Neurophysiological Approaches to Lie Detection: A Systematic Review
Abstract
1. Introduction
2. Literature Review
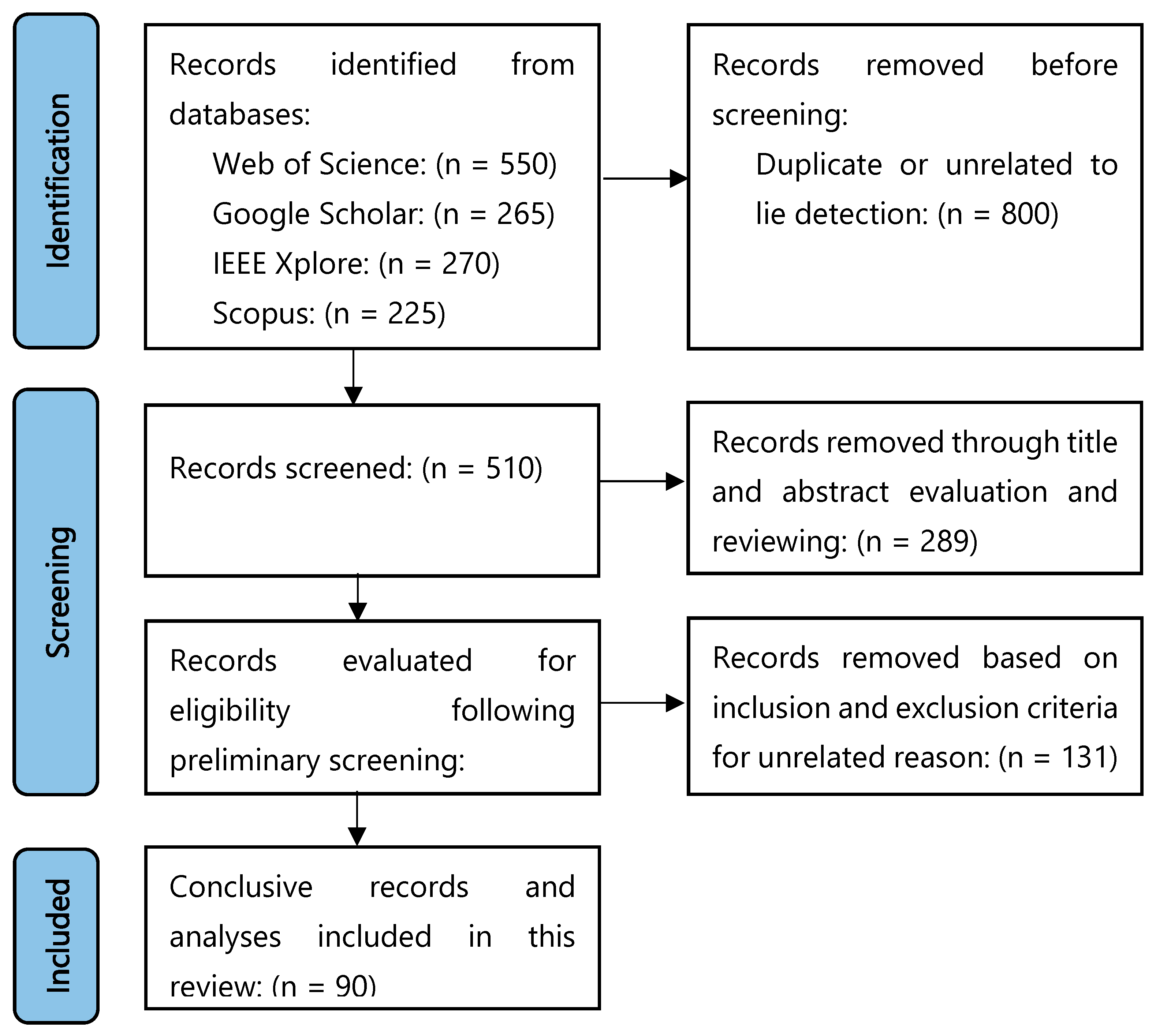
3. Methodology
Study Inclusion and Exclusion Criteria
- English journal articles and conference proceedings analyzing lie detection based on EEG signals were included as the inclusion criteria.
- The selection was made according to the feature extraction methods based on signal processing studies.
- Articles focusing on the accuracy metrics of the models’ performance were taken into consideration.
- Studies analyzing brain responses to recognized and unrecognized faces, particularly involving the ERP P300 component, were included.
- Studies that included experimental protocols (e.g., CIT, GKT, DIT) in which EEG signals obtained from humans were included.
- Studies using different approaches (e.g., fMRI, polygraph, peripheral bio signals) other than EEG were not evaluated.
- Studies that were not directly related to lie detection (e.g., only measuring attention, stress, depression) even if EEG was used, were excluded.
- Theoretical studies that only described EEG collection methods, electrode placement, or general EEG signal properties were excluded.
- Research that did not report or compare metrics such as classification accuracy, F1 score, etc. was excluded.
- Studies, whose full text could not be accessed, even if the title and abstract were appropriate, were excluded.
