging [79]. The heterogeneity of fatigue manifestations means that population-based algorithms may not adequately capture individual patient experiences.
- Actionable insights gap: While smartphones can collect extensive data on fatigue patterns, translating these metrics into actionable clinical interventions remains problematic. Newland et al. [99] found that although continuous monitoring detected fatigue fluctuations in MS patients, clinicians lacked clear guidelines on how to interpret and respond to digital biomarker data in real-time clinical practice.
- Clinical outcome disconnects: Current evidence primarily demonstrates correlations between digital biomarkers and traditional clinical measures rather than causal relationships with disability outcomes. A systematic review by Block et al. [8] found that while digital monitoring could track symptoms, the evidence for improved disability outcomes through smartphone-guided interventions was lacking.
8. Conclusions and Future Research Roadmap
8.1. Neuroimaging Validation Protocols
8.2. Regulatory Qualification Pathways
8.3. Technical Implementation Standards
8.4. Clinical Integration and Precision Medicine
Funding
Conflicts of Interest
References
- Penner, I.K.; Paul, F. Fatigue as a symptom or comorbidity of neurological diseases. Nat. Rev. Neurol. 2017, 13, 662–675. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. eClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef] [PubMed]
- Krupp, L.B.; Serafin, D.J.; Christodoulou, C. Multiple sclerosis-associated fatigue. Expert Rev. Neurother. 2010, 10, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Rudroff, T.; Kindred, J.H.; Koo, P.J.; Karki, R.; Hebert, J.R. Asymmetric glucose uptake in leg muscles of patients with Multiple Sclerosis during walking detected by [18F]-FDG PET/CT. NeuroRehabilitation 2014, 35, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Rudroff, T. Frontal-striatal glucose metabolism and fatigue in patients with multiple sclerosis, long COVID, and COVID-19 recovered controls. Exp. Brain Res. 2024, 242, 2125–2136. [Google Scholar] [CrossRef]
- Piau, A.; Wild, K.; Mattek, N.; Kaye, J. Current State of Digital Biomarker Technologies for Real-Life, Home-Based Monitoring of Cognitive Function for Mild Cognitive Impairment to Mild Alzheimer Disease and Implications for Clinical Care: Systematic Review. J. Med. Internet Res. 2019, 21, e12785. [Google Scholar] [CrossRef]
- Simblett, S.; Greer, B.; Matcham, F.; Curtis, H.; Polhemus, A.; Ferrão, J.; Gamble, P.; Wykes, T. Barriers to and Facilitators of Engagement with Remote Measurement Technology for Managing Health: Systematic Review and Content Analysis of Findings. J. Med. Internet Res. 2018, 20, e10480. [Google Scholar] [CrossRef]
- Block, V.J.; Bove, R.; Nourbakhsh, B. The Role of Remote Monitoring in Evaluating Fatigue in Multiple Sclerosis: A Review. Front. Neurol. 2022, 13, 878313. [Google Scholar] [CrossRef]
- Rudroff, T.; Rainio, O.; Klén, R.; Tuulari, J.J. The untapped potential of dimension reduction in neuroimaging: AI-driven multimodal analysis of Long COVID fatigue. Brain Sci. 2024, 14, 1209. [Google Scholar] [CrossRef]
