Comorbidity Between Hikikomori and Autistic Traits May Be Identified as a Phenotypical Presentation Characterized by Greater Severity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sample and Procedures
2.2. Measures
2.2.1. Adult Autism Spectrum Questionnaire (AdAS Spectrum)
2.2.2. Hikikomori Questionnaire—25 (HQ—25)
2.3. Statistical Analysis
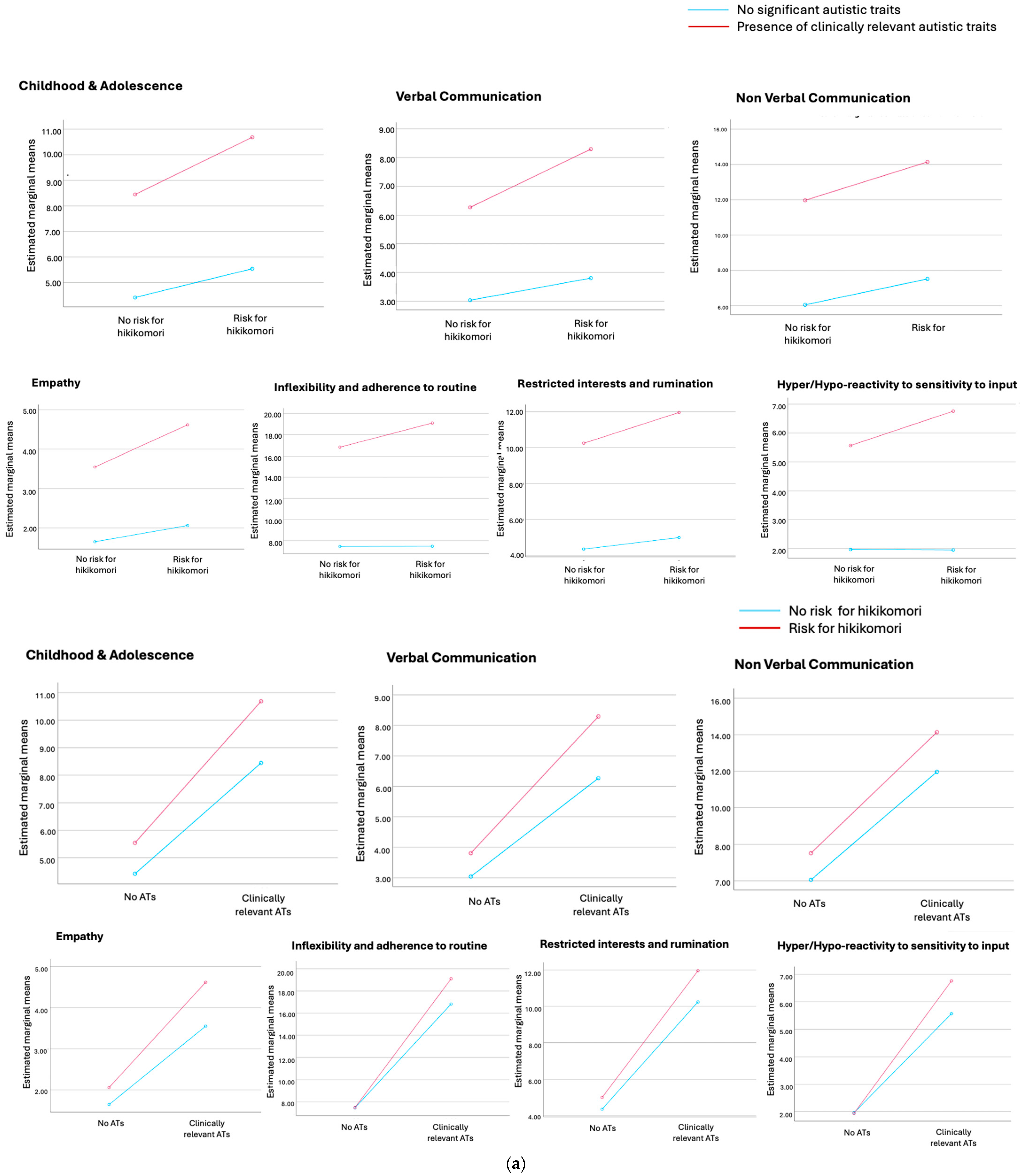
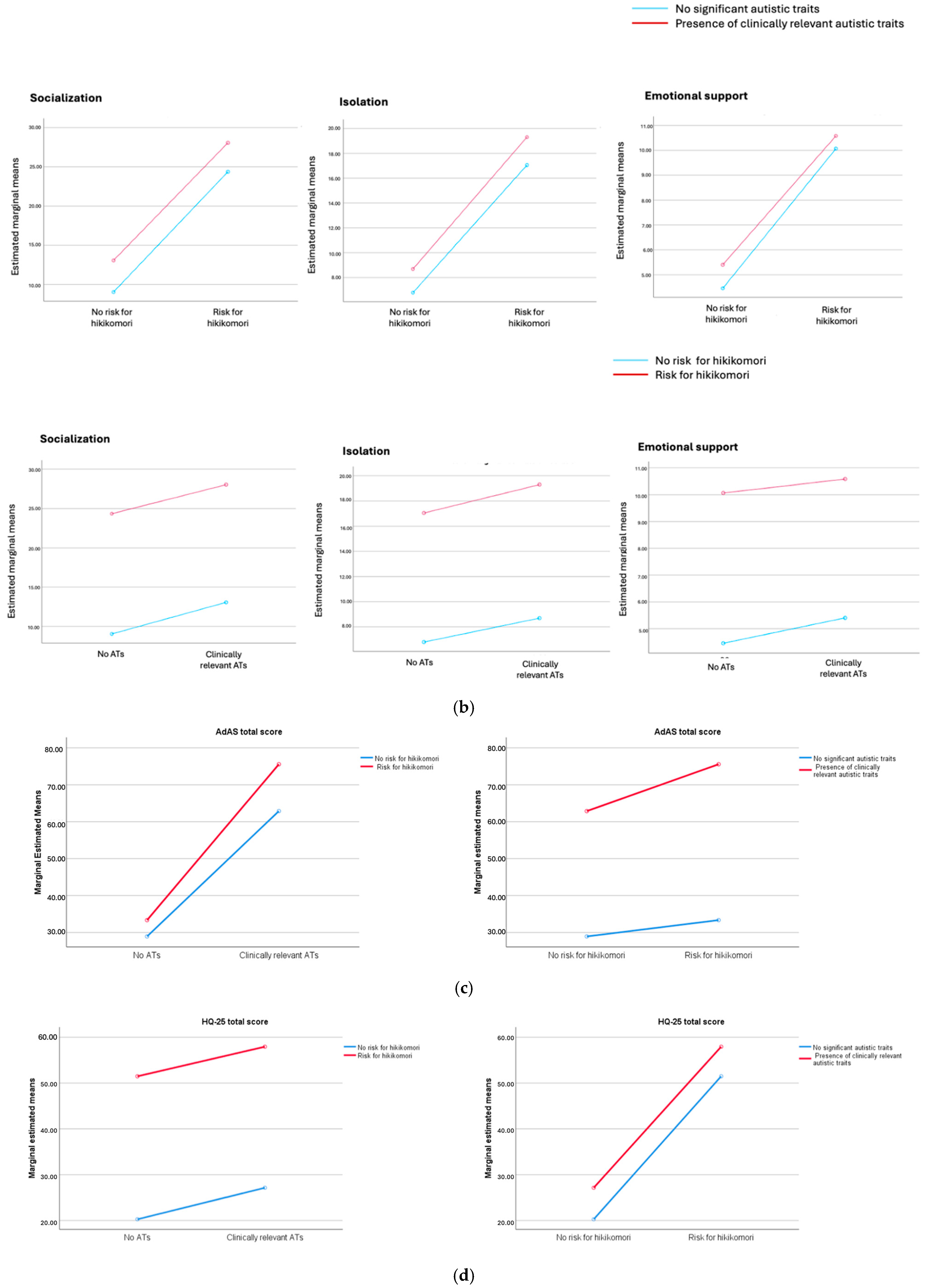
