Autobiographical Memory: A Scoping Meta-Review of Neuroimaging Data Enlightens the Inconsistencies Between Theory and Experimentation
Abstract
:1. Introduction
1.1. Background and Rationale
1.2. Objectives
2. Materials and Methods
2.1. Protocol Registration and Methodology
2.2. Ethical Approval Statement
2.3. Research Questions, Eligibility Criteria, and Search Strategy
2.4. Study Selection
2.5. Data Charting
2.6. Data Analysis and Reporting of Results
3. Results
3.1. Study Inclusion
3.2. Charted Results of Individual Sources of Evidence
3.3. Analysis and Synthesis of the Results
3.3.1. The Relation Between AM, EAM, and SAM
3.3.2. Brain Regions Associated with EAM
3.3.3. Brain Regions Associated with SAM
4. Discussion
Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Date | Database | Query | Results |
|---|---|---|---|
| 3 May 2023 | PsycInfo/PsycArticles | AB autobiogr* AND AB meta-analys* AND AB (imaging or fmri) | 13 |
| 3 May 2023 | PsycInfo/PsycArticles | AB autobiogr* AND AB meta-analys* AND AB (positron or pet) | 2 |
| 3 May 2023 | PubMed | ((“autobiogr*”[Title/Abstract]) AND (meta-analys*[Title/Abstract])) AND (imaging or fmri[Title/Abstract]) | 29 |
| 3 May 2023 | PubMed | ((“autobiogr*”[Title/Abstract]) AND (meta-analys*[Title/Abstract])) AND (positron or pet[Title/Abstract]) | 2 |
| 3 May 2023 | Web of Science (all databases) | AB = (autobiogr* AND meta-analys* AND (imaging or fmri)) | 28 |
| 3 May 2023 | Web of Science (all databases) | AB = (autobiogr* AND meta-analys* AND (positron or pet)) | 0 |
| 3 May 2023 | Scopus | (TITLE-ABS-KEY (autobiogr*) AND TITLE-ABS-KEY (meta-analys*) AND TITLE-ABS-KEY (imaging OR fmri)) | 42 |
| 3 May 2023 | Scopus | (TITLE-ABS-KEY (autobiogr*) AND TITLE-ABS-KEY (meta-analys*) AND TITLE-ABS-KEY (positron OR pet)) | 8 |
References
- Baddeley, A.; Eysenck, M.W.; Anderson, M.C. Memory, 3rd ed.; Psychology Press, Routledge: London, UK, 2020. [Google Scholar] [CrossRef]
- Conway, M.A. Memory and the self. J. Mem. Lang. 2005, 53, 594–628. [Google Scholar] [CrossRef]
- Conway, M.A.; Pleydell-Pearce, C.W. The Construction of Autobiographical Memories in the Self-Memory System. Psychol. Rev. 2000, 107, 261–288. [Google Scholar] [CrossRef] [PubMed]
- McDermott, K.B.; Szpunar, K.K.; Christ, S.E. Laboratory-based and autobiographical retrieval tasks differ substantially in their neural substrates. Neuropsychologia 2009, 47, 2290–2298. [Google Scholar] [CrossRef]
- Conway, M.A.; Williams, H.L. Autobiographical memory. In Learning and Memory: A Comprehensive Reference; Byrne, J.H., Ed.; Elsevier Ltd.: Oxford, UK, 2008; pp. 893–909. [Google Scholar]
