Enhancing Hand Sensorimotor Function in Individuals with Cervical Spinal Cord Injury: A Novel Tactile Discrimination Feedback Approach Using a Multiple-Baseline Design
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Introduction
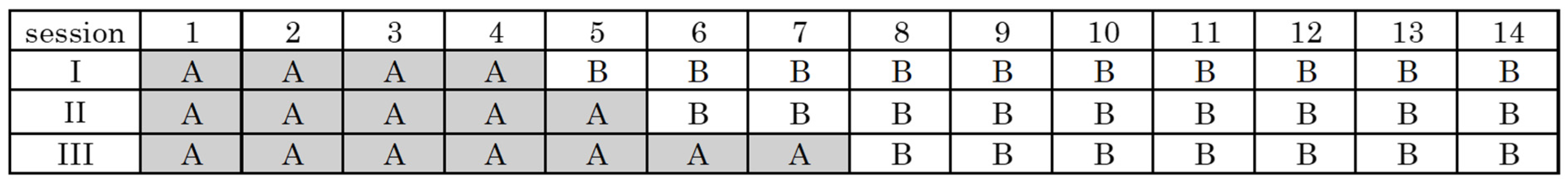
2.2. Intervention Methods
2.3. Analysis Method
3. Results
4. Discussion
4.1. Limitations
4.2. Future Challenges
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SCI | Spinal cord injury |
| EEG | Electroencephalogram |
| ASIA | American Spinal Injury Association |
| MRI | Magnetic Resonance Imaging |
| C | cervical |
| NRS | Numerical Rating Scale |
| Peg Test | Purdue Pegboard Test |
| Yubireco | Finger Recorder |
| MBD | Multiple Baseline Design |
| MAL-14 | Motor Activity Log-14 |
| AoU | Amount of use |
| QoM | Quality of movement |
| SoA | Sense of agency |
| ICA | Independent component analysis |
| dB | Decibel |
| PSD | Power spectral density |
| μV2/Hz | Microvolts squared per Hertz |
| AVG | Average |
| SE | Standard Error |
| I | Case I |
| II | Case II |
| III | Case III |
| Combined | Combined cases I–III |
References
- van Den Hauwe, L.; Sundgren, P.C.; Flanders, A.E. Spinal trauma and spinal cord injury (SCI). In Diseases of the Brain, Head and Neck, Diagnostic Imaging; Hodler, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 231–240. [Google Scholar] [CrossRef]
- Fakhoury, J.; Dowling, T.J. Cervical degenerative disc disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Kadaňka, Z.; Bednařík, J.; Novotný, O.; Urbánek, I.; Dušek, L. Cervical spondylotic myelopathy: Conservative surgical versus surgical treatment after 10 years. Eur. Spine J. 2011, 20, 1533–1538. [Google Scholar] [CrossRef] [PubMed]
- Divi, S.N.; Schroeder, G.D.; Mangan, J.J.; Tadley, M.; Ramey, W.L.; Badhiwala, J.H.; Fehlings, M.G.; Oner, F.C.; Kandziora, F.; Benneker, L.M.; et al. Management of acute traumatic central cord syndrome: A narrative review. Glob. Spine J. 2019, 9 (Suppl. S1), 89S–97S. [Google Scholar] [CrossRef] [PubMed]
- Simpson, L.A.; Eng, J.J.; Hsieh, J.T.C.; Wolfe, D.L.; Spinal Cord Injury Rehabilitation Evidence Scire Research Team. The health and life priorities of individuals with spinal cord injury: A systematic review. J. Neurotrauma 2012, 29, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Jia, L. Acute central cervical cord injury presenting with only upper extremity involvement. Int. Orthop. 1997, 21, 380–382. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Walker, J.R.; Detloff, M.R. Plasticity in cervical motor circuits following spinal cord injury and rehabilitation. Biology 2021, 10, 976. [Google Scholar] [CrossRef]
- Rehman, M.U.; Sneed, D.; Sutor, T.W.; Hoenig, H.; Gorgey, A.S. Optimization of Transspinal Stimulation Applications for Motor Recovery After Spinal Cord Injury: Scoping Review. J. Clin. Med. 2023, 12, 854. [Google Scholar] [CrossRef]
- Jiang, Z.; Davies, B.; Zipser, C.; Margetis, K.; Martin, A.; Matsoukas, S.; Zipser-Mohammadzada, F.; Kheram, N.; Boraschi, A.; Zakin, E.; et al. Diagnostic criteria incubator. The frequency of symptoms in patients with a diagnosis of degenerative cervical myelopathy: Results of a scoping review. Glob. Spine J. 2024, 14, 1395–1421. [Google Scholar] [CrossRef]
- Sarlegna, F.R.; Sainburg, R.L. The roles of vision and proprioception in the planning of reaching movements. Adv. Exp. Med. Biol. 2009, 629, 317–335. [Google Scholar] [CrossRef]