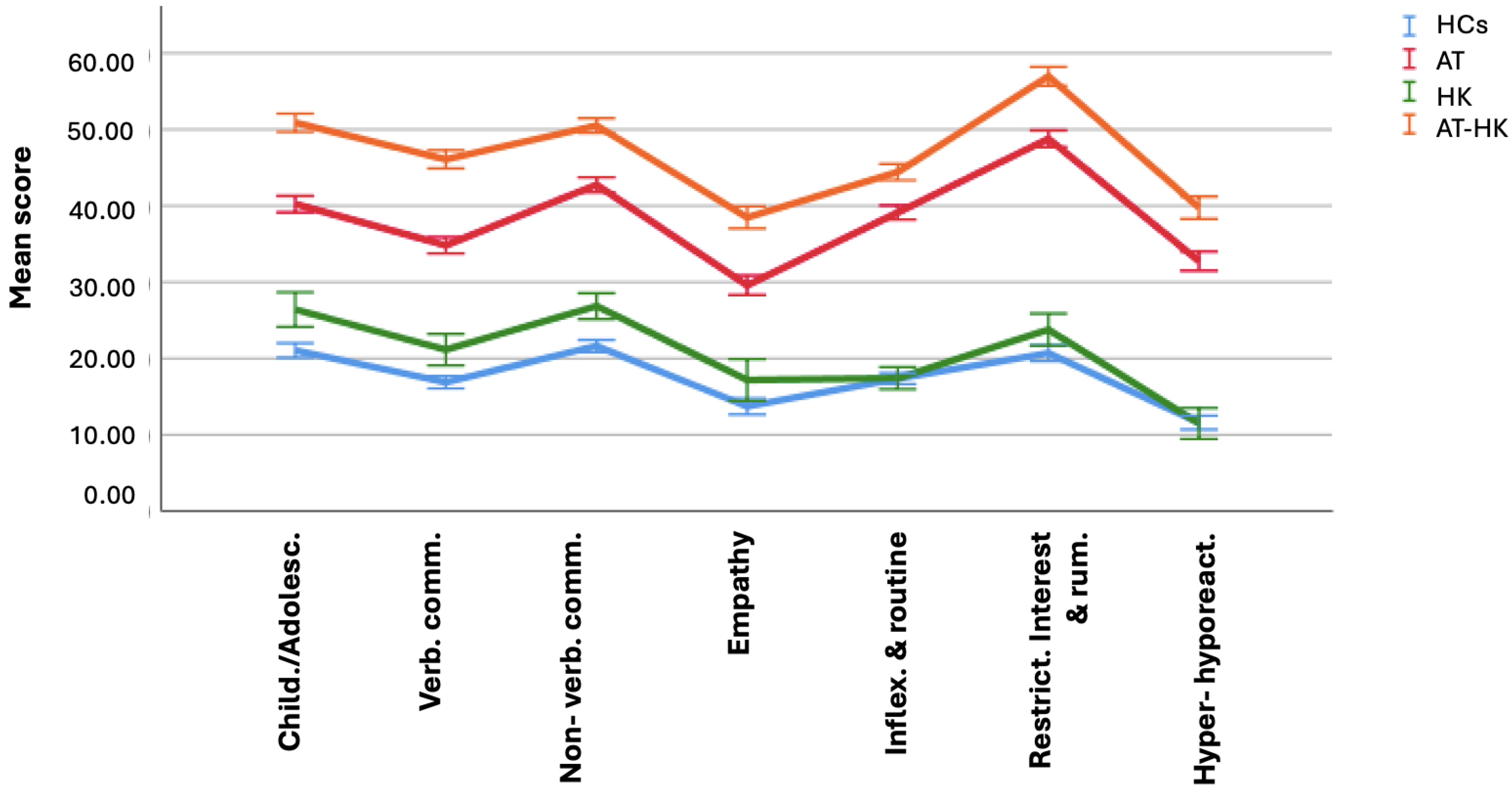
3. Results
- A total of 550 subjects without clinically relevant ATs and at no risk of hikikomori (HCs);
- A total of 118 subjects with significant hikikomori tendencies but without significant ATs (HKs);
- A total of 818 subjects with significant ATs but without hikikomori (ATs);
- A total of 821 subjects who had significant ATs and also showed hikikomori tendencies (AT-HKs).