- Manta, C.; Patrick-Lake, B.; Goldsack, J.C. Digital Measures That Matter to Patients: A Framework to Guide the Selection and Development of Digital Measures of Health. Digit. Biomark. 2020, 4, 69–77. [Google Scholar] [CrossRef]
- Kluger, B.M.; Krupp, L.B.; Enoka, R.M. Fatigue and fatigability in neurologic illnesses: Proposal for a unified taxonomy. Neurology 2013, 80, 409–416. [Google Scholar] [CrossRef]
- Gandevia, S.C. Spinal and supraspinal factors in human muscle fatigue. Physiol. Rev. 2001, 81, 1725–1789. [Google Scholar] [CrossRef] [PubMed]
- Rocca, M.A.; Meani, A.; Riccitelli, G.C.; Colombo, B.; Rodegher, M.; Falini, A.; Comi, G.; Filippi, M. Abnormal adaptation over time of motor network recruitment in multiple sclerosis patients with fatigue. Mult. Scler. 2016, 22, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Kuppuswamy, A. Role of selective attention in fatigue in neurological disorders. Eur. J. Neurol. 2023, 30, 1453–1458. [Google Scholar] [CrossRef] [PubMed]
- Haffke, M.; Freitag, H.; Rudolf, G.; Seifert, M.; Doehner, W.; Scherbakov, N.; Hanitsch, L.; Wittke, K.; Bauer, S.; Konietschke, F.; et al. Endothelial dysfunction and altered endothelial biomarkers in patients with post-COVID-19 syndrome and chronic fatigue syndrome (ME/CFS). J. Transl. Med. 2022, 20, 138. [Google Scholar] [CrossRef]
- Krishna, B.A.; Lim, E.Y.; Metaxaki, M.; Jackson, S.; Mactavous, L.; NIHR BioResource; Lyons, P.A.; Doffinger, R.; Bradley, J.R.; Smith, K.G.C.; et al. Spontaneous, persistent, T cell-dependent IFN-γ release in patients who progress to Long COVID. Sci. Adv. 2024, 10, eadi9379. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; Heijnen, C.J.; Kavelaars, A.; Laye, S.; Capuron, L. The neuroimmune basis of fatigue. Trends Neurosci. 2014, 37, 39–46. [Google Scholar] [CrossRef]
- Dobryakova, E.; DeLuca, J.; Genova, H.M.; Wylie, G.R. Neural correlates of cognitive fatigue: Cortico-striatal circuitry and effort-reward imbalance. J. Int. Neuropsychol. Soc. 2013, 19, 849–853. [Google Scholar] [CrossRef]
- Greenhouse-Tucknott, A.; Butterworth, J.B.; Wrightson, J.G.; Smeeton, N.J.; Critchley, H.D.; Dekerle, J.; Harrison, N.A. Toward the unity of pathological and exertional fatigue: A predictive processing model. Cogn. Affect. Behav. Neurosci. 2022, 22, 215–228. [Google Scholar] [CrossRef]
- Powell, D.J.H.; Liossi, C.; Schlotz, W.; Moss-Morris, R. Tracking daily fatigue fluctuations in multiple sclerosis: Ecological momentary assessment provides unique insights. J. Behav. Med. 2017, 40, 772–783. [Google Scholar] [CrossRef]
- Rudroff, T. Long COVID Fatigue: Clinical Sciences, Artificial Intelligence and the Future of Brain Health; Springer: Cham, Switzerland, 2025. [Google Scholar]
- English, C.; Simpson, D.B.; Billinger, S.A.; Churilov, L.; Coupland, K.G.; Drummond, A.; Kuppuswamy, A.; Kutlubaev, M.A.; Lerdal, A.; Mahmood, A.; et al. A roadmap for research in post-stroke fatigue: Consensus-based core recommendations from the third Stroke Recovery and Rehabilitation Roundtable. Int. J. Stroke 2024, 19, 133–144. [Google Scholar] [CrossRef]
- Northwestern Medicine. In COVID-19 Patients, Neurological Symptoms Last up to Three Years. Northwestern Now. 2024. Available online: https://news.northwestern.edu/stories/2024/august/in-covid-19-patients-neurological-symptoms-last-up-to-three-years/ (accessed on 1 March 2025).