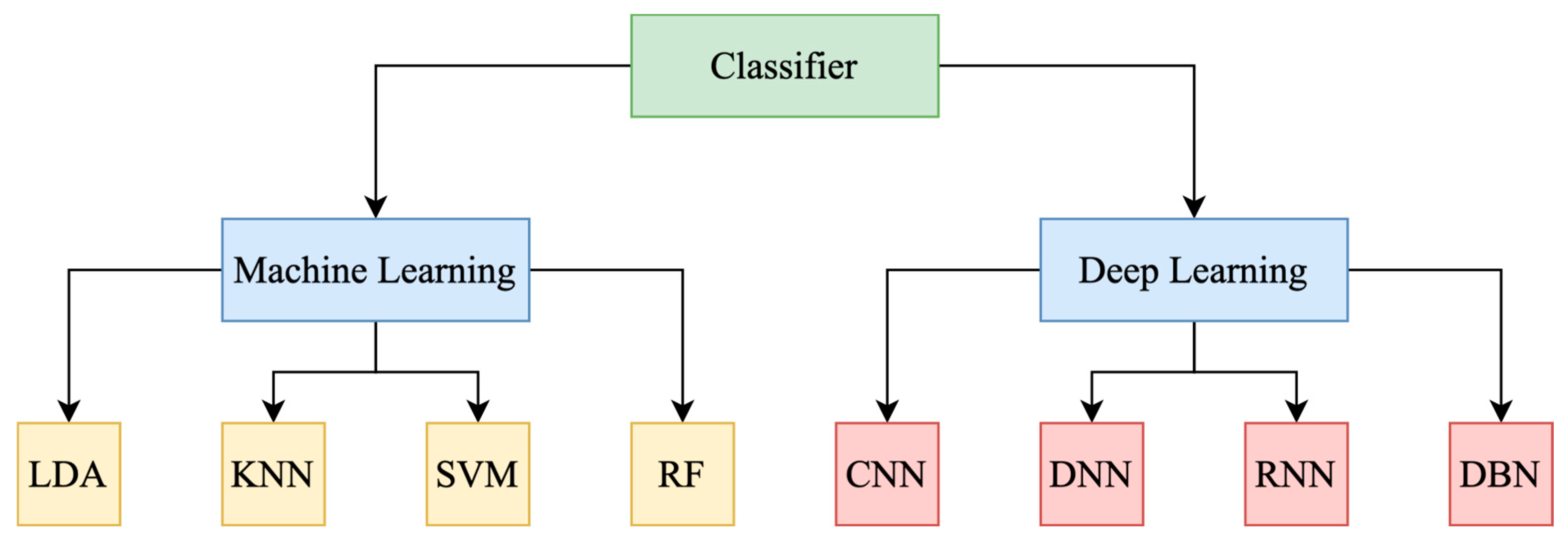
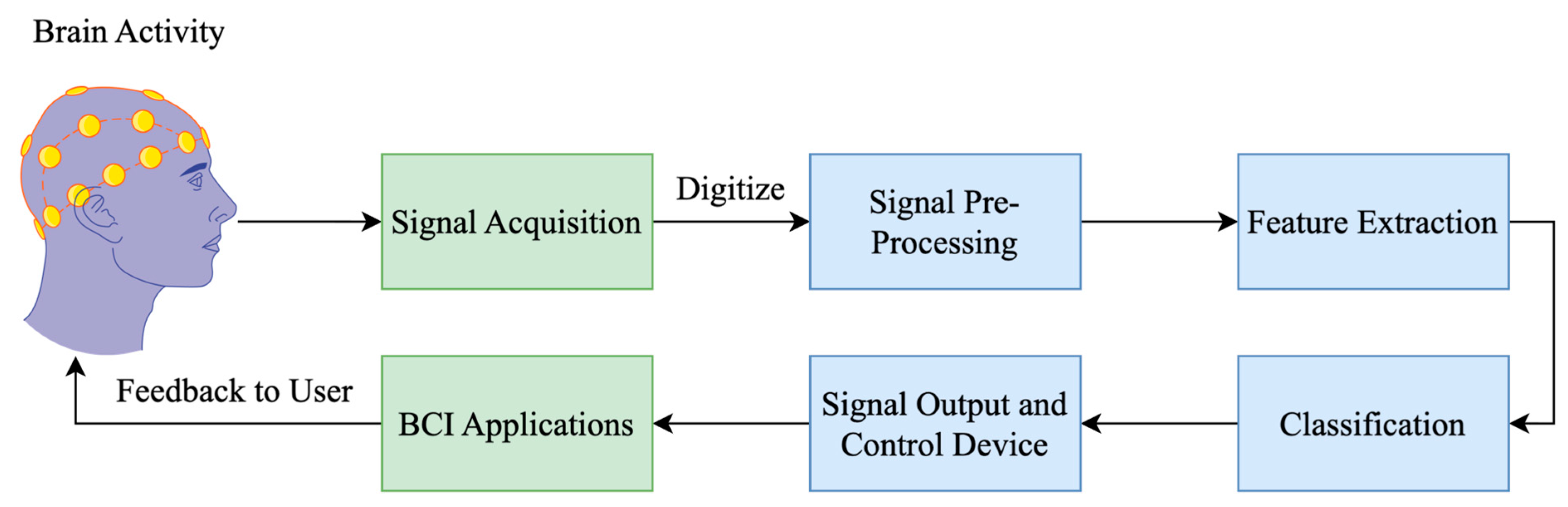
4. EEG-Based Lie Detection
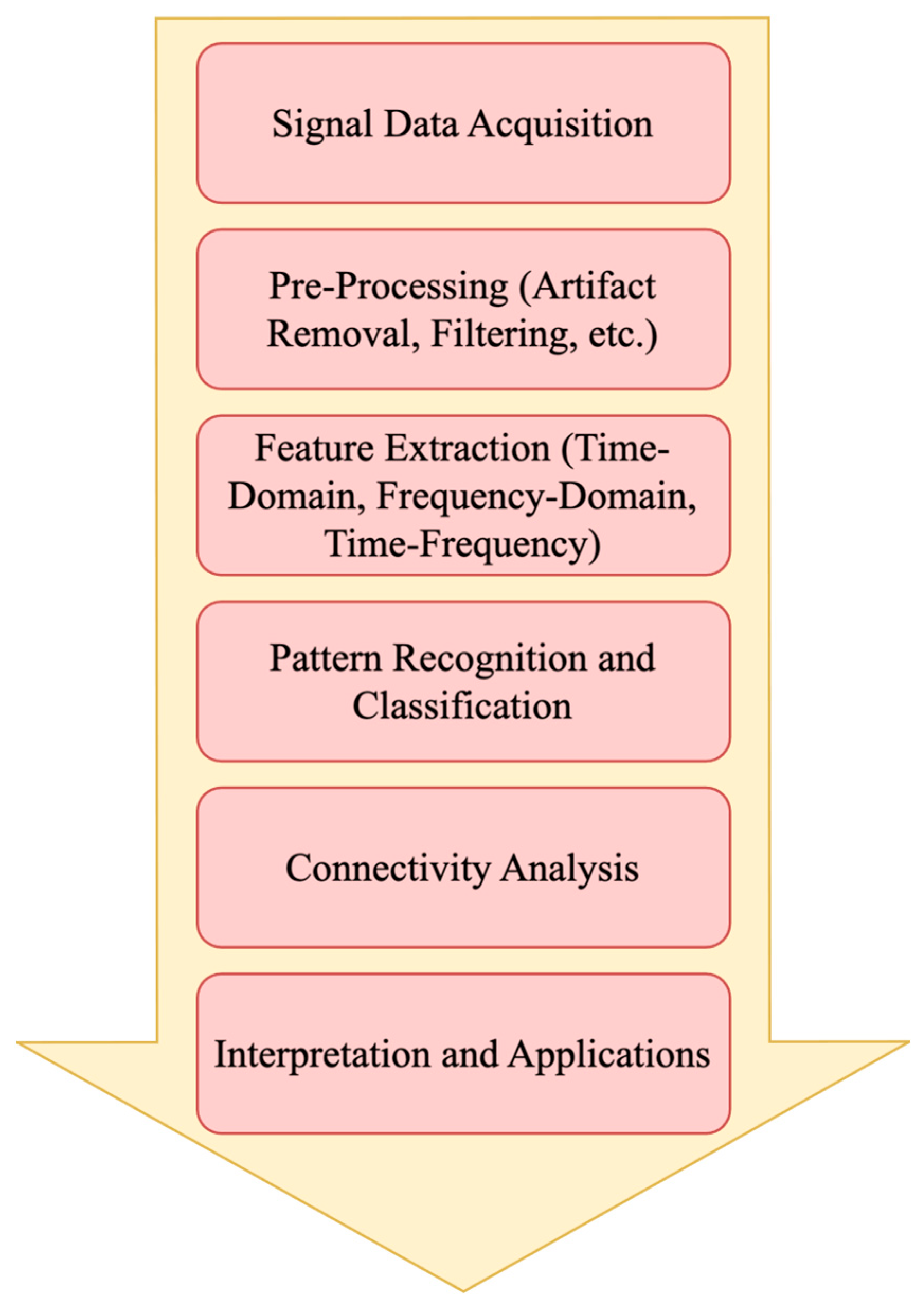
4.1. EEG Data Analysis
4.1.1. Data Acquisition
4.1.2. Preprocessing
4.1.3. Feature Extraction
4.1.4. Pattern Recognition and Classification
4.1.5. Connectivity Analysis
4.1.6. Interpretation and Applications
4.2. EEG Characteristics
- i.
- Delta (0.5–4 Hz): When people are deeply and unconsciously asleep, the slowest EEG waves are usually seen. At this point, delta waves have sizable amplitudes (75–200 volts (V)) and are thus thought to represent the person’s unconscious mind. As our sensitivity to the external environment declines, delta waves become more pronounced [75].
- ii.
- Theta (4–8 Hz): Experiencing waves is best produced when one is calm and focused. Additionally, one may see them while relaxing, in light sleep, recalling memories, and even on certain tests for short-term memory. They can also appear in some pathological circumstances. An amplitude of 100V is typical for theta waves [1,75].
- iii.
- iv.
- v.
- Gamma (Above 30Hz): These are formed during extended high-level information processing. With amplitudes of less than 2V, these waves are very tiny. When it comes to visual inputs, cognitive functioning, and image understanding, gamma waves are recognized to have more important electrical impulses [73,75].
