Epitranscriptomic Analysis of the Ventral Hippocampus in a Mouse Model of Post-Traumatic Stress Disorder Following Deep Brain Stimulation Treatment of the Basolateral Amygdala
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrode Implantation
2.2. Inescapable Foot Shock
2.3. Deep Brain Stimulation
2.4. m6A Epitranscriptomic Microarray Analysis
2.5. Western Blot
3. Results
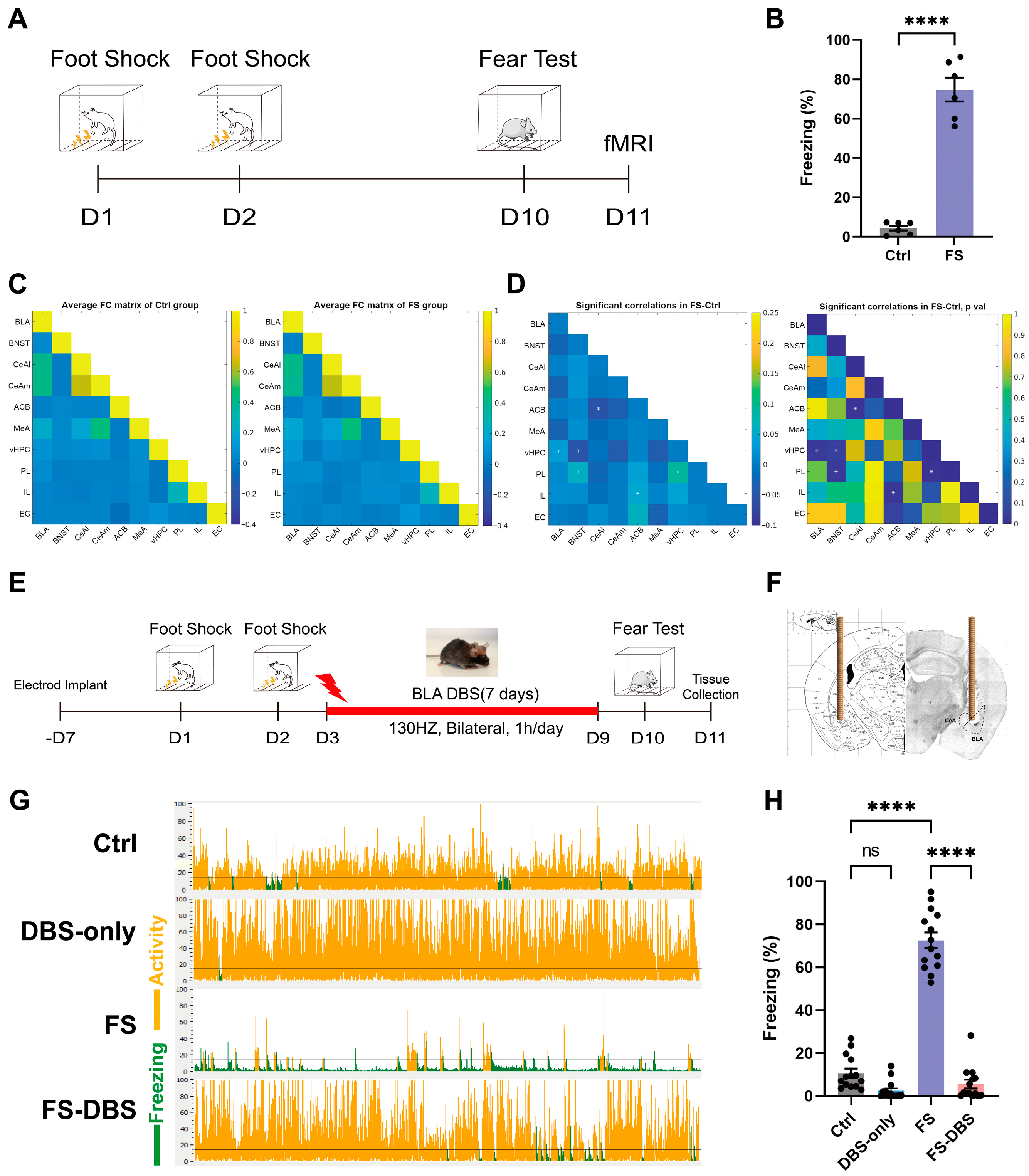
3.1. vHPC Is Potentially Intricately Associated with PTSD Mice Model
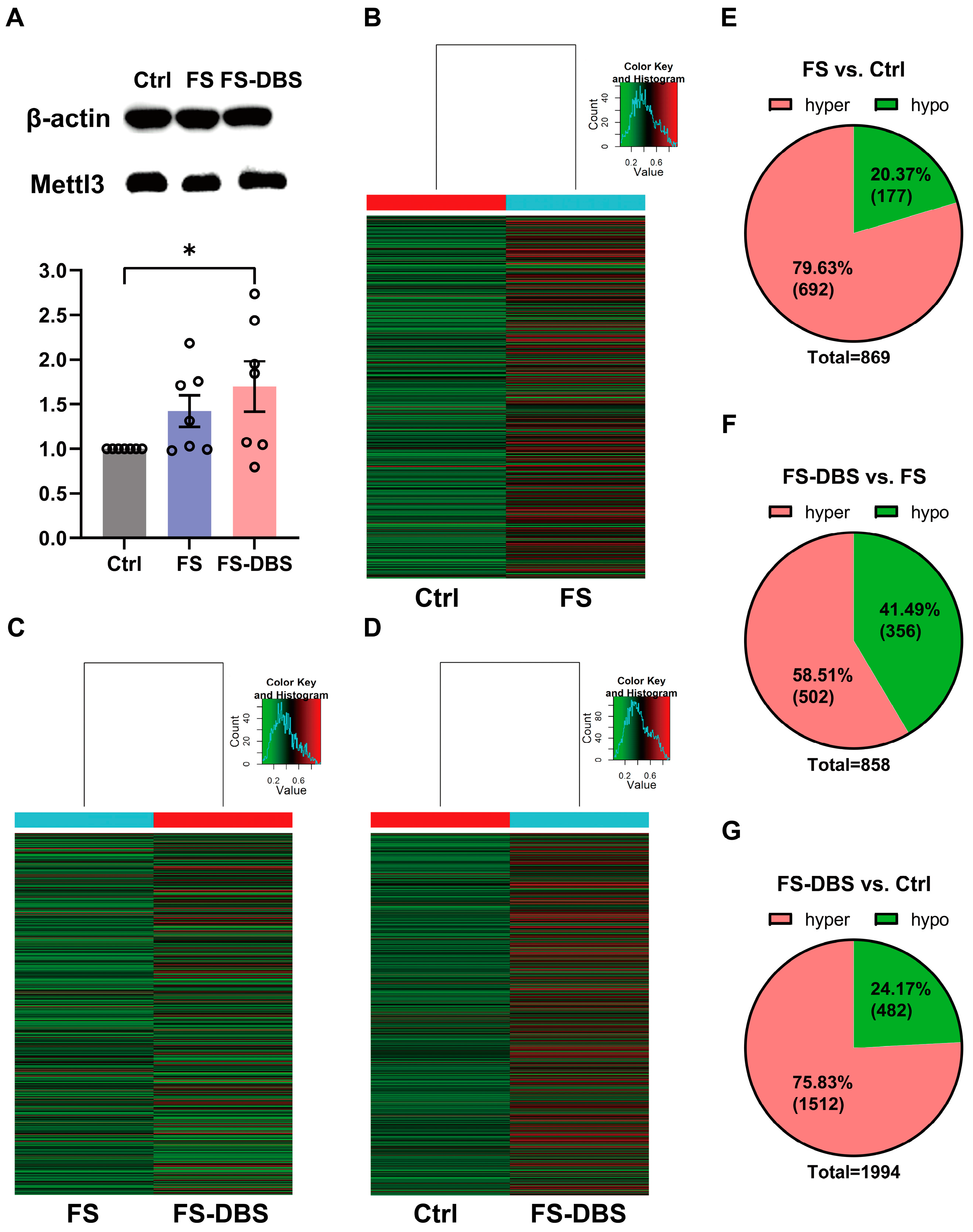
3.2. FS and DBS Induced Alterations in m6A Methylation Levels in vHPC
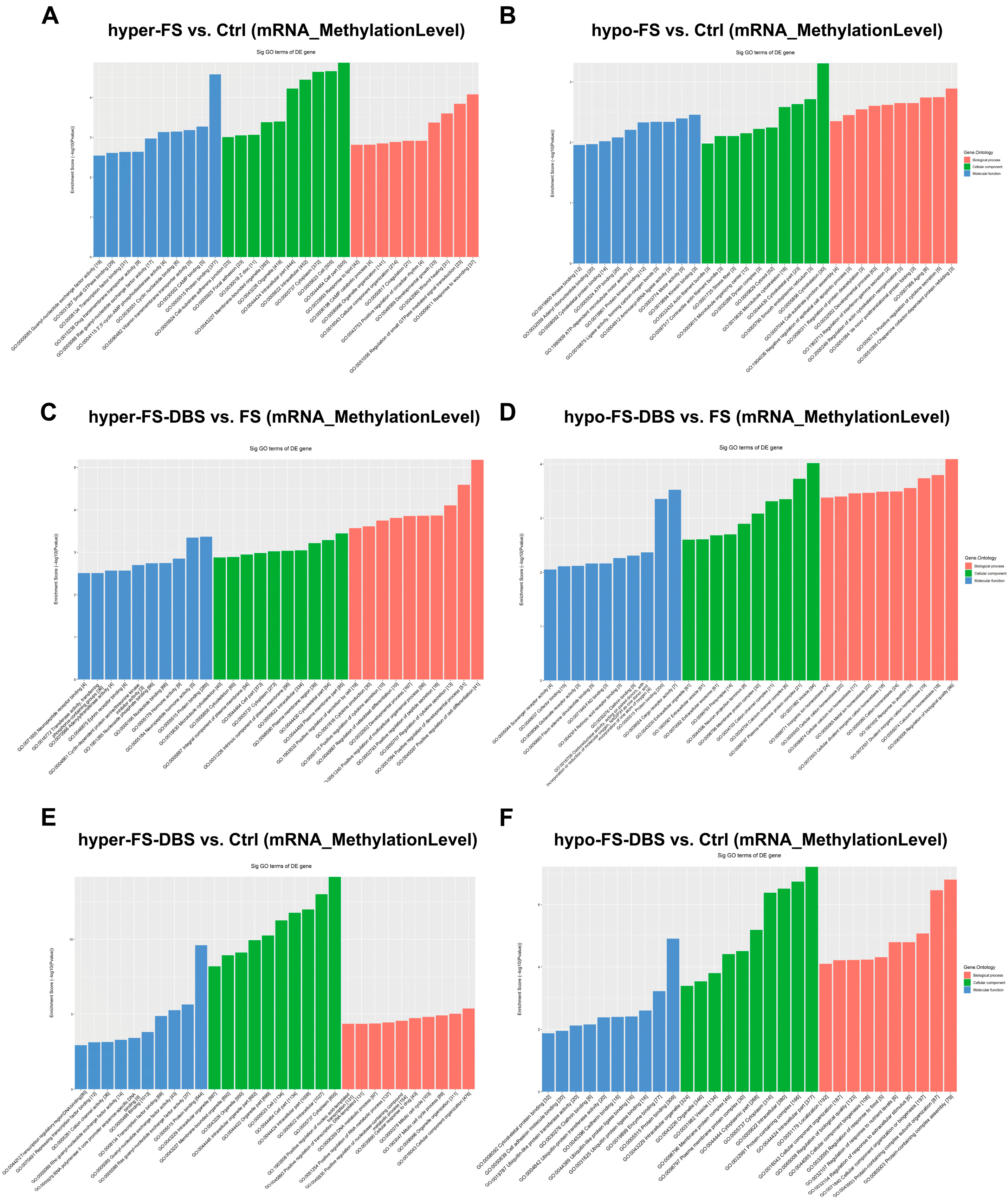
3.3. Gene Ontology Analysis of m6A Modifications in vHPC
3.4. BLA DBS Attenuated m6A Hypermethylation Caused by FS
3.5. BLA DBS Enhanced m6A Hypomethylation Caused by FS
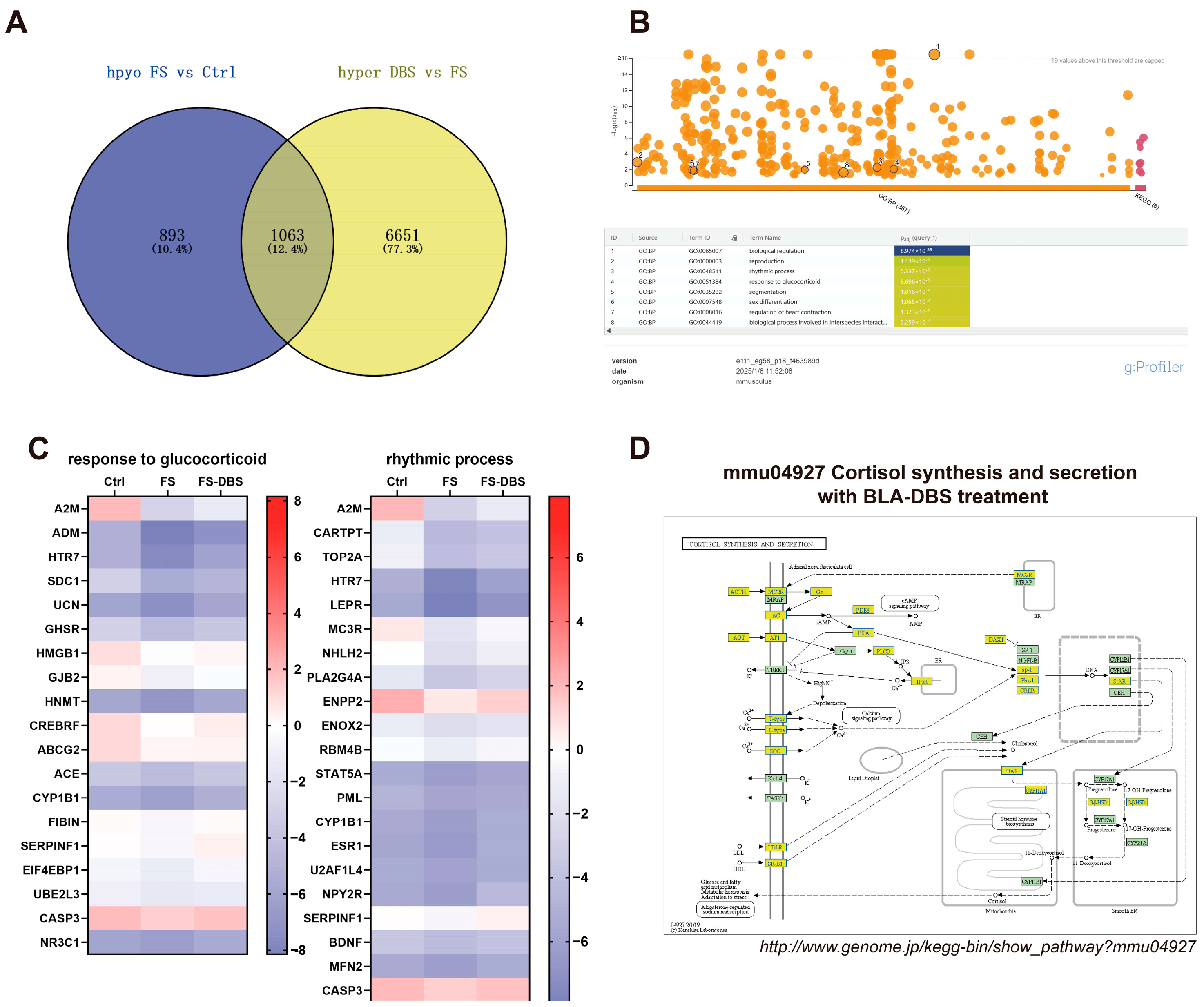
3.6. Detection of PTSD-Related Genes Using MeRIP Real-Time Quantitative PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shalev, A.; Liberzon, I.; Marmar, C. Post-Traumatic Stress Disorder. N. Engl. J. Med. 2017, 376, 2459–2469. [Google Scholar] [CrossRef] [PubMed]
- De Voogd, L.D.; Kanen, J.W.; Neville, D.A.; Roelofs, K.; Fernández, G.; Hermans, E.J. Eye-Movement Intervention Enhances Extinction via Amygdala Deactivation. J. Neurosci. 2018, 38, 8694–8706. [Google Scholar] [CrossRef] [PubMed]
- Merians, A.N.; Spiller, T.; Harpaz-Rotem, I.; Krystal, J.H.; Pietrzak, R.H. Post-traumatic Stress Disorder. Med. Clin. N. Am. 2023, 107, 85–99. [Google Scholar] [CrossRef]
- Lozano, A.M.; Lipsman, N.; Bergman, H.; Brown, P.; Chabardès, S.; Chang, J.; Matthews, K.; McIntyre, C.; Schlaepfer, T.; Schulder, M.; et al. Deep brain stimulation: Current challenges and future directions. Nat. Rev. Neurol. 2019, 15, 148–160. [Google Scholar] [CrossRef]
- Zhang, K.K.; Matin, R.; Gorodetsky, C.; Ibrahim, G.M.; Gouveia, F.V. Systematic review of rodent studies of deep brain stimulation for the treatment of neurological, developmental and neuropsychiatric disorders. Transl. Psychiatry 2024, 14, 186. [Google Scholar] [CrossRef] [PubMed]