- Chudzik, A.; Śledzianowski, A.; Przybyszewski, A.W. Machine Learning and Digital Biomarkers Can Detect Early Stages of Neurodegenerative Diseases. Sensors 2024, 24, 1572. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Sosnoff, J.J. Novel sensing technology in fall risk assessment in older adults: A systematic review. BMC Geriatr. 2018, 18, 14. [Google Scholar] [CrossRef] [PubMed]
- Boukhvalova, A.K.; Kowalczyk, E.; Harris, T.; Kosa, P.; Wichman, A.; Sandford, M.A.; Memon, A.; Bielekova, B. Identifying and Quantifying Neurological Disability via Smartphone. Front. Neurol. 2019, 10, 838. [Google Scholar] [CrossRef] [PubMed]
- Giannouli, E.; Bock, O.; Zijlstra, W. Cognitive functioning is more closely related to real-life mobility than to laboratory-based mobility parameters. Eur. J. Ageing 2018, 15, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.; Ranjan, Y.; Conde, P.; Sun, S.; Zhang, Y.; Rashid, Z.; Sankesara, H.; Cummins, N.; Laiou, P.; Bai, X.; et al. Physiological presentation and risk factors of long COVID in the UK using smartphones and wearable devices: A longitudinal, citizen science, case-control study. Lancet Digit. Health 2024, 6, e640–e650. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Behan, P.O. Fatigue in neurological disorders. Lancet 2004, 363, 978–988. [Google Scholar] [CrossRef]
- Pratap, A.; Neto, E.C.; Snyder, P.; Stepnowsky, C.; Elhadad, N.; Grant, D.; Mohebbi, M.H.; Mooney, S.; Suver, C.; Wilbanks, J.; et al. Indicators of retention in remote digital health studies: A cross-study evaluation of 100,000 participants. NPJ Digit. Med. 2020, 3, 21. [Google Scholar] [CrossRef]
- Bhattarai, J.J.; Patel, K.S.; Dunn, K.M.; Brown, A.; Opelt, B.; Hughes, A.J. Sleep disturbance and fatigue in multiple sclerosis: A systematic review and meta-analysis. Mult. Scler. J. Exp. Transl. Clin. 2023, 9, 20552173231194352. [Google Scholar] [CrossRef]
- Miller, D.M.; Moore, S.M.; Fox, R.J.; Atreja, A.; Fu, A.Z.; Lee, J.C.; Saupe, W.; Stadtler, M.; Chakraborty, S.; Harris, C.M.; et al. Web-based self-management for patients with multiple sclerosis: A practical, randomized trial. Telemed. J. E Health 2011, 17, 5–13. [Google Scholar] [CrossRef]
- Kavaliunas, A.; Danylaite Karrenbauer, V.; Gyllensten, H.; Manouchehrinia, A.; Glaser, A.; Olsson, T.; Alexanderson, K.; Hillert, J. Cognitive function is a major determinant of income among multiple sclerosis patients in Sweden acting independently from physical disability. Mult. Scler. 2019, 25, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Palotai, M.; Wallack, M.; Kujbus, G.; Dalnoki, A.; Guttmann, C. Usability of a Mobile App for Real-Time Assessment of Fatigue and Related Symptoms in Patients with Multiple Sclerosis: Observational Study. JMIR Mhealth Uhealth 2021, 9, e19564. [Google Scholar] [CrossRef] [PubMed]
- Leavitt, V.M.; Wylie, G.; Genova, H.M.; Chiaravalloti, N.D.; DeLuca, J. Altered effective connectivity during performance of an information processing speed task in multiple sclerosis. Mult. Scler. 2012, 18, 409–417. [Google Scholar] [CrossRef]
- Lai, Y.J.; Liu, S.H.; Manachevakul, S.; Lee, T.A.; Kuo, C.T.; Bello, D. Biomarkers in long COVID-19: A systematic review. Front. Med. 2023, 10, 1085988. [Google Scholar] [CrossRef] [PubMed]
- Robotti, C.; Costantini, G.; Saggio, G.; Cesarini, V.; Calastri, A.; Maiorano, E.; Piloni, D.; Perrone, T.; Sabatini, U.; Ferretti, V.V.; et al. Machine Learning-based Voice Assessment for the Detection of Positive and Recovered COVID-19 Patients. J. Voice 2024, 38, 796.e1–796.e13. [Google Scholar] [CrossRef] [PubMed]