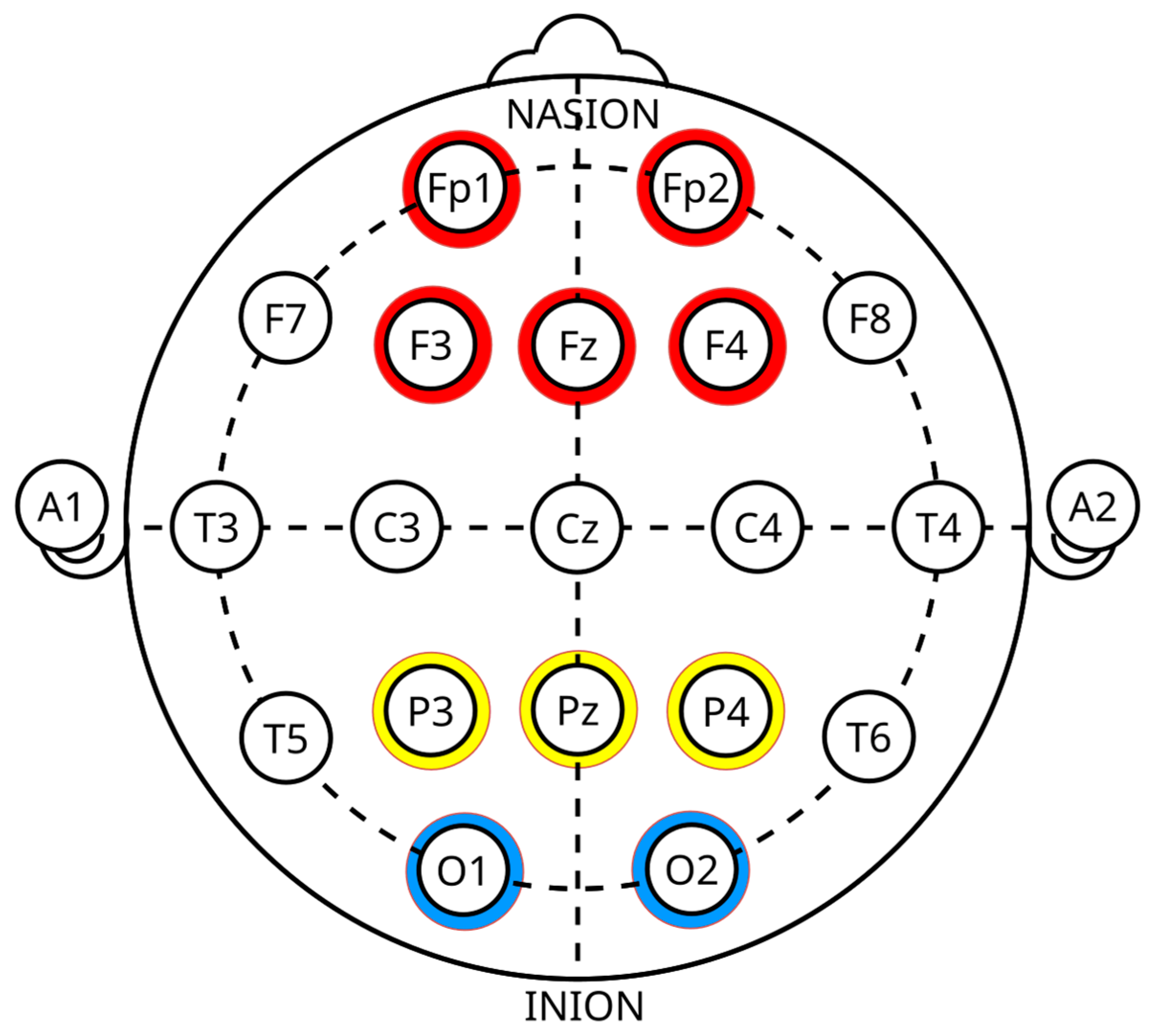
4.3. Effective EEG Channels for Lie Detection
4.4. Visual ERP P300
5. Results
6. Discussion
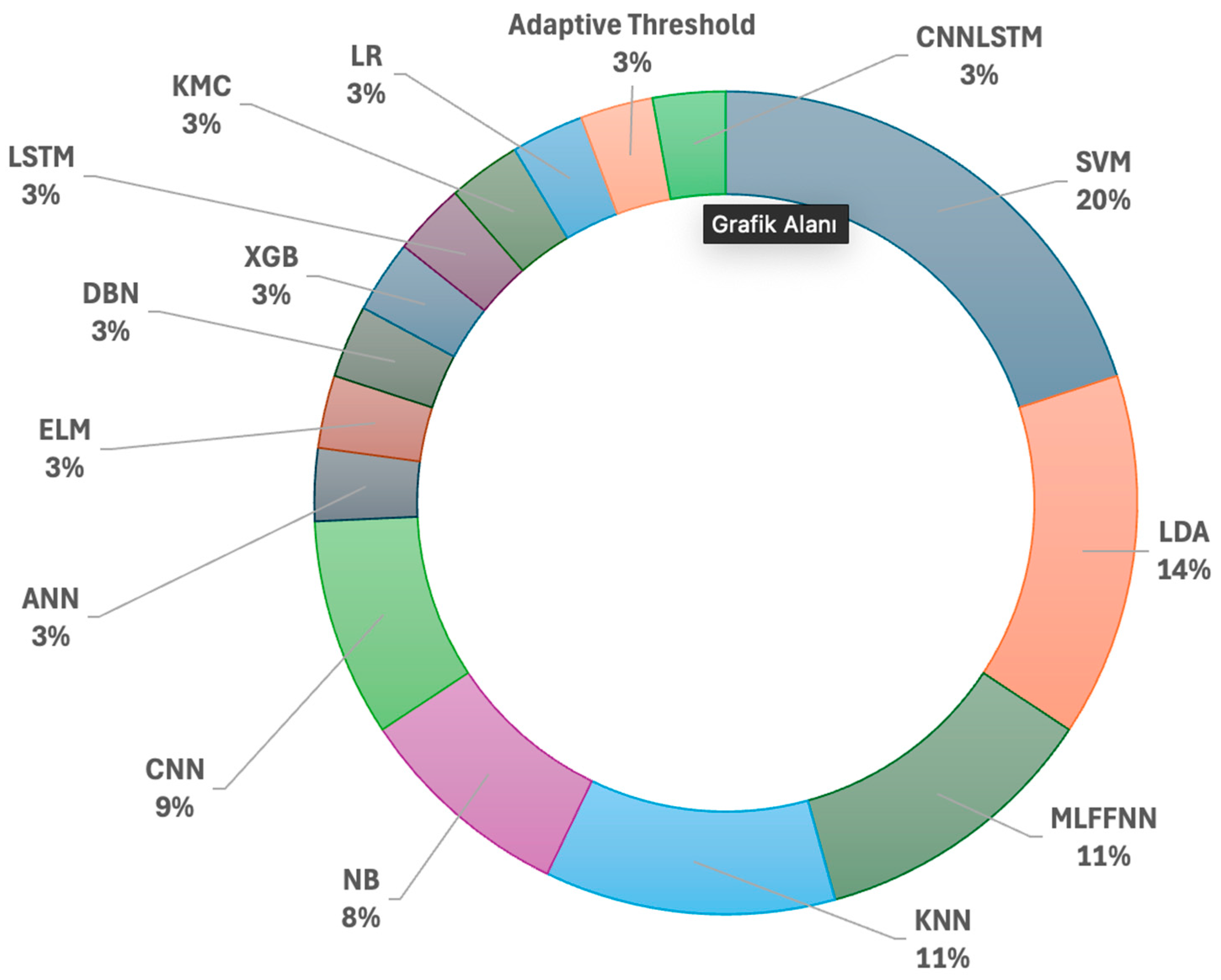
6.1. Evaluating and Discussing Feature Extraction Algorithms for Lie Detection Studies
6.2. Shortcomings of EEG-Based Lie Detection Studies
- i.
