Effects of Matched and Mismatched Visual Flow and Gait Speeds on Human Electrocortical Spectral Power
Abstract
1. Introduction
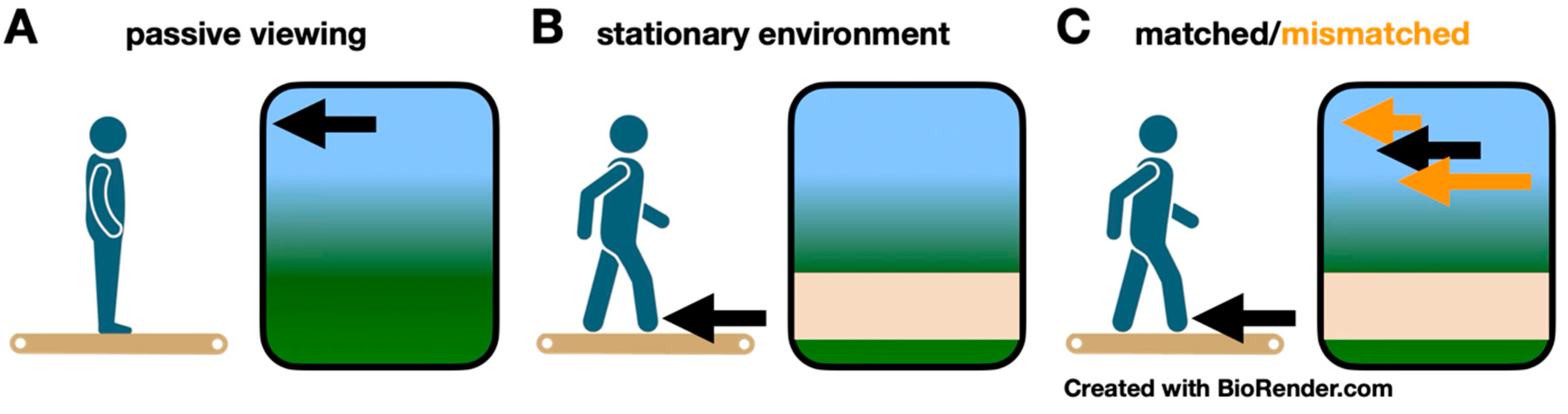
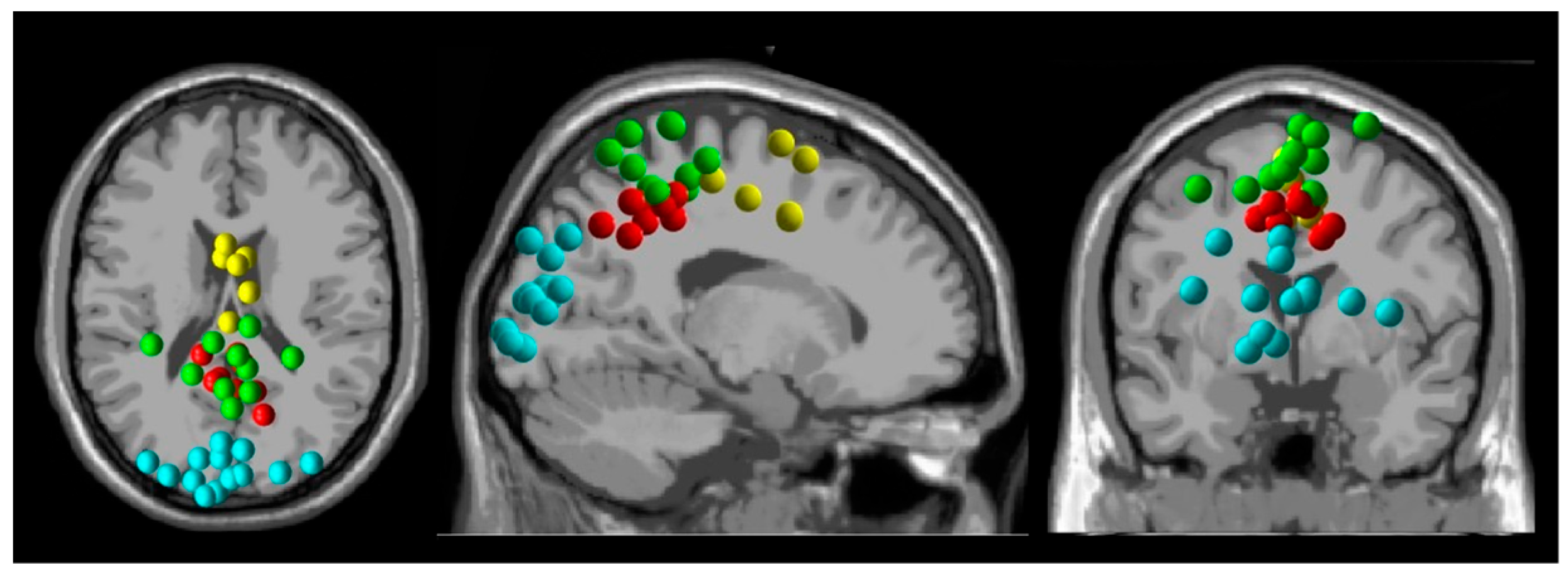
2. Materials and Methods
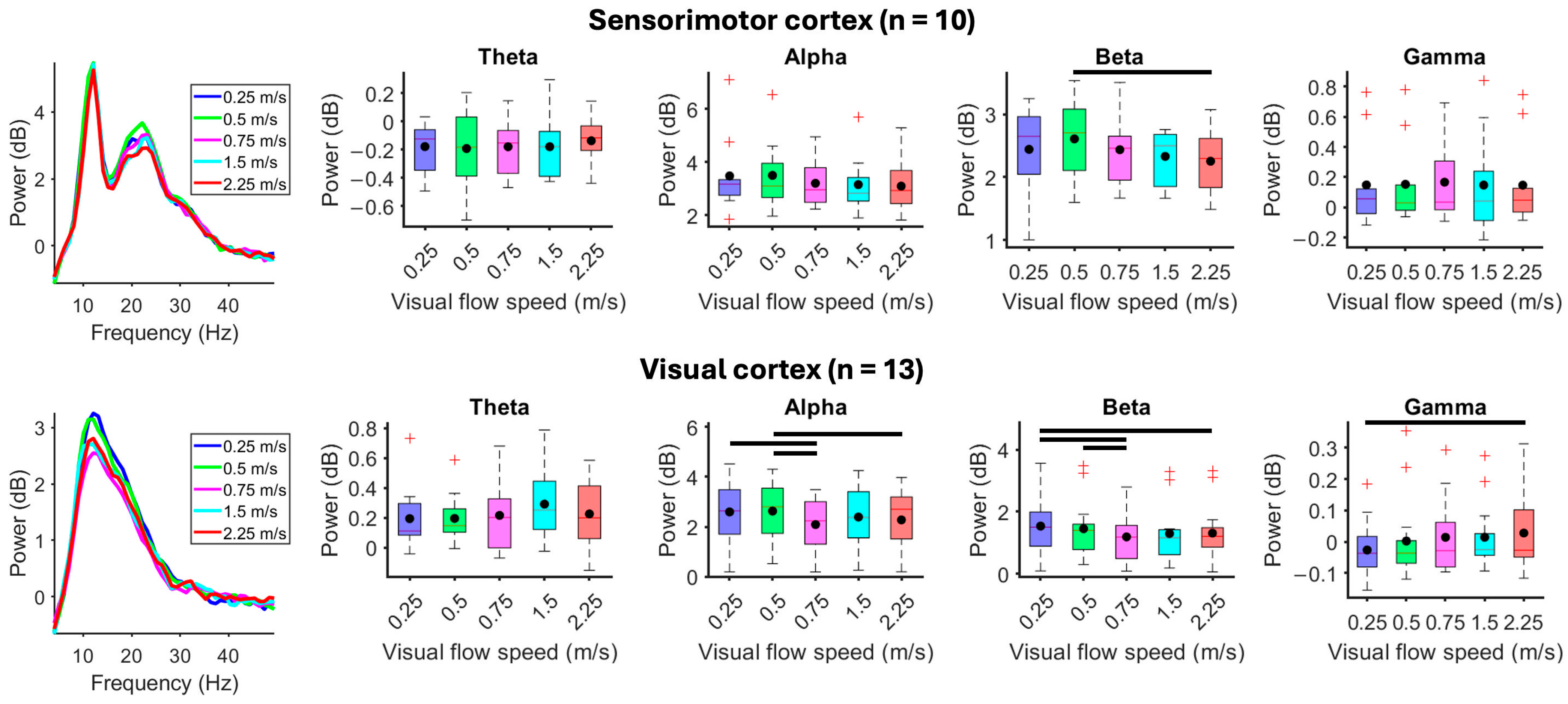
3. Results
4. Discussion
4.1. Effects of Gait Speed and Visual Flow Speed
4.2. Effects of Mismatched Gait and Visual Flow Speeds
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EEG | electroencephalography |
| VR | virtual reality |
References
- Drew, T.; Andujar, J.-E.; Lajoie, K.; Yakovenko, S. Cortical mechanisms involved in visuomotor coordination during precision walking. Brain Res. Rev. 2008, 57, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, M.W. Human cortical areas underlying the perception of optic flow: Brain imaging studies. Int. Rev. Neurobiol. 2000, 44, 269–292. [Google Scholar] [PubMed]
- Thorpe, S.; Fize, D.; Marlot, C. Speed of processing in the human visual system. Nature 1996, 381, 520–522. [Google Scholar] [CrossRef]
- Keller, G.B.; Bonhoeffer, T.; Hübener, M. Sensorimotor mismatch signals in primary visual cortex of the behaving mouse. Neuron 2012, 74, 809–815. [Google Scholar] [CrossRef]
- Liebscher, S.; Keller, G.B.; Goltstein, P.M.; Bonhoeffer, T.; Hübener, M. Selective persistence of sensorimotor mismatch signals in visual cortex of behaving Alzheimer’s disease mice. Curr. Biol. 2016, 26, 956–964. [Google Scholar] [CrossRef]
- Zmarz, P.; Keller, G.B. Mismatch receptive fields in mouse visual cortex. Neuron 2016, 92, 766–772. [Google Scholar] [CrossRef]
- Attinger, A.; Wang, B.; Keller, G.B. Visuomotor coupling shapes the functional development of mouse visual cortex. Cell 2017, 169, 1291–1302.e1214. [Google Scholar] [CrossRef]
- Horrocks, E.A.; Mareschal, I.; Saleem, A.B. Walking humans and running mice: Perception and neural encoding of optic flow during self-motion. Philos. Trans. R. Soc. B 2023, 378, 20210450. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, T.A.; Margrie, T.W. Cortical circuits for integration of self-motion and visual-motion signals. Curr. Opin. Neurobiol. 2020, 60, 122–128. [Google Scholar] [CrossRef]
- Bischof, W.F.; Boulanger, P. Spatial navigation in virtual reality environments: An EEG analysis. Cyberpsychology Behav. 2003, 6, 487–495. [Google Scholar] [CrossRef]
- Drewes, J.; Feder, S.; Einhäuser, W. Gaze during locomotion in virtual reality and the real world. Front. Neurosci. 2021, 15, 656913. [Google Scholar] [CrossRef]