- Elbéji, A.; Zhang, L.; Higa, E.; Fischer, A.; Despotovic, V.; Nazarov, P.V.; Aguayo, G.; Fagherazzi, G. Vocal biomarker predicts fatigue in people with COVID-19: Results from the prospective Predi-COVID cohort study. BMJ Open 2022, 12, e062463. [Google Scholar] [CrossRef]
- Jain, S.H.; Powers, B.W.; Hawkins, J.B.; Brownstein, J.S. The digital phenotype. Nat. Biotechnol. 2015, 33, 462–463. [Google Scholar] [CrossRef] [PubMed]
- Shajari, S.; Kuruvinashetti, K.; Komeili, A.; Sundararaj, U. The Emergence of AI-Based Wearable Sensors for Digital Health Technology: A Review. Sensors 2023, 23, 9498. [Google Scholar] [CrossRef]
- Calderone, A.; Latella, D.; Bonanno, M.; Quartarone, A.; Mojdehdehbaher, S.; Celesti, A.; Calabrò, R.S. Towards Transforming Neurorehabilitation: The Impact of Artificial Intelligence on Diagnosis and Treatment of Neurological Disorders. Biomedicines 2024, 12, 2415. [Google Scholar] [CrossRef] [PubMed]
- Graves, A.; Mohamed, A.R.; Hinton, G. Speech recognition with deep recurrent neural networks. In Proceedings of the 2013 IEEE International Conference on Acoustics, Speech and Signal Processing, Vancouver, BC, Canada, 26–31 May 2013; pp. 6645–6649. [Google Scholar]
- Zheng, V.W.; Cao, B.; Zheng, Y.; Xie, X.; Yang, Q. Collaborative Filtering Meets Mobile Recommendation: A User-Centered Approach. In Proceedings of the AAAI Conference on Artificial Intelligence, Atlanta, GA, USA, 11–15 July 2010; Volume 24, pp. 236–241. [Google Scholar]
- Pan, S.J.; Yang, Q. A Survey on Transfer Learning. IEEE Trans. Knowl. Data Eng. 2010, 22, 1345–1359. [Google Scholar] [CrossRef]
- Elnakib, A.; Khalifa, F.; Soliman, A.; Shalaby, A.; Elhosseini, M. Editorial: Emerging artificial intelligence technologies for neurological and neuropsychiatric research. Front. Neurosci. 2024, 18, 1518442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Shen, D.; Alzheimer’s Disease Neuroimaging Initiative. Multi-modal multi-task learning for joint prediction of multiple regression and classification variables in Alzheimer’s disease. Neuroimage 2012, 59, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Lillie, E.O.; Patay, B.; Diamant, J.; Issell, B.; Topol, E.J.; Schork, N.J. The n-of-1 clinical trial: The ultimate strategy for individualizing medicine? Per. Med. 2011, 8, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Rieke, N.; Hancox, J.; Li, W.; Milletari, F.; Roth, H.R.; Albarqouni, S.; Bakas, S.; Galtier, M.N.; Landman, B.A.; Maier-Hein, K.; et al. The future of digital health with federated learning. NPJ Digit. Med. 2020, 3, 119. [Google Scholar] [CrossRef]
- AbuAlrob, M.A.; Mesraoua, B. Harnessing artificial intelligence for the diagnosis and treatment of neurological emergencies: A comprehensive review of recent advances and future directions. Front. Neurol. 2024, 15, 1485799. [Google Scholar] [CrossRef] [PubMed]
- Aminikhanghahi, S.; Cook, D.J. A survey of methods for time series change point detection. Knowl. Inf. Syst. 2017, 51, 339–367. [Google Scholar] [CrossRef] [PubMed]
- Lansdall-Welfare, T.; Sudhahar, S.; Thompson, J.; Lewis, J.; Team, F.N.; Cristianini, N. Content analysis of 150 years of British periodicals. Proc. Natl. Acad. Sci. USA 2017, 114, E457–E465. [Google Scholar] [CrossRef]
- Rudin, C. Stop explaining black box machine learning models for high stakes decisions and use interpretable models instead. Nat. Mach. Intell. 2019, 1, 206–215. [Google Scholar] [CrossRef]
