Could Traumatic Brain Injury Be a Risk Factor for Bruxism and Temporomandibular Disorders? A Scoping Review
Abstract
1. Introduction
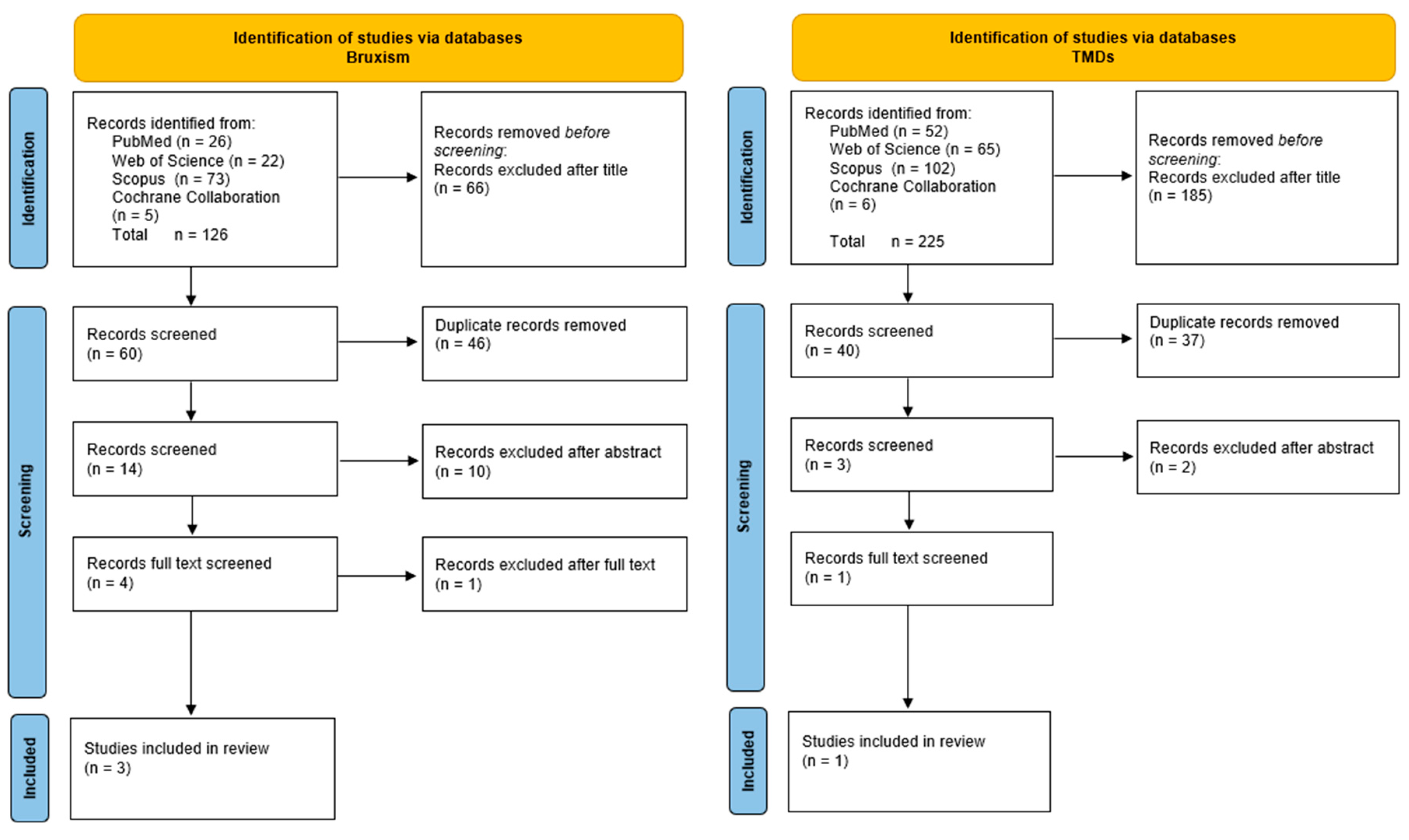
2. Materials and Methods
The Data Collection Process
3. Results
| Author | Group Details | Diagnosis of TBI | Diagnosis of the Stomatognathic System | Suggested Mechanism |
|---|---|---|---|---|
| Bruxism | ||||
| Chaudhuri [23] | 12 participants (2 females, 10 males) Age range: 3 to 58 years | The author describes the use of the Glasgow Coma Scale (GCS) [36]. However, the description in Table 1 in [23] suggests the use of imaging studies. | In this study, bruxism was examined based on clinical observations and physical symptoms, such as teeth grinding and masseter muscle spasms, which were visible in all patients. | The main mechanism linking TBI and bruxism is the damage to CPGs in the brainstem, which are responsible for controlling rhythmic jaw movements. TBI may lead to dysfunction of these structures and disturbances in the dopaminergic system, resulting in uncontrolled clenching and teeth grinding. Pramipexole, as a dopamine agonist, may help normalize these processes, thereby reducing the symptoms of bruxism. |
| Kothari et al. [33] | 10 participants, 4 classified as TBI, (1 female, 4 males) Age range: 20 to 63 years | The author describes the use of the Early Functional Ability [37] and Ranchos Los Amigos Scale [38], but no other studies were mentioned. The patients were treated at the Neurorehabilitation and University Research Clinic, Hammel, Denmark, which suggests additional studies related to TBI that are not described in the paper. | An electromyographic study recorded EMG activity of the anterior temporalis muscles for two hours in two different sessions, in patients in a state most similar to sleep. The EMG device detected “bruxism-like behavior” [39] using a moving average algorithm, analyzing episodes of muscle activity exceeding three times the background level [40]. | TBI can lead to bruxism through disturbances in the functioning of the central pattern generator in the brainstem and dysfunction of the autonomic nervous system, resulting in increased jaw muscle activity. This may serve a compensatory function, helping to maintain airway patency and mucosal hydration. However, it also leads to negative outcomes, such as excessive tooth wear and muscle overload, and its treatment in TBI patients is particularly challenging. |
| Suzuki et al. [34] | n = 24 with mTBI (9 females, 15 males), control group n = 20 (12 female, 8 men) Mean age of mTBI group: 38 ± 11, mean age of control group: 31 ± 9 | The confirmation of TBI was based on an examination by a neurosurgeon, who conducted an assessment according to the diagnosis criteria of the World Health Organization Task Force [41]. The diagnosis included a GCS score of 13–15 at the time of medical care admission. The period of loss of consciousness, mental disorientation, and/or post-traumatic amnesia did not exceed 30 min after the injury. | A polysomnographic study assessed sleep stages based on 20-s epochs of electroencephalography (EEG), electrooculography (EOG), and EMG of the mentalis muscle, following the modified Rechtschaffen and Kales criteria [42]. Sleep parameters such as total sleep time, sleep efficiency, REM sleep latency (time from lights off to the onset of REM sleep), micro-arousals (MAI—micro-arousal index), apnea–hypopnea index (AHI, frequency of apnea and hypopnea episodes), and periodic limb movement index (PLMI, frequency of involuntary limb movements) were analyzed. The frequency of rhythmic masticatory muscle activity (RMMA) during sleep was assessed based on EMG recordings of the masseter muscle and video recordings, classifying episodes as phasic (a series of at least three bursts within 2 s), tonic (a sustained contraction lasting more than 2 s), or mixed. Additionally, the amplitude and frequency of RMMA episodes were calculated relative to total sleep time. | Current findings suggest that the occurrence of mild traumatic brain injury (mTBI) is not associated with an increased risk of bruxism. However, in patients with mTBI who experience persistent and difficult-to-manage headaches, it is advisable to conduct a diagnostic evaluation for bruxism, as it may influence their symptoms. |
| Temporomandibular Disorders | ||||
| Karpuz et al. [35] | 30 patients with TBI (10 females, 10 males) and 30 in the control group, both women and men Mean age of control group: 35.33 ± 9.6, mean age of TBI group: 37.47 ± 9.295 | A physical examination for the presence of complications related to TBI. | The examination for TMDs was conducted using the Fonseca Questionnaire [43] for temporomandibular joint dysfunction, the range of motion of the temporomandibular joint, the mandibular angle, and the pain pressure threshold of the temporalis and masseter muscles. | TBI leads to TMDs through the activation of neuronal mechanisms, such as central sensitization and synaptic plasticity within the trigeminal system. Damage to neural structures, including the facial and trigeminal nerves, can cause sensory disturbances, muscular asymmetry, and chronic pain through excessive activation of microglia and increased pain conduction in the central nervous system. Additionally, alterations in pain processing within the brainstem and sensory cortex may exacerbate TMD symptoms, which explains the high prevalence of this condition in TBI patients, particularly those with post-traumatic headaches. |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- BIAA. Adopts New TBI Definition—Brain Injury Association of America. Available online: https://www.biausa.org/public-affairs/public-awareness/news/biaa-adopts-new-tbi-definition (accessed on 12 November 2024).
- National Academies of Sciences, Medicine, Medicine Division, Board on Health Care Services, and Committee on the Review of the Department of Veterans Affairs Examinations for Traumatic Brain Injury. Evaluation of the Disability Determination Process for Traumatic Brain Injury in Veterans; National Academies Press (US): Cambridge, MA, USA, 2019. [Google Scholar]
- CDC. TBI Data. Available online: https://www.cdc.gov/traumatic-brain-injury/data-research/index.html (accessed on 12 November 2024).