- Enders, L.R.; Smith, R.J.; Gordon, S.M.; Ries, A.J.; Touryan, J. Gaze behavior during navigation and visual search of an open-world virtual environment. Front. Psychol. 2021, 12, 681042. [Google Scholar] [CrossRef] [PubMed]
- Mavros, P.; Wälti, M.J.; Nazemi, M.; Ong, C.H.; Hölscher, C. A mobile EEG study on the psychophysiological effects of walking and crowding in indoor and outdoor urban environments. Sci. Rep. 2022, 12, 18476. [Google Scholar] [CrossRef]
- van der Meer, A.L.; Fallet, G.; Van der Weel, F. Perception of structured optic flow and random visual motion in infants and adults: A high-density EEG study. Exp. Brain Res. 2008, 186, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Vilhelmsen, K.; Agyei, S.B.; Van der Weel, F.; Van der Meer, A.L. A high-density EEG study of differentiation between two speeds and directions of simulated optic flow in adults and infants. Psychophysiology 2019, 56, e13281. [Google Scholar] [CrossRef] [PubMed]
- Rasulo, S.; Vilhelmsen, K.; Van der Weel, F.; van der Meer, A.L. Development of motion speed perception from infancy to early adulthood: A high-density EEG study of simulated forward motion through optic flow. Exp. Brain Res. 2021, 239, 3143–3154. [Google Scholar] [CrossRef]
- Vilhelmsen, K.; Van der Weel, F.; Van der Meer, A.L. A high-density EEG study of differences between three high speeds of simulated forward motion from optic flow in adult participants. Front. Syst. Neurosci. 2015, 9, 146. [Google Scholar] [CrossRef]
- Ehgoetz Martens, K.A.; Ellard, C.G.; Almeida, Q.J. Does manipulating the speed of visual flow in virtual reality change distance estimation while walking in Parkinson’s disease? Exp. Brain Res. 2015, 233, 787–795. [Google Scholar] [CrossRef]
- Melnik, A.; Hairston, W.D.; Ferris, D.P.; Konig, P. EEG correlates of sensorimotor processing: Independent components involved in sensory and motor processing. Sci. Rep. 2017, 7, 4461. [Google Scholar] [CrossRef]
- Gwin, J.T.; Gramann, K.; Makeig, S.; Ferris, D.P. Electrocortical activity is coupled to gait cycle phase during treadmill walking. Neuroimage 2011, 54, 1289–1296. [Google Scholar] [CrossRef]
- Nordin, A.D.; Hairston, W.D.; Ferris, D.P. Faster Gait Speeds Reduce Alpha and Beta EEG Spectral Power From Human Sensorimotor Cortex. IEEE Trans. Biomed. Eng. 2019, 67, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Gwin, J.T.; Gramann, K.; Makeig, S.; Ferris, D.P. Removal of movement artifact from high-density EEG recorded during walking and running. J. Neurophysiol. 2010, 103, 3526–3534. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.S.; Schlink, B.R.; Hairston, W.D.; Konig, P.; Ferris, D.P. Restricted vision increases sensorimotor cortex involvement in human walking. J. Neurophysiol. 2017, 118, 1943–1951. [Google Scholar] [CrossRef] [PubMed]