- Staudt, M.D.; Herring, E.Z.; Gao, K.; Miller, J.P.; Sweet, J.A. Evolution in the Treatment of Psychiatric Disorders: From Psychosurgery to Psychopharmacology to Neuromodulation. Front. Neurosci. 2019, 13, 108. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Ruiz, J.D.; Carrillo-Márquez, J.R.; Beltrán, J.Q.; Jiménez-Ponce, F.; García-Muñoz, L.; Navarro-Olvera, J.L.; Márquez-Franco, R.; Velasco, F. Innovative perspectives in limbic surgery using deep brain stimulation. Front. Neurosci. 2023, 17, 1167244. [Google Scholar] [CrossRef]
- Sgambato-Faure, V.; Alonso, P.; Cuadras, D.; Denys, D.; Goodman, W.; Greenberg, B.D.; Jimenez-Ponce, F.; Kuhn, J.; Lenartz, D.; Mallet, L.; et al. Deep Brain Stimulation for Obsessive-Compulsive Disorder: A Meta-Analysis of Treatment Outcome and Predictors of Response. PLoS ONE 2015, 10, e0133591. [Google Scholar]
- Hitti, F.L.; Yang, A.I.; Cristancho, M.A.; Baltuch, G.H. Deep Brain Stimulation Is Effective for Treatment-Resistant Depression: A Meta-Analysis and Meta-Regression. J. Clin. Med. 2020, 9, 2796. [Google Scholar] [CrossRef]
- Langevin, J.P.; Koek, R.J.; Schwartz, H.N.; Chen, J.W.Y.; Sultzer, D.L.; Mandelkern, M.A.; Kulick, A.D.; Krahl, S.E. Deep Brain Stimulation of the Basolateral Amygdala for Treatment-Refractory Posttraumatic Stress Disorder. Biol. Psychiatry 2016, 79, e82–e84. [Google Scholar] [CrossRef]
- Langevin, J.P.; Chen, J.W.; Koek, R.J.; Sultzer, D.L.; Mandelkern, M.A.; Schwartz, H.N.; Krahl, S.E. Deep Brain Stimulation of the Basolateral Amygdala: Targeting Technique and Electrodiagnostic Findings. Brain Sci. 2016, 6, 28. [Google Scholar] [CrossRef]
- Smith, M.E. Bilateral hippocampal volume reduction in adults with post-traumatic stress disorder: A meta-analysis of structural MRI studies. Hippocampus 2005, 15, 798–807. [Google Scholar] [CrossRef]
