Lactoferrin as a Candidate Multifunctional Therapeutic in Synucleinopathies
Abstract
1. Introduction
2. Lactoferrin as a Novel Frontier in Synucleinopathy Therapies
2.1. Evolutionary Versatility
2.2. Lactoferrin Structure and Iron Binding
3. Alpha-Synuclein Aggregation: A Target Ripe for Disruption
Iron and Alpha-Synuclein Aggregation
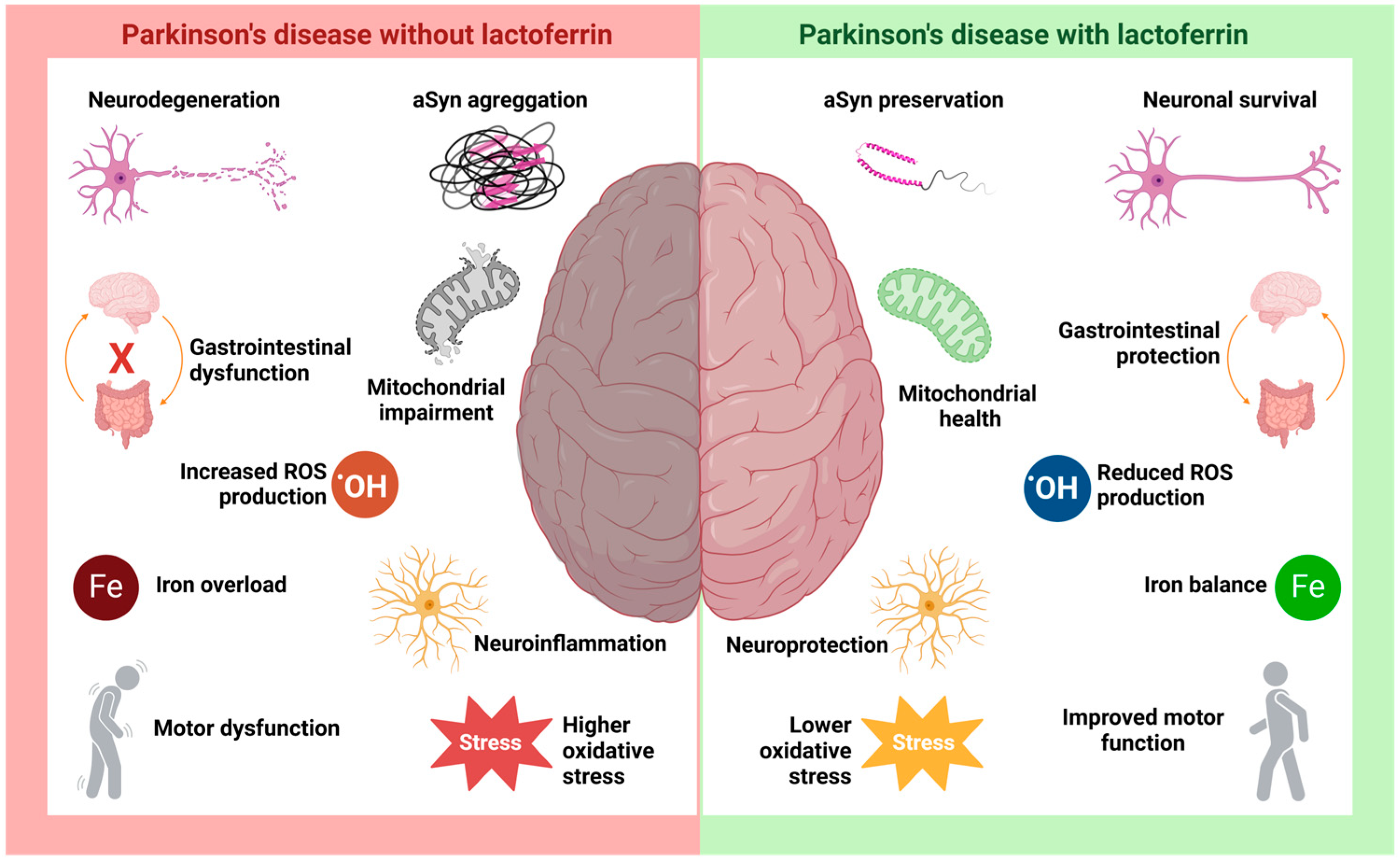
4. Lactoferrin in Parkinson’s Disease
5. Lf in Other Neurodegenerative Disorders
5.1. Lf in Alzheimer’s Disease
5.2. Lf in Prion Disease
6. Beyond the Brain: The Gut-Brain Axis
7. Innovative Applications: Delivery Systems and Combinatorial Strategies
7.1. Target Delivery
7.2. Therapeutic Synergy
8. From Bench to Beside: Bridging Preclinical Promise with Clinical Reality
9. Redefining Therapeutics: Beyond Parkinson’s Disease
9.1. Broader Applications in Synucleionophaties
9.2. Preventive Potential
10. Challenges and Opportunities in the Lactoferrin Landscape: Overcoming Bioavailability Barriers
11. Conclusions: Lactoferrin’s Promise as a Disruptive Force in Neurodegeneration
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Lf | Lactoferrin |
| ROS | Reactive oxygen species |
| PD | Parkinson’s disease |
| Tfs | Serum transferrins |
| oTf | Ovotransferrin |
| COVID-19 | Coronavirus disease 2019 |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| Fe | Iron |
| Cu | Copper |
| Zn | Zinc |
| Mn | Manganese |
| Al | Aluminum |
| Ga | Gallium |
| Co | Cobalt |
| Holo-Lf | Holo-lactoferrin |
| Apo-Lf | Apo-lactoferrin |
| pI | Isoeletric point |
| LRP1 | Low-density lipoprotein receptor-related protein 1 |
| AD | Alzheimer’s disease |
| aSyn | Alpha-synuclein |
| DLB | Lewy bodies |
| MAS | Multiple system atrophy |
| DFP | Deferiprone |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| MPP+ | 1-methyl-4-phenylpyridine |
| DMT1 | Divalent metal transporter |
| TFR | Transferrin receptor |
| MMP | Mitochondrial membrane potential |
| RBR | RING-between-RING |
| FAC | Ammonium ferric citrate |
| GPX4 | Glutathione peroxidase 4 |
| HSC70 | Heat shock cognate protein 70 |
| APP | Amyloid precursor protein |
| Aβ | Amyloid-β |
| CSF | Cerebrospinal fluid |
| PrPC | Cellular prion protein |
| PrPSc | Scrapie prion protein |
| HIF-1α | Hypoxia-inducible factor 1 alpha |
| BBB | Blood-brain barrier |
| LfRs | Lactoferrin receptors |
| HBMECs | Human brain microvascular endothelial cells |
| HAs | Human astrocytes |
| TEER | Transepithelial electrical resistance |
| CD | Carbon dot |
| NO | Nitric oxide |
| PEG | Polyethylene glycol |
| ASX RSV | Astaxanthin Resveratrol |
| LPs | Lipossomes |
| PLGA | Poly(lactic-co-glycolic acid) |
| CAY | CAY10603 |
| PBL | Peripheral blood lymphocytes |
| HSP | Heat shock protein |
| MMSE | Mini-Mental State Examination |
| ADAS-COG 11 | Alzheimer’s Disease Assessment Scale-Cognitive Subscale 11-item |
| aMCI | Mild cognitive impairment |
| MMSE | Mini-Mental State Examination |
| ADAS-COG 11 | Alzheimer’s Disease Assessment Scale-Cognitive Subscale 11-item |
References
- Sorensen, M.; Sorensen, S.P.L. The Proteins in Whey. Compte Rendu Trav. Lab. Carlsberg Ser. Chim. 1940, 123, 55–99. [Google Scholar]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Lactoferrin: Structure, Function, Denaturation and Digestion. Crit. Rev. Food Sci. Nutr. 2019, 59, 580–596. [Google Scholar] [CrossRef]
- Vieira, D.d.S.; Polveiro, R.C.; Butler, T.J.; Hackett, T.A.; Braga, C.P.; Puniya, B.L.; Teixeira, W.F.P.; de Padilha, P.M.; Adamec, J.; Feitosa, F.L.F. An in Silico, Structural, and Biological Analysis of Lactoferrin of Different Mammals. Int. J. Biol. Macromol. 2021, 187, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Du, X.; Lönnerdal, B. Effects of Forming Lactoferrin–Milk Protein Complexes on Lactoferrin Functionality and Intestinal Development in Infancy. Nutrients 2024, 16, 4077. [Google Scholar] [CrossRef]
- Conesa, C.; Bellés, A.; Grasa, L.; Sánchez, L. The Role of Lactoferrin in Intestinal Health. Pharmaceutics 2023, 15, 1569. [Google Scholar] [CrossRef] [PubMed]
- Naidu, S.A.G.; Wallace, T.C.; Davies, K.J.A.; Naidu, A.S. Lactoferrin for Mental Health: Neuro-Redox Regulation and Neuroprotective Effects across the Blood-Brain Barrier with Special Reference to Neuro-COVID-19. J. Diet. Suppl. 2023, 20, 218–253. [Google Scholar] [CrossRef]
- Rascón-Cruz, Q.; Siqueiros-Cendón, T.S.; Siañez-Estrada, L.I.; Villaseñor-Rivera, C.M.; Ángel-Lerma, L.E.; Olivas-Espino, J.A.; León-Flores, D.B.; Espinoza-Sánchez, E.A.; Arévalo-Gallegos, S.; Iglesias-Figueroa, B.F. Antioxidant Potential of Lactoferrin and Its Protective Effect on Health: An Overview. Int. J. Mol. Sci. 2024, 26, 125. [Google Scholar] [CrossRef]
- Kawakami, H.; Park, H.; Park, S.; Kuwata, H.; Shephard, R.J.; Aoyagi, Y. Effects of Enteric-Coated Lactoferrin Supplementation on the Immune Function of Elderly Individuals: A Randomised, Double-Blind, Placebo-Controlled Trial. Int. Dairy J. 2015, 47, 79–85. [Google Scholar] [CrossRef]
- Altwaijry, N.; Somani, S.; Parkinson, J.A.; Tate, R.J.; Keating, P.; Warzecha, M.; Mackenzie, G.R.; Leung, H.Y.; Dufès, C. Regression of Prostate Tumors after Intravenous Administration of Lactoferrin-Bearing Polypropylenimine Dendriplexes Encoding TNF-α, TRAIL, and Interleukin-12. Drug Deliv. 2018, 25, 679–689. [Google Scholar] [CrossRef]
- González-Chávez, S.A.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin: Structure, Function and Applications. Int. J. Antimicrob. Agents 2009, 33, 301.e1–301.e8. [Google Scholar] [CrossRef]
- Anderson, B.F.; Baker, H.M.; Norris, G.E.; Rice, D.W.; Baker, E.N. Structure of Human Lactoferrin: Crystallographic Structure Analysis and Refinement at 2.8 A Resolution. J. Mol. Biol. 1989, 209, 711–734. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, P.D.; Bewley, M.C.; MacGillivray, R.T.; Mason, A.B.; Woodworth, R.C.; Baker, E.N. Ligand-Induced Conformational Change in Transferrins: Crystal Structure of the Open Form of the N-Terminal Half-Molecule of Human Transferrin. Biochemistry 1998, 37, 13978–13986. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, H.; Mikami, B.; Hirose, M. Crystal Structure of Diferric Hen Ovotransferrin at 2.4 A Resolution. J. Mol. Biol. 1995, 254, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, H.; Dewan, J.C.; Mikami, B.; Sacchettini, J.C.; Hirose, M. Crystal Structure of Hen Apo-Ovotransferrin. Both Lobes Adopt an Open Conformation Upon Loss of Iron. J. Biol. Chem. 1999, 274, 28445–28452. [Google Scholar] [CrossRef]
- Moore, S.A.; Anderson, B.F.; Groom, C.R.; Haridas, M.; Baker, E.N. Three-Dimensional Structure of Diferric Bovine Lactoferrin at 2.8 Å Resolution. J. Mol. Biol. 1997, 274, 222–236. [Google Scholar] [CrossRef]
- Baker, E.; Baker, H. A Structural Framework for Understanding the Multifunctional Character of Lactoferrin. Biochimie 2009, 91, 3–10. [Google Scholar] [CrossRef]