4. Discussion
Limits
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kato, T.A.; Katsuki, R.; Kubo, H.; Shimokawa, N.; Sato-Kasai, M.; Hayakawa, K.; Kuwano, N.; Umene-Nakano, W.; Tateno, M.; Setoyama, D.; et al. Development and validation of the 22-item Tarumi’s Modern-Type Depression Trait Scale: Avoidance of Social Roles, Complaint, and Low Self-Esteem (TACS-22). Psychiatry Clin. Neurosci. 2019, 73, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.A.; Kanba, S.; Teo, A.R. A 39-Year-Old “Adultolescent”: Understanding Social Withdrawal in Japan. Am. J. Psychiatry 2016, 173, 112–114. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.A.; Kanba, S.; Teo, A.R. Defining pathological social withdrawal: Proposed diagnostic criteria for hikikomori. World Psychiatry 2020, 19, 116–117. [Google Scholar] [CrossRef]
- Kato, T.A.; Tateno, M.; Shinfuku, N.; Fujisawa, D.; Teo, A.R.; Sartorius, N.; Akiyama, T.; Ishida, T.; Choi, T.Y.; Balhara, Y.P.; et al. Does the ‘hikikomori’ syndrome of social withdrawal exist outside Japan? A preliminary international investigation. Soc. Psychiatry Psychiatr. Epidemiol. 2012, 47, 1061–1075. [Google Scholar] [CrossRef]
- Liu, L.L.; Li, T.M.; Teo, A.R.; Kato, T.A.; Wong, P.W. Harnessing Social Media to Explore Youth Social Withdrawal in Three Major Cities in China: Cross-Sectional Web Survey. JMIR Ment. Health 2018, 5, e34. [Google Scholar] [CrossRef]
- Wong, J.C.M.; Wan, M.J.S.; Kroneman, L.; Kato, T.A.; Lo, T.W.; Wong, P.W.C.; Chan, G.H. Hikikomori Phenomenon in East Asia: Regional Perspectives, Challenges, and Opportunities for Social Health Agencies. Front. Psychiatry 2019, 10, 512. [Google Scholar] [CrossRef]
- Harding, C. Hikikomori. Lancet Psychiatry 2018, 5, 28–29. [Google Scholar] [CrossRef]
- Orsolini, L.; Bellagamba, S.; Volpe, U.; Kato, T.A. Hikikomori and modern-type depression in Italy: A new phenotypical trans-cultural characterization? Int. J. Soc. Psychiatry 2022, 68, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.A.; Kanba, S.; Teo, A.R. Hikikomori: Multidimensional understanding, assessment, and future international perspectives. Psychiatry Clin. Neurosci. 2019, 73, 427–440. [Google Scholar] [CrossRef]
- Uchida, Y.; Norasakkunkit, V. The NEET and Hikikomori spectrum: Assessing the risks and consequences of becoming culturally marginalized. Front. Psychol. 2015, 6, 1117. [Google Scholar] [CrossRef]
- Carpita, B.; Bonelli, C.; Giovannoni, F.; Parri, F.; Gambini, M.; Nardi, B.; Amatori, G.; Cremone, I.M.; Pini, S.; Dell’Osso, L. Case report: Hikikomori syndrome in Italy and its link with autistic traits and internet gaming disorder. Front. Psychiatry 2024, 15, 1378572. [Google Scholar] [CrossRef]
- World Health Organization. ICD-11: International Classification of Diseases, 11th ed.; World Health Organization: Geneva, Switzerland, 2022.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2022. [Google Scholar]
- Koyama, A.; Miyake, Y.; Kawakami, N.; Tsuchiya, M.; Tachimori, H.; Takeshima, T.; World Mental Health Japan Survey Group 2002–2006. Lifetime prevalence, psychiatric comorbidity and demographic correlates of “hikikomori” in a community population in Japan. Psychiatry Res. 2010, 176, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Japan Cabinet Office. Wakamono No Seikatsu ni Kansuru Chousa-Houkokusho [Investigation on Life of Youth]; Japan Cabinet Office: Tokyo, Japan, 2016.