- Mcnerney, M.W.; Sheng, T.; Nechvatal, J.M.; Lee, A.G.; Lyons, D.M.; Soman, S.; Liao, C.-P.; O’Hara, R.; Hallmayer, J.; Taylor, J.; et al. Integration of neural and epigenetic contributions to posttraumatic stress symptoms: The role of hippocampal volume and glucocorticoid receptor gene methylation. PLoS ONE 2018, 13, e0192222. [Google Scholar] [CrossRef] [PubMed]
- Ben-Zion, Z.; Korem, N.; Fine, N.B.; Katz, S.; Siddhanta, M.; Funaro, M.C.; Duek, O.; Spiller, T.R.; Danböck, S.K.; Levy, I.; et al. Structural Neuroimaging of Hippocampus and Amygdala Subregions in Posttraumatic Stress Disorder: A Scoping Review. Biol. Psychiatry Glob. Open Sci. 2024, 4, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Postel, C.; Mary, A.; Dayan, J.; Fraisse, F.; Vallée, T.; Guillery-Girard, B.; Viader, F.; de la Sayette, V.; Peschanski, D.; Eustache, F.; et al. Variations in response to trauma and hippocampal subfield changes. Neurobiol. Stress 2021, 15, 100346. [Google Scholar] [CrossRef] [PubMed]
- Karl, A.; Schaefer, M.; Malta, L.S.; Dörfel, D.; Rohleder, N.; Werner, A. A meta-analysis of structural brain abnormalities in PTSD. Neurosci. Biobehav. Rev. 2006, 30, 1004–1031. [Google Scholar] [CrossRef]
- Fanselow, M.S.; Dong, H.-W. Are the Dorsal and Ventral Hippocampus Functionally Distinct Structures? Neuron 2010, 65, 7–19. [Google Scholar] [CrossRef]
- Acheson, D.T.; Gresack, J.E.; Risbrough, V.B. Hippocampal dysfunction effects on context memory: Possible etiology for posttraumatic stress disorder. Neuropharmacology 2012, 62, 674–685. [Google Scholar] [CrossRef]
- Chaposhloo, M.; Nicholson, A.A.; Becker, S.; McKinnon, M.C.; Lanius, R.; Shaw, S.B.; Alzheimer’s Disease Neuroimaging Initiative. Altered Resting-State functional connectivity in the anterior and posterior hippocampus in Post-traumatic stress disorder: The central role of the anterior hippocampus. Neuroimage Clin. 2023, 38, 103417. [Google Scholar] [CrossRef]
