Real-World Use of COMT Inhibitors in the Management of Patients with Parkinson’s Disease in Spain Who Present Early Motor Fluctuations: Interim Results from the REONPARK Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Assessments
2.3. Statistical Analysis
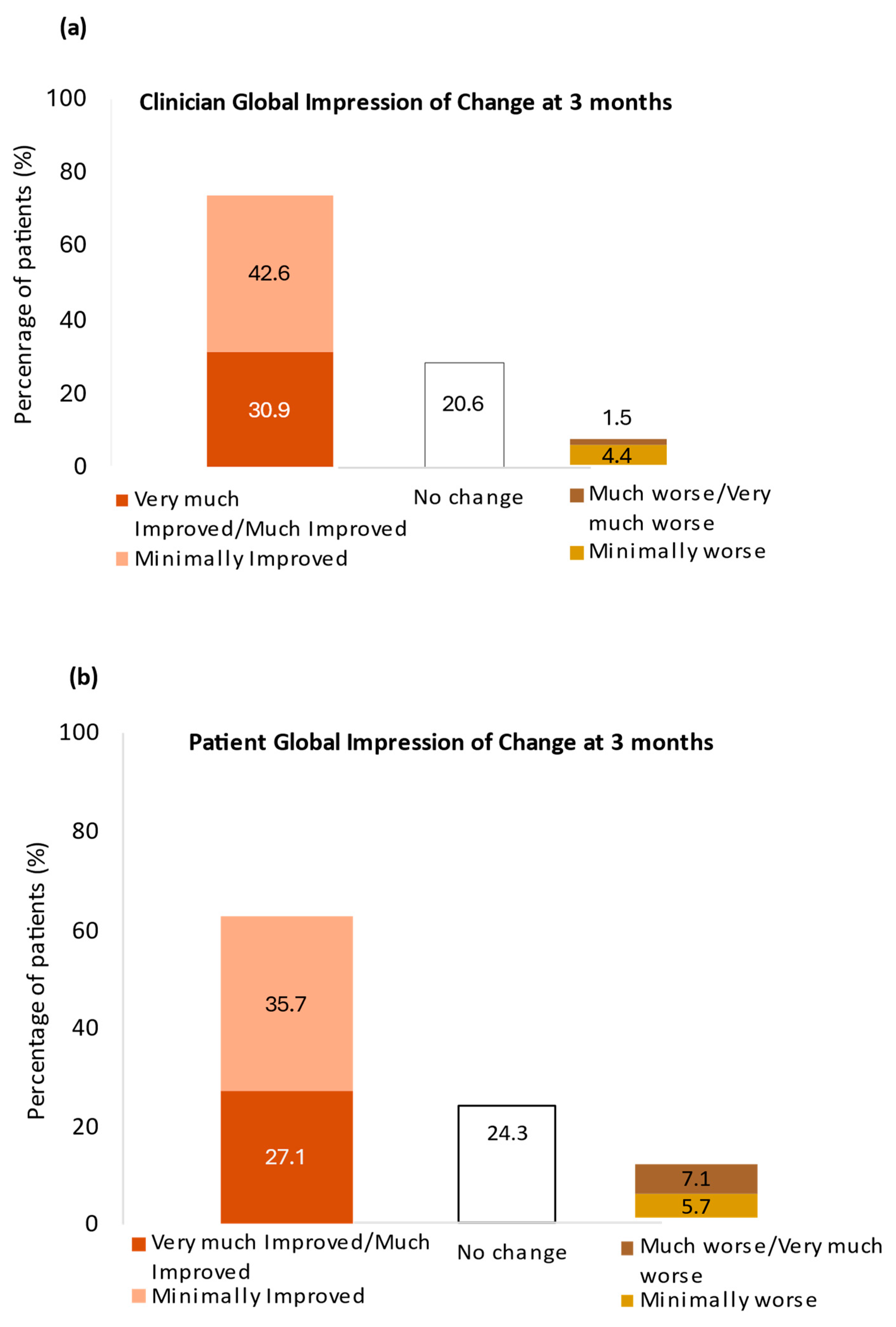
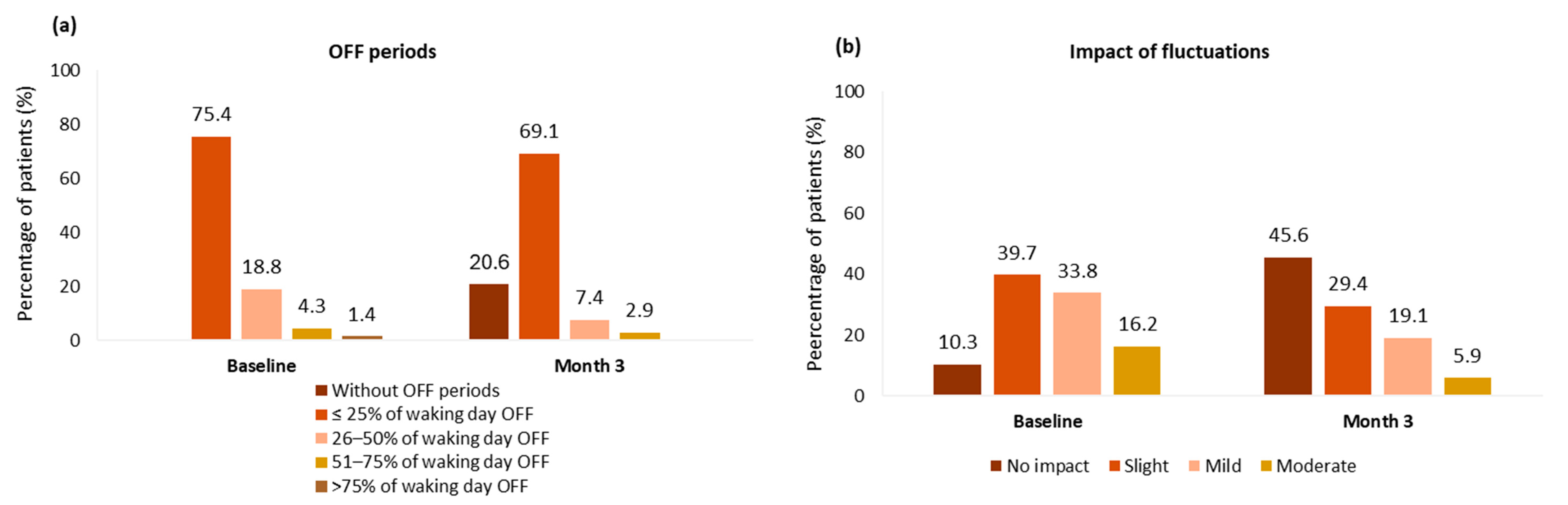
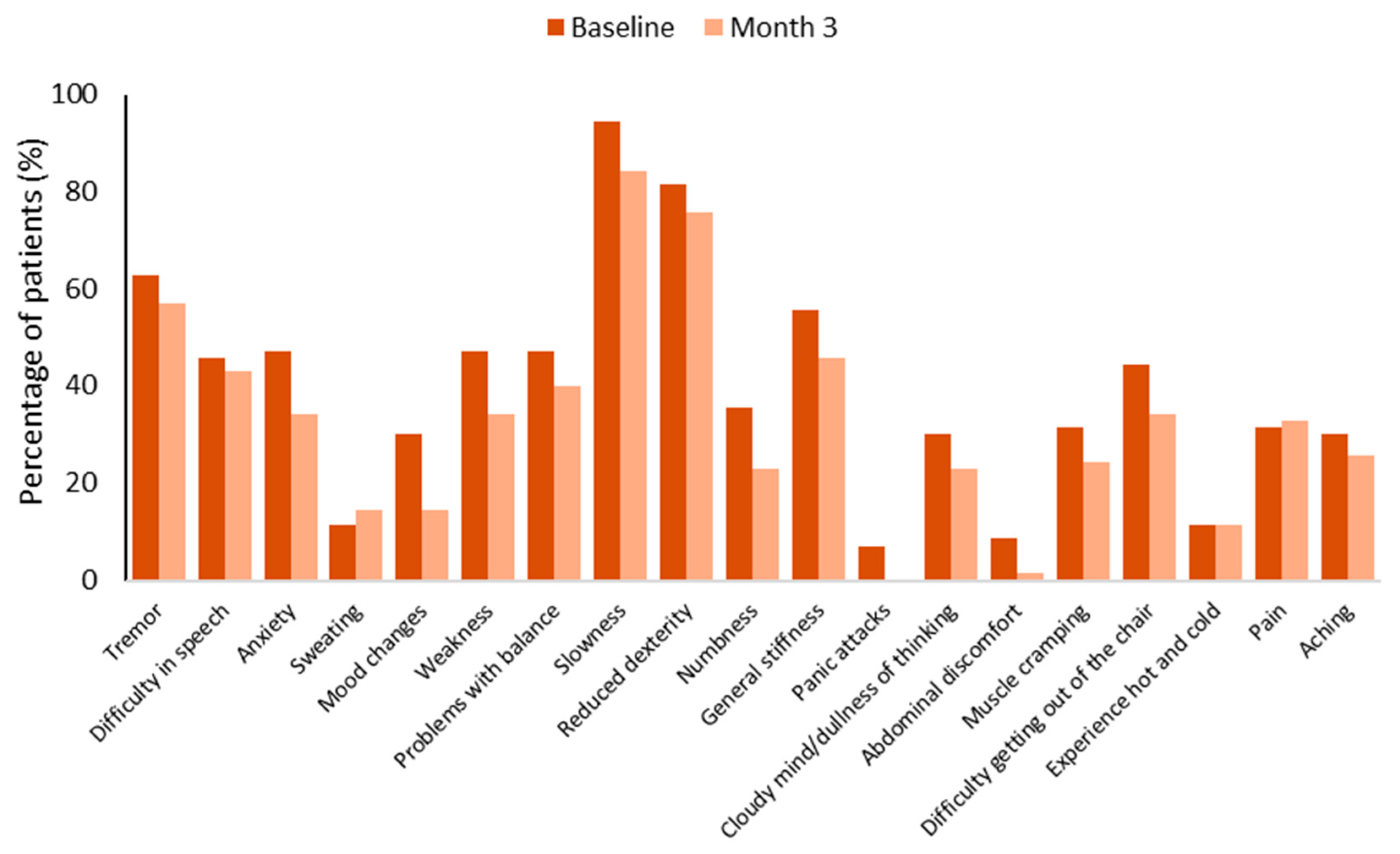
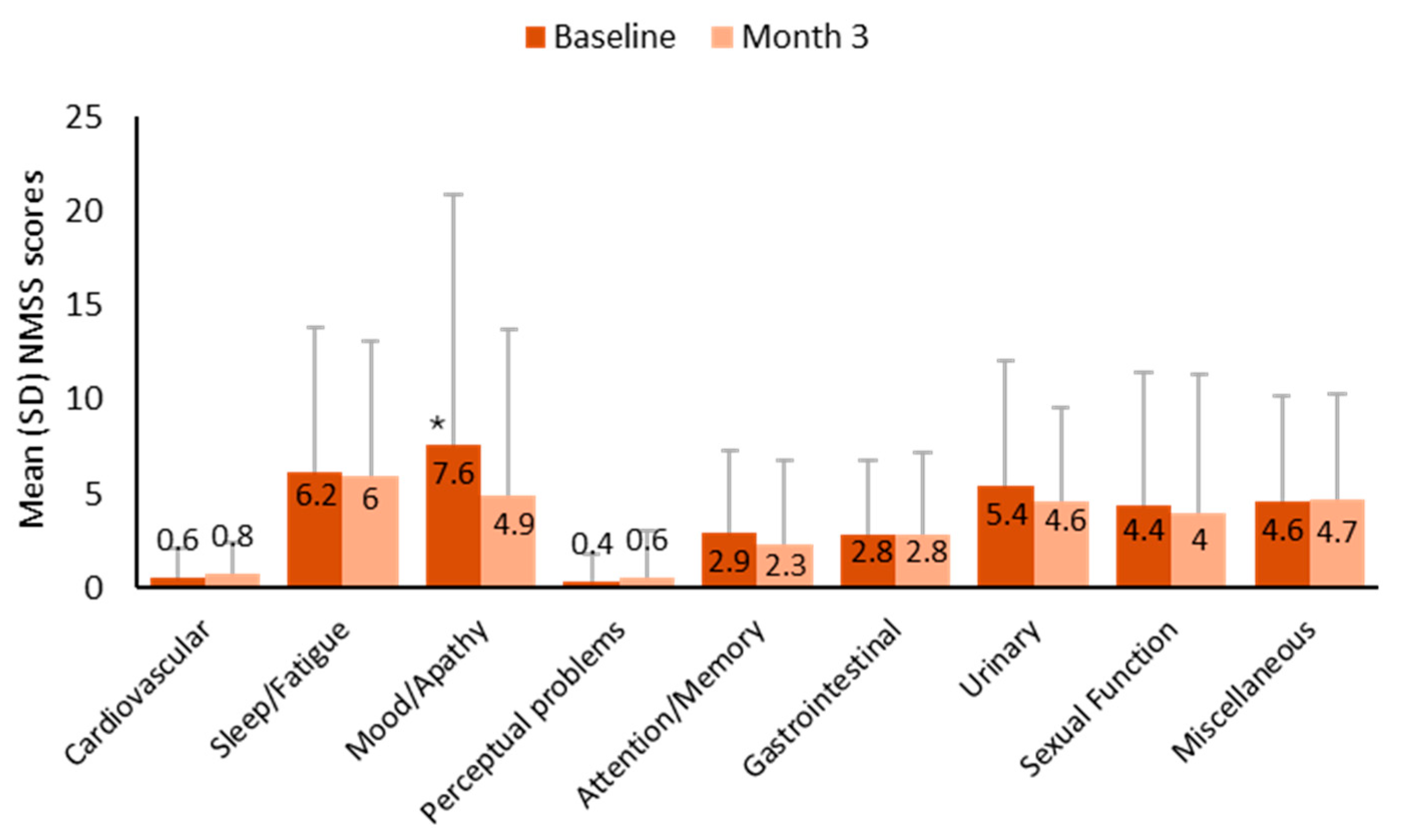
3. Results
3.1. Patient Characteristics
3.2. Changes in Levodopa Dosing and Other Anti-Parkinsonian Medications
3.3. Effectiveness
3.4. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | Adverse event |
| CGI-C | Clinician Global Impression of Change |
| CGI-S | Clinical Global Impression Severity |
| CI | Confidence Interval |
| COMT | Catechol O-methyltransferase |
| IQR | Interquartile range |
| LEDD | Levodopa equivalent daily dose |
| MAOI | Monoamine oxidase inhibitors |
| MDS-UPDRS | Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale |
| NMSS | Non-Motor Symptoms Scale |
| PGI-C | Patient Global Impression of Change |
| PD | Parkinson’s disease |
| PDQ-8 | Parkinson’s Disease Questionnaire-8 |
| QoL | Quality of life |
| SD | Standard deviation |
| WOQ-19 | Wearing-off questionnaire |
References
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef] [PubMed]
- Olanow, C.W. Levodopa is the best symptomatic therapy for PD: Nothing more, nothing less. Mov. Disord. 2019, 34, 812–815. [Google Scholar] [CrossRef] [PubMed]
- Aquino, C.C.; Fox, S.H. Clinical spectrum of levodopa-induced complications. Mov. Disord. 2015, 30, 80–89. [Google Scholar] [CrossRef]
- Cilia, R.; Siri, C.; Canesi, M.; Zecchinelli, A.L.; De Gaspari, D.; Natuzzi, F.; Tesei, S.; Meucci, N.; Mariani, C.B.; Sacilotto, G.; et al. Dopamine dysregulation syndrome in Parkinson’s disease: From clinical and neuropsychological characterisation to management and long-term outcome. J. Neurol. Neurosurg. Psychiatry 2014, 85, 311–318. [Google Scholar] [CrossRef]