- Khojasteh, B.; Janko, M.; Visell, Y. Complexity, rate, and scale in sliding friction dynamics between a finger and textured surface. Sci. Rep. 2018, 8, 13710. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kanzler, C.M.; Schwarz, A.; Held, J.P.O.; Luft, A.R.; Gassert, R.; Lambercy, O. Technology-aided assessment of functionally relevant sensorimotor impairments in arm and hand of post-stroke individuals. J. Neuroeng. Rehabil. 2020, 17, 128. [Google Scholar] [CrossRef]
- Doyle, S.; Bennett, S.; Fasoli, S.E.; McKenna, K.T. Interventions for sensory impairment in the upper limb after stroke. Cochrane Database Syst. Rev. 2010, 2010, CD006331. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakagawa, H.; Ninomiya, T.; Yamashita, T.; Takada, M. Treatment with the neutralizing antibody against repulsive guidance molecule-A promotes recovery from impaired manual dexterity in a primate model of spinal cord injury. Cereb. Cortex 2019, 29, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Hoogewoud, F.; Hamadjida, A.; Wyss, A.F.; Mir, A.; Schwab, M.E.; Belhaj-Saif, A.; Rouiller, E.M. Comparison of functional recovery of manual dexterity after unilateral spinal cord lesion or motor cortex lesion in adult macaque monkeys. Front. Neurol. 2013, 4, 101. [Google Scholar] [CrossRef]
- Kitai, K.; Ueda, T.; Yamauchi, R.; Mizushima, Y.; Murata, S.; Nakano, H.; Inoue, M.; Nagano, H.; Kodama, T. Effectiveness of sensory compensation approach for hand sensory-motor dysfunction following central cervical spinal cord injury. Int. J. Phys. Med. Rehabil. 2022, 10, 649. [Google Scholar]
- Kitai, K.; Ito, D.; Murata, S.; Katayama, O.; Kodama, T. Verifying the efficacy of a tactile perceptual discrimination stimulation approach for individuals with finger sensorimotor dysfunction: A case report and literature review. Ann. Case Rep. 2024, 9, 1950. [Google Scholar] [CrossRef]
- Kalsi-Ryan, S.; Beaton, D.; Curt, A.; Duff, S.; Popovic, M.R.; Rudhe, C.; Fehlings, M.G.; Verrier, M.C. The Graded Redefined Assessment of Strength Sensibility and prehension: Reliability and validity. J. Neurotrauma 2012, 29, 905–914. [Google Scholar] [CrossRef]
- Gruenwald, J.; Znobishchev, A.; Kapeller, C.; Kamada, K.; Scharinger, J.; Guger, C. Time-variant linear discriminant analysis improves hand gesture and finger movement decoding for invasive brain-computer interfaces. Front. Neurosci. 2019, 13, 901. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salis, F.; Costaggiu, D.; Mandas, A. Mini-Mental State Examination: Optimal cut-off levels for mild and severe cognitive impairment. Geriatrics 2023, 8, 12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kitai, K.; Odagiri, M.; Yamauchi, R.; Kodama, T. Evaluation of intervention effectiveness of sensory compensatory training with tactile discrimination feedback on sensorimotor dysfunction of the hand after stroke. Brain Sci. 2021, 11, 1314. [Google Scholar] [CrossRef]
- Irie, K.; Iseki, H.; Okamoto, K.; Nishimura, S.; Kagechika, K. Introduction of the purdue pegboard test for fine assessment of severity of cervical myelopathy before and after surgery. J. Phys. Ther. Sci. 2020, 32, 210–214. [Google Scholar] [CrossRef]
- Tanaka, Y.; Ueda, Y.; Sano, A. Roughness evaluation by wearable tactile sensor utilizing human active sensing. Mech. Eng. J. 2016, 3, 15–460. [Google Scholar] [CrossRef]
- Kinugasa, T.; Cerin, E.; Hooper, S. Single-subject research designs and data analyses for assessing elite athletes’ conditioning. Sports Med. 2004, 34, 1035–1050. [Google Scholar] [CrossRef] [PubMed]
- Kratochwill, T.R.; Hitchcock, J.; Horner, R.H.; Levin, J.R.; Odom, S.L.; Rindskopf, D.M.; Shadish, W.R. Single-Case Designs Technical Documentation. 2010. Available online: https://files.eric.ed.gov/fulltext/ED510743.pdf (accessed on 3 January 2025).