- Kim, H. A dual-system model of the brain’s default network: Self-referential processing, memory retrieval processes, and autobiographical memory retrieval. Neuroimage 2012, 61, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Tulving, E.; Craik, F.I.M. (Eds.) The Oxford Handbook of Memory; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Tulving, E. Episodic and semantic memory. In Organization of Memory; Tulving, E., Donaldson, W., Eds.; Academic Press: New York, NY, USA, 1972; pp. 381–403. [Google Scholar]
- Tulving, E. Memory and Consciousness. Can. Psychol. 1985, 26, 1–12. [Google Scholar] [CrossRef]
- Tulving, E. Episodic Memory: From Mind to Brain. Annu. Rev. Psychol. 2002, 53, 1–25. [Google Scholar] [CrossRef]
- Wheeler, M.A.; Stuss, D.T.; Tulving, E. Toward a Theory of Episodic Memory: The Frontal Lobes and Autonoetic Consciousness. Psychol. Bull. 1997, 121, 331–354. [Google Scholar] [CrossRef]
- Renoult, L.; Irish, M.; Moscovitch, M.; Rugg, D. From Knowing to Remembering: The Semantic-Episodic Distinction. Trends Cogn. Sci. 2019, 23, 1041–1057. [Google Scholar] [CrossRef]
- Levine, B.; Turner, G.R.; Tisserand, D.; Hevenor, S.J.; Graham, S.J.; McIntosh, A.R. The Functional Neuroanatomy of Episodic and Semantic Autobiographical Remembering: A Prospective Functional MRI Study. J. Cogn. Neurosci. 2004, 16, 1633–1646. [Google Scholar] [CrossRef]
- Maguire, E.A.; Mummery, C.J. Differential Modulation of a Common Memory Retrieval Network Revealed by Positron Emission Tomography. Hippocampus 1999, 9, 54–61. [Google Scholar] [CrossRef]
- Martinelli, P.; Sperduti, M.; Piolino, P. Neural Substrates of the Self-Memory System: New Insights from a Meta-Analysis. Hum. Brain Mapp. 2013, 34, 1515–1529. [Google Scholar] [CrossRef] [PubMed]
- Addis, D.R.; McIntosh, A.R.; Moscovitch, M.; Crawley, A.P.; McAndrews, M.P. Characterizing spatial and temporal features of autobiographical memory retrieval networks: A partial least squares approach. Neuroimage 2004, 23, 1460–1471. [Google Scholar] [CrossRef]
- Cabeza, R.; St Jacques, P. Functional neuroimaging of autobiographical memory. Trends Cogn. Sci. 2007, 11, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Donix, M.; Poettrich, K.; Weiss, P.H.; Werner, A.; von Kummer, R.; Fink, G.R.; Holthoff, V.A. Age-Dependent Differences in the Neural Mechanisms Supporting Long-Term Declarative Memories. Arch. Clin. Neuropsychol. 2010, 25, 383–395. [Google Scholar] [CrossRef]
- Markowitsch, H.J.; Staniloiu, A. Memory, autonoetic consciousness, and the self. Conscious. Cogn. 2011, 20, 16–39. [Google Scholar] [CrossRef]
- Piolino, P.; Desgranges, B.; Clarys, D.; Guillery-Girard, B.; Taconnat, L.; Isingrini, M.; Eustache, F. Autobiographical memory, autonoetic consciousness, and self-perspective in ageing. Psychol. Aging 2006, 21, 510–525. [Google Scholar] [CrossRef]