- Lange, J.; Oostenveld, R.; Fries, P. Reduced occipital alpha power indexes enhanced excitability rather than improved visual perception. J. Neurosci. 2013, 33, 3212–3220. [Google Scholar] [CrossRef]
- Khajuria, A.; Sharma, R.; Joshi, D. EEG Dynamics of Locomotion and Balancing: Solution to Neuro-Rehabilitation. Clin. EEG Neurosci. 2024, 55, 143–163. [Google Scholar] [CrossRef]
- Ergenoglu, T.; Demiralp, T.; Bayraktaroglu, Z.; Ergen, M.; Beydagi, H.; Uresin, Y. Alpha rhythm of the EEG modulates visual detection performance in humans. Cogn. Brain Res. 2004, 20, 376–383. [Google Scholar] [CrossRef]
- Darch, H.T.; Cerminara, N.L.; Gilchrist, I.D.; Apps, R. Pre-movement changes in sensorimotor beta oscillations predict motor adaptation drive. Sci. Rep. 2020, 10, 17946. [Google Scholar] [CrossRef]
- Crone, N.E.; Miglioretti, D.L.; Gordon, B.; Sieracki, J.M.; Wilson, M.T.; Uematsu, S.; Lesser, R.P. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. I. Alpha and beta event-related desynchronization. Brain J. Neurol. 1998, 121, 2271–2299. [Google Scholar] [CrossRef]
- van Driel, J.; Ridderinkhof, K.R.; Cohen, M.X. Not all errors are alike: Theta and alpha EEG dynamics relate to differences in error-processing dynamics. J. Neurosci. 2012, 32, 16795–16806. [Google Scholar] [CrossRef]
- Pastötter, B.; Hanslmayr, S.; Bäuml, K.-H.T. Conflict processing in the anterior cingulate cortex constrains response priming. NeuroImage 2010, 50, 1599–1605. [Google Scholar] [CrossRef]
- Ehinger, B.V.; Fischer, P.; Gert, A.L.; Kaufhold, L.; Weber, F.; Pipa, G.; König, P. Kinesthetic and vestibular information modulate alpha activity during spatial navigation: A mobile EEG study. Front. Hum. Neurosci. 2014, 8, 71. [Google Scholar] [CrossRef]
- Bigdely-Shamlo, N.; Mullen, T.; Kothe, C.; Su, K.-M.; Robbins, K.A. The PREP pipeline: Standardized preprocessing for large-scale EEG analysis. Front. Neuroinformatics 2015, 9, 16. [Google Scholar] [CrossRef]
- Safieddine, D.; Kachenoura, A.; Albera, L.; Birot, G.; Karfoul, A.; Pasnicu, A.; Biraben, A.; Wendling, F.; Senhadji, L.; Merlet, I. Removal of muscle artifact from EEG data: Comparison between stochastic (ICA and CCA) and deterministic (EMD and wavelet-based) approaches. EURASIP J. Adv. Signal Process. 2012, 2012, 127. [Google Scholar] [CrossRef]