- García-Montoya, I.A.; Cendón, T.S.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin a Multiple Bioactive Protein: An Overview. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 226–236. [Google Scholar] [CrossRef]
- Brock, J.H. Lactoferrin—50 Years On. Biochem. Cell Biol. 2012, 90, 245–251. [Google Scholar] [CrossRef]
- Amini, A.; Nair, L. Lactoferrin: A Biologically Active Molecule for Bone Regeneration. Curr. Med. Chem. 2011, 18, 1220–1229. [Google Scholar] [CrossRef]
- Giansanti, F.; Panella, G.; Leboffe, L.; Antonini, G. Lactoferrin from Milk: Nutraceutical and Pharmacological Properties. Pharmaceuticals 2016, 9, 61. [Google Scholar] [CrossRef]
- Silva, A.M.V.d.; Machado, T.L.; Nascimento, R.D.S.; Rodrigues, M.P.M.D.; Coelho, F.S.; Tubarão, L.N.; da Rosa, L.C.; Bayma, C.; Rocha, V.P.; Frederico, A.B.T.; et al. Immunomodulatory Effect of Bovine Lactoferrin during SARS-CoV-2 Infection. Front. Immunol. 2024, 15, 1456634. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Li, M.; Luo, C.; Wang, J.-Q.; Zheng, N. Lactoferrin Exerts Antitumor Effects by Inhibiting Angiogenesis in a HT29 Human Colon Tumor Model. J. Agric. Food Chem. 2017, 65, 10464–10472. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, S.; Ju, R.; Xiao, Y.; Wang, X.; Song, X.; Gu, L.; Cheng, L.; Li, X.; Chen, G. Antitumor Efficacy of Lf Modified Daunorubicin plus Honokiol Liposomes in Treatment of Brain Glioma. Eur. J. Pharm. Sci. 2017, 106, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Rocha, V.P.; Campos, S.P.C.; Barros, C.A.; Trindade, P.; Souza, L.R.Q.; Silva, T.G.; Gimba, E.R.P.; Teodoro, A.J.; Gonçalves, R.B. Bovine Lactoferrin Induces Cell Death in Human Prostate Cancer Cells. Oxid. Med. Cell Longev. 2022, 2022, 2187696. [Google Scholar] [CrossRef] [PubMed]
- Serrano, G.; Kochergina, I.; Albors, A.; Diaz, E.; Oroval, M.; Hueso, G.; Serrano, J.M. Liposomal Lactoferrin as Potential Preventative and Cure for COVID-19. Int. J. Res. Health Sci. 2020, 8, 8–15. [Google Scholar] [CrossRef]
- Campione, E.; Lanna, C.; Cosio, T.; Rosa, L.; Conte, M.P.; Iacovelli, F.; Romeo, A.; Falconi, M.; Del Vecchio, C.; Franchin, E.; et al. Lactoferrin Against SARS-CoV-2: In Vitro and In Silico Evidences. Front. Pharmacol. 2021, 12, 666600. [Google Scholar] [CrossRef]
- Hu, K.; Li, J.; Shen, Y.; Lu, W.; Gao, X.; Zhang, Q.; Jiang, X. Lactoferrin-Conjugated PEG–PLA Nanoparticles with Improved Brain Delivery: In Vitro and in Vivo Evaluations. J. Control. Release 2009, 134, 55–61. [Google Scholar] [CrossRef]
- Denani, C.B.; Real-Hohn, A.; de Carvalho, C.A.M.; Gomes, A.M.d.O.; Gonçalves, R.B. Lactoferrin Affects Rhinovirus B-14 Entry into H1-HeLa Cells. Arch. Virol. 2021, 166, 1203–1211. [Google Scholar] [CrossRef]
- Carvalho, C.A.M.; Sousa, I.P.; Silva, J.L.; Oliveira, A.C.; Gonçalves, R.B.; Gomes, A.M.O. Inhibition of Mayaro Virus Infection by Bovine Lactoferrin. Virology 2014, 452–453, 297–302. [Google Scholar] [CrossRef]
- Carvalho, C.A.M.; Casseb, S.M.M.; Gonçalves, R.B.; Silva, E.V.P.; Gomes, A.M.O.; Vasconcelos, P.F.C. Bovine Lactoferrin Activity against Chikungunya and Zika Viruses. J. Gen. Virol. 2017, 98, 1749–1754. [Google Scholar] [CrossRef]
- Marchetti, M.; Trybala, E.; Superti, F.; Johansson, M.; Bergström, T. Inhibition of Herpes Simplex Virus Infection by Lactoferrin Is Dependent on Interference with the Virus Binding to Glycosaminoglycans. Virology 2004, 318, 405–413. [Google Scholar] [CrossRef]
- Superti, F.; Agamennone, M.; Pietrantoni, A.; Ammendolia, M. Bovine Lactoferrin Prevents Influenza A Virus Infection by Interfering with the Fusogenic Function of Viral Hemagglutinin. Viruses 2019, 11, 51. [Google Scholar] [CrossRef]
- Chen, J.-M.; Fan, Y.-C.; Lin, J.-W.; Chen, Y.-Y.; Hsu, W.-L.; Chiou, S.-S. Bovine Lactoferrin Inhibits Dengue Virus Infectivity by Interacting with Heparan Sulfate, Low-Density Lipoprotein Receptor, and DC-SIGN. Int. J. Mol. Sci. 2017, 18, 1957. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, N.; Singh, A.; Singh, P.K.; Tyagi, T.K.; Pandey, S.; Shin, K.; Kaur, P.; Sharma, S.; Singh, T.P. Structure of Iron Saturated C-Lobe of Bovine Lactoferrin at PH 6.8 Indicates a Weakening of Iron Coordination. Proteins Struct. Funct. Bioinform. 2016, 84, 591–599. [Google Scholar] [CrossRef]
- Lönnerdal, B.; Iyer, S. Lactoferrin: Molecular Structure and Biological Function. Annu. Rev. Nutr. 1995, 15, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Barros, C.A.; Sanches, D.; Marques de Carvalho, C.A.; Santos, R.A.; Ferraz de Souza, T.L.; Macena Leite, V.L.; Pereira da Costa Campos, S.; Cheble de Oliveira, A.; Gonçalves, R.B. Influence of Iron Binding in the Structural Stability and Cellular Internalization of Bovine Lactoferrin. Heliyon 2021, 7, e08087. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wu, H.; Zhu, N.; Xu, Z.; Wang, Y.; Qu, Y.; Wang, J. Lactoferrin Protects against Iron Dysregulation, Oxidative Stress, and Apoptosis in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced Parkinson’s Disease in Mice. J. Neurochem. 2020, 152, 397–415. [Google Scholar] [CrossRef]
- Bonaccorsi di Patti, M.C.; Cutone, A.; Polticelli, F.; Rosa, L.; Lepanto, M.S.; Valenti, P.; Musci, G. The Ferroportin-Ceruloplasmin System and the Mammalian Iron Homeostasis Machine: Regulatory Pathways and the Role of Lactoferrin. BioMetals 2018, 31, 399–414. [Google Scholar] [CrossRef]
- Willnow, T.E.; Goldstein, J.L.; Orth, K.; Brown, M.S.; Herz, J. Low Density Lipoprotein Receptor-Related Protein and Gp330 Bind Similar Ligands, Including Plasminogen Activator-Inhibitor Complexes and Lactoferrin, an Inhibitor of Chylomicron Remnant Clearance. J. Biol. Chem. 1992, 267, 26172–26180. [Google Scholar] [CrossRef]
- Ito, S.; Ohtsuki, S.; Kamiie, J.; Nezu, Y.; Terasaki, T. Cerebral Clearance of Human Amyloid-β Peptide (1–40) across the Blood–Brain Barrier Is Reduced by Self-aggregation and Formation of Low-density Lipoprotein Receptor-related Protein-1 Ligand Complexes. J. Neurochem. 2007, 103, 2482–2490. [Google Scholar] [CrossRef]
- Petralla, S.; Panayotova, M.; Franchina, E.; Fricker, G.; Puris, E. Low-Density Lipoprotein Receptor-Related Protein 1 as a Potential Therapeutic Target in Alzheimer’s Disease. Pharmaceutics 2024, 16, 948. [Google Scholar] [CrossRef]
- Brás, I.C.; Dominguez-Meijide, A.; Gerhardt, E.; Koss, D.; Lázaro, D.F.; Santos, P.I.; Vasili, E.; Xylaki, M.; Outeiro, T.F. Synucleinopathies: Where We Are and Where We Need to Go. J. Neurochem. 2020, 153, 433–454. [Google Scholar] [CrossRef]
- Kao, A.W.; Racine, C.A.; Quitania, L.C.; Kramer, J.H.; Christine, C.W.; Miller, B.L. Cognitive and Neuropsychiatric Profile of the Synucleinopathies. Alzheimer Dis. Assoc. Disord. 2009, 23, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Stefanova, N.; Bücke, P.; Duerr, S.; Wenning, G.K. Multiple System Atrophy: An Update. Lancet Neurol. 2009, 8, 1172–1178. [Google Scholar] [CrossRef]
- Marques, O.; Outeiro, T.F. Alpha-Synuclein: From Secretion to Dysfunction and Death. Cell Death Dis. 2012, 3, e350. [Google Scholar] [CrossRef] [PubMed]