- Kalani, M.; Anjankar, A. Revolutionizing Neurology: The Role of Artificial Intelligence in Advancing Diagnosis and Treatment. Cureus 2024, 16, e61706. [Google Scholar] [CrossRef]
- Pearl, J.; Mackenzie, D. The Book of Why: The New Science of Cause and Effect; Basic Books; Hachette Book Group: New York, NY, USA, 2018. [Google Scholar]
- Chadaga, K.; Prabhu, S.; Sampathila, N.; Chadaga, R.; Umakanth, S.; Bhat, D.; G S, S.K. Explainable artificial intelligence approaches for COVID-19 prognosis prediction using clinical markers. Sci. Rep. 2024, 14, 1783. [Google Scholar] [CrossRef]
- Zhai, Y.; Nasseri, N.; Pöttgen, J.; Gezhelbash, E.; Heesen, C.; Stellmann, J.P. Smartphone Accelerometry: A Smart and Reliable Measurement of Real-Life Physical Activity in Multiple Sclerosis and Healthy Individuals. Front. Neurol. 2020, 11, 688. [Google Scholar] [CrossRef] [PubMed]
- Arteaga-Bracho, E.; Cosne, G.; Kanzler, C.; Karatsidis, A.; Mazzà, C.; Penalver-Andres, J.; Zhu, C.; Shen, C.; Erb, M.K.; Freigang, M.; et al. Smartphone-Based Assessment of Mobility and Manual Dexterity in Adult People with Spinal Muscular Atrophy. J. Neuromuscul. Dis. 2024, 11, 1049–1065. [Google Scholar] [CrossRef]
- Onnela, J.P.; Rauch, S.L. Harnessing Smartphone-Based Digital Phenotyping to Enhance Behavioral and Mental Health. Neuropsychopharmacology 2016, 41, 1691–1696. [Google Scholar] [CrossRef] [PubMed]
- Goldsack, J.C.; Coravos, A.; Bakker, J.P.; Bent, B.; Dowling, A.V.; Fitzer-Attas, C.; Godfrey, A.; Godino, J.G.; Gujar, N.; Izmailova, E.; et al. Verification, analytical validation, and clinical validation (V3): The foundation of determining fit-for-purpose for Biometric Monitoring Technologies (BioMeTs). NPJ Digit. Med. 2020, 3, 55. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, G.; Nitsche, M.A.; Bergmann, T.O.; Towhidkhah, F.; Violante, I.R.; Lorenz, R.; Kuplicki, R.; Tsuchiyagaito, A.; Mulyana, B.; Mayeli, A.; et al. Closing the loop between brain and electrical stimulation: Towards precision neuromodulation treatments. Transl. Psychiatry 2023, 13, 279. [Google Scholar] [CrossRef] [PubMed]
- Sosnoff, J.J.; Socie, M.J.; Boes, M.K.; Sandroff, B.M.; Pula, J.H.; Suh, Y.; Weikert, M.; Balantrapu, S.; Morrison, S.; Motl, R.W. Mobility, balance and falls in persons with multiple sclerosis. PLoS ONE 2011, 6, e28021. [Google Scholar] [CrossRef]
- Veauthier, C.; Paul, F. Sleep disorders in multiple sclerosis and their relationship to fatigue. Sleep Med. 2014, 15, 5–14. [Google Scholar] [CrossRef]
- Kos, D.; Kerckhofs, E.; Carrea, I.; Verza, R.; Ramos, M.; Jansa, J. Evaluation of the Modified Fatigue Impact Scale in four different European countries. Mult. Scler. 2005, 11, 76–80. [Google Scholar] [CrossRef]
- Lavallee, D.C.; Chenok, K.E.; Love, R.M.; Petersen, C.; Holve, E.; Segal, C.D.; Franklin, P.D. Incorporating Patient-Reported Outcomes into Health Care to Engage Patients and Enhance Care. Health Aff. 2016, 35, 575–582. [Google Scholar] [CrossRef]
- Amtmann, D.; Bamer, A.M.; Noonan, V.; Lang, N.; Kim, J.; Cook, K.F. Comparison of the psychometric properties of two fatigue scales in multiple sclerosis. Rehabil. Psychol. 2012, 57, 159–166. [Google Scholar] [CrossRef]
- Newland, P.; Starkweather, A.; Sorenson, M. Central fatigue in multiple sclerosis: A review of the literature. J. Spinal Cord Med. 2016, 39, 386–399. [Google Scholar] [CrossRef] [PubMed]