- Low Spatial Resolution: EEG records brain activity at the scalp level and provides high temporal resolution but poor spatial resolution, and therefore cannot localize frontal lobe activity precisely, making it difficult to pinpoint the exact brain regions responsible for deception. Unlike hybrid EEGs, which could address spatial resolution issues, and fMRI, which can localize activity with greater precision, EEG signals are averaged across broad cortical areas, limiting the ability to distinguish specific neural correlates of lying [16,17,18,19,20,21,22,23].
- ii.
- iii.
- Individual Differences: EEG signals vary significantly between individuals due to differences in brain structure, cognitive strategies, and psychological states. What constitutes a deception-related brain response in one person may not be the same in another, limiting the generalizability of findings and making it challenging to develop a universal deception detection model [18,19,20,21,22,23,24,25].
- iv.
- Cognitive Complexity of Deception: Lying is a complex cognitive and emotional process that involves multiple brain functions, including those influenced by memory, executive control, intention, personality traits, and emotional regulation. EEG may capture neural responses associated with these processes, but it cannot differentiate between deception and other cognitive activities like (anxiety, stress, or recall difficulty), leading to false positives [19,20,21,22,23,24,25,26].
- v.
- Inconsistency across Studies: Many EEG-based lie detection studies show inconsistent results, partly due to differences in study design, task paradigms, and analysis methods. Variability in the stimuli used to induce deception and the classification methods can lead to contradictory findings, reducing the reliability of conclusions [20,21,22,23,24,25,26,27].
- vi.
- Difficulty in Establishing Reliable Biomarkers: Identifying universal EEG-based lie detection remains a challenge. While certain waveforms like (P300 and N400) have been linked to deception, these markers are also associated with other cognitive processes, making it difficult to establish a definitive neural indicator of lying [21,22,23,24,25,26,27,29].
- vii.
- Limited Ecological Validity: Most EEG-based deception studies are conducted in controlled lab environments with artificial tasks, which may not reflect real-world deception. Differences in motivation, emotional states, and stakes influence brain activity. The stakes are often low in experimental settings, meaning the neural mechanisms involved in deception may differ from those in high-stakes, real-life situations [22,23,24,25,26,27,28,29].
- viii.
- Ethical and Legal Concerns: Using EEG for lie detection raises ethical concerns, including privacy violations and the risk of misclassifying innocent individuals. The legal admissibility of EEG-based lie detection is also questionable, as the reliability of brainwave-based deception detection is not yet robust enough for forensic or legal contexts, applications and inadmissibility in courts [8,23,24,25,26,27,28,29].
6.3. Limitation of High-Accuracy Claims
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
List of Abbreviations and Symbols
| ALAR | Absolute Latency/Amplitude Ratio |
| ADC | Analog to Digital Conversion |
| ACC | Anterior Cingulate Cortex |
| ANN | Artificial Neural Network |
| ADHD | Attention Deficit and Hyperactivity Disorder |
| ATAR | Automated and Adjustable Artefact Removal |
| BPF | Bandpass Filter |
| BAD | Bootstrapped Amplitude Difference |
| BCD | Bootstrapped Correlation Difference |
| BWD | Burg’s Wavelet Decomposition |
| CAR | Common Average Reference |
| CSP | Common Spatial Pattern |
| CIT | Concealed Information Test |
| CF | Connectivity Features |
| CQT | Control Question Test |
| CNN | Convolutional Neural Networks |
| DIT | Deceit Identification Test |
| DBN | Deep Belief Network |
| DL | Deep Learning |
| DWT | Discrete Wavelet Transform |
| DCM | Dynamic Causal Modeling |
| EEG | Electroencephalography |
| EOG | Electrooculography |
| ER | Emotion Recognition |
| EMD | Empirical Mode Decomposition |
| ERP | Event-Related Potential |
| EPs | Evoked Potentials |
| ELM | Extreme Learning Machine |
| FFT | Fast Fourier Transform |
| FD | Fractal Dimension |
| fMRI | functional Magnetic Resonance Imaging |
| GA | Genetic Algorithm |
| GDA | Gradient Descent Algorithm |
| GKT | Guilty Knowledge Test |
| HT | Hilbert Transform |
| HSP | Hjorth’s Statistical Parameters |
| ICA | Independent Component Analysis |
| IMFs | Intrinsic Mode Functions |
| KNN | K-Nearest Neighbor |
| LC | Linear Classifier |
| LDA | Linear Discriminant Analysis |
| LR | Logistic Regression |
| LSTM | Long/Short Term Memory |
| ML | Machine Learning |
| MRI | Magnetic Resonance Imaging |
| mPFC | medial Prefrontal Cortex |
| MI | Motor Imagery |
| MLFFNN | Multilayer Feed Forward Neural Network |
| NB | Naive Bayes |
| OSW | Overlapping Sliding Window |
| P2P | Peak-to-Peak |
| P2PT | Peak-to-Peak Time |
| PLV | Phase Locking Value |
| PS | Phase Synchronization |
| PSD | Power Spectral Density |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PFC | Prefrontal Cortex |
| PCA | Principal Component Analysis |
| RF | Random Forest |
| ReLU | Rectified Linear Unit |
| RNN | Recurrent Neural Network |
| STFT | Short-Time Fourier Transform |
| SNR | Signal-to-Noise Ratio |
| SSA | Slope Signal Alteration |
| SSPT | Smart Signal Processing Techniques |
| SDA | Spatial Denoising Algorithm |
| SDF | Spatial Domain Feature |
| SM | Statistical Method |
| SSVEPs | Steady State Visually Evoked Potentials |
| SVM | Support Vector Machine |
| TST | Three-Stimulus Technique |
| WPT | Wavelet Packet Transform |
| WT | Wavelet Transform |
| WV | Weighted Voting |
References
- Kohan, M.D.; NasrAbadi, A.M.; Sharifi, A.; Shamsollahi, M.B. Interview based connectivity analysis of EEG in order to detect deception. Med. Hypotheses 2020, 136, 109–517. [Google Scholar] [CrossRef]
- Lin, X.; Sai, L.; Yuan, Z. Detecting Concealed Information with Fused Electroencephalography and Functional Near-infrared Spectroscopy. Neuroscience 2018, 386, 284–294. [Google Scholar] [CrossRef]
- Yang, S.; Deravi, F. On the Usability of Electroencephalographic Signals for Biometric Recognition: A Survey. IEEE Trans. Hum.-Mach. Syst. 2017, 47, 958–969. [Google Scholar] [CrossRef]
- Craik, A.; He, Y.; Contreras-Vidal, J.L. Deep learning for electroencephalogram (EEG) classification tasks: A review. J. Neural Eng. 2019, 16, 031001. [Google Scholar] [CrossRef] [PubMed]
- Maiorana, E. Deep learning for EEG-based biometric recognition. Neurocomputing 2020, 410, 374–386. [Google Scholar] [CrossRef]
- Mao, Z.; Yao, W.X.; Huang, Y. EEG-based biometric identification with deep learning. In Proceedings of the 2017 8th International IEEE/EMBS Conference on Neural Engineering (NER), Shanghai, China, 25–28 May 2017; pp. 609–612. [Google Scholar] [CrossRef]
- Chen, J.X.; Mao, Z.J.; Yao, W.X.; Huang, Y.F. EEG-based biometric identification with convolutional neural network. Multimed. Tools Appl. 2020, 79, 10655–10675. [Google Scholar] [CrossRef]
- Dodia, S.; Edla, D.R.; Bablani, A.; Cheruku, R. Lie detection using extreme learning machine: A concealed information test based on short-time Fourier transform and binary bat optimization using a novel fitness function. Comput. Intell. 2020, 36, 637–658. [Google Scholar] [CrossRef]
- Haider, S.K.; Daud, M.I.; Jiang, A.; Khan, Z. Evaluation of P300 based Lie Detection Algorithm Electr. Electron. Eng. 2017, 2017, 69–76. [Google Scholar]
- Turnip, A.; Amri, M.F.; Suhendra, M.A.; Kusumandari, D.E. Lie detection based EEG-P300 signal classified by ANFIS method. J. Telecommun. Electron. Comput. Eng. 2017, 9, 107–110. [Google Scholar]
- Khalil, M.A.; George, K. Using Neural Network Models for BCI Based Lie Detection. In Proceedings of the 2022 IEEE 13th Annual Ubiquitous Computing, Electronics & Mobile Communication Conference (UEMCON), New York, NY, USA, 26–29 October 2022; pp. 505–509. [Google Scholar] [CrossRef]
- Movahedi, F.; Coyle, J.L.; Sejdic, E. Deep belief networks for electroencephalography: A review of recent contributions and future outlooks. IEEE J. Biomed. Health Inform. 2017, 22, 642–652. [Google Scholar] [CrossRef]
- Das, B.B.; Kumar, P.; Kar, D.; Ram, S.K.; Babu, K.S.; Mohapatra, R.K. A spatio-temporal model for EEG-based person identification. Multimed. Tools Appl. 2019, 78, 28157–28177. [Google Scholar] [CrossRef]
- The McGill Physiology Virtual Lab [Online]. Available online: http://www.medicine.mcgill.ca/physio/vlab/biomed_signals/EEG_n.htm (accessed on 13 May 2025).