- Emin, D.; Zhang, Y.P.; Lobanova, E.; Miller, A.; Li, X.; Xia, Z.; Dakin, H.; Sideris, D.I.; Lam, J.Y.L.; Ranasinghe, R.T.; et al. Small Soluble α-Synuclein Aggregates Are the Toxic Species in Parkinson’s Disease. Nat. Commun. 2022, 13, 5512. [Google Scholar] [CrossRef]
- Ray, S.; Singh, N.; Kumar, R.; Patel, K.; Pandey, S.; Datta, D.; Mahato, J.; Panigrahi, R.; Navalkar, A.; Mehra, S.; et al. α-Synuclein Aggregation Nucleates through Liquid–Liquid Phase Separation. Nat. Chem. 2020, 12, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Rocha, E.M.; De Miranda, B.; Sanders, L.H. Alpha-Synuclein: Pathology, Mitochondrial Dysfunction and Neuroinflammation in Parkinson’s Disease. Neurobiol. Dis. 2018, 109, 249–257. [Google Scholar] [CrossRef]
- Vicente Miranda, H.; Szegő, É.M.; Oliveira, L.M.A.; Breda, C.; Darendelioglu, E.; de Oliveira, R.M.; Ferreira, D.G.; Gomes, M.A.; Rott, R.; Oliveira, M.; et al. Glycation Potentiates α-Synuclein-Associated Neurodegeneration in Synucleinopathies. Brain 2017, 140, 1399–1419. [Google Scholar] [CrossRef]
- Abeyawardhane, D.L.; Fernández, R.D.; Murgas, C.J.; Heitger, D.R.; Forney, A.K.; Crozier, M.K.; Lucas, H.R. Iron Redox Chemistry Promotes Antiparallel Oligomerization of α-Synuclein. J. Am. Chem. Soc. 2018, 140, 5028–5032. [Google Scholar] [CrossRef]
- Sant, V.; Matthes, D.; Mazal, H.; Antonschmidt, L.; Wieser, F.; Movellan, K.T.; Xue, K.; Nimerovsky, E.; Stampolaki, M.; Nathan, M.; et al. Lipidic Folding Pathway of α-Synuclein via a Toxic Oligomer. Nat. Commun. 2025, 16, 760. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Spillantini, M.G.; Del Tredici, K.; Braak, H. 100 Years of Lewy Pathology. Nat. Rev. Neurol. 2013, 9, 13–24. [Google Scholar] [CrossRef]
- Vijiaratnam, N.; Simuni, T.; Bandmann, O.; Morris, H.R.; Foltynie, T. Progress towards Therapies for Disease Modification in Parkinson’s Disease. Lancet Neurol. 2021, 20, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Gholam Azad, M.; Dharmasivam, M.; Richardson, V.; Quinn, R.J.; Feng, Y.; Pountney, D.L.; Tonissen, K.F.; Mellick, G.D.; Yanatori, I.; et al. Parkinson’s Disease: Alterations in Iron and Redox Biology as a Key to Unlock Therapeutic Strategies. Redox Biol. 2021, 41, 101896. [Google Scholar] [CrossRef]
- Lee, S.; Kovacs, G.G. The Irony of Iron: The Element with Diverse Influence on Neurodegenerative Diseases. Int. J. Mol. Sci. 2024, 25, 4269. [Google Scholar] [CrossRef] [PubMed]
- Naderi, S.; Khodagholi, F.; Janahmadi, M.; Motamedi, F.; Torabi, A.; Batool, Z.; Heydarabadi, M.F.; Pourbadie, H.G. Ferroptosis and Cognitive Impairment: Unraveling the Link and Potential Therapeutic Targets. Neuropharmacology 2025, 263, 110210. [Google Scholar] [CrossRef]
- Xiao, Z.; Wang, X.; Pan, X.; Xie, J.; Xu, H. Mitochondrial Iron Dyshomeostasis and Its Potential as a Therapeutic Target for Parkinson’s Disease. Exp. Neurol. 2024, 372, 114614. [Google Scholar] [CrossRef]
- He, N.; Ling, H.; Ding, B.; Huang, J.; Zhang, Y.; Zhang, Z.; Liu, C.; Chen, K.; Yan, F. Region-specific Disturbed Iron Distribution in Early Idiopathic Parkinson’s Disease Measured by Quantitative Susceptibility Mapping. Hum. Brain Mapp. 2015, 36, 4407–4420. [Google Scholar] [CrossRef]
- Guan, X.; Xuan, M.; Gu, Q.; Xu, X.; Huang, P.; Wang, N.; Shen, Z.; Xu, J.; Luo, W.; Zhang, M. Influence of Regional Iron on the Motor Impairments of Parkinson’s Disease: A Quantitative Susceptibility Mapping Study. J. Magn. Reson. Imaging 2017, 45, 1335–1342. [Google Scholar] [CrossRef]
- Guan, X.; Xuan, M.; Gu, Q.; Huang, P.; Liu, C.; Wang, N.; Xu, X.; Luo, W.; Zhang, M. Regionally Progressive Accumulation of Iron in Parkinson’s Disease as Measured by Quantitative Susceptibility Mapping. NMR Biomed. 2017, 30, e3489. [Google Scholar] [CrossRef]
- Martin-Bastida, A.; Lao-Kaim, N.P.; Loane, C.; Politis, M.; Roussakis, A.A.; Valle-Guzman, N.; Kefalopoulou, Z.; Paul-Visse, G.; Widner, H.; Xing, Y.; et al. Motor Associations of Iron Accumulation in Deep Grey Matter Nuclei in Parkinson’s Disease: A Cross-sectional Study of Iron-related Magnetic Resonance Imaging Susceptibility. Eur. J. Neurol. 2017, 24, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Chen, Y.; Zhang, Y.; Wang, F.; Yu, H.; Zhang, C.; Jiang, Z.; Luo, W. Iron Deposition in Parkinson’s Disease by Quantitative Susceptibility Mapping. BMC Neurosci. 2019, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Straumann, N.; Combes, B.F.; Dean Ben, X.L.; Sternke-Hoffmann, R.; Gerez, J.A.; Dias, I.; Chen, Z.; Watts, B.; Rostami, I.; Shi, K.; et al. Visualizing Alpha-synuclein and Iron Deposition in M83 Mouse Model of Parkinson’s Disease in Vivo. Brain Pathol. 2024, 34, e13288. [Google Scholar] [CrossRef] [PubMed]
- Febbraro, F.; Giorgi, M.; Caldarola, S.; Loreni, F.; Romero-Ramos, M. α-Synuclein Expression Is Modulated at the Translational Level by Iron. Neuroreport 2012, 23, 576–580. [Google Scholar] [CrossRef]
- Agostini, F.; Bubacco, L.; Chakrabarti, S.; Bisaglia, M. α-Synuclein Toxicity in Drosophila Melanogaster Is Enhanced by the Presence of Iron: Implications for Parkinson’s Disease. Antioxidants 2023, 12, 261. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, X.; Huang, S.; Li, G.; Mo, M.; Zhang, L.; Chen, C.; Guo, W.; Zhou, M.; Wu, Z.; et al. Iron Promotes A-synuclein Aggregation and Transmission by Inhibiting TFEB-mediated Autophagosome-lysosome Fusion. J. Neurochem. 2018, 145, 34–50. [Google Scholar] [CrossRef]
- Uversky, V.N.; Li, J.; Fink, A.L. Metal-Triggered Structural Transformations, Aggregation, and Fibrillation of Human α-Synuclein. J. Biol. Chem. 2001, 276, 44284–44296. [Google Scholar] [CrossRef]
- La Vitola, P.; Szegö, E.M.; Pinto-Costa, R.; Rollar, A.; Harbachova, E.; Schapira, A.H.; Ulusoy, A.; Di Monte, D.A. Mitochondrial Oxidant Stress Promotes α-Synuclein Aggregation and Spreading in Mice with Mutated Glucocerebrosidase. npj Park. Dis. 2024, 10, 233. [Google Scholar] [CrossRef]
- Paxinou, E.; Chen, Q.; Weisse, M.; Giasson, B.I.; Norris, E.H.; Rueter, S.M.; Trojanowski, J.Q.; Lee, V.M.-Y.; Ischiropoulos, H. Induction of α-Synuclein Aggregation by Intracellular Nitrative Insult. J. Neurosci. 2001, 21, 8053–8061. [Google Scholar] [CrossRef]
- Mahoney-Sanchez, L.; Bouchaoui, H.; Boussaad, I.; Jonneaux, A.; Timmerman, K.; Berdeaux, O.; Ayton, S.; Krüger, R.; Duce, J.A.; Devos, D.; et al. Alpha Synuclein Determines Ferroptosis Sensitivity in Dopaminergic Neurons via Modulation of Ether-Phospholipid Membrane Composition. Cell Rep. 2022, 40, 111231. [Google Scholar] [CrossRef]
- Angelova, P.R.; Choi, M.L.; Berezhnov, A.V.; Horrocks, M.H.; Hughes, C.D.; De, S.; Rodrigues, M.; Yapom, R.; Little, D.; Dolt, K.S.; et al. Alpha Synuclein Aggregation Drives Ferroptosis: An Interplay of Iron, Calcium and Lipid Peroxidation. Cell Death Differ. 2020, 27, 2781–2796. [Google Scholar] [CrossRef]