- Eickhoff, S.B.; Bzdok, D.; Laird, A.R.; Kurth, F.; Fox, P.T. Activation Likelihood Estimation meta-analysis revisited. Neuroimage 2011, 59, 2349–2361. [Google Scholar] [CrossRef] [PubMed]
- Ahn, E.J.; Kang, H. Introduction to systematic review and meta-analysis. Korean J. Anesthesiol. 2018, 71, 103–112. [Google Scholar] [CrossRef]
- Tawfik, G.M.; Dila, K.A.S.; Mohamed, M.Y.F.; Tam, D.N.H.; Kien, N.D.; Ahmed, A.M.; Huy, N.T. A step by step guide for conducting a systematic review and meta-analysis with simulation data. Trop. Med. Health 2019, 47, 46. [Google Scholar] [CrossRef]
- Sarrami-Foroushani, P.; Travaglia, J.; Debono, D.; Clay-Williams, R.; Braithwaite, J. Scoping Meta-Review: Introducing a New Methodology. Clin. Transl. Sci. 2015, 8, 77–81. [Google Scholar] [CrossRef]
- Peters, M.D.J.; Godfrey, C.; McInerney, P.; Munn, Z.; Tricco, A.C.; Khalil, H. Chapter 11: Scoping Reviews. In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; JBI: Adelaide, Australia, 2020. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Daudt, H.M.L.; van Mossel, C.; Scott, S.J. Enhancing the scoping study methodology: A large, inter-professional team’s experience with Aksey and O’Malley’s framework. BMC Med. Res. Methodol. 2013, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Arigo, D.; Brown, M.M.; Pasko, K.; Suls, J. Social comparison features in physical activity promotion apps: Scoping meta-review. J. Med. Internet Res. 2020, 22, e15642. [Google Scholar] [CrossRef]
- Ortiz, K.; Nash, J.; Shea, L.; Oetzel, J.; Garoutte, J.; Sanchez-Youngman, S.; Wallerstein, N. Partnerships, processes, and outcomes: A health equity–focused scoping meta-review of community-engaged scholarship. Annu. Rev. Public Health 2020, 41, 177–199. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef]
- Svoboda, E.; McKinnon, M.C.; Levine, B. The functional neuroanatomy of autobiographical memory: A meta-analysis. Neuropsychologia 2006, 44, 2189–2208. [Google Scholar] [CrossRef]
- Spreng, R.N.; Mar, R.A.; Kim, A.S.N. The Common Neural Basis of Autobiographical Memory, Prospection, Navigation, Theory of Mind, and the Default Mode: A Quantitative Meta-analysis. J. Cogn. Neurosci. 2009, 21, 489–510. [Google Scholar] [CrossRef]
- Kim, H. Encoding and Retrieval Along the Long Axis of the Hippocampus and Their Relationships with Dorsal Attention and Default Mode Networks: The HERNET Model. Hippocampus 2015, 25, 500–510. [Google Scholar] [CrossRef]
- Bréchet, L.; Grivaz, P.; Gauthier, B.; Blanke, O. Common Recruitment of Angular Gyrus in Episodic Autobiographical Memory and Bodily Self-Consciousness. Front. Behav. Neurosci. 2018, 12, 270. [Google Scholar] [CrossRef]