- Taub, E.; Uswatte, G.; King, D.K.; Morris, D.; Crago, J.E.; Chatterjee, A. A placebo-controlled trial of constraint-induced movement therapy for upper extremity after stroke. Stroke 2006, 37, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Hyvärinen, A.; Oja, E. Independent component analysis: Algorithms and applications. Neural Netw. 2000, 13, 411–430. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Bell, A.J.; Sejnowski, T.J. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995, 7, 1129–1159. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, Z.; Zhang, B.; Feng, B.; Yu, T.; Li, Z. The CSP-based new features plus non-convex log sparse feature selection for motor imagery EEG classification. Sensors 2020, 20, 4749. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parker, R.I.; Vannest, K.J.; Davis, J.L.; Sauber, S.B. Combining nonoverlap and trend for single–case research: Tau–U. Behav. Ther. 2011, 42, 284–299. [Google Scholar] [CrossRef]
- Ninci, J.; Vannest, K.J.; Willson, V.; Zhang, N. Interrater agreement between visual analysts of single-case data: A meta-analysis. Behav. Modif. 2015, 39, 510–541. [Google Scholar] [CrossRef]
- Vannest, K.J.; Parker, R.I.; Gonen, O.; Adiguzel, T. Single Case Res.: Web-Based Calculators for SCR Analysis. (Version 2.0); [Web-Based Application]; Texas A&M University: College Station, TX, USA, 2016; Available online: https://singlecaseresearch.org/ (accessed on 24 March 2024).
- Quandt, F.; Bönstrup, M.; Schulz, R.; Timmermann, J.E.; Zimerman, M.; Nolte, G.; Hummel, F.C. Spectral variability in the aged brain during fine motor control. Front. Aging Neurosci. 2016, 8, 305. [Google Scholar] [CrossRef]
- Weersink, J.B.; Gefferie, S.R.; van Laar, T.; Maurits, N.M.; de Jong, B.M. Pre-Movement Cortico-Muscular Dynamics Underlying Improved Parkinson Gait Initiation After Instructed Arm Swing. J. Park. Dis. 2020, 10, 1675–1693. [Google Scholar] [CrossRef] [PubMed]
- Botrel, L.; Kreilinger, A.; Müller, M.; Pfeiffer, M.; Scheu, V.; Vowinkel, N.; Zechner, R.; Käthner, I.; Kübler, A. The influence of time and visualization on neurofeedback-guided parietal alpha downregulation and sense of presence in virtual reality. Front. Neurosci. 2025, 19, 1476264. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Balbi, M.; Xiao, D.; Jativa Vega, M.; Hu, H.; Vanni, M.P.; Bernier, L.P.; LeDue, J.; MacVicar, B.; Murphy, T.H. Gamma frequency activation of inhibitory neurons in the acute phase after stroke attenuates vascular and behavioral dysfunction. Cell Rep. 2021, 34, 108696. [Google Scholar] [CrossRef]
- Amo Usanos, C.; Boquete, L.; de Santiago, L.; Barea Navarro, R.; Cavaliere, C. Induced gamma-band activity during actual and imaginary movements: EEG analysis. Sensors 2020, 20, 1545. [Google Scholar] [CrossRef] [PubMed Central]
- Uhlhaas, P.J.; Haenschel, C.; Nikolić, D.; Singer, W. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr. Bull. 2008, 34, 927–943. [Google Scholar] [CrossRef]
- Tallon-Baudry, C.; Bertrand, O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn. Sci. 1999, 3, 151–162. [Google Scholar] [CrossRef]