- Nahum-Shani, I.; Smith, S.N.; Spring, B.J.; Collins, L.M.; Witkiewitz, K.; Tewari, A.; Murphy, S.A. Just-in-Time Adaptive Interventions (JITAIs) in Mobile Health: Key Components and Design Principles for Ongoing Health Behavior Support. Ann. Behav. Med. 2018, 52, 446–462. [Google Scholar] [CrossRef] [PubMed]
- Bove, R.; White, C.C.; Giovannoni, G.; Glanz, B.; Golubchikov, V.; Hujol, J.; Jennings, C.; Langdon, D.; Lee, M.; Legedza, A.; et al. Evaluating more naturalistic outcome measures: A 1-year smartphone study in multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2015, 2, e162. [Google Scholar] [CrossRef] [PubMed]
- Schwid, S.R.; Covington, M.; Segal, B.M.; Goodman, A.D. Fatigue in multiple sclerosis: Current understanding and future directions. J. Rehabil. Res. Dev. 2002, 39, 211–224. [Google Scholar] [PubMed]
- Lipsmeier, F.; Taylor, K.I.; Kilchenmann, T.; Wolf, D.; Scotland, A.; Schjodt-Eriksen, J.; Cheng, W.Y.; Fernandez-Garcia, I.; Siebourg-Polster, J.; Jin, L.; et al. Evaluation of smartphone-based testing to generate exploratory outcome measures in a phase 1 Parkinson’s disease clinical trial. Mov. Disord. 2018, 33, 1287–1297. [Google Scholar] [CrossRef]
- Berry, S.M.; Connor, J.T.; Lewis, R.J. The platform trial: An efficient strategy for evaluating multiple treatments. JAMA 2015, 313, 1619–1620. [Google Scholar] [CrossRef]
- Rudroff, T.; Rainio, O.; Klén, R. Leveraging Artificial Intelligence to Optimize Transcranial Direct Current Stimulation for Long COVID Management: A Forward-Looking Perspective. Brain Sci. 2024, 14, 831. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Glidden, A.M.; Holloway, M.R.; Birbeck, G.L.; Schwamm, L.H. Teleneurology and mobile technologies: The future of neurological care. Nat. Rev. Neurol. 2018, 14, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Gold, S.M.; Heesen, C. Raising the bar: The challenges of applying the MAGNIMS consensus guidelines for future MS trials. Nat. Rev. Neurol. 2018, 14, 708–709. [Google Scholar]
- Babrak, L.M.; Menetski, J.; Rebhan, M.; Nisato, G.; Zinggeler, M.; Brasier, N.; Baerenfaller, K.; Brenzikofer, T.; Baltzer, L.; Vogler, C.; et al. Traditional and Digital Biomarkers: Two Worlds Apart? Digit. Biomark. 2019, 3, 92–102. [Google Scholar] [CrossRef]
- Martinez-Martin, N.; Insel, T.R.; Dagum, P.; Greely, H.T.; Cho, M.K. Data mining for health: Staking out the ethical territory of digital phenotyping. NPJ Digit. Med. 2018, 1, 68. [Google Scholar] [CrossRef] [PubMed]
- Marelli, L.; Lievevrouw, E.; Van Hoyweghen, I. Fit for purpose? The GDPR and the governance of European digital health. Policy Stud. 2020, 42, 616–635. [Google Scholar] [CrossRef]
- Bot, B.M.; Suver, C.; Neto, E.C.; Kellen, M.; Klein, A.; Bare, C.; Doerr, M.; Pratap, A.; Wilbanks, J.; Dorsey, E.R.; et al. The mPower study, Parkinson disease mobile data collected using ResearchKit. Sci. Data 2016, 3, 160011. [Google Scholar] [CrossRef] [PubMed]
- Woelfle, T.; Pless, S.; Wiencierz, A.; Kappos, L.; Naegelin, Y.; Lorscheider, J. Practice Effects of Mobile Tests of Cognition, Dexterity, and Mobility on Patients with Multiple Sclerosis: Data Analysis of a Smartphone-Based Observational Study. J. Med. Internet Res. 2021, 23, e30394. [Google Scholar] [CrossRef] [PubMed]
- Creagh, A.P.; Simillion, C.; Scotland, A.; Lipsmeier, F.; Bernasconi, C.; Belachew, S.; van Beek, J.; Baker, M.; Gossens, C.; Lindemann, M.; et al. Smartphone-based remote assessment of upper extremity function for multiple sclerosis using the Draw a Shape Test. Physiol. Meas. 2020, 41, 054002. [Google Scholar] [CrossRef] [PubMed]