- Boccia, M.; Teghil, A.; Guariglia, C. Looking into recent and remote past: Meta-analytic evidence for cortical reorganization of episodic autobiographical memories. Neurosci. Biobehav. Rev. 2019, 107, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Fenerci, C.; Gurguryan, L.; Spreng, R.N.; Sheldon, S. Comparing neural activity during autobiographical memory retrieval between younger and older adults: An ALE meta-analysis. Neurobiol. Aging 2022, 119, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Stark, C.E.; Squire, L.R. When zero is not zero: The problem of ambiguous baseline conditions in fMRI. Proc. Natl. Acad. Sci. USA 2001, 98, 12760–12766. [Google Scholar] [CrossRef]
- Fox, P.T.; Parsons, L.M.; Lancaster, J.L. Beyond the single study: Function/location metanalysis in cognitive neuroimaging. Curr. Opin. Neurobiol. 1998, 8, 178–187. [Google Scholar] [CrossRef]
- Turkeltaub, P.E.; Eden, G.F.; Jones, K.M.; Zeffiro, T.A. Meta-analysis of the functional neuroanatomy of single-word reading: Method and validation. Neuroimage 2002, 16, 765–780. [Google Scholar] [CrossRef]
- Laird, A.R.; Fox, P.M.; Price, C.J.; Glahn, D.C.; Uecker, A.M.; Lancaster, J.L.; Turkeltaub, P.E.; Kochunov, P.; Fox, P.T. ALE meta-analysis: Controlling the false discovery rate and performing statistical contrasts. Hum. Brain Mapp. 2005, 25, 155–164. [Google Scholar] [CrossRef]
- Eickhoff, S.B.; Laird, A.R.; Grefkes, C.; Wang, L.E.; Zilles, K.; Fox, P.T. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 2009, 30, 2907–2926. [Google Scholar] [CrossRef]
- Conway, M.A.; Justice, L.V.; D’Argembeau, A. The self-memory system revisited: Past, present, and future. In The Organization and Structure of Autobiographical Memory; Mace, J.H., Ed.; Oxford University Press: Oxford, UK, 2019; pp. 28–51. [Google Scholar]
- Renoult, L.; Davidson, P.S.R.; Palombo, D.J.; Moscovitch, M.; Levine, B. Personal semantics: At the crossroads of semantic and episodic memory. Trends Cogn. Sci. 2012, 16, 550–558. [Google Scholar] [CrossRef]
- Addis, D.R.; Szpunar, K.K. Beyond the episodic–semantic continuum: The multidimensional model of mental representations. Phil. Trans. R. Soc. B 2024, 379, 20230408. [Google Scholar] [CrossRef]
- Conway, M.A.; Jobson, L. On the nature of autobiographical memory. In Understanding Autobiographical Memory: Theories and Approaches; Berntsen, D., Rubin, D.C., Eds.; Cambridge University Press: Cambridge, UK, 2012; pp. 54–69. [Google Scholar]
- Kalenzaga, S.; Sperduti, M.; Anssens, A.; Martinelli, P.; Devauchelle, A.D.; Gallarda, T.; Delhommeau, M.; Lion, S.; Amado, I.; Krebs, M.O.; et al. Episodic memory and self-reference via semantic autobiographical memory: Insights from an fMRI study in younger and older adults. Front. Behav. Neurosci. 2015, 8, 449. [Google Scholar] [CrossRef]