- Ortega, R.; Carmona, A.; Roudeau, S.; Perrin, L.; Dučić, T.; Carboni, E.; Bohic, S.; Cloetens, P.; Lingor, P. α-Synuclein Over-Expression Induces Increased Iron Accumulation and Redistribution in Iron-Exposed Neurons. Mol. Neurobiol. 2016, 53, 1925–1934. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-J.; Yue, F.; Song, D.-Y.; Bousset, L.; Liang, X.; Tang, J.; Yuan, L.; Li, W.; Melki, R.; Tang, Y.; et al. Intranasal Administration of α-Synuclein Preformed Fibrils Triggers Microglial Iron Deposition in the Substantia Nigra of Macaca Fascicularis. Cell Death Dis. 2021, 12, 81. [Google Scholar] [CrossRef] [PubMed]
- Deas, E.; Cremades, N.; Angelova, P.R.; Ludtmann, M.H.R.; Yao, Z.; Chen, S.; Horrocks, M.H.; Banushi, B.; Little, D.; Devine, M.J.; et al. Alpha-Synuclein Oligomers Interact with Metal Ions to Induce Oxidative Stress and Neuronal Death in Parkinson’s Disease. Antioxid. Redox Signal 2016, 24, 376–391. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Prudent, M.; Fauvet, B.; Lashuel, H.A.; Girault, H.H. Phosphorylation of α-Synuclein at Y125 and S129 Alters Its Metal Binding Properties: Implications for Understanding the Role of α-Synuclein in the Pathogenesis of Parkinson’s Disease and Related Disorders. ACS Chem. Neurosci. 2011, 2, 667–675. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Wang, S.; Yang, D.; Zhang, Y.; Xu, L.; Ma, L.; Zheng, J.; Petersen, R.B.; Zheng, L.; et al. Copper and Iron Ions Accelerate the Prion-like Propagation of α-Synuclein: A Vicious Cycle in Parkinson’s Disease. Int. J. Biol. Macromol. 2020, 163, 562–573. [Google Scholar] [CrossRef]
- Dexter, D.T.; Statton, S.A.; Whitmore, C.; Freinbichler, W.; Weinberger, P.; Tipton, K.F.; Della Corte, L.; Ward, R.J.; Crichton, R.R. Clinically Available Iron Chelators Induce Neuroprotection in the 6-OHDA Model of Parkinson’s Disease after Peripheral Administration. J. Neural Transm. 2011, 118, 223–231. [Google Scholar] [CrossRef]
- Grosso Jasutkar, H.; Oh, S.E.; Mouradian, M.M. Therapeutics in the Pipeline Targeting α-Synuclein for Parkinson’s Disease. Pharmacol. Rev. 2022, 74, 207–237. [Google Scholar] [CrossRef]
- Martin-Bastida, A.; Ward, R.J.; Newbould, R.; Piccini, P.; Sharp, D.; Kabba, C.; Patel, M.C.; Spino, M.; Connelly, J.; Tricta, F.; et al. Brain Iron Chelation by Deferiprone in a Phase 2 Randomised Double-Blinded Placebo Controlled Clinical Trial in Parkinson’s Disease. Sci. Rep. 2017, 7, 1398. [Google Scholar] [CrossRef]
- Negida, A.; Hassan, N.M.; Aboeldahab, H.; Zain, Y.E.; Negida, Y.; Cadri, S.; Cadri, N.; Cloud, L.J.; Barrett, M.J.; Berman, B. Efficacy of the Iron-chelating Agent, Deferiprone, in Patients with Parkinson’s Disease: A Systematic Review and Meta-analysis. CNS Neurosci. Ther. 2024, 30, e14607. [Google Scholar] [CrossRef]
- Fillebeen, C.; Descamps, L.; Dehouck, M.; Fenart, L.; Benaïssa, M.; Spik, G.; Cecchelli, R.; Pierce, A. Receptor-Mediated Transcytosis of Lactoferrin through the Blood-Brain Barrier. J. Biol. Chem. 1999, 274, 7011–7017. [Google Scholar] [CrossRef] [PubMed]
- Kawamata, T.; Tooyama, I.; Yamada, T.; Walker, D.G.; McGeer, P.L. Lactotransferrin Immunocytochemistry in Alzheimer and Normal Human Brain. Am. J. Pathol. 1993, 142, 1574–1585. [Google Scholar]
- Wang, J.; Bi, M.; Liu, H.; Song, N.; Xie, J. The Protective Effect of Lactoferrin on Ventral Mesencephalon Neurons against MPP+ Is Not Connected with Its Iron Binding Ability. Sci. Rep. 2015, 5, 10729. [Google Scholar] [CrossRef]
- Rousseau, E.; Michel, P.P.; Hirsch, E.C. The Iron-Binding Protein Lactoferrin Protects Vulnerable Dopamine Neurons from Degeneration by Preserving Mitochondrial Calcium Homeostasis. Mol. Pharmacol. 2013, 84, 888–898. [Google Scholar] [CrossRef]
- Xu, S.-F.; Zhang, Y.-H.; Wang, S.; Pang, Z.-Q.; Fan, Y.-G.; Li, J.-Y.; Wang, Z.-Y.; Guo, C. Lactoferrin Ameliorates Dopaminergic Neurodegeneration and Motor Deficits in MPTP-Treated Mice. Redox Biol. 2019, 21, 101090. [Google Scholar] [CrossRef]
- Kopaeva, M.Y.; Cherepov, A.B.; Nesterenko, M.V.; Zarayskaya, I.Y. Pretreatment with Human Lactoferrin Had a Positive Effect on the Dynamics of Mouse Nigrostriatal System Recovery after Acute MPTP Exposure. Biology 2021, 10, 24. [Google Scholar] [CrossRef]
- Xu, S.-F.; Cui, J.-H.; Liu, X.; Pang, Z.-Q.; Bai, C.-Y.; Jiang, C.; Luan, C.; Li, Y.-P.; Zhao, Y.; You, Y.-M.; et al. Astrocytic Lactoferrin Deficiency Augments MPTP-Induced Dopaminergic Neuron Loss by Disturbing Glutamate/Calcium and ER-Mitochondria Signaling. Free Radic. Biol. Med. 2024, 225, 374–387. [Google Scholar] [CrossRef]
- Yong, S.J.; Veerakumarasivam, A.; Teoh, S.L.; Lim, W.L.; Chew, J. Lactoferrin Protects Against Rotenone-Induced Toxicity in Dopaminergic SH-SY5Y Cells through the Modulation of Apoptotic-Associated Pathways. J. Mol. Neurosci. 2024, 74, 88. [Google Scholar] [CrossRef]
- Meredith, G.E.; Rademacher, D.J. MPTP Mouse Models of Parkinson’s Disease: An Update. J. Park. Dis. 2011, 1, 19–33. [Google Scholar] [CrossRef]
- Huang, R.; Ke, W.; Liu, Y.; Wu, D.; Feng, L.; Jiang, C.; Pei, Y. Gene Therapy Using Lactoferrin-Modified Nanoparticles in a Rotenone-Induced Chronic Parkinson Model. J. Neurol. Sci. 2010, 290, 123–130. [Google Scholar] [CrossRef]
- Sindhu, K.M.; Saravanan, K.S.; Mohanakumar, K.P. Behavioral Differences in a Rotenone-Induced Hemiparkinsonian Rat Model Developed Following Intranigral or Median Forebrain Bundle Infusion. Brain Res. 2005, 1051, 25–34. [Google Scholar] [CrossRef]
- Zakharova, E.T.; Sokolov, A.V.; Pavlichenko, N.N.; Kostevich, V.A.; Abdurasulova, I.N.; Chechushkov, A.V.; Voynova, I.V.; Elizarova, A.Y.; Kolmakov, N.N.; Bass, M.G.; et al. Erythropoietin and Nrf2: Key Factors in the Neuroprotection Provided by Apo-Lactoferrin. BioMetals 2018, 31, 425–443. [Google Scholar] [CrossRef] [PubMed]
- Hamm-Alvarez, S.F.; Janga, S.R.; Edman, M.C.; Feigenbaum, D.; Freire, D.; Mack, W.J.; Okamoto, C.T.; Lew, M.F. Levels of Oligomeric α-Synuclein in Reflex Tears Distinguish Parkinson’S Disease Patients From Healthy Controls. Biomark. Med. 2019, 13, 1447–1457. [Google Scholar] [CrossRef]
- Takase, K. Reactions of Denatured Proteins with Other Cellular Components to Form Insoluble Aggregates and Protection by Lactoferrin. FEBS Lett. 1998, 441, 271–274. [Google Scholar] [CrossRef]
- Nilsson, M.R.; Dobson, C.M. In Vitro Characterization of Lactoferrin Aggregation and Amyloid Formation. Biochemistry 2003, 42, 375–382. [Google Scholar] [CrossRef]
- Mizuno, Y.; Hattori, N.; Mori, H.; Suzuki, T.; Tanaka, K. Parkin and Parkinson’s Disease. Curr. Opin. Neurol. 2001, 14, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Seirafi, M.; Kozlov, G.; Gehring, K. Parkin Structure and Function. FEBS J. 2015, 282, 2076–2088. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, K.; Hashimoto, M.; Bar-On, P.; Ho, G.J.; Crews, L.; Mizuno, H.; Rockenstein, E.; Imam, S.Z.; Masliah, E. α-Synuclein Aggregates Interfere with Parkin Solubility and Distribution. J. Biol. Chem. 2008, 283, 6979–6987. [Google Scholar] [CrossRef]