- Nonaka, S.; Takeda, T.; Sakai, M. Who are hikikomori? Demographic and clinical features of hikikomori (prolonged social withdrawal): A systematic review. Aust. N. Z. J. Psychiatry 2022, 56, 1542–1554. [Google Scholar] [CrossRef]
- Kondo, N.; Sakai, M.; Kuroda, Y.; Kiyota, Y.; Kitabata, Y.; Kurosawa, M. General condition of hikikomori (prolonged social withdrawal) in Japan: Psychiatric diagnosis and outcome in mental health welfare centres. Int. J. Soc. Psychiatry 2013, 59, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Tateno, M.; Park, T.W.; Kato, T.A.; Umene-Nakano, W.; Saito, T. Hikikomori as a possible clinical term in psychiatry: A questionnaire survey. BMC Psychiatry 2012, 12, 169. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N. Shishunki no hikikomori to hattatsu shougai [social withdrawal in adolescence and developmental disorders]. Jpn. J. Psychosom. Med. 2010, 50, 285. [Google Scholar]
- Yamada, M.; Kato, T.A.; Katsuki, R.I.; Yokoi, H.; Igarashi, M.; Komine, Y.; Kamata, Y.; Kato, N.; Iwanami, A.; Ohta, H. Pathological social withdrawal in autism spectrum disorder: A case control study of hikikomori in Japan. Front. Psychiatry 2023, 14, 1114224. [Google Scholar] [CrossRef]
- Dell’Osso, L.; Dalle Luche, R.; Maj, M. Adult autism spectrum as a transnosographic dimension. CNS Spectr. 2016, 21, 131–133. [Google Scholar] [CrossRef]
- Carpita, B.; Nardi, B.; Bonelli, C.; Massimetti, E.; Amatori, G.; Cremone, I.M.; Pini, S.; Dell’Osso, L. Presence and correlates of autistic traits among patients with social anxiety disorder. Front. Psychiatry 2024, 14, 1320558. [Google Scholar] [CrossRef]
- Dell’Osso, L.; Cremone, I.M.; Carpita, B.; Fagiolini, A.; Massimetti, G.; Bossini, L.; Vita, A.; Barlati, S.; Carmassi, C.; Gesi, C. Correlates of autistic traits among patients with borderline personality disorder. Compr. Psychiatry 2018, 83, 7–11. [Google Scholar] [CrossRef]
- Dell’Osso, L.; Amatori, G.; Bonelli, C.; Nardi, B.; Massimetti, E.; Cremone, I.M.; Carpita, B. Autistic traits underlying social anxiety, obsessive-compulsive, and panic disorders. CNS Spectr. 2024, 1–34. [Google Scholar] [CrossRef]
- Ishizuka, K.; Ishiguro, T.; Nomura, N.; Inada, T. Autistic traits as predictors of persistent depression. Eur. Arch. Psychiatry Clin. Neurosci. 2022, 272, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Richards, G.; Kenny, R.; Griffiths, S.; Allison, C.; Mosse, D.; Holt, R.; O’Connor, R.C.; Cassidy, S.; Baron-Cohen, S. Autistic traits in adults who have attempted suicide. Mol. Autism 2019, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Dell’Osso, L.; Nardi, B.; Bonelli, C.; Amatori, G.; Pereyra, M.A.; Massimetti, E.; Cremone, I.M.; Pini, S.; Carpita, B. Autistic Traits as Predictors of Increased Obsessive-Compulsive Disorder Severity: The Role of Inflexibility and Communication Impairment. Brain Sci. 2024, 14, 64. [Google Scholar] [CrossRef]
- Marazziti, D.; Abelli, M.; Baroni, S.; Carpita, B.; Piccinni, A.; Dell’Osso, L. Recent findings on the pathophysiology of social anxiety disorder. Clin. Neuropsychiatry 2014, 11, 91–100. [Google Scholar]
- Dell’Osso, L.; Carpita, B.; Muti, D.; Morelli, V.; Salarpi, G.; Salerni, A.; Scotto, J.; Massimetti, G.; Gesi, C.; Ballerio, M.; et al. Mood symptoms and suicidality across the autism spectrum. Compr. Psychiatry 2019, 91, 34–38. [Google Scholar] [CrossRef]
- Carpita, B.; Muti, D.; Muscarella, A.; Dell’Oste, V.; Diadema, E.; Massimetti, G.; Signorelli, M.S.; Fusar Poli, L.; Gesi, C.; Aguglia, E.; et al. Sex Differences in the Relationship between PTSD Spectrum Symptoms and Autistic Traits in a Sample of University Students. Clin. Pract. Epidemiol. Ment. Health 2019, 15, 110–119. [Google Scholar] [CrossRef]
- Dell’Osso, L.; Lorenzi, P.; Carpita, B. Autistic Traits and Illness Trajectories. Clin. Pract. Epidemiol. Ment. Health 2019, 15, 94–98. [Google Scholar] [CrossRef]
- Flegenheimer, C.; Scherf, K.S. College as a Developmental Context for Emerging Adulthood in Autism: A Systematic Review of What We Know and Where We Go from Here. J. Autism Dev. Disord. 2022, 52, 2075–2097. [Google Scholar] [CrossRef]