- Olanow, C.W.; Kieburtz, K.; Rascol, O.; Poewe, W.; Schapira, A.H.; Emre, M.; Nissinen, H.; Leinonen, M.; Stocchi, F.; Stalevo Reduction in Dyskinesia Evaluation in Parkinson’s Disease (STRIDE-PD) Investigators. Factors predictive of the development of Levodopa-induced dyskinesia and wearing-off in Parkinson’s disease. Mov. Disord. 2013, 28, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Stocchi, F.; Antonini, A.; Barone, P.; Tinazzi, M.; Zappia, M.; Onofrj, M.; Ruggieri, S.; Morgante, L.; Bonuccelli, U.; Lopiano, L.; et al. Early DEtection of wEaring off in Parkinson disease: The DEEP study. Park. Relat. Disord. 2014, 20, 204–211. [Google Scholar] [CrossRef]
- Bjornestad, A.; Forsaa, E.B.; Pedersen, K.F.; Tysnes, O.B.; Larsen, J.P.; Alves, G. Risk and course of motor complications in a population-based incident Parkinson’s disease cohort. Park. Relat. Disord. 2016, 22, 48–53. [Google Scholar] [CrossRef]
- Kim, H.J.; Mason, S.; Foltynie, T.; Winder-Rhodes, S.; Barker, R.A.; Williams-Gray, C.H. Motor complications in Parkinson’s disease: 13-year follow-up of the CamPaIGN cohort. Mov. Disord. 2020, 35, 185–190. [Google Scholar] [CrossRef]
- Jenner, P.; Rocha, J.F.; Ferreira, J.J.; Rascol, O.; Soares-da-Silva, P. Redefining the strategy for the use of COMT inhibitors in Parkinson’s disease: The role of opicapone. Expert. Rev. Neurother. 2021, 21, 1019–1033. [Google Scholar] [CrossRef]
- Muller, T. Catechol-O-methyltransferase inhibitors in Parkinson’s disease. Drugs 2015, 75, 157–174. [Google Scholar] [CrossRef]