- Gholkar, A.A.; Schmollinger, S.; Velasquez, E.F.; Lo, Y.-C.; Cohn, W.; Capri, J.; Dharmarajan, H.; Deardorff, W.J.; Gao, L.W.; Abdusamad, M.; et al. Regulation of Iron Homeostasis through Parkin-Mediated Lactoferrin Ubiquitylation. Biochemistry 2020, 59, 2916–2921. [Google Scholar] [CrossRef]
- Wang, L.; Sato, H.; Zhao, S.; Tooyama, I. Deposition of Lactoferrin in Fibrillar-Type Senile Plaques in the Brains of Transgenic Mouse Models of Alzheimer’s Disease. Neurosci. Lett. 2010, 481, 164–167. [Google Scholar] [CrossRef]
- Mohamed, W.A.; Salama, R.M.; Schaalan, M.F. A Pilot Study on the Effect of Lactoferrin on Alzheimer’s Disease Pathological Sequelae: Impact of the p-Akt/PTEN Pathway. Biomed. Pharmacother. 2019, 111, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.-G.; Ge, R.-L.; Ren, H.; Jia, R.-J.; Wu, T.-Y.; Lei, X.-F.; Wu, Z.; Zhou, X.-B.; Wang, Z.-Y. Astrocyte-Derived Lactoferrin Inhibits Neuronal Ferroptosis by Reducing Iron Content and GPX4 Degradation in APP/PS1 Transgenic Mice. Pharmacol. Res. 2024, 209, 107404. [Google Scholar] [CrossRef] [PubMed]
- Tsatsanis, A.; McCorkindale, A.N.; Wong, B.X.; Patrick, E.; Ryan, T.M.; Evans, R.W.; Bush, A.I.; Sutherland, G.T.; Sivaprasadarao, A.; Guennewig, B.; et al. The Acute Phase Protein Lactoferrin Is a Key Feature of Alzheimer’s Disease and Predictor of Aβ Burden through Induction of APP Amyloidogenic Processing. Mol. Psychiatry 2021, 26, 5516–5531. [Google Scholar] [CrossRef] [PubMed]
- Carro, E.; Bartolomé, F.; Bermejo-Pareja, F.; Villarejo-Galende, A.; Molina, J.A.; Ortiz, P.; Calero, M.; Rabano, A.; Cantero, J.L.; Orive, G. Early Diagnosis of Mild Cognitive Impairment and Alzheimer’s Disease Based on Salivary Lactoferrin. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2017, 8, 131–138. [Google Scholar] [CrossRef]
- González-Sánchez, M.; Bartolome, F.; Antequera, D.; Puertas-Martín, V.; González, P.; Gómez-Grande, A.; Llamas-Velasco, S.; Herrero-San Martín, A.; Pérez-Martínez, D.; Villarejo-Galende, A.; et al. Decreased Salivary Lactoferrin Levels Are Specific to Alzheimer’s Disease. EBioMedicine 2020, 57, 102834. [Google Scholar] [CrossRef]
- Gleerup, H.S.; Jensen, C.S.; Høgh, P.; Hasselbalch, S.G.; Simonsen, A.H. Lactoferrin in Cerebrospinal Fluid and Saliva Is Not a Diagnostic Biomarker for Alzheimer’s Disease in a Mixed Memory Clinic Population. EBioMedicine 2021, 67, 103361. [Google Scholar] [CrossRef]
- Gleerup, H.S.; Hasselbalch, S.G.; Simonsen, A.H. Biomarkers for Alzheimer’s Disease in Saliva: A Systematic Review. Dis. Markers 2019, 2019, 4761054. [Google Scholar] [CrossRef]
- Farah, R.; Haraty, H.; Salame, Z.; Fares, Y.; Ojcius, D.M.; Said Sadier, N. Salivary Biomarkers for the Diagnosis and Monitoring of Neurological Diseases. Biomed. J. 2018, 41, 63–87. [Google Scholar] [CrossRef]
- Iwamaru, Y.; Shimizu, Y.; Imamura, M.; Murayama, Y.; Endo, R.; Tagawa, Y.; Ushiki-Kaku, Y.; Takenouchi, T.; Kitani, H.; Mohri, S.; et al. Lactoferrin Induces Cell Surface Retention of Prion Protein and Inhibits Prion Accumulation. J. Neurochem. 2008, 107, 636–646. [Google Scholar] [CrossRef]
- Park, Y.-G.; Jeong, J.-K.; Lee, J.-H.; Lee, Y.-J.; Seol, J.-W.; Kim, S.-J.; Hur, T.-Y.; Jung, Y.-H.; Kang, S.-J.; Park, S.-Y. Lactoferrin Protects against Prion Protein-Induced Cell Death in Neuronal Cells by Preventing Mitochondrial Dysfunction. Int. J. Mol. Med. 2013, 31, 325–330. [Google Scholar] [CrossRef]
- Park, Y.-G.; Moon, J.-H.; Park, S.-Y. Lactoferrin from Bovine Colostrum Regulates Prolyl Hydroxylase 2 Activity and Prevents Prion Protein-Mediated Neuronal Cell Damage via Cellular Prion Protein. Neuroscience 2014, 274, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R.; Wallen, Z.D.; Won, W.-J.; Standaert, D.G.; Payami, H.; Harms, A.S. Alpha Synuclein Overexpression Can Drive Microbiome Dysbiosis in Mice. Sci. Rep. 2025, 15, 4014. [Google Scholar] [CrossRef] [PubMed]
- Hartke, A.-S.; Schreiber, C.S.; Lau, K.; Wiesweg, I.; Waltl, I.; Kalinke, U.; Richter, F.; Käufer, C. Alpha-Synuclein Pathology Enhances Peripheral and CNS Immune Responses to Bacterial Endotoxins. Neurobiol. Dis. 2025, 205, 106773. [Google Scholar] [CrossRef]
- Cersosimo, M.G.; Raina, G.B.; Pecci, C.; Pellene, A.; Calandra, C.R.; Gutiérrez, C.; Micheli, F.E.; Benarroch, E.E. Gastrointestinal Manifestations in Parkinson’s Disease: Prevalence and Occurrence before Motor Symptoms. J. Neurol. 2013, 260, 1332–1338. [Google Scholar] [CrossRef]
- Liu, N.; Feng, G.; Zhang, X.; Hu, Q.; Sun, S.; Sun, J.; Sun, Y.; Wang, R.; Zhang, Y.; Wang, P.; et al. The Functional Role of Lactoferrin in Intestine Mucosal Immune System and Inflammatory Bowel Disease. Front. Nutr. 2021, 8, 759507. [Google Scholar] [CrossRef]
- Massier, L.; Blüher, M.; Kovacs, P.; Chakaroun, R.M. Impaired Intestinal Barrier and Tissue Bacteria: Pathomechanisms for Metabolic Diseases. Front. Endocrinol. 2021, 12, 616506. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Human Gut Microbiota/Microbiome in Health and Diseases: A Review. Antonie van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- Singhal, R.; Shah, Y.M. Oxygen Battle in the Gut: Hypoxia and Hypoxia-Inducible Factors in Metabolic and Inflammatory Responses in the Intestine. J. Biol. Chem. 2020, 295, 10493–10505. [Google Scholar] [CrossRef]
- Mahbub, N.U.; Islam, M.M.; Hong, S.-T.; Chung, H.-J. Dysbiosis of the Gut Microbiota and Its Effect on α-Synuclein and Prion Protein Misfolding: Consequences for Neurodegeneration. Front. Cell Infect. Microbiol. 2024, 14, 1348279. [Google Scholar] [CrossRef]
- Zhou, H.-H.; Wang, G.; Luo, L.; Ding, W.; Xu, J.-Y.; Yu, Z.; Qin, L.-Q.; Wan, Z. Dietary Lactoferrin Has Differential Effects on Gut Microbiota in Young versus Middle-Aged APPswe/PS1dE9 Transgenic Mice but No Effects on Cognitive Function. Food Nutr. Res. 2021, 65, 10–29219. [Google Scholar] [CrossRef]
- Hirotani, Y.; Ikeda, K.; Kato, R.; Myotoku, M.; Umeda, T.; Ijiri, Y.; Tanaka, K. Protective Effects of Lactoferrin against Intestinal Mucosal Damage Induced by Lipopolysaccharide in Human Intestinal Caco-2 Cells. Yakugaku Zasshi 2008, 128, 1363–1368. [Google Scholar] [CrossRef] [PubMed]
- Ran, L.; Shi, J.; Lin, Y.; Xu, C.; Han, Z.; Tian, S.; Qin, X.; Li, Q.; Zhang, T.; Li, H.; et al. Evaluation of the Protective Bioactivity and Molecular Mechanism Verification of Lactoferrin in an Alzheimer’s Mouse Model with Ulcerative Enteritis. J. Dairy Sci. 2024, 107, 8796–8810. [Google Scholar] [CrossRef]
- Rajkovaca Latic, I.; Popovic, Z.; Mijatovic, K.; Sahinovic, I.; Pekic, V.; Vucic, D.; Cosic, V.; Miskic, B.; Tomic, S. Association of Intestinal Inflammation and Permeability Markers with Clinical Manifestations of Parkinson’s Disease. Park. Relat. Disord. 2024, 123, 106948. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The Blood–Brain Barrier: Structure, Regulation and Drug Delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fang, R.; Li, Y.; Jin, J.; Yang, F.; Chen, J. Encapsulation of Au(III) Complex Using Lactoferrin Nanoparticles to Combat Glioma. Mol. Pharm. 2023, 20, 3632–3644. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiang, M.; Kang, T.; Miao, D.; Gu, G.; Song, Q.; Yao, L.; Hu, Q.; Tu, Y.; Pang, Z.; et al. Lactoferrin-Modified PEG-Co-PCL Nanoparticles for Enhanced Brain Delivery of NAP Peptide Following Intranasal Administration. Biomaterials 2013, 34, 3870–3881. [Google Scholar] [CrossRef]