- Felix-Ortiz Ada c Beyeler, A.; Seo, C.; Leppla, C.A.; Wildes, C.P.; Tye, K.M. BLA to vHPC Inputs Modulate Anxiety-Related Behaviors. Neuron 2013, 79, 658–664. [Google Scholar] [CrossRef]
- Vinkers, C.H.; Geuze, E.; Van Rooij, S.J.H.; Kennis, M.; Schür, R.R.; Nispeling, D.M.; Smith, A.K.; Nievergelt, C.M.; Uddin, M.; Rutten, B.P.F.; et al. Successful treatment of post-traumatic stress disorder reverses DNA methylation marks. Mol. Psychiatry 2019, 26, 1264–1271. [Google Scholar] [CrossRef]
- Engel, M.; Eggert, C.; Kaplick, P.M.; Eder, M.; Röh, S.; Tietze, L.; Namendorf, C.; Arloth, J.; Weber, P.; Rex-Haffner, M.; et al. The Role of m6A/m-RNA Methylation in Stress Response Regulation. Neuron 2018, 99, 389–403.e389. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.-F.; Han, Q.-Q.; Chen, F.-F.; Shen, T.-T.; Li, Y.-H.; Cao, Y.; Chen, J.-G.; Wang, F. Erasing m6A-dependent transcription signature of stress-sensitive genes triggers antidepressant actions. Neurobiol. Stress 2021, 15, 100390. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Fan, J.; Liu, J.-M.; Kong, P.-L.; Ren, J.; Mo, J.-W.; Lu, C.-L.; Zhong, Q.-L.; Chen, L.-Y.; Jiang, H.-T.; et al. Astrocytic ALKBH5 in stress response contributes to depressive-like behaviors in mice. Nat. Commun. 2024, 15, 4347. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, X.; Weng, Y.-L.; Lu, Z.; Liu, Y.; Lu, Z.; Li, J.; Hao, P.; Zhang, Y.; Zhang, F.; et al. m6A facilitates hippocampus-dependent learning and memory through YTHDF1. Nature 2018, 563, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Ochi, S.; Dwivedi, Y. M6A RNA Methylation-Based Epitranscriptomic Modifications in Plasticity-Related Genes via miR-124-C/EBPα-FTO-Transcriptional Axis in the Hippocampus of Learned Helplessness Rats. Int. J. Neuropsychopharmacol. 2022, 25, 1037–1049. [Google Scholar] [CrossRef]
- Zhou, Y.; Yuan, X.; Guo, M. Unlocking NAC’s potential ATF4 and m6A dynamics in rescuing cognitive impairments in PTSD. Metab. Brain Dis. 2025, 40, 129. [Google Scholar] [CrossRef]