- Roy, V.; Shukla, S.; Shukla, P.K.; Rawat, P. Gaussian Elimination-Based Novel Canonical Correlation Analysis Method for EEG Motion Artifact Removal. J. Healthc. Eng. 2017, 2017, 9674712. [Google Scholar] [CrossRef]
- Palmer, J.A.; Kreutz-Delgado, K.; Makeig, S. AMICA: An Adaptive Mixture of Independent Component Analyzers with Shared Components; Technical Report; Swartz Center for Computatonal Neursoscience, University of California San Diego: San Diego, CA, USA, 2012. [Google Scholar]
- Pion-Tonachini, L.; Kreutz-Delgado, K.; Makeig, S. ICLabel: An automated electroencephalographic independent component classifier, dataset, and website. NeuroImage 2019, 198, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Donoghue, T.; Haller, M.; Peterson, E.J.; Varma, P.; Sebastian, P.; Gao, R.; Noto, T.; Lara, A.H.; Wallis, J.D.; Knight, R.T. Parameterizing neural power spectra into periodic and aperiodic components. Nat. Neurosci. 2020, 23, 1655–1665. [Google Scholar] [CrossRef]
- Bulea, T.C.; Kim, J.; Damiano, D.L.; Stanley, C.J.; Park, H.-S. Prefrontal, posterior parietal and sensorimotor network activity underlying speed control during walking. Front. Hum. Neurosci. 2015, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.M.; Ferris, D.P. Differentiation in theta and beta electrocortical activity between visual and physical perturbations to walking and standing balance. eNeuro 2018, 5, 4. [Google Scholar] [CrossRef]
- Wagner, J.; Solis-Escalante, T.; Scherer, R.; Neuper, C.; Müller-Putz, G. It’s how you get there: Walking down a virtual alley activates premotor and parietal areas. Front. Hum. Neurosci. 2014, 8, 93. [Google Scholar] [CrossRef]
- Liska, J.P.; Rowley, D.P.; Nguyen, T.T.K.; Muthmann, J.-O.; Butts, D.A.; Yates, J.; Huk, A.C. Running modulates primate and rodent visual cortex differently. eLife 2024, 12, RP87736. [Google Scholar] [CrossRef]
- Talluri, B.C.; Kang, I.; Lazere, A.; Quinn, K.R.; Kaliss, N.; Yates, J.L.; Butts, D.A.; Nienborg, H. Activity in primate visual cortex is minimally driven by spontaneous movements. Nat. Neurosci. 2023, 26, 1953–1959. [Google Scholar] [CrossRef] [PubMed]
- Hoogenboom, N.; Schoffelen, J.-M.; Oostenveld, R.; Parkes, L.M.; Fries, P. Localizing human visual gamma-band activity in frequency, time and space. NeuroImage 2006, 29, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Popov, T.; Kastner, S.; Jensen, O. FEF-controlled alpha delay activity precedes stimulus-induced gamma-band activity in visual cortex. J. Neurosci. 2017, 37, 4117–4127. [Google Scholar] [CrossRef]