- Cecotti, H.; Eckstein, M.P.; Giesbrecht, B. Single-Trial Classification of Event-Related Potentials in Rapid Serial Visual Presentation Tasks Using Supervised Spatial Filtering. IEEE Trans. Neural Netw. Learn. Syst. 2014, 25, 2030–2042. [Google Scholar] [CrossRef] [PubMed]
- Aslan, M.; Baykara, M.; Alakus, T.B. LieWaves: Dataset for lie detection based on EEG signals and wavelets. Med. Biol. Eng. Comput. 2024, 62, 1571–1588. [Google Scholar] [CrossRef] [PubMed]
- Bablani, A.; Edla, D.R.; Tripathi, D.; Kuppili, V. An efficient Concealed Information Test: EEG feature extraction and ensemble classification for lie identification. Mach. Vis. Appl. 2019, 30, 813–832. [Google Scholar] [CrossRef]
- Anwar, S.; Batool, T.; Majid, M. Event Related Potential (ERP) based Lie Detection using a Wearable EEG headset. In Proceedings of the 2019 16th International Bhurban Conference on Applied Sciences and Technology (IBCAST), Islamabad, Pakistan, 8–12 January 2019; pp. 543–547. [Google Scholar] [CrossRef]
- Bablani, A.; Edla, D.R.; Tripathi, D.; Dodia, S.; Chintala, S. A Synergistic Concealed Information Test With Novel Approach for EEG Channel Selection and SVM Parameter Optimization. IEEE Trans. Inf. Forensics Secur. 2019, 14, 3057–3068. [Google Scholar] [CrossRef]
- Kulasinghe, Y.; Kulasinghe, D.H.Y. Using EEG and Machine Learning to Perform Lie Detection Kinect Sensor for Skeleton Abnormality Detection View Project Software Effort Estimation Model Based on Story Points for Agile Development View project Using EEG and Machine Learning to Perform Lie D. [Online]. Available online: https://www.researchgate.net/publication/335095404 (accessed on 13 May 2025).
- Mehrnam, A.H.; Nasrabadi, A.M.; Ghodousi, M.; Mohammadian, A.; Torabi, S. A new approach to analyze data from EEG-based concealed face recognition system. Int. J. Psychophysiol 2017, 116, 1–8. [Google Scholar] [CrossRef]
- Xiong, Y.; Gu, L.; Gao, J. Phase synchrony and its application to lie detection. In Proceedings of the 2020 IEEE International Conference on Power, Intelligent Computing and Systems (ICPICS), Shenyang, China, 28–30 July 2020; pp. 726–729. [Google Scholar] [CrossRef]
- Bablani, A.; Edla, D.R.; Dodia, S. Classification of EEG Data using k-Nearest Neighbor approach for Concealed Information Test. Procedia Comput. Sci. 2018, 143, 242–249. [Google Scholar] [CrossRef]
- Baghel, N.; Singh, D.; Dutta, M.K.; Burget, R.; Myska, V. Truth Identification from EEG Signal by Using Convolution Neural Network: Lie Detection. In Proceedings of the 2020 43rd International Conference on Telecommunications and Signal Processing (TSP), Milan, Italy, 7–9 July 2020; Available online: https://ieeexplore.ieee.org/document/9163497 (accessed on 13 May 2025).
- Bablani, A.; Edla, D.R.; Kuppili, V.; Ramesh, D. A Multi Stage EEG data classification using k-Means and Feed Forward Neural Network. Clin. Epidemiol. Glob. Health 2020, 8, 718–724. [Google Scholar] [CrossRef]
- Saini, N.; Bhardwaj, S.; Agarwal, R. Classification of EEG signals using a hybrid combination of features for lie detection. Neural. Comput. Appl. 2020, 32, 3777–3787. [Google Scholar] [CrossRef]
- Bablani, A.; Edla, D.R.; Kuppili, V.; Dharavath, R. Lie Detection Using Fuzzy Ensemble Approach with Novel Defuzzification Method for Classification of EEG Signals. IEEE Trans. Instrum. Meas. 2021, 70, 2509413. [Google Scholar] [CrossRef]
- Dodia, S.; Edla, D.R.; Bablani, A.; Ramesh, D.; Kuppili, V. An Efficient EEG based Deceit Identification Test using Wavelet Packet Transform and Linear Discriminant Analysis. J. Neurosci. Methods 2019, 314, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Bablani, A.; Edla, D.R.; Kuppili, V. Deceit Identification Test on EEG Data Using Deep Belief Network. In Proceedings of the 2018 9th International Conference on Computing, Communication and Networking Technologies (ICCCNT), Bengaluru, India, 10–12 July 2018; pp. 1–6. Available online: https://ieeexplore.ieee.org/stamp/stamp.jsp?tp=&arnumber=8494124 (accessed on 13 May 2025).