- Deslauriers, J.; Toth, M.; Der-Avakian, A.; Risbrough, V.B. Current Status of Animal Models of Posttraumatic Stress Disorder: Behavioral and Biological Phenotypes, and Future Challenges in Improving Translation. Biol. Psychiatry 2018, 83, 895–907. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, D.; Zhang, H.; Zheng, D.; Du, J.; Yuan, C.; Ma, M.; Yin, Y.; Wang, J.; Zhang, X.; et al. BLA DBS improves anxiety and fear by correcting weakened synaptic transmission from BLA to adBNST and CeL in a mouse model of foot shock. Cell Rep. 2024, 43, 113766. [Google Scholar] [CrossRef]
- Verbitsky, A.; Dopfel, D.; Zhang, N. Rodent models of post-traumatic stress disorder: Behavioral assessment. Transl. Psychiatry 2020, 10, 132. [Google Scholar] [CrossRef]
- Zhang, L.-M.; Yao, J.-Z.; Li, Y.; Li, K.; Chen, H.-X.; Zhang, Y.-Z.; Li, Y.-F. Anxiolytic Effects of Flavonoids in Animal Models of Posttraumatic Stress Disorder. Evid.-Based Complement. Altern. Med. 2012, 2012, 623753. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liao, Y.; Dong, Y.; Li, X.; Li, J.; Cheng, Y.; Cheng, J.; Yuan, Z. Microglial deletion and inhibition alleviate behavior of post-traumatic stress disorder in mice. J. Neuroinflamm. 2021, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Fenster, R.J.; Lebois La, M.; Ressler, K.J.; Suh, J. Brain circuit dysfunction in post-traumatic stress disorder: From mouse to man. Nat. Rev. Neurosci. 2018, 19, 535–551. [Google Scholar] [CrossRef] [PubMed]
- Reznikov, R.; Hamani, C. Posttraumatic Stress Disorder: Perspectives for the Use of Deep Brain Stimulation. Neuromodulation Technol. Neural Interface 2017, 20, 7–14. [Google Scholar] [CrossRef]
- Meeres, J.; Hariz, M. Deep Brain Stimulation for Post-Traumatic Stress Disorder: A Review of the Experimental and Clinical Literature. Stereotact. Funct. Neurosurg. 2022, 100, 143–155. [Google Scholar] [CrossRef]