- Compère, L.; Charron, S.; Gallarda, T.; Rari, E.; Lion, S.; Nys, M.; Anssens, A.; Coussinoux, S.; Machefaux, S.; Oppenheim, C.; et al. Gender identity better than sex explains individual differences in episodic and semantic components of autobiographical memory: An fMRI study. Neuroimage 2021, 225, 117507. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.B. The self: As a construct in psychology and neuropsychological evidence for its multiplicity. WIREs Cogn. Sci. 2010, 1, 172–183. [Google Scholar] [CrossRef]
- Grilli, M.D.; Bercel, J.J.; Wank, A.A.; Rapcsak, S.Z. The contribution of the left anterior ventrolateral temporal lobe to the retrieval of personal semantics. Neuropsychologia 2018, 117, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Tanguay, A.F.N.; Palombo, D.J.; Love, B.; Glikstein, R.; Davidson, P.S.R.; Renoult, L. The shared and unique neural correlates of personal semantic, general semantic, and episodic memory. eLife 2022, 12, e83645. [Google Scholar] [CrossRef] [PubMed]
- Foster, B.L.; Koslov, S.R.; Aponik-Gremillon, L.; Monko, M.E.; Hayden, B.Y.; Heilbronner, S.R. A tripartite view of the posterior cingulate cortex. Nat. Rev. Neurosci. 2023, 24, 173–189. [Google Scholar] [CrossRef]
- Leech, R.; Smallwood, J. The posterior cingulate cortex: Insights from structure and function. In Handbook of Clinical Neurology; Vogt, B.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 166, pp. 73–85. [Google Scholar] [CrossRef]
- Leech, R.; Sharp, D.J. The role of the posterior cingulate cortex in cognition and disease. Brain 2014, 137, 12–32. [Google Scholar] [CrossRef]
- Brewer, J.A.; Garrison, K.A.; Whitfield-Gabrieli, S. What about the “self” is processed in the posterior cingulate cortex? Front. Hum. Neurosci. 2013, 7, 647. [Google Scholar] [CrossRef]
- Bird, C.M.; Burgess, N. The hippocampus and memory: Insights from spatial processing. Nat. Rev. Neurosci. 2008, 9, 182–194. [Google Scholar] [CrossRef]
- Holland, A.C.; Addis, D.R.; Kensinger, E.A. The neural correlates of specific versus general autobiographical memory construction and elaboration. Neuropsychologia 2011, 49, 3164–3177. [Google Scholar] [CrossRef]
- Maguire, E.A.; Mullally, S.L. The Hippocampus: A Manifesto for Change. J. Exp. Psychol. Gen. 2013, 142, 1180–1189. [Google Scholar] [CrossRef]
- Opitz, B. Memory Function and the Hippocampus. Front. Neurol. Neurosci. 2014, 34, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Aminoff, E.M.; Kveraga, K.; Bar, M. The role of the parahippocampal cortex in cognition. Trends Cogn. Sci. 2013, 17, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Cavanna, A.E.; Trimble, M.R. The precuneus: A review of its functional anatomy and behavioural correlates. Brain 2006, 129, 564–583. [Google Scholar] [CrossRef] [PubMed]