- Ferreira, J.J.; Lees, A.; Rocha, J.F.; Poewe, W.; Rascol, O.; Soares-da-Silva, P.; Bi-Park, I. Opicapone as an adjunct to levodopa in patients with Parkinson’s disease and end-of-dose motor fluctuations: A randomised, double-blind, controlled trial. Lancet Neurol. 2016, 15, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Lees, A.J.; Ferreira, J.; Rascol, O.; Poewe, W.; Rocha, J.F.; McCrory, M.; Soares-da-Silva, P.; Investigators, B.-S. Opicapone as Adjunct to Levodopa Therapy in Patients with Parkinson Disease and Motor Fluctuations: A Randomized Clinical Trial. JAMA Neurol. 2017, 74, 197–206. [Google Scholar] [CrossRef]
- Reichmann, H.; Lees, A.; Rocha, J.-F.; Magalhães, D.; Soares-Da-Silva, P.; OPTIPARK investigators. Effectiveness and safety of opicapone in Parkinson’s disease patients with motor fluctuations: The OPTIPARK open-label study. Transl. Neurodegener. 2020, 9, 9. [Google Scholar] [CrossRef]
- Santos Garcia, D.; Fernandez Pajarin, G.; Oropesa-Ruiz, J.M.; Escamilla Sevilla, F.; Rahim Lopez, R.R.A.; Munoz Enriquez, J.G. Opicapone Improves Global Non-Motor Symptoms Burden in Parkinson’s Disease: An Open-Label Prospective Study. Brain Sci. 2022, 12, 383. [Google Scholar] [CrossRef] [PubMed]
- Schofield, C.; Chaudhuri, K.R.; Carroll, C.; Sharma, J.C.; Pavese, N.; Evans, J.; Foltynie, T.; Reichmann, H.; Zurowska, L.; Soares-da-Silva, P.; et al. Opicapone in UK clinical practice: Effectiveness, safety and cost analysis in patients with Parkinson’s disease. Neurodegener. Dis. Manag. 2022, 12, 77–91. [Google Scholar] [CrossRef]
- Ferreira, J.J.; Poewe, W.; Rascol, O.; Stocchi, F.; Antonini, A.; Moreira, J.; Guimaraes, B.; Rocha, J.F.; Soares-da-Silva, P. Effect of Opicapone on Levodopa Pharmacokinetics in Patients with Fluctuating Parkinson’s Disease. Mov. Disord. 2022, 37, 2272–2283. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.J.; Lees, A.; Rocha, J.F.; Poewe, W.; Rascol, O.; Soares-da-Silva, P. Long-term efficacy of opicapone in fluctuating Parkinson’s disease patients: A pooled analysis of data from two phase 3 clinical trials and their open-label extensions. Eur. J. Neurol. 2019, 26, 953–960. [Google Scholar] [CrossRef]
- Rocha, J.F.; Ebersbach, G.; Lees, A.; Tolosa, E.; Ferreira, J.J.; Poewe, W.; Rascol, O.; Stocchi, F.; Antonini, A.; Magalhaes, D.; et al. The Added Benefit of Opicapone When Used Early in Parkinson’s Disease Patients with Levodopa-Induced Motor Fluctuations: A Post-hoc Analysis of BIPARK-I and -II. Front. Neurol. 2021, 12, 754016. [Google Scholar] [CrossRef]
- Lee, J.Y.; Ma, H.I.; Ferreira, J.J.; Rocha, J.F.; Sung, Y.H.; Song, I.U.; Ahn, T.B.; Kwon, D.Y.; Cheon, S.M.; Kim, J.M.; et al. Opicapone to Treat Early Wearing-off in Parkinson’s Disease Patients: The Korean ADOPTION Trial. Mov. Disord. Clin. Pract. 2024, 11, 655–665. [Google Scholar] [CrossRef]
- Goetz, C.G.; Stebbins, G.T.; Tilley, B.C. Calibration of unified Parkinson’s disease rating scale scores to Movement Disorder Society-unified Parkinson’s disease rating scale scores. Mov. Disord. 2012, 27, 1239–1242. [Google Scholar] [CrossRef]
- Jenkinson, C.; Fitzpatrick, R.; Peto, V. The PDQ-8: Development and validation of a short-form Parkinson’s Disease Questionnaire. Psychol. Health. 1997, 12, 805–814. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Martinez-Martin, P.; Brown, R.G.; Sethi, K.; Stocchi, F.; Odin, P.; Ondo, W.; Abe, K.; Macphee, G.; Macmahon, D.; et al. The metric properties of a novel non-motor symptoms scale for Parkinson’s disease: Results from an international pilot study. Mov. Disord. 