- Yehuda, R.; Hoge, C.W.; Mcfarlane, A.C.; Vermetten, E.; Lanius, R.A.; Nievergelt, C.M.; Hobfoll, S.E.; Koenen, K.C.; Neylan, T.C.; Hyman, S.E. Post-traumatic stress disorder. Nat. Rev. Dis. Primers 2015, 1, 15057. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Zhao, N.; Qiao, Y.; Feng, Z.-J.; Hu, Y.-S.; Ge, Q.; Zhang, T.-Q.; Zhang, Z.-Q.; Wang, J.; Zang, Y.-F. Higher reliability and validity of Wavelet-ALFF of resting-state fMRI: From multicenter database and application to rTMS modulation. Human. Brain Mapp. 2022, 44, 1105–1117. [Google Scholar] [CrossRef]
- Gutierrez-Barragan, D.; Singh, N.A.; Alvino, F.G.; Coletta, L.; Rocchi, F.; De Guzman, E.; Galbusera, A.; Uboldi, M.; Panzeri, S.; Gozzi, A. Unique spatiotemporal fMRI dynamics in the awake mouse brain. Curr. Biol. 2022, 32, 631–644.e6. [Google Scholar] [CrossRef]
- Hayes, J.P.; Hayes, S.M.; Mikedis, A.M. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol. Mood Anxiety Disord. 2012, 2, 9. [Google Scholar] [CrossRef]
- Fani, N.; King, T.Z.; Shin, J.; Srivastava, A.; Brewster, R.C.; Jovanovic, T.; Bradley, B.; Ressler, K.J. Structural and Functional Connectivity in Posttraumatic Stress Disorder: Associations with Fkbp5. Depress. Anxiety 2016, 33, 300–307. [Google Scholar] [CrossRef]
- Li, B.; Piriz, J.; Mirrione, M.; Chung, C.; Proulx, C.D.; Schulz, D.; Henn, F.; Malinow, R. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature 2011, 470, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Toews, M.L.; Prinster, S.C.; Schulte, N.A. Regulation of alpha-1B adrenergic receptor localization, trafficking, function, and stability. Life Sci. 2003, 74, 379–389. [Google Scholar] [CrossRef]
- Gao, B.; Sekido, Y.; Maximov, A.; Saad, M.; Forgacs, E.; Latif, F.; Wei, M.H.; Lerman, M.; Lee, J.H.; Perez-Reyes, E.; et al. Functional properties of a new voltage-dependent calcium channel alpha(2)delta auxiliary subunit gene (CACNA2D2). J. Biol. Chem. 2000, 275, 12237–12242. [Google Scholar] [CrossRef]