- Hermes, D.; Miller, K.; Wandell, B.; Winawer, J. Stimulus dependence of gamma oscillations in human visual cortex. Cereb. Cortex 2015, 25, 2951–2959. [Google Scholar] [CrossRef]
- Marshall, T.R.; O’Shea, J.; Jensen, O.; Bergmann, T.O. Frontal eye fields control attentional modulation of alpha and gamma oscillations in contralateral occipitoparietal cortex. J. Neurosci. 2015, 35, 1638–1647. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, B.R.; Foxe, J.J.; Joshi, S.; Verghese, J.; Mahoney, J.R.; Molholm, S.; De Sanctis, P. Aging-related changes in cortical mechanisms supporting postural control during base of support and optic flow manipulations. Eur. J. Neurosci. 2021, 54, 8139–8157. [Google Scholar] [CrossRef]
- Guerraz, M.; Bronstein, A.M. Mechanisms underlying visually induced body sway. Neurosci. Lett. 2008, 443, 12–16. [Google Scholar] [CrossRef]
- Mergner, T.; Schweigart, G.; Maurer, C.; Blümle, A. Human postural responses to motion of real and virtual visual environments under different support base conditions. Exp. Brain Res. 2005, 167, 535–556. [Google Scholar] [CrossRef]
- Lestienne, F.; Soechting, J.; Berthoz, A. Postural readjustments induced by linear motion of visual scenes. Exp. Brain Res. 1977, 28, 363–384. [Google Scholar] [CrossRef]
- Slobounov, S.M.; Teel, E.; Newell, K.M. Modulation of cortical activity in response to visually induced postural perturbation: Combined VR and EEG study. Neurosci. Lett. 2013, 547, 6–9. [Google Scholar] [CrossRef]
- Kagawa, T.; Makeig, S.; Miyakoshi, M. Electroencephalographic study on sensory integration in visually induced postural sway. J. Cogn. Neurosci. 2021, 33, 482–498. [Google Scholar] [CrossRef] [PubMed]
- Salminen, J.; Liu, C.; Pliner, E.M.; Tenerowicz, M.; Roy, A.; Richer, N.; Hwang, J.; Hass, C.J.; Clark, D.J.; Seidler, R.D. Gait Speed Related Changes in Electrocortical Activity in Younger and Older Adults. J. Neurophysiol. 2025, in press. [CrossRef] [PubMed]
- Gramann, K.; Onton, J.; Riccobon, D.; Mueller, H.J.; Bardins, S.; Makeig, S. Human brain dynamics accompanying use of egocentric and allocentric reference frames during navigation. J. Cogn. Neurosci. 2010, 22, 2836–2849. [Google Scholar] [CrossRef]