- Houssein, E.H.; Hammad, A.; Ali, A.A. Human emotion recognition from EEG-based brain–computer interface using machine learning: A comprehensive review. Neural Comput. Appl. 2022, 34, 12527–12557. [Google Scholar]
- Min, B.K.; Suk, H.I.; Ahn, M.H.; Lee, M.H.; Lee, S.W. Individual identification using cognitive electroencephalographic neurodynamics. IEEE Trans. Inf. Forensics Secur. 2017, 12, 2159–2167. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, D.; Zhang, Y.; Zhang, P. Emotion recognition from spatiotemporal EEG representations with hybrid convolutional recurrent neural networks via wearable multi-channel headset. Comput. Commun 2020, 154, 58–65. [Google Scholar] [CrossRef]
- Varbu, K.; Muhammad, N.; Muhammad, Y. Past, present, and future of EEG-based BCI applications. Sensors 2022, 22, 3331. [Google Scholar] [CrossRef]
- Sun, S.; Zhou, J. A review of adaptive feature extraction and classification methods for EEG-based brain-computer interfaces. In Proceedings of the 2014 International Joint Conference on Neural Networks (IJCNN), Beijing, China, 6–11 July 2014; pp. 1746–1753. [Google Scholar] [CrossRef]
- Virgilio G, C.D.; Sossa A, J.H.; Antelis, J.M.; Falcon, L.E. Spiking Neural Networks applied to the classification of motor tasks in EEG signals. Neural Netw. 2020, 122, 130–143. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, C.; Wu, J.; Qin, W.; Xu, M.; Yin, E. Temporal combination pattern optimization based on feature selection method for motor imagery BCIs. Front. Hum. Neurosci. 2020, 14, 231. [Google Scholar] [CrossRef]
- Baig, M.Z.; Aslam, N.; Shum, H.P.H.; Zhang, L. Differential Evolution Algorithm as a Tool for optimal feature subset selection in motor imagery EEG. Expert Syst. Appl. 2017, 90, 184–195. [Google Scholar] [CrossRef]
- Belwafi, K.; Gannouni, S.; Aboalsamh, H.; Mathkour, H.; Belghith, A. A dynamic and self-adaptive classification algorithm for motor imagery EEG signals. J. Neurosci. Methods 2019, 327, 108346. [Google Scholar] [CrossRef]
- Lee, S.B.; Kim, H.J.; Kim, H.; Jeong, J.H.; Lee, S.W.; Kim, D.J. Comparative analysis of features extracted from EEG spatial. spectral and temporal domains for binary and multiclass motor imagery classification. Inf. Sci. 2019, 502, 190–200. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, S.; Vaish, A. Feature extraction using emprical mode decomposition for biometric system in Signal Propagation and Computer Technology (ICSPCT). In Proceedings of the 2014 International Conference on Signal Propagation and Computer Technology (ICSPCT 2014), Ajmer, India, 12–13 July 2014; pp. 283–287. Available online: https://ieeexplore.ieee.org/document/6885030 (accessed on 13 May 2025).
- Subasi, A.; Qaisar, S.M. The ensemble machine learning-based classification of motor imagery tasks in brain-computer interface. J. Heal. Eng. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Ji, N.; Ma, L.; Dong, H.; Zhang, X. EEG signals feature extraction based on DWT and EMD combined with approximate entropy. Brain Sci. 2019, 9, 201. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chum, P.; Sim, K.B. Analysis the effect of PCA for feature reduction in nonstationary EEG based motor imagery of BCI system. Optik 2014, 125, 1498–1502. [Google Scholar] [CrossRef]
- Carrera-Leon, O.; Ramirez, J.M.; Alarcon-Aquino, V.; Baker, M.; Croz-Baron, D.D.; Gomez-Gil, P. A Motor Imagery BCI Experiment Using Wavelet Analysis and Spatial Patterns Feature Extraction. In Proceedings of the 2012 Workshop On Engineering Applications, Bogota, Colombia, 2–4 May 2012; Volume 2012, pp. 18–20. [Google Scholar] [CrossRef]
- Aydemir, O.; Kayikcioglu, T. Decision tree structure based classification of EEG signals recorded during two dimensional cursormovement imagery. J. Neurosci. Methods 2014, 229, 68–75. [Google Scholar] [CrossRef]
- Khan, P.; Kader, F.; Islam, S.M.R.; Rahman, A.B.; Kamal, S.; Toha, M.U.; Kwak, K.-S. Machine learning and deep learning approaches for brain disease diagnosis: Principles and recent advances. IEEE Access 2021, 9, 37622–37655. [Google Scholar] [CrossRef]
- Naseer, N.; Qureshi, N.K.; Noori, F.M.; Hong, K.S. Analysis of different classification techniques for two-class functional near-infrared spectroscopy-based brain-computer interface. Comput. Intell. Neurosci. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Antony, M.J.; Sankaralingam, B.P.; Mahendran, R.K.; Gardezi, A.A.; Shafiq, M.; Choi, J.-G.; Hamam, H. Classification of EEG using adaptive SVM classifier with CSP and online recursive independent component analysis. Sensors 2022, 22, 7596. [Google Scholar] [CrossRef]
- Rodriguez-Bermudez, G.; Garcia-Laencina, P.J. Automatic and adaptive classification of electroencephalographic signals for brain computer interfaces. J. Med. Syst. 2012, 36 (Suppl. 1), 51–63. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Z.; Zheng, H.; Li, T.; Chen, K.; Wang, X.; Liu, C.; Xu, L.; Wu, X.; Lin, D.; et al. The combination of brain-computer interfaces and artificial intelligence: Applications and challenges. Ann. Transl. Med. 2020, 8, 712. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning Book, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 978-0387848570. Available online: https://link.springer.com/book/10.1007/978-0-387-84858-7 (accessed on 13 May 2025).
- Bishop, C.M. Pattern Recognition and Machine Learning Book; Springer: Berlin/Heidelberg, Germany, 2006; ISBN 978-0387310732. Available online: https://link.springer.com/book/9780387310732 (accessed on 13 May 2025).
- Sagee, G.S.; Hema, S. EEG feature extraction and classification in multiclass multiuser motor imagery brain computer interface u sing Bayesian Network and ANN. In Proceedings of the 2017 International Conference on Intelligent Computing, Instrumentation and Control Technologies (ICICICT), Kerala, India, 6–7 July 2017; pp. 938–943. [Google Scholar] [CrossRef]