- Spooner, R.K.; Wilson, T.W. Cortical theta-gamma coupling governs the adaptive control of motor commands. Brain Commun. 2022, 4, fcac249. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Struber, L.; Baumont, M.; Barraud, P.A.; Nougier, V.; Cignetti, F. Brain oscillatory correlates of visuomotor adaptive learning. NeuroImage 2021, 245, 118645. [Google Scholar] [CrossRef]
- Gomes-Osman, J.; Field-Fote, E.C. Cortical vs. afferent Stimulation as an Adjunct to Functional Task Practice Training: A Randomized, Comparative Pilot Study in People with Cervical Spinal Cord Injury. Clin. Rehabil. 2015, 29, 771–782. [Google Scholar] [CrossRef]
- Wright, D.J.; Holmes, P.S.; Di Russo, F.; Loporto, M.; Smith, D. Differences in cortical activity related to motor planning between experienced guitarists and non-musicians during guitar playing. Hum. Mov. Sci. 2012, 31, 567–577. [Google Scholar] [CrossRef]
- Leech, K.A.; Roemmich, R.T.; Gordon, J.; Reisman, D.S.; Cherry-Allen, K.M. Updates in motor learning: Implications for physical therapist practice and education. Phys. Ther. 2022, 102, pzab250. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Pala, A.; Pedrido, L.; Kremer, Y.; Welker, E.; Petersen, C.C.H. Membrane potential dynamics of neocortical projection neurons driving target-specific signals. Neuron 2013, 80, 1477–1490. [Google Scholar] [CrossRef]
- Yanagisawa, T.; Hirata, M.; Saitoh, Y.; Kishima, H.; Matsushita, K.; Goto, T.; Fukuma, R.; Yokoi, H.; Kamitani, Y.; Yoshimine, T. Electrocorticographic control of a prosthetic arm in paralyzed patients. Ann. Neurol. 2012, 71, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Cohen, L.G. Recovery of motor function after stroke. Dev. Psychobiol. 2012, 54, 254–262. [Google Scholar] [CrossRef]
- Synofzik, M.; Vosgerau, G.; Newen, A. Beyond the comparator model: A multifactorial two-step account of agency. Conscious. Cogn. 2008, 17, 219–239. [Google Scholar] [CrossRef]
- Chen, L.; Meng, F.; Huo, C.; Shao, G.; Pan, G.; Zhang, X.; Zhang, S.; Li, Z. Effects of tactile feedback in post-stroke hand rehabilitation on functional connectivity and cortical activation: An fNIRS study. Biomed. Opt. Express 2025, 16, 643. [Google Scholar] [CrossRef]
- Mishra, B.; Sudheer, P.; Agarwal, A.; Srivastava, M.V.P.; Nilima, V.; Vishnu, V.Y. Minimal clinically important difference (MCID) in patient-reported outcome measures for neurological conditions: Review of concept and methods. Ann. Indian Acad. Neurol. 2023, 26, 334–343. [Google Scholar] [CrossRef]
- Reuter, E.M.; Booms, A.; Leow, L.A. Using EEG to study sensorimotor adaptation. Neurosci. Biobehav. Rev. 2022, 134, 104520. [Google Scholar] [CrossRef]

| Cases | I | II | III |
|---|---|---|---|
| Gender, Age (yr) | Woman, 83 | Man, 51 | Man, 53 |
| Height (cm), weight (kg), Body Mass Index | 154.0, 42.6, 18.0 | 174.0, 55.5, 18.3 | 162.0, 59.1, 22.5 |
| Diagnosis | Cervical spondylotic myelopathy (C3/4) | Cervical spondylotic myelopathy (C3–6) | Cervical spinal cord injury (C7) |
| Operative History | Cervical laminectomy (C3/4) | Cervical laminoplasty (C3–6) | None |
| Numbness Numerical Rating Scale | Right fingers 1 | Right fingers 8 | Right fingers 4 |