- Bi, C.; Wang, A.; Chu, Y.; Liu, S.; Mu, H.; Liu, W.; Wu, Z.; Sun, K.; Li, Y. Intranasal Delivery of Rotigotine to the Brain with Lactoferrin-Modified PEG-PLGA Nanoparticles for Parkinson’s Disease Treatment. Int. J. Nanomed. 2016, 11, 6547–6559. [Google Scholar] [CrossRef]
- Mudshinge, S.R.; Deore, A.B.; Patil, S.; Bhalgat, C.M. Nanoparticles: Emerging Carriers for Drug Delivery. Saudi Pharm. J. 2011, 19, 129–141. [Google Scholar] [CrossRef]
- Agrawal, M.; Ajazuddin; Tripathi, D.K.; Saraf, S.; Saraf, S.; Antimisiaris, S.G.; Mourtas, S.; Hammarlund-Udenaes, M.; Alexander, A. Recent Advancements in Liposomes Targeting Strategies to Cross Blood-Brain Barrier (BBB) for the Treatment of Alzheimer’s Disease. J. Control. Release 2017, 260, 61–77. [Google Scholar] [CrossRef]
- Agwa, M.M.; Sabra, S. Lactoferrin Coated or Conjugated Nanomaterials as an Active Targeting Approach in Nanomedicine. Int. J. Biol. Macromol. 2021, 167, 1527–1543. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Kondapi, A.K. Lactoferrin Nanoparticle Mediated Targeted Delivery of 5-Fluorouracil for Enhanced Therapeutic Efficacy. Int. J. Biol. Macromol. 2017, 95, 232–237. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Cheng, S.-J. Brain Targeted Delivery of Carmustine Using Solid Lipid Nanoparticles Modified with Tamoxifen and Lactoferrin for Antitumor Proliferation. Int. J. Pharm. 2016, 499, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Swami, R.; Pooja, D.; Jeengar, M.K.; Khan, W.; Sistla, R. Lactoferrin Bioconjugated Solid Lipid Nanoparticles: A New Drug Delivery System for Potential Brain Targeting. J. Drug Target. 2016, 24, 212–223. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, B.; Kebebe, D.; Guo, L.; Guo, H.; Li, N.; Pi, J.; Qi, D.; Guo, P.; Liu, Z. Preparation, Optimization and Cellular Uptake Study of Tanshinone I Nanoemulsion Modified with Lactoferrin for Brain Drug Delivery. Pharm. Dev. Technol. 2019, 24, 982–991. [Google Scholar] [CrossRef]
- Ouyang, Q.; Meng, Y.; Zhou, W.; Tong, J.; Cheng, Z.; Zhu, Q. New Advances in Brain-Targeting Nano-Drug Delivery Systems for Alzheimer’s Disease. J. Drug Target. 2022, 30, 61–81. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Ji, M.; Li, Y.; Qian, M.; Qin, Y.; Li, W.; Nie, H.; Lv, W.; Jiang, G.; Huang, R.; et al. Iron Ions-Sequestrable and Antioxidative Carbon Dot-Based Nano-Formulation with Nitric Oxide Release for Parkinson’s Disease Treatment. Biomaterials 2024, 309, 122622. [Google Scholar] [CrossRef]
- Nguyen, T.-D.; Khanal, S.; Lee, E.; Choi, J.; Bohara, G.; Rimal, N.; Choi, D.-Y.; Park, S. Astaxanthin-Loaded Brain-Permeable Liposomes for Parkinson’s Disease Treatment via Antioxidant and Anti-Inflammatory Responses. J. Nanobiotechnol. 2025, 23, 78. [Google Scholar] [CrossRef]
- Pham, K.-Y.; Khanal, S.; Bohara, G.; Rimal, N.; Song, S.-H.; Nguyen, T.T.K.; Hong, I.-S.; Cho, J.; Kang, J.-S.; Lee, S.; et al. HDAC6 Inhibitor-Loaded Brain-Targeted Nanocarrier-Mediated Neuroprotection in Methamphetamine-Driven Parkinson’s Disease. Redox Biol. 2025, 79, 103457. [Google Scholar] [CrossRef]
- Mittal, S.; Shah, S.; Yadav, H.N.; Ali, J.; Gupta, M.M.; Baboota, S. Quality by Design Engineered, Enhanced Anticancer Activity of Temozolomide and Resveratrol Coloaded NLC and Brain Targeting via Lactoferrin Conjugation in Treatment of Glioblastoma. Eur. J. Pharm. Biopharm. 2023, 191, 175–188. [Google Scholar] [CrossRef]
- Janjua, T.I.; Ahmed-Cox, A.; Meka, A.K.; Mansfeld, F.M.; Forgham, H.; Ignacio, R.M.C.; Cao, Y.; McCarroll, J.A.; Mazzieri, R.; Kavallaris, M.; et al. Facile Synthesis of Lactoferrin Conjugated Ultra Small Large Pore Silica Nanoparticles for the Treatment of Glioblastoma. Nanoscale 2021, 13, 16909–16922. [Google Scholar] [CrossRef] [PubMed]
- Madugulla, L.; Ravula, A.R.; Kondapi, A.K.; Yenugu, S. Evaluation of the Reproductive Toxicity of Antiretroviral Drug Loaded Lactoferrin Nanoparticles. Syst. Biol. Reprod. Med. 2019, 65, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Megeed, R.M.; Abdel-Hamid, A.-H.Z.; Kadry, M.O. Titanium Nanostructure Mitigating Doxorubicin–Induced Testicular Toxicity in Rats via Regulating Major Autophagy Signaling Pathways. Toxicol. Rep. 2025, 14, 101869. [Google Scholar] [CrossRef]
- Chen, H.; Tang, L.; Qin, Y.; Yin, Y.; Tang, J.; Tang, W.; Sun, X.; Zhang, Z.; Liu, J.; He, Q. Lactoferrin-Modified Procationic Liposomes as a Novel Drug Carrier for Brain Delivery. Eur. J. Pharm. Sci. 2010, 40, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Kamalinia, G.; Khodagholi, F.; Atyabi, F.; Amini, M.; Shaerzadeh, F.; Sharifzadeh, M.; Dinarvand, R. Enhanced Brain Delivery of Deferasirox–Lactoferrin Conjugates for Iron Chelation Therapy in Neurodegenerative Disorders: In Vitro and in Vivo Studies. Mol. Pharm. 2013, 10, 4418–4431. [Google Scholar] [CrossRef]
- Meng, F.; Asghar, S.; Gao, S.; Su, Z.; Song, J.; Huo, M.; Meng, W.; Ping, Q.; Xiao, Y. A Novel LDL-Mimic Nanocarrier for the Targeted Delivery of Curcumin into the Brain to Treat Alzheimer’s Disease. Colloids Surf. B Biointerfaces 2015, 134, 88–97. [Google Scholar] [CrossRef]
- Yu, Y.; Pang, Z.; Lu, W.; Yin, Q.; Gao, H.; Jiang, X. Self-Assembled Polymersomes Conjugated with Lactoferrin as Novel Drug Carrier for Brain Delivery. Pharm. Res. 2012, 29, 83–96. [Google Scholar] [CrossRef]
- Fernandes, K.E.; Weeks, K.; Carter, D.A. Lactoferrin Is Broadly Active against Yeasts and Highly Synergistic with Amphotericin B. Antimicrob. Agents Chemother. 2020, 64, 10–1128. [Google Scholar] [CrossRef]
- Fernandes, K.E.; Carter, D.A. The Antifungal Activity of Lactoferrin and Its Derived Peptides: Mechanisms of Action and Synergy with Drugs against Fungal Pathogens. Front. Microbiol. 2017, 8, 2. [Google Scholar] [CrossRef]
- Viani, R.M.; Gutteberg, T.J.; Lathey, J.L.; Spector, S.A. Lactoferrin Inhibits HIV-1 Replication in Vitro and Exhibits Synergy When Combined with Zidovudine. AIDS 1999, 13, 1273. [Google Scholar] [CrossRef]
- Kaito, M.; Iwasa, M.; Fujita, N.; Kobayashi, Y.; Kojima, Y.; Ikoma, J.; Imoto, I.; Adachi, Y.; Hamano, H.; Yamauchi, K. Effect of Lactoferrin in Patients with Chronic Hepatitis C: Combination Therapy with Interferon and Ribavirin. J. Gastroenterol. Hepatol. 2007, 22, 1894–1897. [Google Scholar] [CrossRef]