- Orban, M.; Elsamanty, M.; Guo, K.; Zhang, S.; Yang, H. A review of brain activity and EEG-based brain–computer interfaces for rehabilitation application. Bioengineering 2022, 9, 768. [Google Scholar] [CrossRef]
- Mohamed, E.A.; Yusoff, M.Z.B.; Selman, N.K.; Malik, A.S. Enhancing EEG signals in brain computer interface using wavelet transform. Int. J. Inf. Electron. Eng. 2014, 4, 234–238. [Google Scholar] [CrossRef]
- Williams, S.C.; Horsfall, H.L.; Funnell, J.P.; Hanrahan, J.G.; Schaefer, A.T.; Muirhead, W.; Marcus, H.J. Neurosurgical team acceptability of brain–computer interfaces: A two-stage international cross-sectional survey. World Neurosurg. 2022, 164, e884–e898. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, R.; Saini, J.S.; Priyanka. Genetic algorithms tuned expert model for detection of epileptic seizures from EEG signatures. Appl. Soft Comput. 2014, 19, 8–17. [Google Scholar] [CrossRef]
- Reis, P.M.R.; Lochmann, M. Using a motion capture system for spatial localization of EEG electrodes. Front. Neurosci 2015, 9, 130. [Google Scholar] [CrossRef][Green Version]
- Michel, C.M.; Brunet, D. EEG source imaging: A practical review of the analysis steps. Front. Neurol. 2019, 10, 325. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, R.; Sun, Y.; Helian, N.; Davey, N.; Mayor, D.; Steffert, T. The correlation between EEG signals as measured in different positions on scalp varying with distance. Procedia Comput. Sci. 2018, 123, 92–97. [Google Scholar] [CrossRef]
- Fiedler, P.; Fonseca, C.; Supriyanto, E.; Zanow, F.; Haueisen, J. A high-density 256-channel cap for dry electroencephalography. Hum. Brain Mapp. 2022, 43, 1295–1308. [Google Scholar] [CrossRef]
- Do, H.; Truong, V.; George, K.; Shirke, B. EEG-Based Biometrics Utilizing Image Recognition for Patient Identification. In Proceedings of the 2019 IEEE 10th Annual Ubiquitous Computing, Electronics & Mobile Communication Conference (UEMCON), New York, NY, USA, 10–12 October 2019; pp. 591–599. [Google Scholar] [CrossRef]
- Chen, Y.; Atnafu, A.D.; Schlattner, I.; Weldtsadik, W.T.; Roh, M.-C.; Kim, H.J. A high-security EEG-based login system with RSVP stimuli and dry electrodes. IEEE Trans. Inf. Forensics Secur. 2016, 11, 2635–2647. [Google Scholar] [CrossRef]
- Del Pozo-Banos, M.; Alonso, J.B.; Ticay-Rivas, J.R.; Travieso, C.M. Electroencephalogram subject identification: A review. Expert Syst. Appl. 2014, 41, 6537–6554. [Google Scholar] [CrossRef]
- La Rocca, D.; Campisi, P.; Vegso, B.; Cserti, P.; Kozmann, G.; Babiloni, F.; Fallani, F.D.V. Human brain distinctiveness based on EEG spectral coherence connectivity. IEEE Trans. Biomed. Eng. 2014, 61, 2406–2412. [Google Scholar] [CrossRef]
- Syam, S.H.F.; Lakany, H.; Ahmad, R.B.; Conway, B.A. Comparing common average referencing to laplacian referencing in detecting imagination and intention of movement for brain computer interface. MATEC Web Conf. 2017, 140, 01028. [Google Scholar] [CrossRef][Green Version]
- Stancin, I.; Cifrek, M.; Jovic, A. A review of eeg signal features and their application in driver drowsiness detection systems. Sensors 2021, 21, 3786. [Google Scholar] [CrossRef] [PubMed]
- Lujan, M.A.; Jimeno, M.V.; Sotos, J.M.; Ricarte, J.J.; Borja, A.L. A survey on EEG signal processing techniques and machine learning: Applications to the neurofeedback of autobiographical memory deficits in schizophrenia. Electronics 2021, 10, 3037. [Google Scholar] [CrossRef]
- Vidaurre, C.; Kramer, N.; Blankertz, B.; Schlogl, A. Time domain parameters as a feature for EEG-based brain-computer interfaces. Neural Netw. 2009, 22, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhou, B.; Lv, Z.; Zhang, C. To explore the potentials of independent component analysis in brain-computer interface of motor imagery. IEEE J. Biomed. Heal. Inform. 2020, 24, 775–787. [Google Scholar] [CrossRef]
- Al-Fahoum, A.S.; Al-Fraihat, A.A. Methods of EEG signal features extraction using linear analysis in frequency and time-frequency domains. ISRN Neurosci. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Dressler, O.; Schneider, G.; Stockmanns, G.; Kochs, E.F. Awareness and the EEG power spectrum: Analysis of frequencies. Br. J. Anaesth. 2004, 93, 806–809. [Google Scholar] [CrossRef]
- Lima, C.A.M.; Coelho, A.L.V.; Eisencraft, M. Tackling EEG Signal Classification with Least Squares Support Vector Machines: A Sensitivity Analysis Study’s. Trans. Comput. Biol. Med. 2010, 40, 705–714. [Google Scholar] [CrossRef]
- Wang, K.; Tian, F.; Xu, M.; Zhang, S.; Xu, L.; Ming, D. Resting-state EEG in alpha rhythm may Be indicative of the performance of motor imagery-based brain–computer interface. Entropy 2022, 24, 1556. [Google Scholar] [CrossRef]
- Subasi, A.; Gursoy, I.M. Signal Classification Using PCA, ICA, LDA, and Support Vector Machines. Trans. Expert Syst. Appl. 2010, 37, 865–866. [Google Scholar] [CrossRef]
- Farwell, L.A. Brain fingerprinting: A comprehensive tutorial review of detection of concealed information with event-related brain potentials. Cogn. Neurodynamics 2012, 6, 115–154. [Google Scholar] [CrossRef]
- Tsenov, G.T.; Mladenov, V.M. EEG alphabet speller with a Neural Network classifier for P300 signal detection. In Proceedings of the 2018 14th Symposium on Neural Networks and Applications (NEUREL), Belgrade, Serbia, 20–21 November 2018; pp. 1–6. Available online: https://ieeexplore.ieee.org/document/8587033 (accessed on 13 May 2025).
- Nashed, N.N.; Eldawlatly, S.; Aly, G.M. A deep learning approach to single-trial classification for P300 spellers. In Proceedings of the 2018 IEEE 4th Middle East Conference on Biomedical Engineering (MECBME), Tunis, Tunisia, 28–30 March 2018; pp. 6–11. Available online: https://ieeexplore.ieee.org/document/8402397 (accessed on 13 May 2025).
- Abdulrahman, A.; Baykara, M. A Comprehensive Review for Emotion Detection Based on EEG Signals: Challenges, Applications, and Open Issues. Int. Inf. Eng. Technol. Assoc. 2021, 38, 1189–1200. [Google Scholar] [CrossRef]