- Mowry, E.M.; Bermel, R.A.; Williams, J.R.; Benzinger, T.L.S.; de Moor, C.; Fisher, E.; Hersh, C.M.; Hyland, M.H.; Izbudak, I.; Jones, S.E.; et al. Harnessing Real-World Data to Inform Decision-Making: Multiple Sclerosis Partners Advancing Technology and Health Solutions (MS PATHS). Front. Neurol. 2020, 11, 632. [Google Scholar] [CrossRef] [PubMed]
- Price, W.N., II; Cohen, I.G. Privacy in the age of medical big data. Nat. Med. 2019, 25, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Vayena, E.; Blasimme, A. Biomedical Big Data: New Models of Control Over Access, Use and Governance. J. Bioeth. Inq. 2017, 14, 501–513. [Google Scholar] [CrossRef]
- Perrin, A.; Atske, S. Americans with Disabilities Less Likely than Those Without to Own Some Digital Devices; Pew Research Center: Washington, DC, USA, 2021. [Google Scholar]
- Tarricone, R.; Petracca, F.; Ciani, O.; Cucciniello, M. Distinguishing features in the assessment of mHealth apps. Expert Rev. Pharmacoecon. Outcomes Res. 2021, 21, 521–526. [Google Scholar] [CrossRef]
- Gould, C.E.; Loup, J.; Kuhn, E.; Beaudreau, S.A.; Ma, F.; Goldstein, M.K.; Wetherell, J.L.; Zapata, A.M.; Choe, P.; O’Hara, R. Technology Use and Preferences for Mental Health Self-Management Interventions Among Older Veterans. Int. J. Geriatr. Psychiatry 2020, 35, 321–330. [Google Scholar] [CrossRef]
- Nouri, S.S.; Avila-Garcia, P.; Cemballi, A.G.; Sarkar, U.; Aguilera, A.; Lyles, C.R. Assessing Mobile Phone Digital Literacy and Engagement in User-Centered Design in a Diverse, Safety-Net Population: Mixed Methods Study. JMIR Mhealth Uhealth 2019, 7, e14250. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Digital Health Software Precertification (Pre-Cert) Program. Updated September. 2019. Available online: https://www.fda.gov/medical-devices/digital-health-center-excellence/digital-health-software-precertification-pre-cert-pilot-program (accessed on 1 March 2025).
- Muehlematter, U.J.; Daniore, P.; Vokinger, K.N. Approval of artificial intelligence and machine learning-based medical devices in the USA and Europe (2015–2020): A comparative analysis. Lancet Digit. Health 2021, 3, e195–e203. [Google Scholar]
- Cohen, A.B.; Dorsey, E.R.; Mathews, S.C.; Bates, D.W.; Safavi, K. A digital health industry cohort across the health continuum. NPJ Digit. Med. 2020, 3, 68. [Google Scholar] [CrossRef] [PubMed]
- Bietz, M.J.; Bloss, C.S.; Calvert, S.; Godino, J.G.; Gregory, J.; Claffey, M.P.; Sheehan, J.; Patrick, K. Opportunities and challenges in the use of personal health data for health research. J. Am. Med. Inform. Assoc. 2016, 23, e42–e48. [Google Scholar] [CrossRef] [PubMed]
- Rice, D.R.; Kaplan, T.B.; Hotan, G.C.; Vogel, A.C.; Matiello, M.; Gillani, R.L.; Hutto, S.K.; Ham, A.S.; Klawiter, E.C.; George, I.C.; et al. Electronic pill bottles to monitor and promote medication adherence for people with multiple sclerosis: A randomized, virtual clinical trial. J. Neurol. Sci. 2021, 428, 117612. [Google Scholar] [CrossRef] [PubMed]
- Bove, R.M.; Rush, G.; Zhao, C.; Rowles, W.; Garcha, P.; Morrissey, J.; Schembri, A.; Alailima, T.; Langdon, D.; Possin, K.; et al. Videogame-Based Digital Therapeutic to Improve Processing Speed in People with Multiple Sclerosis: A Feasibility Study. Neurol. Ther. 2019, 8, 135–145. [Google Scholar] [CrossRef]
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef]