- Utevsky, A.V.; Smith, D.V.; Huettel, S.A. Precuneus Is a Functional Core of the Default-Mode Network. J. Neurosci. 2014, 34, 932–940. [Google Scholar] [CrossRef]
- Carter, R.M.; Huettel, S.A. A nexus model of the temporal-parietal junction. Trends Cogn. Sci. 2013, 17, 328–336. [Google Scholar] [CrossRef]
- Quesque, F.; Brass, M. The Role of the Temporoparietal Junction in Self-Other Distinction. Brain Topogr. 2019, 32, 943–955. [Google Scholar] [CrossRef]
- Seghier, M.L. The Angular Gyrus: Multiple Functions and Multiple Subdivisions. Neuroscientist 2013, 19, 43–61. [Google Scholar] [CrossRef]
- Ramanan, S.; Piguet, O.; Irish, M. Rethinking the Role of the Angular Gyrus in Remembering the Past and Imagining the Future: The Contextual Integration Model. Neuroscientist 2018, 24, 342–352. [Google Scholar] [CrossRef]
- Etkin, A.; Egner, T.; Kalisch, R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 2011, 15, 85–93. [Google Scholar] [CrossRef]
- Euston, D.R.; Gruber, A.J.; McNaughton, B.L. The Role of Medial Prefrontal Cortex in Memory and Decision Making. Neuron 2012, 76, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.D.; Haxby, J.V.; Heatherton, T.F. The representation of self and person knowledge in the medial prefrontal cortex. WIREs Cogn. Sci. 2012, 3, 451–470. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, M.D.; Straccia, M.A.; Meyer, M.L.; Du, M.; Tan, K.M. Social, self, (situational), and affective processes in medial prefrontal cortex (MPFC): Causal, multivariate, and reverse inference evidence. Neurosci. Biobehav. Rev. 2019, 99, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Vogt, B.A. The cingulate cortex in neurologic diseases: History, Structure, Overview. In Handbook of Clinical Neurology; Vogt, B.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 166, pp. 3–21. [Google Scholar] [CrossRef]
- Thome, J.; Terpou, J.; McKinnon, M.C.; Lanius, R.A. The neural correlates of trauma-related autobiographical memory in posttraumatic stress disorder: A meta-analysis. Depress. Anxiety 2019, 37, 321–345. [Google Scholar] [CrossRef]
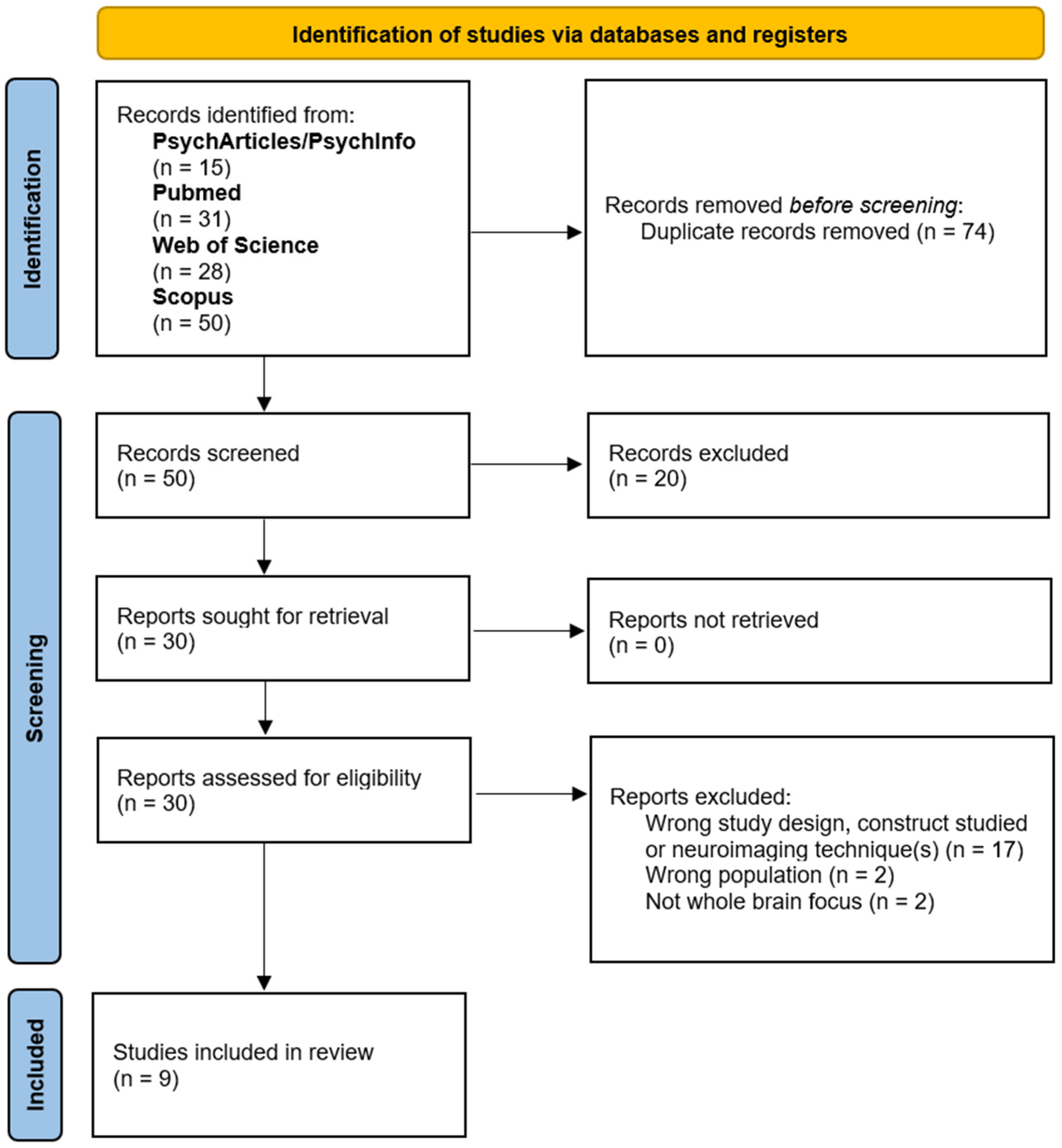
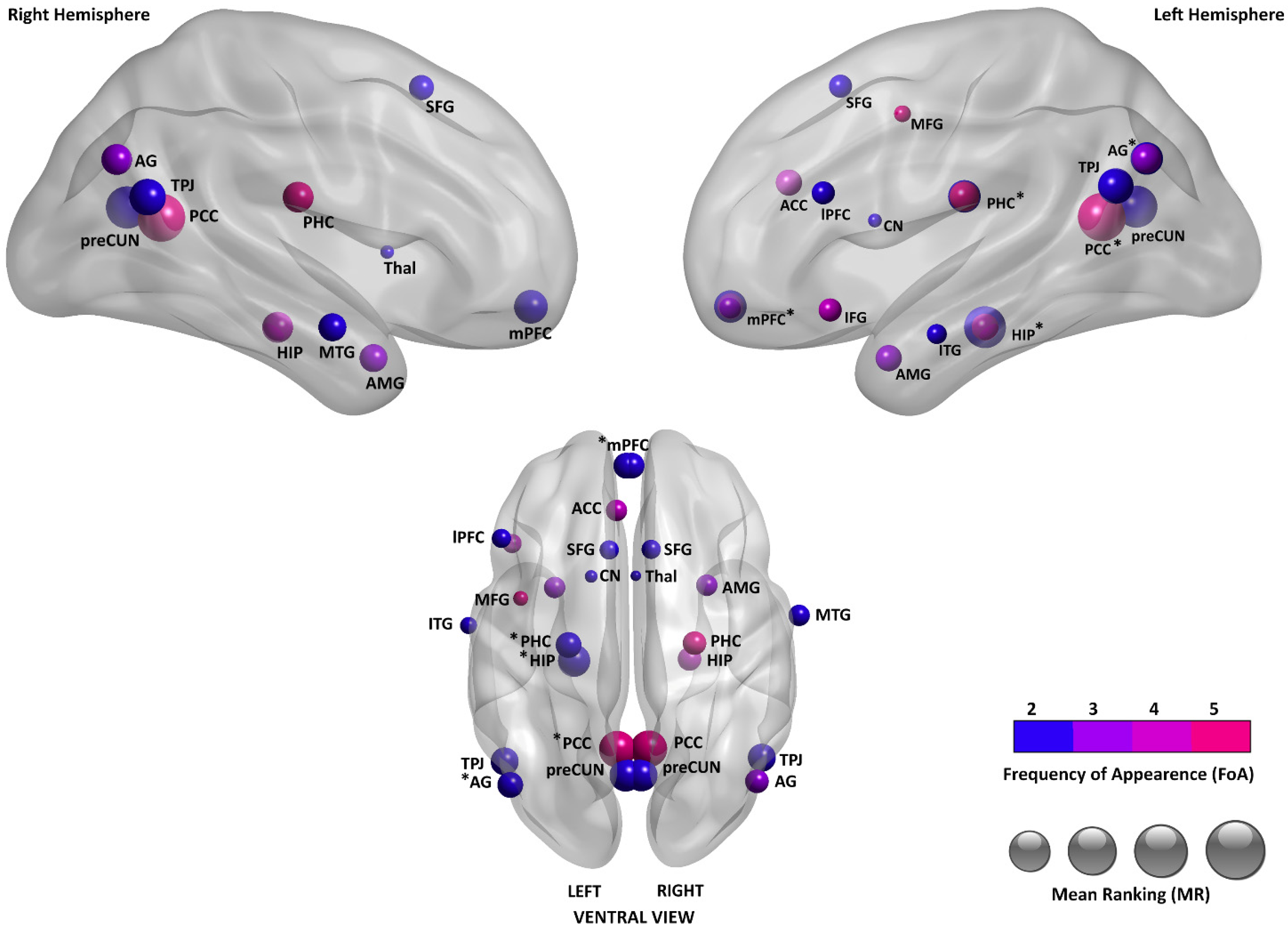
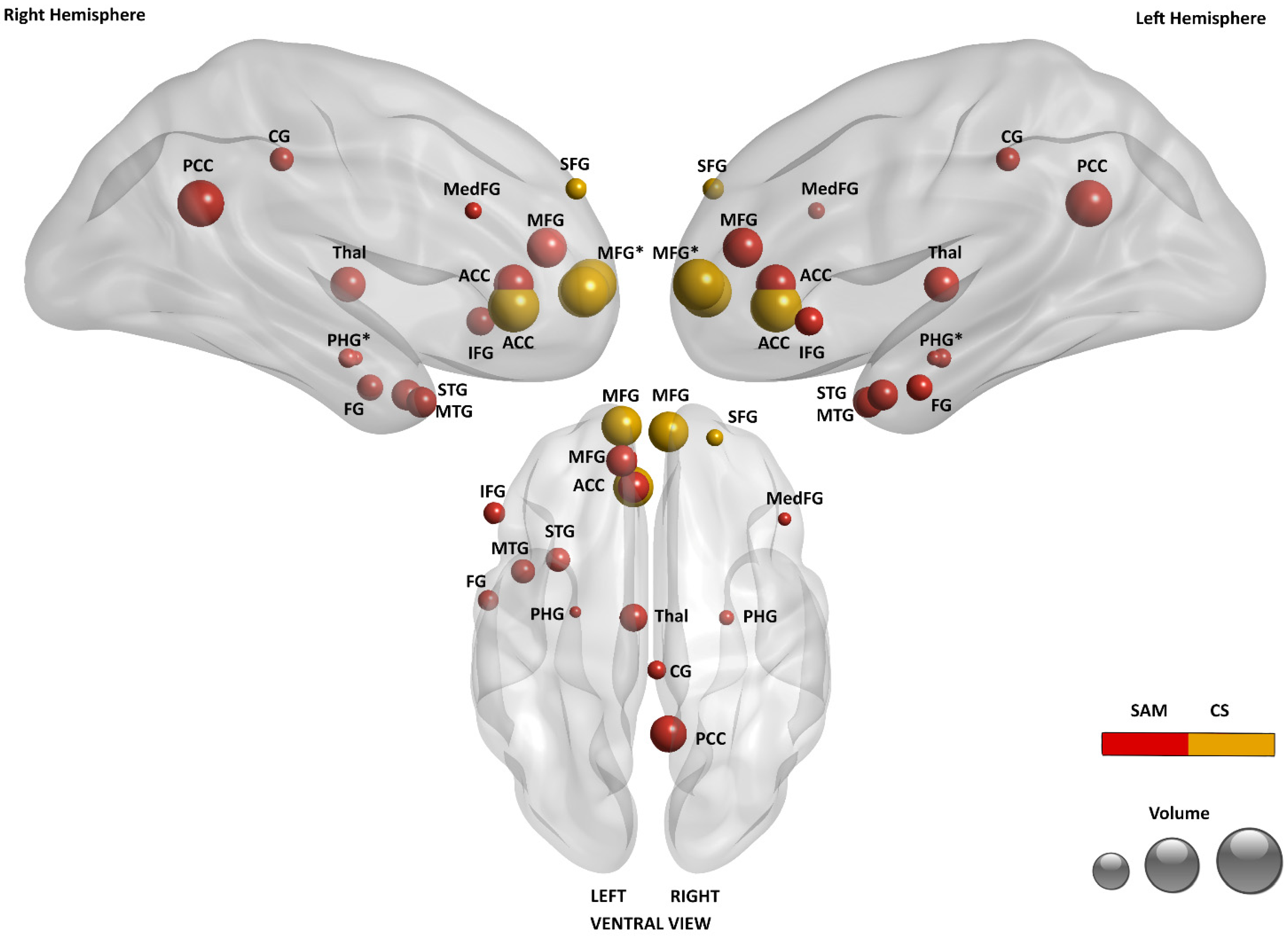
| ID | Author(s) | Year | Neuroimaging Technique(s) | Memory Construct(s) | Total Number of Participants |
|---|---|---|---|---|---|
| 1 | Svoboda et al. [32] | 2006 | PET, fMRI | AM | 243 |
| 2 | McDermott et al. [4] | 2009 | PET, fMRI | AM | n/a |
| 3 | Spreng et al. [33] | 2009 | PET, fMRI | AM | 228 |
| 4 | Kim [6] | 2012 | PET, fMRI | AM | 494 |
| 5 | Martinelli et al. [15] | 2013 | PET, fMRI | EAM, SAM | 153, 186 |
| 6 | Kim [34] | 2015 | PET, fMRI | AM | 656 |
| 7 | Bréchet et al. [35] | 2018 | PET, fMRI | EAM, SAM | 813, 396 |