- Kyriatzis, G.; Khrestchatisky, M.; Ferhat, L.; Chatzaki, E.A. Neurotensin and Neurotensin Receptors in Stress-related Disorders: Pathophysiology & Novel Drug Targets. Curr. Neuropharmacol. 2024, 22, 916–934. [Google Scholar] [PubMed]
- Harbauer, A.B.; Hees, J.T.; Wanderoy, S.; Segura, I.; Gibbs, W.; Cheng, Y.; Ordonez, M.; Cai, Z.; Cartoni, R.; Ashrafi, G.; et al. Neuronal mitochondria transport Pink1 mRNA via synaptojanin 2 to support local mitophagy. Neuron 2022, 110, 1516–1531.e9. [Google Scholar] [CrossRef]
- Sah, R.; Geracioti, T.D. Neuropeptide Y and posttraumatic stress disorder. Mol. Psychiatry 2013, 18, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Jordan, C.J.; Xi, Z.X. Progress in brain cannabinoid CB(2) receptor research: From genes to behavior. Neurosci. Biobehav. Rev. 2019, 98, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Bakoyiannis, I.; Ducourneau, E.G.; Parkes, S.L.; Ferreira, G. Pathway specific interventions reveal the multiple roles of ventral hippocampus projections in cognitive functions. Rev. Neurosci. 2023, 34, 825–838. [Google Scholar] [CrossRef]
- Mendez, P.; Stefanelli, T.; Flores, C.E.; Muller, D.; Lüscher, C. Homeostatic Plasticity in the Hippocampus Facilitates Memory Extinction. Cell Rep. 2018, 22, 1451–1461. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, S.; Li, X.-N.; Li, W.-G. Memory Trace for Fear Extinction: Fragile yet Reinforceable. Neurosci. Bull. 2023, 40, 777–794. [Google Scholar] [CrossRef]
- Almeida, F.B.; Pinna, G.; Barros, H.M.T. The Role of HPA Axis and Allopregnanolone on the Neurobiology of Major Depressive Disorders and PTSD. Int. J. Mol. Sci. 2021, 22, 5495. [Google Scholar] [CrossRef] [PubMed]
- Michopoulos, V.; Vester, A.; Neigh, G. Posttraumatic stress disorder: A metabolic disorder in disguise? Exp. Neurol. 2016, 284, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, B.W.; Wong, A. The hypothalamic-pituitary-adrenal axis in PTSD: Pathophysiology and treatment interventions. Progress. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 89, 361–379. [Google Scholar] [CrossRef] [PubMed]