2007, 22, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Martin, P.; Tolosa, E.; Hernandez, B.; Badia, X.; Valid, Q.S.G. Validation of the “QUICK” questionnaire--a tool for diagnosis of “wearing-off” in patients with Parkinson’s disease. Mov. Disord. 2008, 23, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Jost, S.T.; Kaldenbach, M.A.; Antonini, A.; Martinez-Martin, P.; Timmermann, L.; Odin, P.; Katzenschlager, R.; Borgohain, R.; Fasano, A.; Stocchi, F.; et al. Levodopa Dose Equivalency in Parkinson’s Disease: Updated Systematic Review and Proposals. Mov. Disord. 2023, 38, 1236–1252. [Google Scholar] [CrossRef]
- Kerr, C.; Lloyd, E.J.; Kosmas, C.E.; Smith, H.T.; Cooper, J.A.; Johnston, K.; McIntosh, E.; Lloyd, A.J. Health-related quality of life in Parkinson’s: Impact of ‘off’ time and stated treatment preferences. Qual. Life Res. 2016, 25, 1505–1515. [Google Scholar] [CrossRef]
- Horvath, K.; Aschermann, Z.; Acs, P.; Deli, G.; Janszky, J.; Komoly, S.; Balazs, E.; Takacs, K.; Karadi, K.; Kovacs, N. Minimal clinically important difference on the Motor Examination part of MDS-UPDRS. Park. Relat. Disord. 2015, 21, 1421–1426. [Google Scholar] [CrossRef]
- Makkos, A.; Kovacs, M.; Pinter, D.; Janszky, J.; Kovacs, N. Minimal clinically important difference for the historic parts of the Unified Dyskinesia Rating Scale. Park. Relat. Disord. 2019, 58, 79–82. [Google Scholar] [CrossRef]
- Santos Garcia, D.; Labandeira Guerra, C.; Yanez Bana, R.; Cimas Hernando, M.I.; Cabo Lopez, I.; Paz Gonalez, J.M.; Alonso Losada, M.G.; Gonzalez Palmas, M.J.; Martinez Miro, C. Safinamide Improves Non-Motor Symptoms Burden in Parkinson’s Disease: An Open-Label Prospective Study. Brain Sci. 2021, 11, 316. [Google Scholar] [CrossRef]