| 8 | Boccia et al. [36] | 2019 | fMRI | EAM | 1409 |
| 9 | Fenerci et al. [37] | 2022 | PET, fMRI | AM | 656 |
| ID | Experimental Conditions (AM Retrieval) | Control Conditions (Baseline) | Procedure | Template | Brain Areas |
|---|---|---|---|---|---|
| 1 | AM recall (not specified if cued or free) | AM retrieval Distracting non-memory tasks Resting state Semantic memory retrieval | “effect-location” method | TAL | mPFC; vlPFC; OFC; FEF; M1; ACC; RSC; PCC; PreCUN; TPJ; MTL; FFG; TL; INS; OL; AMG; BG; Thal; Brainstem; CBM. |
| 2 | Cued recall (visual; auditory; true/false judgments) | Distracting nonmemory tasks Resting state Semantic memory retrieval | ALE method | MNI | Left mPFC; Left ACC; Left PMC; Left IFG; Left MFG; Bil AG; Bil PHC; Right aHIP; Left PCC; Thal; CN. |
| 3 | Cued recall (visual; auditory; true/false judgments) | Distracting nonmemory tasks Semantic memory retrieval | ALE method | TAL | Bil preCUN; Bil PCC; Bil HIP; Bil PHC; Bil AMG; Bil TPJ; Left mPFC; Left ACC; Left STS; Left MTG; Left ITG; Bil vlPFC; Bil TL; Bil MFG; Left lPFC; Left FP; Right TP; Right STS; Right MTG; Left TP; Left OL; Left dlPFC; Right Thal; Left SFG. |
| 4 | Cued recall (visual; auditory; true/false judgments) AM visualization Free AM retrieval | Distracting nonmemory task Laboratory-based episodic memory retrieval Resting state Semantic memory retrieval | ALE method | TAL | Bil amPFC; Left SMA; Left MFG; Left IFG; Bil INS; Bil HIP; Bil PHC; Bil AMG; Bil LTC; Bil PCC; Left IPL; Right SOG; Left Thal; Left CN. |
| 6 | Cued recall (visual; auditory) Free AM retrieval | Distracting nonmemory task Laboratory-based episodic memory retrieval Resting state Semantic memory retrieval | ALE method | TAL | Bil PCC; Bil RSC; Bil AMG; Bil HIP; Bil PHC; Bil AG; Bil amPFC; Left aLTC; Bil SFG; Left IFG; Left MFG; Right Thal; Left CN. |
| 9 | AM recall (not specified if cued or free) | AM retrieval Distracting nonmemory tasks Resting state Semantic memory retrieval | ALE method | MNI | ----younger---- Left PCC; Left HIP; Left AG; Left mPFC; Left lPFC ----older---- Bil HIP; Bil AG; Right MTG; Left PCC; Bil mPFC. |
| ID | Experimental Conditions (EAM Retrieval) | Control Conditions (Baseline) | Procedure | Template | Brain Area |
|---|---|---|---|---|---|
| 5 | EAM recall (not specified if cued or free) | Laboratory-based episodic memory retrieval Memory tasks Semantic memory retrieval | ALE | TAL | Bil PHC; Bil CBM; Left HIP; Bil preCUN; Left MTG; Right PCC; Left MFG. |
| 7 | EAM recall (not specified if cued or free) | Distracting nonmemory tasks Memory tasks Resting state Semantic memory retrieval | ALE | MNI | Bil TPJ; Bil PCC; Left ACC; Left PHC; Left ITG; Left IFG; Bil SFG; Left MFG. |