- Dell’Osso, L.; Gesi, C.; Massimetti, E.; Cremone, I.M.; Barbuti, M.; Maccariello, G.; Moroni, I.; Barlati, S.; Castellini, G.; Luciano, M.; et al. Adult Autism Subthreshold Spectrum (AdAS Spectrum): Validation of a questionnaire investigating subthreshold autism spectrum. Compr. Psychiatry 2017, 73, 61–83. [Google Scholar] [CrossRef]
- Donati, M.A.; Berrocal, C.; Primi, C.; Petracchi, G.; Carpita, B.; Cosci, F.; Ruiz, A.; Carmassi, C.; Dell’Osso, L. Measuring subthreshold autistic traits in the general population: Psychometric properties of the Adult Autism Subthreshold Spectrum (AdAS Spectrum) scale. Psychiatry Res. 2019, 281, 112576. [Google Scholar] [CrossRef] [PubMed]
- Dell’Osso, L.; Carmassi, C.; Cremone, I.M.; Muti, D.; Salerni, A.; Barberi, F.M.; Massimetti, E.; Gesi, C.; Politi, P.; Aguglia, E.; et al. Defining the Optimal Threshold Scores for Adult Autism Subthreshold Spectrum (AdAS Spectrum) in Clinical and General Population. Clin. Pract. Epidemiol. Ment. Health 2020, 16, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Teo, A.R.; Chen, J.I.; Kubo, H.; Katsuki, R.; Sato-Kasai, M.; Shimokawa, N.; Hayakawa, K.; Umene-Nakano, W.; Aikens, J.E.; Kanba, S.; et al. Development and validation of the 25-item Hikikomori Questionnaire (HQ-25). Psychiatry Clin. Neurosci. 2018, 72, 780–788. [Google Scholar] [CrossRef]
- Amendola, S.; Presaghi, F.; Teo, A.R.; Cerutti, R. Psychometric Properties of the Italian Version of the 25-Item Hikikomori Questionnaire. Int. J. Environ. Res. Public Health 2022, 19, 13552. [Google Scholar] [CrossRef] [PubMed]
- Katsuki, R.; Tateno, M.; Kubo, H.; Kurahara, K.; Hayakawa, K.; Kuwano, N.; Kanba, S.; Kato, T.A. Autism spectrum conditions in hikikomori: A pilot case-control study. Psychiatry Clin. Neurosci. 2020, 74, 652–658. [Google Scholar] [CrossRef]
- Brosnan, M.; Gavin, J. The impact of higher levels of autistic traits on risk of hikikomori (pathological social withdrawal) in young adults. PLoS ONE 2023, 18, e0281833. [Google Scholar] [CrossRef]
- Carpita, B.; Nardi, B.; Giovannoni, F.; Parri, F.; Cerofolini, G.; Bonelli, C.; Amatori, G.; Massimetti, G.; Cremone, I.M.; Pini, S.; et al. Exploring the relationship among hikikomori tendencies, autistic traits, computer game use and eating disorder symptoms. CNS Spectr. 2024, 29, 670–681. [Google Scholar] [CrossRef]
- Dell’Osso, L.; Amatori, G.; Muti, D.; Giovannoni, F.; Parri, F.; Violi, M.; Cremone, I.M.; Carpita, B. Autism Spectrum, Hikikomori Syndrome and Internet Gaming Disorder: Is There a Link? Brain Sci. 2023, 13, 1116. [Google Scholar] [CrossRef]
- Alonso-Esteban, Y.; López-Ramón, M.F.; Moreno-Campos, V.; Navarro-Pardo, E.; Alcantud-Marín, F. A Systematic Review on the Impact of the Social Confinement on People with Autism Spectrum Disorder and Their Caregivers during the COVID-19 Pandemic. Brain Sci. 2021, 11, 1389. [Google Scholar] [CrossRef]
- Kwan, C.; Gitimoghaddam, M.; Collet, J.P. Effects of Social Isolation and Loneliness in Children with Neurodevelopmental Disabilities: A Scoping Review. Brain Sci. 2020, 10, 786. [Google Scholar] [CrossRef]
- Hall, J.A.; Horgan, T.G.; Murphy, N.A. Nonverbal Communication. Annu. Rev. Psychol. 2019, 70, 271–294. [Google Scholar] [CrossRef] [PubMed]
- Krieg, A.; Dickie, J.R. Attachment and hikikomori: A psychosocial developmental model. Int. J. Soc. Psychiatry 2013, 59, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Zhang, X. Bullying Victimization and Quality of Life among Chinese Adolescents: An Integrative Analysis of Internet Addiction and Social Withdrawal. Int. J. Environ. Res. Public Health 2022, 19, 16973. [Google Scholar] [CrossRef] [PubMed]
- Wakuta, M.; Nishimura, T.; Osuka, Y.; Tsukui, N.; Takahashi, M.; Adachi, M.; Suwa, T.; Katayama, T. Adverse childhood experiences: Impacts on adult mental health and social withdrawal. Front. Public Health 2023, 11, 1277766. [Google Scholar] [CrossRef]