- Jost, S.T.; Visser-Vandewalle, V.; Rizos, A.; Loehrer, P.A.; Silverdale, M.; Evans, J.; Samuel, M.; Petry-Schmelzer, J.N.; Sauerbier, A.; Gronostay, A.; et al. Non-motor predictors of 36-month quality of life after subthalamic stimulation in Parkinson disease. npj Park. Dis. 2021, 7, 48. [Google Scholar] [CrossRef]
- Rocha, J.F.; Ebersbach, G.; Lees, A.; Tolosa, E.; Ferreira, J.J.; Poewe, W.; Rascol, O.; Stocchi, F.; Antonini, A.; Magalhaes, D.; et al. The safety/tolerability of opicapone when used early in Parkinson’s disease patients with levodopa-induced motor fluctuations: A post-hoc analysis of BIPARK-I and II. Front. Neurol. 2022, 13, 994114. [Google Scholar] [CrossRef]
| Characteristic | Value |
|---|---|
| Age (years), mean ± SD | 64.4 ± 10 |
| Sex, n (%) Male Female | 48 (68.6) 22 (31.4) |
| Time since PD diagnosis (years), mean ± SD | 4.8 ± 3.1 |
| Time since onset of motor fluctuations (years), mean ± SD | 0.6 ± 0.6 |
| Hoehn and Yahr stage, n (%) I II III | 15 (21.4) 49 (70) 6 (8.6) |
| PD subtype at diagnosis, n (%) a Mild motor-predominant PD Diffuse malignant PD Intermediate | 46 (71.9) 5 (7.8) 13 (20.3) |
| Clinical Global Impression Severity of illness (CGI-S), n (%) b Borderline Mild Moderate Marked Severe | 7 (10.1) 25 (36.2) 25 (36.2) 11 (15.9) 1 (1.4) |
| History of dyskinesias, n (%) | 11 (15.7) |
| MDS-UPDRS Part III (motor) score, mean ± SD | 28.6 ± 13.9 |
| MDS-UPDRS Part IV (dyskinesia) score, mean ± SD | 4.4 ± 1.7 |
| Off time Absolute time (hours) Percentage of total awake time (%) | 3.7 ± 2.6 22.3 ±15.8 |
| Wearing-off symptoms (WOQ-19 score), mean ± SD | 5.1 ± 2.4 |
| NMSS score, mean ± SD | 33.3 ± 34.7 |
| Levodopa daily dose at inclusion, (mg) mean ± SD c Median (IQR) | 484.8 ± 212.5 450 (300–600) |
| Number of daily doses of levodopa, n (%) d 2 3 4 5 6 | 3 (4.3) 44 (63.8) 16 (23.2) 3 (4.3) 2 (2.9) |
| LEDD, (mg) mean ± SD e Median (IQR) | 665.0 ± 257.4 605.0 (501.2–775.0) |