| 8 | Cued recall (auditory; visual; olfactory) Generation of mental images True/false judgements | Distracting nonmemory tasks Laboratory-based episodic memory retrieval Other EAM conditions (specific vs. general; recent vs. remote; and vivid vs. nonvivid) Semantic memory retrieval | ALE | MNI | Bil PCC; Left PHC; Left AG; Right PHC; Left ACC; Left vmPFC; Right AG; Right CBM; Right MTG. |
| ID | Experimental Conditions (EAM Retrieval) | Control Conditions (Baseline) | Procedure | Template | Brain Area |
|---|---|---|---|---|---|
| 5 | SAM recall (not specified if cued or free): retrieval of general personal events or personal information, no self-trait judgments | Laboratory-based episodic memory | ALE | TAL | Right PCC; Left ACC; Bil MFG; Thal; Left STG; Left MTG; Left IFG; Left FFG; Bil PHC. |
| 7 | SAM recall (not specified if cued or free): retrieval of general personal events; personal information or also self-trait judgments | Nonpersonal semantic memory | ALE | MNI | Bil ACC; Bil PCC. |
| Mean Rank (MR) | Frequency of Appearance (FoA) | Area Name and Laterality |
|---|---|---|
| 1.2 | 5 | Bil PCC |
| 1.33 | 3 | Left PCC |
| 1.5 | 2 | Left HIP |
| 1.5 | 2 | Bil PreCUN |
| 2.5 | 2 | Bil TPJ |
| 3 | 2 | Left AG |
| 3 | 2 | Bil amPFC |
| 3 | 2 | Left PHC |
| 4 | 3 | Bil AG |
| 4 | 4 | Bil HIP |
| 4 | 5 | Bil PHC |
| 4.67 | 3 | Left mPFC |
| 4.75 | 4 | Left ACC |
| 5 | 3 | Bil AMG |
| 5 | 2 | Right MTG |
| 5.5 | 2 | Bil SFG |
| 6.25 | 4 | Left IFG |
| 6.5 | 2 | Left lPFC |
| 7 | 2 | Left ITG |
| 8 | 5 | Left MFG |
| 10 | 2 | Left CN |
| 11 | 2 | Right Thal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donarelli, E.; Civilotti, C.; Di Fini, G.; Gandino, G.; Celeghin, A. Autobiographical Memory: A Scoping Meta-Review of Neuroimaging Data Enlightens the Inconsistencies Between Theory and Experimentation. Brain Sci. 2025, 15, 515. https://doi.org/10.3390/brainsci15050515
Donarelli E, Civilotti C, Di Fini G, Gandino G, Celeghin A. Autobiographical Memory: A Scoping Meta-Review of Neuroimaging Data Enlightens the Inconsistencies Between Theory and Experimentation. Brain Sciences. 2025; 15(5):515. https://doi.org/10.3390/brainsci15050515
Chicago/Turabian StyleDonarelli, Edoardo, Cristina Civilotti, Giulia Di Fini, Gabriella Gandino, and Alessia Celeghin. 2025. "Autobiographical Memory: A Scoping Meta-Review of Neuroimaging Data Enlightens the Inconsistencies Between Theory and Experimentation" Brain Sciences 15, no. 5: 515. https://doi.org/10.3390/brainsci15050515
APA StyleDonarelli, E., Civilotti, C., Di Fini, G., Gandino, G., & Celeghin, A. (2025). Autobiographical Memory: A Scoping Meta-Review of Neuroimaging Data Enlightens the Inconsistencies Between Theory and Experimentation. Brain Sciences, 15(5), 515. https://doi.org/10.3390/brainsci15050515