- Schultz, R.T.; Gauthier, I.; Klin, A.; Fulbright, R.K.; Anderson, A.W.; Volkmar, F.; Skudlarski, P.; Lacadie, C.; Cohen, D.J.; Gore, J.C. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Arch. Gen. Psychiatry 2000, 57, 331–340. [Google Scholar] [CrossRef]
- Bachevalier, J.; Loveland, K.A. The orbitofrontal-amygdala circuit and self-regulation of social-emotional behavior in autism. Neurosci. Biobehav. Rev. 2006, 30, 97–117. [Google Scholar] [CrossRef]
- Scott-Van Zeeland, A.A.; Dapretto, M.; Ghahremani, D.G.; Poldrack, R.A.; Bookheimer, S.Y. Reward processing in autism. Autism Res. 2010, 3, 53–67. [Google Scholar] [CrossRef]
- Chevallier, C.; Kohls, G.; Troiani, V.; Brodkin, E.S.; Schultz, R.T. The social motivation theory of autism. Trends Cogn. Sci. 2012, 16, 231–239. [Google Scholar] [CrossRef]
- Modi, M.E.; Young, L.J. The oxytocin system in drug discovery for autism: Animal models and novel therapeutic strategies. Horm. Behav. 2012, 61, 340–350. [Google Scholar] [CrossRef]
- Teo, A.R. Social isolation associated with depression: A case report of hikikomori. Int. J. Soc. Psychiatry 2013, 59, 339–341. [Google Scholar] [CrossRef]
- Taylor, M. Strategies of dissociation: A mimetic dimension to social problems in Japan. Anthropoetics 2006, 12, 11. [Google Scholar]
- Lee, Y.S.; Lee, J.Y.; Choi, T.Y.; Choi, J.T. Home visitation program for detecting, evaluating and treating socially withdrawn youth in Korea. Psychiatry Clin. Neurosci. 2013, 67, 193–202. [Google Scholar] [CrossRef] [PubMed]

|
HCs Mean ± SD n = 550 |
HK Mean ± SD n = 118 |
AT Mean ± SD n = 818 |
AT-HK Mean ± SD n = 821 | F | p | ||
|---|---|---|---|---|---|---|---|
| Age | 25.23 ± 6.35 | 26.08 ± 9.28 | 23.87 ± 5.25 | 24.76 ± 5.85 | 8.344 | <0.001 * | |
| n(%) | n(%) | n(%) | n(%) | Chi-square | p | ||
| Gender | F | 316 a (57.5%) | 61 b (51.7%) | 449 b (54.9%) | 410 a (49.9%) | 8.428 | 0.038 * |
| M | 234 b (42.5%) | 57 b (48.3%) | 369 b (45.1%) | 411 b (50.1%) | |||
| (a) | ||||||
| Source | Dependent Variable | Type III Sum of Squares | df | Mean Square | F | p |
| Corrected Model | AdAS Spectrum | |||||
| Child./adolesc. | 13,812.181 | 3 | 4604.060 | 445.208 | <0.001 * | |
| Verb. comm. | 9756.571 | 3 | 3252.190 | 451.629 | <0.001 * | |
| Non verb. comm. | 23,747.788 | 3 | 7915.929 | 606.636 | <0.001 * | |
| Empathy | 3137.983 | 3 | 1045.994 | 217.743 | <0.001 * | |
| Inflex. and routine | 54,413.152 | 3 | 18,137.717 | 566.395 | <0.001 * | |
| Restrict. interest and rum. | 22,224.705 | 3 | 7408.235 | 672.611 | <0.001 * | |
| Hyper-hyporeact. | 8942.784 | 3 | 2980.928 | 326.227 | <0.001 * | |
| HQ-25 | ||||||
| Socialization | 152,004.066 | 3 | 50,668.022 | 1234.124 | <0.001 * | |
| Isolation | 70,214.727 | 3 | 23,404.909 | 1134.953 | <0.001 * | |
| Emotional support | 17,129.499 | 3 | 5709.833 | 381.433 | <0.001 * | |
| Intercept | AdAS Spectrum | |||||
| Child./adolesc. | 66,469.476 | 1 | 66,469.476 | 6427.534 | <0.001 * | |
| Verb. comm. | 35,970.629 | 1 | 35,970.629 | 4995.214 | <0.001 * | |
| Non verb. comm. | 123,677.907 | 1 | 123,677.907 | 9478.033 | <0.001 * | |
| Empathy | 11,065.245 | 1 | 11,065.245 | 2302.440 | <0.001 * | |
| Inflex. and routine | 203,188.264 | 1 | 203,188.264 | 6345.060 | <0.001 * | |
| Restrict. interest and rum. | 78,145.272 | 1 | 78,145.272 | 7094.997 | <0.001 * | |
| Hyper-hyporeact. | 20,717.238 | 1 | 20,717.238 | 2267.254 | <0.001 * | |
| HQ-25 | ||||||
| Socialization | 435,825.126 | 1 | 435,825.126 | 10,615.419 | <0.001 * | |
| Isolation | 211,012.747 | 1 | 211,012.747 | 10,232.446 | <0.001 * | |
| Emotional support | 73,062.762 | 1 | 73,062.762 | 4880.799 | <0.001 * | |
| Hikikomori | AdAS Spectrum | |||||
| Child./adolesc. | 888.591 | 1 | 888.591 | 85.926 | <0.001 * | |
| Verb. comm. | 613.847 | 1 | 613.847 | 85.244 | <0.001 * | |
| Non verb. comm. | 1037.606 | 1 | 1037.606 | 79.517 | <0.001 * | |
| Empathy | 172.322 | 1 | 172.322 | 35.872 | <0.001 * | |
| Inflex. and routine | 414.861 | 1 | 414.861 | 12.955 | <0.001 * | |
| Restrict. interest and rum. | 439.493 | 1 | 439.493 | 39.903 | <0.001 * | |
| Hyper-hyporeact. | 107.811 | 1 | 107.811 | 11.799 | 0.001 * | |
| HQ-25 | ||||||