- Corbetta, M.; Shulman, G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002, 3, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Benedek, M.; Schickel, R.J.; Jauk, E.; Fink, A.; Neubauer, A.C. Alpha power increases in right parietal cortex reflects focused internal attention. Neuropsychologia 2014, 56, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Arnal, L.H.; Giraud, A.-L. Cortical oscillations and sensory predictions. Trends Cogn. Sci. 2012, 16, 390–398. [Google Scholar] [CrossRef]
- Straka, H.; Simmers, J.; Chagnaud, B.P. A new perspective on predictive motor signaling. Curr. Biol. 2018, 28, R232–R243. [Google Scholar] [CrossRef]
- Mohler, B.J.; Thompson, W.B.; Creem-Regehr, S.H.; Pick, H.L., Jr.; Warren, W.H., Jr. Visual flow influences gait transition speed and preferred walking speed. Exp. Brain Res. 2007, 181, 221–228. [Google Scholar] [CrossRef]
- Prokop, T.; Schubert, M.; Berger, W. Visual influence on human locomotion Modulation to changes in optic flow: Modulation to changes in optic flow. Exp. Brain Res. 1997, 114, 63–70. [Google Scholar] [CrossRef]
- Lamontagne, A.; Fung, J.; McFadyen, B.J.; Faubert, J. Modulation of walking speed by changing optic flow in persons with stroke. J. Neuroeng. Rehabil. 2007, 4, 22. [Google Scholar] [CrossRef]
- Lewis, M.M.; Waltz, C.; Scelina, L.; Scelina, K.; Owen, K.M.; Hastilow, K.; Zimmerman, E.M.; Rosenfeldt, A.B.; Miller Koop, M.; Alberts, J.L. Gait patterns during overground and virtual omnidirectional treadmill walking. J. Neuroeng. Rehabil. 2024, 21, 29. [Google Scholar] [CrossRef] [PubMed]
- Salinas, M.M.; Wilken, J.M.; Dingwell, J.B. How humans use visual optic flow to regulate stepping during walking. Gait Posture 2017, 57, 15–20. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.-P.; Nordin, A.D. Effects of Matched and Mismatched Visual Flow and Gait Speeds on Human Electrocortical Spectral Power. Brain Sci. 2025, 15, 531. https://doi.org/10.3390/brainsci15050531
Cheng Y-P, Nordin AD. Effects of Matched and Mismatched Visual Flow and Gait Speeds on Human Electrocortical Spectral Power. Brain Sciences. 2025; 15(5):531. https://doi.org/10.3390/brainsci15050531
Chicago/Turabian StyleCheng, Yu-Po, and Andrew D. Nordin. 2025. "Effects of Matched and Mismatched Visual Flow and Gait Speeds on Human Electrocortical Spectral Power" Brain Sciences 15, no. 5: 531. https://doi.org/10.3390/brainsci15050531
APA StyleCheng, Y.-P., & Nordin, A. D. (2025). Effects of Matched and Mismatched Visual Flow and Gait Speeds on Human Electrocortical Spectral Power. Brain Sciences, 15(5), 531. https://doi.org/10.3390/brainsci15050531