- Abdulrahman, A.; Baykara, M. Feature Extraction Approach Based on Statistical Methods and Wavelet Packet Decomposition for Emotion Recognition using EEG Signals. In Proceedings of the 2021 International Conference on INnovations in Intelligent SysTems and Applications (INISTA), Kocaeli, Turkey, 25–27 August 2021. [Google Scholar] [CrossRef]
- Abdulrahman, A.; Baykara, M.; Alakus, T.B. A Novel Approach for Emotion Recognition Based on EEG Signal Using Deep Learning. Appl. Sci. 2022, 12, 10028. [Google Scholar] [CrossRef]
- Aslan, M.; Baykara, M.; Alakus, T.B. LSTMNCP: Lie detection from EEG signals with novel hybrid deep learning method. Multimed. Tools Appl. 2024, 83, 31655–31671. [Google Scholar] [CrossRef]
- Schwartz, R.; Stanovsky, G. On the Limitations of Dataset Balancing: The Lost Battle Against Spurious Correlations. arXiv 2022, arXiv:2204.12708v1. [Google Scholar] [CrossRef]
- Power, A.; Burda, Y.; Edwards, H.; Babuschkin, I.; Misra, V. Grokking: Generalization Beyond Overfitting on Small Algorithmic Datasets. arXiv 2022, arXiv:2201.02177v1. [Google Scholar] [CrossRef]
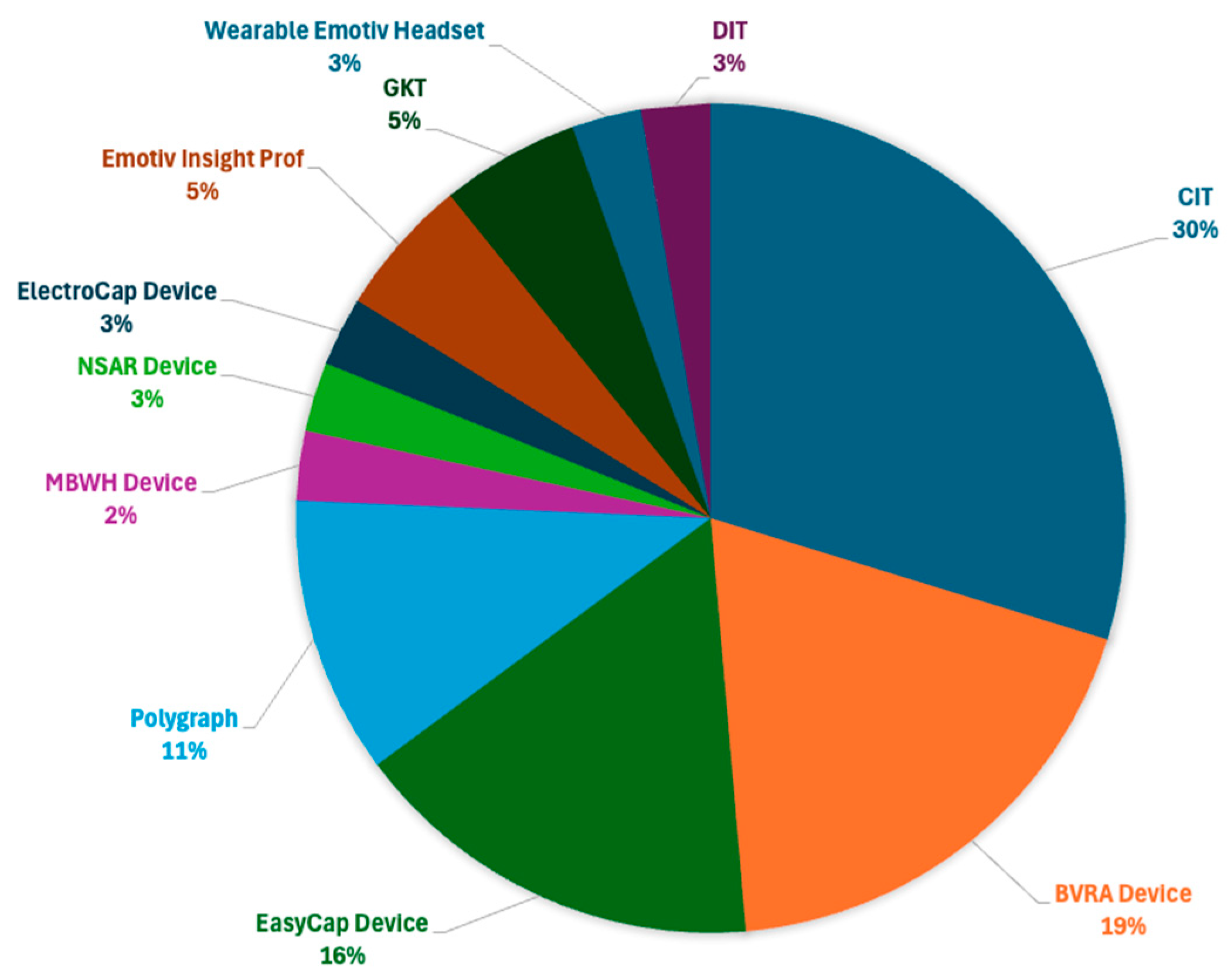


| Used Device and Protocol | Dataset (no. Participants) | Preprocessing Approaches | Feature Extraction Approaches | Classification Methods | Accuracy Scores | Referece |
|---|---|---|---|---|---|---|
| Emotive Insight Pro | LieWaves (27) | ATAR and OSW | DWT + FFT + SM | CNN, LSTM, CNN-LSTM | 99.88% | [16] |
| Polygraph and Brain Vision Recorder and Analyzer Devices with GKT and CIT | Own data (10) | BPF | DWT, FFT, and Hjorth’s Statistical Parameters (HSP) | LDA, SVM, MLFFNN, KNN, and NB | 92.40% | [17] |
| Wearable EMOTIV Headeset and Brain Vision Recorder and Analyzer Devices | Knowledge (30) | Principal Component Analysis (PCA), and Smart Signal Processing Techniques (SSPT) | DWT, entropy, amplitude, average amplitude, peak-to-peak, peak-to-peak time, and approximate entropy | SVM | 83.00% | [18] |
| Polygraph and Brain Vision Recorder and Analyzer Devices with CIT | Own data (10) | BPF Device, Binary Bat, and Conventional Bat | FFT, EMD, and WT | LDA, SVM, KNN, NB, and MLFNN | 96.80% | [19] |
| ElectroCap Device with CIT | Own data (20) | BPF Device | FFT, STFT, and Independent Component Analysis (ICA) | LC, K-means Clustering, XGBoost, SVM, and ANN | 86.00% | [20] |
| Polygraph and Brain Vision Recorder and Analyzer Devices with GKT and CIT | Own data (49) | BPF Device, and Visual Inspection of EOG data | DWT, FFT, GA, Slope Signal Alteration (SSA), and Absolute Latency/Amplitude Ratio (ALAR) | LDA | 91.83% | [21] |
| Neuroscan Synamps Amplifier Recording Device with CIT | Own data (20) | BPF Device, EEG LAB, and PS | PLV | SVM | 88.05% | [22] |
| EasyCap Device with CIT | Own data (10) | BPF Device | HSP | KNN | 96.70% | [23] |
| Mobile Brain Wear Headset Device and Emotive Insight Pro | Dryad and Lie Detection datasets (30) | BPF Device | Rectified Linear Unit (ReLU) Activation Function, DWT, and FFT | CNN | 84.44% for Dryad, and 82.00% for Lie detection datasets | [24] |
| EasyCap Device with CIT | Own data (10) | BPF Device | WT, and Gradient Descent Algorithm (GDA) | MLFFNN | 83.10% | [25] |
| EasyCap Device with CIT | Own data (33) | BPF Device | EMD + Burg’s Wavelet Decomposition (BWD) + Intrinsic Mode Functions (IMF) + Hilbert Transform (HT) | SVM | 99.44% | [26] |
| Polygraph and Brain Vision Recorder and Analyzer Devices with CIT | Own data (10) | BPF Device | CSP | Fuzzy, LDA, MLFFNN, KNN, SVM, and NB | LDA = 96.67%, MLFFNN and SVM = 98.33, and NB, and KNN = 100% | [27] |
| EasyCap Device with CIT | Own data (10) | BPF Device | WT | DBN | 81.03% | [29] |
| EasyCap and Brain Vision Recorder and Analyzer Devices with CIT | Own data (20) | BPF Device | WPT | LDA | 91.67% | [28] |
| EasyCap and Brain Vision Recorder and Analyzer Devices with CIT | Own data (10) | BPF Device | STFT, and Binary Bat | ELM | 88.30% | [8] |
| Algorithm | Avg. Time Complexity | Worst-Case Complexity | Noise Robustness | Real-Time Applicability |
|---|---|---|---|---|
| QuickSort | O(nlogn) | O(n2) | Low–Medium | Medium |
| MergeSort | O(nlogn) | O(nlogn) | Low | Medium |
| Kalman Filter | O(n) | O(n) | High | High |
| Extended Kalman Filter | O(n2) | O(n2) | High | High |
| SVM (linear) | O(n) | O(n2) | Medium–High | Medium |
| SVM (non-linear kernel) | O(n2. d) | Up to O(n3) | Medium | Low |
| CNN (Inference) | Varies (e.g., O(n2)) | Hardware dependent | High | High (if optimized) |
| LDA | O(nd2) | O(nd2) | Medium | High |
| PCA | O(nd2 + d3) | O(nd2 + d3) | Low–Medium | High |
| KNN | O(n. d) for query | O(n. d) for query | Medium–High | Low–Medium |
| K-Means | O(nkdi) | O(n2) | Low–Medium | Low–Medium |
| Naive Bayes | O(nd) | O(nd) | Medium–High | High |
| A* (A-star) Search | O(bd) | O(bd) | Medium | Medium–High |
| Random Forest (RF) | O(nlogn), t = trees | O(nlogn), t = trees | High | Medium–High |
| Logistic Regression (LR) | O(nd) | O(nd) | Medium | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taha, B.N.; Baykara, M.; Alakuş, T.B. Neurophysiological Approaches to Lie Detection: A Systematic Review. Brain Sci. 2025, 15, 519. https://doi.org/10.3390/brainsci15050519
Taha BN, Baykara M, Alakuş TB. Neurophysiological Approaches to Lie Detection: A Systematic Review. Brain Sciences. 2025; 15(5):519. https://doi.org/10.3390/brainsci15050519
Chicago/Turabian StyleTaha, Bewar Neamat, Muhammet Baykara, and Talha Burak Alakuş. 2025. "Neurophysiological Approaches to Lie Detection: A Systematic Review" Brain Sciences 15, no. 5: 519. https://doi.org/10.3390/brainsci15050519
APA StyleTaha, B. N., Baykara, M., & Alakuş, T. B. (2025). Neurophysiological Approaches to Lie Detection: A Systematic Review. Brain Sciences, 15(5), 519. https://doi.org/10.3390/brainsci15050519