| Socialization | 72,216.835 | 1 | 72,216.835 | 1758.990 | <0.001 * | |
| Isolation | 34,208.393 | 1 | 34,208.393 | 1658.836 | <0.001 * | |
| Emotional support | 9145.719 | 1 | 9145.719 | 610.960 | <0.001 * | |
| Significant ATs | AdAS Spectrum | |||||
| Child./adolesc. | 6605.892 | 1 | 6605.892 | 638.783 | <0.001 * | |
| Verb. comm. | 4678.825 | 1 | 4678.825 | 649.745 | <0.001 * | |
| Non verb. comm. | 12,358.944 | 1 | 12,358.944 | 947.125 | <0.001 * | |
| Empathy | 1562.486 | 1 | 1562.486 | 325.261 | <0.001 * | |
| Inflex. and routine | 34,552.256 | 1 | 34,552.256 | 1078.980 | <0.001 * | |
| Restrict. interest and rum. | 13,016.314 | 1 | 13,016.314 | 1181.782 | <0.001 * | |
| Hyper-hyporeact. | 5548.491 | 1 | 5548.491 | 607.16 | <0.001 * | |
| HQ-25 | ||||||
| Socialization | 4686.351 | 1 | 4686.351 | 114.146 | <0.001 * | |
| Isolation | 1356.006 | 1 | 1356.006 | 65.756 | <0.001 * | |
| Emotional support | 167.459 | 1 | 167.459 | 11.187 | 0.001 * | |
| Hikikomori * Significant ATs | AdAS Spectrum | |||||
| Child./adolesc. | 97.697 | 1 | 97.697 | 9.447 | 0.002 * | |
| Verb. comm. | 124.349 | 1 | 124.349 | 17.268 | <0.001 * | |
| Non verb. comm. | 39.189 | 1 | 39.189 | 3.003 | 0.083 | |
| Empathy | 33.545 | 1 | 33.545 | 6.983 | 0.008 * | |
| Inflex. and routine | 399.672 | 1 | 399.672 | 12.481 | <0.001 * | |
| Restrict. interest and rum. | 89.889 | 1 | 89.889 | 8.161 | 0.004 * | |
| Hyper-hyporeact. | 115.274 | 1 | 115.274 | 12.615 | <0.001 * | |
| HQ-25 | ||||||
| Socialization | 8.526 | 1 | 8.526 | 0.208 | 0.649 | |
| Isolation | 9.465 | 1 | 9.465 | 0.459 | 0.498 | |
| Emotional support | 15.115 | 1 | 15.115 | 1.010 | 0.315 | |
| Error | AdAS Spectrum | |||||
| Child./adolesc. | 23,816.163 | 2303 | 10.341 | |||
| Verb. comm. | 16,583.948 | 2303 | 7.201 | |||
| Non verb. comm. | 30,051.617 | 2303 | 13.049 | |||
| Empathy | 11,063.134 | 2303 | 4.804 | |||
| Inflex. and routine | 73,749.114 | 2303 | 32.023 | |||
| Restrict. interest and rum. | 25,365.558 | 2303 | 11.014 | |||
| Hyper-hyporeact. | 21,043.870 | 2303 | 9.138 | |||
| HQ-25 | ||||||
| Socialization | 94,551.640 | 2303 | 41.056 | |||
| Isolation | 47,492.298 | 2303 | 20.622 | |||
| Emotional support | 34,474.587 | 2303 | 14.969 | |||
| Total | AdAS Spectrum | |||||
| Child./adolesc. | 190,278.000 | 2307 | ||||
| Verb. comm. | 111,956.000 | 2307 | ||||
| Non verb. comm. | 338,318.000 | 2307 | ||||
| Empathy | 40,851.000 | 2307 | ||||
| Inflex. and routine | 641,911.000 | 2307 | ||||
| Restrict. interest and rum. | 242,057.000 | 2307 | ||||
| Hyper-hyporeact. | 86,458.000 | 2307 | ||||
| HQ-25 | ||||||
| Socialization | 994,960.000 | 2307 | ||||
| Isolation | 474,846.000 | 2307 | ||||
| Emotional support | 173,087.000 | 2307 | ||||
| Corrected total | AdAS Spectrum | |||||
| Child./adolesc. | 37,628.344 | 2306 | ||||
| Verb. comm. | 26,340.518 | 2306 | ||||
| Non verb. comm. | 53,799.404 | 2306 | ||||
| Empathy | 14,201.117 | 2306 | ||||
| Inflex. and routine | 128,162.266 | 2306 | ||||
| Restrict. interest and rum. | 47,590.264 | 2306 | ||||
| Hyper-hyporeact. | 29,986.654 | 2306 | ||||
| HQ-25 | ||||||
| Socialization | 246,555.707 | 2306 | ||||
| Isolation | 117,707.025 | 2306 | ||||
| Emotional support | 51,604.087 | 2306 | ||||
| (b) | ||||||
| Source | Type III Sum of Squares | df | Mean Square | F | p | |
| Corrected model | 808,948.507 | 3 | 269,649.502 | 1014.480 | 0.000 * | |
| Intercept | 3,163,381.852 | 1 | 3,163,381.852 | 11,901.334 | 0.000 * | |
| Significant AT | 455,405.354 | 1 | 455,405.354 | 1713.334 | 0.000 * | |
| Hikikomori | 22,994.576 | 1 | 22,994.576 | 86.511 | 0.000 * | |
| Significant AT * Hikikomori | 5372.392 | 1 | 5372.392 | 20.212 | 0.000 * | |
| Error | 612,138.823 | 2303 | 265.801 | |||
| Total | 9,123,597.000 | 2307 | ||||
| Corrected total | 1,421,087.330 | 2306 | ||||
| (c) | ||||||
| Source | Type III Sum of Squares | dF | Mean Square | F | p | |
| Corrected model | 616,619.289 | 3 | 205,539.763 | 1889.519 | 0.000 * | |
| Intercept | 1,931,635.792 | 1 | 1,931,635.792 | 17,757.456 | 0.000 * | |