- Andersen, J.H.; Jenssen, H.; Gutteberg, T.J. Lactoferrin and Lactoferricin Inhibit Herpes Simplex 1 and 2 Infection and Exhibit Synergy When Combined with Acyclovir. Antiviral Res. 2003, 58, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, M.E.; de Vries, H.G.; Eikelboom, M.C.; Meijer, D.K.F.; Swart, P.J. Synergistic Fungistatic Effects of Lactoferrin in Combination with Antifungal Drugs against Clinical Candida Isolates. Antimicrob. Agents Chemother. 1999, 43, 2635–2641. [Google Scholar] [CrossRef]
- Bollimpelli, V.S.; Kumar, P.; Kumari, S.; Kondapi, A.K. Neuroprotective Effect of Curcumin-Loaded Lactoferrin Nano Particles against Rotenone Induced Neurotoxicity. Neurochem. Int. 2016, 95, 37–45. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, B.-Q.; Li, Y.-H.; Jiang, Q.-Q.; Cong, W.-H.; Chen, K.-J.; Wen, X.-M.; Wu, Z.-Z. Lactoferrin Modification of Berberine Nanoliposomes Enhances the Neuroprotective Effects in a Mouse Model of Alzheimer’s Disease. Neural Regen. Res. 2023, 18, 226. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Tsao, C.-W. Neuroprotection against Apoptosis of SK-N-MC Cells Using RMP-7- and Lactoferrin-Grafted Liposomes Carrying Quercetin. Int. J. Nanomed. 2017, 12, 2857–2869. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Cucca, F.; Frau, R.; Corrias, F.; Schlich, M.; Caboni, P.; Fadda, A.M.; Bassareo, V. Systemic Administration of Orexin a Loaded Liposomes Potentiates Nucleus Accumbens Shell Dopamine Release by Sucrose Feeding. Front. Psychiatry 2018, 9, 640. [Google Scholar] [CrossRef]
- Guo, X.; Qiao, X.; Li, X.; Zhou, W.; Liu, C.; Yu, F.; Chen, Q.; Pan, M.; Niu, X.; Wang, X.; et al. Lactoferrin-Modified Organic-Inorganic Hybrid Mesoporous Silica for Co-Delivery of Levodopa and Curcumin in the Synergistic Treatment of Parkinson’s Disease. Phytomedicine 2025, 140, 156547. [Google Scholar] [CrossRef]
- Katila, N.; Duwa, R.; Bhurtel, S.; Khanal, S.; Maharjan, S.; Jeong, J.-H.; Lee, S.; Choi, D.-Y.; Yook, S. Enhancement of Blood–Brain Barrier Penetration and the Neuroprotective Effect of Resveratrol. J. Control. Release 2022, 346, 1–19. [Google Scholar] [CrossRef]
- Alster, P.; Madetko-Alster, N.; Otto-Ślusarczyk, D.; Migda, A.; Migda, B.; Struga, M.; Friedman, A. Role of Orexin in Pathogenesis of Neurodegenerative Parkinsonisms. Neurol. Neurochir. Pol. 2023, 57, 335–343. [Google Scholar] [CrossRef]
- Tiwari, R.; Tickell, K.D.; Yoshioka, E.; Otieno, J.; Shah, A.; Richardson, B.A.; Keter, L.; Okello, M.; Nyabinda, C.; Trehan, I.; et al. Lactoferrin and Lysozyme to Promote Nutritional, Clinical and Enteric Recovery: A Protocol for a Factorial, Blinded, Placebo-Controlled Randomised Trial among Children with Diarrhoea and Malnutrition (the Boresha Afya Trial). BMJ Open 2024, 14, e079448. [Google Scholar] [CrossRef] [PubMed]
- Ellakkany, N.; Abdel-Hady, H.; Eita, A.M.; Mosaad, Y.M.; Megahed, A. Influence of Bovine Lactoferrin on Feeding Intolerance and Intestinal Permeability in Preterm Infants: A Randomized Controlled Trial. Eur. J. Pediatr. 2024, 184, 30. [Google Scholar] [CrossRef] [PubMed]
- Björmsjö, M.; Ekström, N.; Silfverdal, S.A.; Hernell, O.; Lönnerdal, B.; Berglund, S.K. Vaccine Response Was Higher in Formula-fed Infants Compared to Breastfed but Not Affected by Lactoferrin or Iron in a Randomised Controlled Trial. Acta Paediatr. 2024, 113, 2266–2274. [Google Scholar] [CrossRef]
- Ochoa, T.J.; Zegarra, J.; Bellomo, S.; Carcamo, C.P.; Cam, L.; Castañeda, A.; Villavicencio, A.; Gonzales, J.; Rueda, M.S.; Turin, C.G.; et al. Randomized Controlled Trial of Bovine Lactoferrin for Prevention of Sepsis and Neurodevelopment Impairment in Infants Weighing Less Than 2000 Grams. J. Pediatr. 2020, 219, 118–125.e5. [Google Scholar] [CrossRef]
- Ochoa, T.J.; Zegarra, J.; Cam, L.; Llanos, R.; Pezo, A.; Cruz, K.; Zea-Vera, A.; Cárcamo, C.; Campos, M.; Bellomo, S. Randomized Controlled Trial of Lactoferrin for Prevention of Sepsis in Peruvian Neonates Less than 2500 g. Pediatr. Infect. Dis. J. 2015, 34, 571–576. [Google Scholar] [CrossRef]
- Young, G.; Berrington, J.E.; Cummings, S.; Dorling, J.; Ewer, A.K.; Frau, A.; Lett, L.; Probert, C.; Juszczak, E.; Kirby, J.; et al. Mechanisms Affecting the Gut of Preterm Infants in Enteral Feeding Trials: A Nested Cohort within a Randomised Controlled Trial of Lactoferrin. Arch. Dis. Child. Fetal Neonatal Ed. 2023, 108, 272–279. [Google Scholar] [CrossRef]
- Mahmoud, R.M.A.; Mohammed, A. Lactoferrin: A Promising New Player in Treatment of Iron Deficiency Anemia in Patients on Regular Hemodialysis: A Randomized Controlled Trial. Saudi J. Kidney Dis. Transplant. 2023, 34, 235–241. [Google Scholar] [CrossRef]
- Kubo, S.; Oda, H.; Tanaka, M.; Koikeda, T.; Tomita, S. Effects of Lactoferrin on Oral and Throat Conditions under Low Humidity Environments: A Randomized, Double-Blind, and Placebo-Controlled Crossover Trial. Nutrients 2023, 15, 4033. [Google Scholar] [CrossRef] [PubMed]
- Oda, H.; Kubo, S.; Tada, A.; Yago, T.; Sugita, C.; Yoshida, H.; Toida, T.; Tanaka, M.; Kurokawa, M. Effects of Bovine Lactoferrin on the Maintenance of Respiratory and Systemic Physical Conditions in Healthy Adults—A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2023, 15, 3959. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Guo, C. A Review on Lactoferrin and Central Nervous System Diseases. Cells 2021, 10, 1810. [Google Scholar] [CrossRef]
- Eker, F.; Bolat, E.; Pekdemir, B.; Duman, H.; Karav, S. Lactoferrin: Neuroprotection against Parkinson’s Disease and Secondary Molecule for Potential Treatment. Front. Aging Neurosci. 2023, 15, 1204149. [Google Scholar] [CrossRef]
- Marogianni, C.; Sokratous, M.; Dardiotis, E.; Hadjigeorgiou, G.M.; Bogdanos, D.; Xiromerisiou, G. Neurodegeneration and Inflammation—An Interesting Interplay in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 8421. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of Neuroinflammation in Neurodegeneration Development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef] [PubMed]
- Schirmbeck, G.H.; Sizonenko, S.; Sanches, E.F. Neuroprotective Role of Lactoferrin during Early Brain Development and Injury through Lifespan. Nutrients 2022, 14, 2923. [Google Scholar] [CrossRef]
- Faucheux, B.A.; Nillesse, N.; Damier, P.; Spik, G.; Mouatt-Prigent, A.; Pierce, A.; Leveugle, B.; Kubis, N.; Hauw, J.J.; Agid, Y. Expression of Lactoferrin Receptors Is Increased in the Mesencephalon of Patients with Parkinson Disease. Proc. Natl. Acad. Sci. USA 1995, 92, 9603–9607. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.I.; Liu, J.; Dutta, P. Bayesian Inference for Parameter Estimation in Lactoferrin-Mediated Iron Transport across Blood-Brain Barrier. Biochim. Biophys. Acta—(BBA) Gen. Subj. 2020, 1864, 129459. [Google Scholar] [CrossRef] [PubMed]
- Dix, C.; Wright, O. Bioavailability of a Novel Form of Microencapsulated Bovine Lactoferrin and Its Effect on Inflammatory Markers and the Gut Microbiome: A Pilot Study. Nutrients 2018, 10, 1115. [Google Scholar] [CrossRef]
- Lin, Y.; Song, S.; Guo, H. Recent Advances in Synergistic Effect of Lactoferrin. Curr. Opin. Food Sci. 2025, 63, 101285. [Google Scholar] [CrossRef]