| Levodopa treatment at inclusion | |
| Levodopa and carbidopa (100/25 mg) Levodopa and carbidopa (250/25 mg) Levodopa and benserazide (200/50 mg) Levodopa-controlled release | 37 (53.6) 9 (13) 22 (31.9) 5 (7.2) |
| Adjunct treatment at inclusion, n (%) | |
| MAOI ¶ DA ¶ Amantadine Anticholinergics (trihexyphenidyl) | 55 (78.6) 41 (58.6) 4 (5.7) 6 (8.6) |
| Main Comorbidities, n (%) | |
| Hypertension Dyslipidemia Depression Anxiety Diabetes | 16 (22.9) 9 (12.9) 6 (8.6) 3 (4.3) 3 (4.3) |
| Baseline | 3 Months | Absolute Change | Percentage of Change | p-Value | |
|---|---|---|---|---|---|
| Part I (non-motor experiences of daily living) | 7.5 ± 5.3 (95% CI: 6.2 to 8.7) | 7.6 ± 6.0 (95% CI: 6.2 to 9.1) | 0.2 ± 4.2 (95% CI: −0.8 to 1.2) | 6.9 ± 66.7 (95% CI: −9.3 to 23) | 0.956 |
| Part II (motor experiences of daily living) | 8.8 ± 5.7 (95% CI: 7.4 to 10.1) | 7.6 ± 5.5 (95% CI: 6.3 to 8.9) | −1.2 ± 4.5 (95% CI: −2.3 to −0.1) | −0.4 ± 93.1 (95% CI: −22.8 to 21.9) | 0.018 |
| Part III (motor symptoms) | 28.6 ± 13.9 (95% CI: 25.2 to 31.9) | 25.2 ± 14.0 (95% CI: 21.9 to 28.6) | −3.3 ± 7.7 (95% CI: −5.2 to −1.5) | −8.3 ± 35.5 (95% CI: −16.8 to 0.2) | <0.001 |
| Part IV a (motor complications) | 4.4 ± 1.7 (95% CI: 3.9 to 4.8) | 3.0 ± 2.0 (95% CI: 2.5 to 3.5) | −1.3 ± 1.7 (95% CI: −1.7 to −0.9) | −31.8 ± 44.1 (95% CI: −42.3 to −21.3) | <0.001 |
| Total score | 49.2 ± 20.2 (95% CI: 44.3 to 54.0) | 43.5 ± 20.9 (95% CI: 38.5 to 48.5) | −5.7 ± 11.4 (95% CI: −8.4 to −2.9) | −11.0 ± 24.4 (95% CI: −16.8 to −5.1) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Manzanares, L.; García Caldentey, J.; Álvarez-Santullano, M.M.; Vilas Rolán, D.; Herreros-Rodríguez, J.; Solano Vila, B.; Cerdán Sánchez, M.; Delgado Ballestero, T.; García-Ramos, R.; Rodríguez-Sanz, A.; et al. Real-World Use of COMT Inhibitors in the Management of Patients with Parkinson’s Disease in Spain Who Present Early Motor Fluctuations: Interim Results from the REONPARK Study. Brain Sci. 2025, 15, 532. https://doi.org/10.3390/brainsci15050532