| Significant AT | 13,976.323 | 1 | 13,976.323 | 128.484 | 0.000 * | |
| Hikikomori | 301,752.755 | 1 | 301,752.755 | 2774.002 | 0.000 * | |
| adascut43 * HQcut | 13.921 | 1 | 13.921 | 0.128 | 0.721 | |
| Error | 250,517.709 | 2303 | 108.779 | |||
| Total | 4,147,795.000 | 2307 | ||||
| Corrected total | 867,136.999 | 2306 | ||||
|
HCs Mean ± SD n = 550 |
HK Mean ± SD n = 118 |
AT Mean ± SD n = 818 |
AT-HK Mean ± SD n = 821 | F | p | |
|---|---|---|---|---|---|---|
| AdAS Spectrum scores | ||||||
| Child./adolesc. | 4.42 ± 2.41 | 5.54 ± 2.61 | 8.45 ± 3.29 | 10.68 ± 3.66 | 445.208 | <0.001 * |
| Verb. comm. | 3.04 ± 1.74 | 3.80 ± 2.04 | 6.27 ± 2.78 | 8.29 ± 3.14 | 451.629 | <0.001 * |
| Non verb. comm. | 6.05 ± 2.66 | 7.52 ± 2.60 | 11.97 ± 3.98 | 14.14 ± 3.89 | 606.636 | <0.001 ° |
| Empathy | 1.64 ± 1.52 | 2.06 ± 1.80 | 3.55 ± 2.25 | 4.62 ± 2.54 | 217.743 | <0.001 * |
| Inflex. and routine | 7.46 ± 3.72 | 7.48 ± 3.43 | 16.82 ± 5.81 | 19.10 ± 6.73 | 566.395 | <0.001 * |
| Restrict. interest and rum. | 4.34 ± 2.61 | 4.99 ± 2.43 | 10.24 ± 3.32 | 11.96 ± 3.81 | 672.611 | <0.001 * |
| Hyper-hyporeact. | 1.97 ± 1.84 | 1.95 ± 1.89 | 5.57 ± 3.09 | 6.76 ± 3.65 | 326.277 | <0.001 * |
| Total score | 28.93 ± 9.45 | 33.35 ± 7.03 | 62.87 ± 16.46 | 75.56 ± 20.24 | 1014.480 | <0.001 * |
| HQ-25 | ||||||
| Socialization | 9.03 ± 5.91 | 24.35 ± 6.11 | 13.06 ± 6.13 | 28.05 ± 7.01 | 1234.124 | <0.001 ^ |
| Isolation | 6.79 ± 4.22 | 17.05 ± 4.38 | 8.69 ± 4.17 | 19.30 ± 5.09 | 1134.953 | <0.001 ^ |
| Emotional support | 4.45 ± 3.21 | 10.07 ± 3.60 | 5.40 ± 3.69 | 10.58 ± 4.44 | 381.433 | <0.001 § |
| Total score | 20.27 ± 10.26 | 51.47 ± 8.66 | 27.15 ± 9.58 | 57.93 ± 11.53 | 1889.519 | <0.001 ^ |
| B (S.E) | BETA | t | p | |
|---|---|---|---|---|
| Constant | 27.405 (3.645) | 7.519 | <0.001 * | |
| Socialization | 1.090 (0.112) | 0.323 | 9.748 | <0.001 * |
| Isolation | 0.547 (0.158) | 0.117 | 3.451 | 0.001 * |
| Emotional support | 0.227 (0.169) | 0.042 | 1.341 | 0.180 |
| B (S.E.) | BETA | t | p | |
|---|---|---|---|---|
| constant | 11.968 (1.618) | 7.399 | <0.001 * | |
| Child./adolesc. | 1.044 (0.132) | 0.204 | 7.913 | <0.001 * |
| Verb. comm. | 1.247 (0.170) | 0.209 | 7.339 | <0.001 * |
| Non verb. comm. | 0.617 (0.123) | 0.135 | 5.030 | <0.001 * |
| Empathy | 0.792 (0.185) | 0.104 | 4.280 | <0.001 * |
| Inflex. and routine | −0.249 (0.089) | −0.085 | −2.787 | 0.005 |
| Restrict. interest and rum. | 0.481 (0.151) | 0.095 | 3.194 | 0.001 |
| Hyper-hyporeact. | −0.104 (0.151) | −0.019 | −0.686 | 0.493 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dell’Osso, L.; Nardi, B.; Muti, D.; De Felice, C.; Tognini, V.; Parri, F.; Giovannoni, F.; Del Grande, F.; Bonelli, C.; Massimetti, G.; et al. Comorbidity Between Hikikomori and Autistic Traits May Be Identified as a Phenotypical Presentation Characterized by Greater Severity. Brain Sci. 2025, 15, 496. https://doi.org/10.3390/brainsci15050496
Dell’Osso L, Nardi B, Muti D, De Felice C, Tognini V, Parri F, Giovannoni F, Del Grande F, Bonelli C, Massimetti G, et al. Comorbidity Between Hikikomori and Autistic Traits May Be Identified as a Phenotypical Presentation Characterized by Greater Severity. Brain Sciences. 2025; 15(5):496. https://doi.org/10.3390/brainsci15050496
Chicago/Turabian StyleDell’Osso, Liliana, Benedetta Nardi, Dario Muti, Chiara De Felice, Valeria Tognini, Francesca Parri, Federico Giovannoni, Filippo Del Grande, Chiara Bonelli, Gabriele Massimetti, and et al. 2025. "Comorbidity Between Hikikomori and Autistic Traits May Be Identified as a Phenotypical Presentation Characterized by Greater Severity" Brain Sciences 15, no. 5: 496. https://doi.org/10.3390/brainsci15050496
APA StyleDell’Osso, L., Nardi, B., Muti, D., De Felice, C., Tognini, V., Parri, F., Giovannoni, F., Del Grande, F., Bonelli, C., Massimetti, G., Pini, S., Fiorillo, A., & Carpita, B. (2025). Comorbidity Between Hikikomori and Autistic Traits May Be Identified as a Phenotypical Presentation Characterized by Greater Severity. Brain Sciences, 15(5), 496. https://doi.org/10.3390/brainsci15050496