- Superti, F. Lactoferrin from Bovine Milk: A Protective Companion for Life. Nutrients 2020, 12, 2562. [Google Scholar] [CrossRef]
- Yao, X.; Bunt, C.; Cornish, J.; Quek, S.-Y.; Wen, J. Oral Delivery of Lactoferrin: A Review. Int. J. Pept. Res. Ther. 2013, 19, 125–134. [Google Scholar] [CrossRef]
- Ong, R.; Cornish, J.; Wen, J. Nanoparticular and Other Carriers to Deliver Lactoferrin for Antimicrobial, Antibiofilm and Bone-Regenerating Effects: A Review. BioMetals 2023, 36, 709–727. [Google Scholar] [CrossRef] [PubMed]



| Study | Model | Lf Dose | Administration Route | Main Results |
|---|---|---|---|---|
| Rousseau et al., 2013 [84] | Midbrain cell cultures | Lf 0.1–30 µM | In Vitro treatment | Lf’s iron binding protects dopamine cells under oxidative stress; Lf accumulation in PD brains may reflect the brain’s protective response. |
| Wang et al., 2015 [83] | Ventral mesencephalon neurons (incubated with MPP+) | Apo- and holo-Lf 100 ng/mL | In Vitro treatment | Apo-Lf and holo-Lf are neuroprotective against MPP+; increase mitochondrial membrane potential; improve Cu/Zn-superoxide dismutase activity; enhance BCL-2 expression. |
| Xu et al., 2019 [85] | Mouse model of PD (MTPT injection) | Human Lf 4 mg/kg body weight | Intraperitoneal injection | Lf promove reduction of MPTP-induced apoptosis of dopaminergic neurons; decrease in neuroinflammation and histological alterations; suppression of excessive iron accumulation; downregulation of DMT1 and TFR; improvement in antioxidant enzyme activity. |
| Liu et al., 2020 [37] | Mouse model of PD (MTPT injection) | Apo- and holo-Lf 5, 10 and 15 mg/kg | Intragastric gavage | Lf treatment downregulated DMT1 and upregulated ferroportin 1; alleviated MPTP-induced accumulation of nigral iron; reduced serum iron and ferritin levels; decreased spleen iron content and spleen weight loss. |
| Kopaeva et al., 2021 [86] | Mouse model of PD (MTPT injection) | Human Lf 4 mg/animal | Intraperitoneal injection | Lf reduced MPTP toxicity; improvement in motor function and exploratory behavior; partial recovery of dopaminergic neurons in the substantia nigra; increase in TH-positive fibers in the striatum; evidence of neuroprotective and compensatory mechanisms. |
| Xu et al., 2024 [87] | Mouse model of PD (MTPT injection) and knockout of the astrocyte Lf gene | - | - | MPTP-treated astrocytic Lf knockout mice exhibited abnormal levels of effects implicated in glutamate and calcium homeostasis; mitochondrial dysfunction; and signs of oxidative stress. |
| Yong et al., 2024 [88] | Cellular model of PD (differentiating SH-SY5Y to dopaminergic neurons and exposure to rotenone) | Lf 1–10 µg/mL | In Vitro treatment | Lactoferrin pre-treatment reduced cell viability loss; prevented mitochondrial membrane potential impairment; decreased ROS generation; reduced pro-apoptotic activities (caspase activation and nuclear condensation); decreased Bax:Bcl2 ratio; increased pAkt expression. |
| Study | Model | Related-Disease | Lf Interaction | Administration Route | Main Results |
|---|---|---|---|---|---|
| Bollimpelli et al.,2016 [154] | Cell line SK-N-SH | PD | Curcumin loaded Lf nano particles prepared by sol-oil chemistry. | In Vitro treatment | Higher intracellular drug uptake; sustained drug retention; greater neuroprotective activity; reduced ROS levels. |
| Kuo & Tsao, 2017 [156] | Cell line SK-N-MC | AD | Quercetin, encapsulated liposomes grafted with RMP-7 and LF. | In Vitro treatment | Inhibited cell apoptosis and the expression of phosphorylated proteins associated with apoptosis; low toxicity; increased viability of SK-N-MC cells and reduced neurotoxicity induced by β-amyloid fibrils. |
| Lai et al., 2018 [157] | Male Sprague– Dawley rats | Not related disease | Orexin A-loaded lactoferrin-conjugated Liposomes. | Intravenous injection | Lactoferrin-conjugated liposomes with orexin A enhanced dopamine release in the nucleus accumbens shell; facilitate orexin A delivery to the central nervous system. |
| Wang et al., 2023 [155] | Mouse model of AD (Aβ-injected) | AD | Lf-modified berberine nanoliposomes. | Injection via caudal vein | Improved mouse behavior; reduced tau over-phosphorylation; inhibited acetylcholinesterase activity; and enhanced neuroprotective effects. |
| Guo et al., 2024 [158] | Mouse model of PD (MTPT injection) | PD | Carbon dots, polyethylene glycol and Lf. | Intravenous injection | Antioxidant; reduction of oxidative stress; reduction of brain inflammation; behavioral improvement. |
| Katila et al., 2022 [159] | Mouse model of PD (MTPT injection); SH-SY5Y and HBMECs cells | PD | Lf-conjugated resveratrol-loaded PLGA nanoparticles conjugated with Lf. | Injection via caudal vein | Increased internalization in SH-SY5Y and brain endothelial cells; Reduction of oxidative stress; improved brain bioavailability; enhanced protective effects in the MPTP-induced PD model. |
| Pham et al., 2025 [139] | HBMECs and SH-SY5Y cells; C57BL/6J mice (methamphetamine PD model) and female BALB/c | PD | Lf-decorated CAY10603-loaded poly(lactic-co-glycolic acid) nanoparticles. | Injection via caudal vein | Enhanced BBB penetration; restoration of acetylation balance; reversed mitochondrial dysfunction; suppressed ROS; inhibited aSyn accumulation; normalized dopamine and tyrosine hydroxylase levels; improved behavioral impairments in the Meth-induced PD mouse model. |
| Nguyen et al., 2025 [138] | Cell line SH-SY5Y and C57BL/6 mice (MTPT injection) | PD | Lf-conjugated astaxanthin-loaded liposomes. | Intravenous injection | Cytoprotective effects in vitro; liposomes demonstrated significantly improved cellular uptake; neuroprotective effects in the MPTP mouse model; alleviated behavioral impairments. |
| Guo et al., 2025 [158] | Mouse model of PD (MTPT injection) and SH-SY5Y cells | PD | LF-modified silica nanoparticles for co-delivery of levodopa and curcumin. | Intraperitoneal injection | Reduction of oxidative stress; lower aSyn accumulation; increased neuronal survival; optimized brain delivery; improvement of motor function; low systemic toxicity. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barros, C.A.; Vieira, T.C.R.G. Lactoferrin as a Candidate Multifunctional Therapeutic in Synucleinopathies. Brain Sci. 2025, 15, 380. https://doi.org/10.3390/brainsci15040380
Barros CA, Vieira TCRG. Lactoferrin as a Candidate Multifunctional Therapeutic in Synucleinopathies. Brain Sciences. 2025; 15(4):380. https://doi.org/10.3390/brainsci15040380
Chicago/Turabian StyleBarros, Caroline A., and Tuane C. R. G. Vieira. 2025. "Lactoferrin as a Candidate Multifunctional Therapeutic in Synucleinopathies" Brain Sciences 15, no. 4: 380. https://doi.org/10.3390/brainsci15040380
APA StyleBarros, C. A., & Vieira, T. C. R. G. (2025). Lactoferrin as a Candidate Multifunctional Therapeutic in Synucleinopathies. Brain Sciences, 15(4), 380. https://doi.org/10.3390/brainsci15040380