- Zach, H.; Dirkx, M.F.; Pasman, J.W.; Bloem, B.R.; Helmich, R.C. The patient’s perspective: The effect of levodopa on Parkinson symptoms. Park. Relat. Disord. 2017, 35, 48–54. [Google Scholar] [CrossRef]
- Page, A.; Yung, N.; Auinger, P.; Venuto, C.; Glidden, A.; Macklin, E.; Omberg, L.; Schwarzschild, M.A.; Dorsey, E.R. A Smartphone Application as an Exploratory Endpoint in a Phase 3 Parkinson’s Disease Clinical Trial: A Pilot Study. Digit. Biomark. 2022, 6, 1–8. [Google Scholar] [CrossRef]
- Midaglia, L.; Mulero, P.; Montalban, X.; Graves, J.; Hauser, S.L.; Julian, L.; Baker, M.; Schadrack, J.; Gossens, C.; Scotland, A.; et al. Adherence and Satisfaction of Smartphone- and Smartwatch-Based Remote Active Testing and Passive Monitoring in People with Multiple Sclerosis: Nonrandomized Interventional Feasibility Study. J. Med. Internet Res. 2022, 24, e38048. [Google Scholar]
- Golan, D.; Sagiv, S.; Glass-Marmor, L.; Miller, A. Mobile phone-based e-diary for assessment and enhancement of medications adherence among patients with multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2020, 6, 2055217320939309. [Google Scholar] [CrossRef] [PubMed]
- Newland, P.K.; Lunsford, V.; Flach, A. The interaction of fatigue, physical activity, and health-related quality of life in adults with multiple sclerosis (MS) and cardiovascular disease (CVD). Appl. Nurs. Res. 2017, 33, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Sieberts, S.K.; Schaff, J.; Duda, M.; Pataki, B.Á.; Sun, M.; Snyder, P.; Daneault, J.F.; Parisi, F.; Costante, G.; Rubin, U.; et al. Crowdsourcing digital health measures to predict Parkinson’s disease severity: The Parkinson’s Disease Digital Biomarker DREAM Challenge. NPJ Digit. Med. 2021, 4, 53. [Google Scholar] [CrossRef] [PubMed]
- Scholz, M.; Haase, R.; Schriefer, D.; Voigt, I.; Ziemssen, T. Electronic Health Interventions in the Case of Multiple Sclerosis: From Theory to Practice. Brain Sci. 2021, 11, 180. [Google Scholar] [CrossRef] [PubMed]
- van Oirschot, P.; Heerings, M.; Wendrich, K.; den Teuling, B.; Martens, M.B.; Jongen, P.J. Symbol Digit Modalities Test Variant in a Smartphone App for Persons with Multiple Sclerosis: Validation Study. JMIR Mhealth Uhealth 2020, 8, e18160. [Google Scholar] [CrossRef]
- Manjaly, Z.M.; Harrison, N.A.; Critchley, H.D.; Do, C.T.; Stefanics, G.; Wenderoth, N.; Lutterotti, A.; Müller, A.; Stephan, K.E. Pathophysiological and cognitive mechanisms of fatigue in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2019, 90, 642–651. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudroff, T. Digital Biomarkers and AI for Remote Monitoring of Fatigue Progression in Neurological Disorders: Bridging Mechanisms to Clinical Applications. Brain Sci. 2025, 15, 533. https://doi.org/10.3390/brainsci15050533
Rudroff T. Digital Biomarkers and AI for Remote Monitoring of Fatigue Progression in Neurological Disorders: Bridging Mechanisms to Clinical Applications. Brain Sciences. 2025; 15(5):533. https://doi.org/10.3390/brainsci15050533
Chicago/Turabian StyleRudroff, Thorsten. 2025. "Digital Biomarkers and AI for Remote Monitoring of Fatigue Progression in Neurological Disorders: Bridging Mechanisms to Clinical Applications" Brain Sciences 15, no. 5: 533. https://doi.org/10.3390/brainsci15050533
APA StyleRudroff, T. (2025). Digital Biomarkers and AI for Remote Monitoring of Fatigue Progression in Neurological Disorders: Bridging Mechanisms to Clinical Applications. Brain Sciences, 15(5), 533. https://doi.org/10.3390/brainsci15050533