| Purdue Pegboard Test (Quantity) | 6.0 | 1.5 | 10.0 |
| The American Spinal Injury Association score | |||
| Motor Function | C6: 3/5 | C6: 2/5 | C6: 4/5 |
| C7: 3/5 | C7: 2/5 | C7: 5/5 | |
| C8: 3/5 | C8: 3/5 | C8: 4/5 | |
| Sensory Function | C6: 2/3 | C6: 1/3 | C6: 1/3 |
| C7: 2/3 | C7: 1/3 | C7: 1/3 | |
| C8: 2/3 | C8: 1/3 | C8: 1/3 | |
| Study start date | X+about 2 months | X+about 2 weeks | X+about 3 months |
| History | Hypertension, chronic gastritis, osteoporosis | None | Hypertension, insomnia |
| Gamma Wave (40–100 Hz) | ||||
|---|---|---|---|---|
| Fz (dB) | Pz (dB) | |||
| A AVG ± SE | B AVG ± SE | A AVG ± SE | B AVG ± SE | |
| I | 0.015 ± 0.008 | 0.016 ± 0.019 | 0.008 ± 0.001 | 0.014 ± 0.013 |
| II | 0.074 ± 0.038 | 0.090 ± 0.036 | 0.007 ± 0.003 | 0.055 ± 0.023 |
| III | 0.744 ± 0.281 | 0.010 ± 0.003 | 0.852 ± 0.322 | 0.033 ± 0.017 |
| A AVG ± SE | B AVG ± SE | Tau-U | |
|---|---|---|---|
| I | 6.50 ± 0.35 | 7.30 ± 0.26 | 0.67 |
| II | 2.30 ± 0.34 | 3.28 ± 0.30 | 0.67 |
| III | 11.9 ± 0.38 | 14.9 ± 0.26 | 1.00 |
| Combined | 0.80 |
| A AVG ± SE | B AVG ± SE | Tau-U | |
|---|---|---|---|
| I | 1.00 ± 0.00 | 1.00 ± 0.00 | 0.00 |
| II | 7.80 ± 0.20 | 7.11 ± 0.31 | 0.52 |
| III | 3.70 ± 0.49 | 1.00 ± 0.58 | 1.00 |
| Combined | 0.65 |
| I | AoU | QoM | SoA |
| Initial | 2.85 | 2.46 | 5.69 |
| A | 3.08 | 2.54 | 5.69 |
| B | 3.62 | 3.23 | 6.92 |
| II | AoU | QoM | SoA |
| Initial | 0.64 | 0.43 | 0.79 |
| A | 1.14 | 1.36 | 1.29 |
| B | 1.50 | 1.43 | 1.64 |
| III | AoU | QoM | SoA |
| Initial | 4.07 | 4.07 | 8.29 |
| A | 4.21 | 4.21 | 8.71 |
| B | 4.71 | 4.71 | 9.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitai, K.; Nishigaya, K.; Mizomoto, Y.; Ito, H.; Yamauchi, R.; Katayama, O.; Morita, K.; Murata, S.; Kodama, T. Enhancing Hand Sensorimotor Function in Individuals with Cervical Spinal Cord Injury: A Novel Tactile Discrimination Feedback Approach Using a Multiple-Baseline Design. Brain Sci. 2025, 15, 352. https://doi.org/10.3390/brainsci15040352
Kitai K, Nishigaya K, Mizomoto Y, Ito H, Yamauchi R, Katayama O, Morita K, Murata S, Kodama T. Enhancing Hand Sensorimotor Function in Individuals with Cervical Spinal Cord Injury: A Novel Tactile Discrimination Feedback Approach Using a Multiple-Baseline Design. Brain Sciences. 2025; 15(4):352. https://doi.org/10.3390/brainsci15040352
Chicago/Turabian StyleKitai, Ken, Kaichi Nishigaya, Yasuhisa Mizomoto, Hiroki Ito, Ryosuke Yamauchi, Osamu Katayama, Kiichiro Morita, Shin Murata, and Takayuki Kodama. 2025. "Enhancing Hand Sensorimotor Function in Individuals with Cervical Spinal Cord Injury: A Novel Tactile Discrimination Feedback Approach Using a Multiple-Baseline Design" Brain Sciences 15, no. 4: 352. https://doi.org/10.3390/brainsci15040352
APA StyleKitai, K., Nishigaya, K., Mizomoto, Y., Ito, H., Yamauchi, R., Katayama, O., Morita, K., Murata, S., & Kodama, T. (2025). Enhancing Hand Sensorimotor Function in Individuals with Cervical Spinal Cord Injury: A Novel Tactile Discrimination Feedback Approach Using a Multiple-Baseline Design. Brain Sciences, 15(4), 352. https://doi.org/10.3390/brainsci15040352