- Neria, Y. Functional Neuroimaging in PTSD: From Discovery of Underlying Mechanisms to Addressing Diagnostic Heterogeneity. Am. J. Psychiatry 2021, 178, 128–135. [Google Scholar] [CrossRef]
- Hofmann, J.; Huber, C.; Novak, B.; Schreckenbach, M.; Schubert, C.F.; Touma, C.; Rutten, B.P.; Schmidt, U. Oxytocin receptor is a potential biomarker of the hyporesponsive HPA axis subtype of PTSD and might be modulated by HPA axis reactivity traits in humans and mice. Psychoneuroendocrinology 2021, 129, 105242. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, M.; Fan, H.; Zhang, H.; Yin, Y.; Wang, Y.; Gao, Y. Epitranscriptomic Analysis of the Ventral Hippocampus in a Mouse Model of Post-Traumatic Stress Disorder Following Deep Brain Stimulation Treatment of the Basolateral Amygdala. Brain Sci. 2025, 15, 473. https://doi.org/10.3390/brainsci15050473
Ma M, Fan H, Zhang H, Yin Y, Wang Y, Gao Y. Epitranscriptomic Analysis of the Ventral Hippocampus in a Mouse Model of Post-Traumatic Stress Disorder Following Deep Brain Stimulation Treatment of the Basolateral Amygdala. Brain Sciences. 2025; 15(5):473. https://doi.org/10.3390/brainsci15050473
Chicago/Turabian StyleMa, Mingxi, Hao Fan, Hui Zhang, Yao Yin, Yizheng Wang, and Yan Gao. 2025. "Epitranscriptomic Analysis of the Ventral Hippocampus in a Mouse Model of Post-Traumatic Stress Disorder Following Deep Brain Stimulation Treatment of the Basolateral Amygdala" Brain Sciences 15, no. 5: 473. https://doi.org/10.3390/brainsci15050473
APA StyleMa, M., Fan, H., Zhang, H., Yin, Y., Wang, Y., & Gao, Y. (2025). Epitranscriptomic Analysis of the Ventral Hippocampus in a Mouse Model of Post-Traumatic Stress Disorder Following Deep Brain Stimulation Treatment of the Basolateral Amygdala. Brain Sciences, 15(5), 473. https://doi.org/10.3390/brainsci15050473