López-Manzanares L, García Caldentey J, Álvarez-Santullano MM, Vilas Rolán D, Herreros-Rodríguez J, Solano Vila B, Cerdán Sánchez M, Delgado Ballestero T, García-Ramos R, Rodríguez-Sanz A, et al. Real-World Use of COMT Inhibitors in the Management of Patients with Parkinson’s Disease in Spain Who Present Early Motor Fluctuations: Interim Results from the REONPARK Study. Brain Sciences. 2025; 15(5):532. https://doi.org/10.3390/brainsci15050532
Chicago/Turabian StyleLópez-Manzanares, Lydia, Juan García Caldentey, Marina Mata Álvarez-Santullano, Dolores Vilas Rolán, Jaime Herreros-Rodríguez, Berta Solano Vila, María Cerdán Sánchez, Tania Delgado Ballestero, Rocío García-Ramos, Ana Rodríguez-Sanz, and et al. 2025. "Real-World Use of COMT Inhibitors in the Management of Patients with Parkinson’s Disease in Spain Who Present Early Motor Fluctuations: Interim Results from the REONPARK Study" Brain Sciences 15, no. 5: 532. https://doi.org/10.3390/brainsci15050532
APA StyleLópez-Manzanares, L., García Caldentey, J., Álvarez-Santullano, M. M., Vilas Rolán, D., Herreros-Rodríguez, J., Solano Vila, B., Cerdán Sánchez, M., Delgado Ballestero, T., García-Ramos, R., Rodríguez-Sanz, A., Olivares Romero, J., Blanco Ameijeiras, J., Pijuan Jiménez, I., & Tegel Ayuela, I., on behalf of the REONPARK Study Group. (2025). Real-World Use of COMT Inhibitors in the Management of Patients with Parkinson’s Disease in Spain Who Present Early Motor Fluctuations: Interim Results from the REONPARK Study. Brain Sciences, 15(5), 532. https://doi.org/10.3390/brainsci15050532