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.-C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the Global Incidence of Traumatic Brain Injury. J. Neurosurg. 2019, 130, 1080–1097. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.H.; Fazli, Y.; Lund, S.; Qazi, S.U.; Tahir, R.; Masood, A.Z.; Qureshi, A.A.; Safdar, S.; Zaheer, H.; Chaychi, M.T. Mortality Trends of Traumatic Brain Injuries in the Adult Population of the United States: A CDC WONDER Analysis from 1999 to 2020. BMC Public Health 2025, 25, 482. [Google Scholar] [CrossRef] [PubMed]
- Kan, V.; Huang, W.; Steigauf-Regan, G.; Anderson, J.; Dang, I.; Darling, C. Injuries and Outcomes of Ground-Level Falls Among Older Patients: A Retrospective Cohort Study. In Western Journal of Emergency Medicine: Integrating Emergency Care with Population Health; University of California: Los Angeles, CA, USA, 2025. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, W.; Zhou, G.; Huang, X.; Xu, M.; Zhao, Q.; Yan, H. Relationship between Alcohol Use and Traumatic Brain Injury: Evidence from Mendelian Randomization. Brain Inj. 2025. [Google Scholar] [CrossRef]
- Thompson, H.J.; McCormick, W.C.; Kagan, S.H. Traumatic Brain Injury in Older Adults: Epidemiology, Outcomes, and Future Implications. J. Am. Geriatr. Soc. 2006, 54, 1590–1595. [Google Scholar] [CrossRef]
- Montero-Odasso, M.; van der Velde, N.; Martin, F.C.; Petrovic, M.; Tan, M.P.; Ryg, J.; Aguilar-Navarro, S.; Alexander, N.B.; Becker, C.; Blain, H.; et al. World Guidelines for Falls Prevention and Management for Older Adults: A Global Initiative. Age Ageing 2022, 51, afac205. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, K.; Gogna, S.; Pee, S.; Samson, D.J.; Con, J.; Latifi, R. Falling Again? Falls in Geriatric Adults—Risk Factors and Outcomes Associated With Recidivism. J. Surg. Res. 2020, 247, 66–76. [Google Scholar] [CrossRef]
- Militi, A.; Bonanno, M.; Calabrò, R.S. It Is Time for a Multidisciplinary Rehabilitation Approach: A Scoping Review on Stomatognathic Diseases in Neurological Disorders. J. Clin. Med. 2023, 12, 3528. [Google Scholar] [CrossRef]
- Jha, S.; Ghewade, P. Management and Treatment of Traumatic Brain Injuries. Cureus 2022, 14, e30617. [Google Scholar] [CrossRef]
- Ivanhoe, C.B.; Lai, J.M.; Francisco, G.E. Bruxism after Brain Injury: Successful Treatment with Botulinum Toxin-A. Arch. Phys. Med. Rehabil. 1997, 78, 1272–1273. [Google Scholar] [CrossRef]
- Meng, Q.; Li, B.; Long, X.; Li, J.; Yan, Q. Ankylosis of Temporomandibular Joint after the Traumatic Brain Injury: A Report of Two Cases. Dent. Traumatol. Off. Publ. Int. Assoc. Dent. Traumatol. 2013, 29, 328–333. [Google Scholar] [CrossRef]
- Phuong, N.T.T.; Ngoc, V.T.N.; Linh, L.M.; Duc, N.M.; Tra, N.T.; Anh, L.Q. Bruxism, Related Factors and Oral Health-Related Quality of Life Among Vietnamese Medical Students. Int. J. Environ. Res. Public Health 2020, 17, 7408. [Google Scholar] [CrossRef] [PubMed]
- Wieckiewicz, M.; Jenca, A.; Seweryn, P.; Orzeszek, S.; Petrasova, A.; Grychowska, N.; Winocur-Arias, O.; Emodi-Perlman, A.; Kujawa, K. Determination of Pain Intensity, Pain-Related Disability, Anxiety, Depression, and Perceived Stress in Polish Adults with Temporomandibular Disorders: A Prospective Cohort Study. Front. Integr. Neurosci. 2022, 16, 1026781. [Google Scholar] [CrossRef] [PubMed]
- Seweryn, P.; Orzeszek, S.M.; Waliszewska-Prosół, M.; Jenča, A.; Osiewicz, M.; Paradowska-Stolarz, A.; Winocur-Arias, O.; Ziętek, M.; Bombała, W.; Więckiewicz, M. Relationship between Pain Severity, Satisfaction with Life and the Quality of Sleep in Polish Adults with Temporomandibular Disorders. Dent. Med. Probl. 2023, 60, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, G.; Pająk-Zielińska, B.; Ginszt, M. A Meta-Analysis of the Global Prevalence of Temporomandibular Disorders. J. Clin. Med. 2024, 13, 1365. [Google Scholar] [CrossRef]
- Zieliński, G.; Pająk, A.; Wójcicki, M. Global Prevalence of Sleep Bruxism and Awake Bruxism in Pediatric and Adult Populations: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 4259. [Google Scholar] [CrossRef]
- Lobbezoo, F.; Ahlberg, J.; Raphael, K.G.; Wetselaar, P.; Glaros, A.G.; Kato, T.; Santiago, V.; Winocur, E.; De Laat, A.; De Leeuw, R.; et al. International Consensus on the Assessment of Bruxism: Report of a Work in Progress. J. Oral Rehabil. 2018, 45, 837–844. [Google Scholar] [CrossRef]
- Uchima Koecklin, K.H.; Aliaga-Del Castillo, A.; Li, P. The Neural Substrates of Bruxism: Current Knowledge and Clinical Implications. Front. Neurol. 2024, 15, 1451183. [Google Scholar] [CrossRef]
- Pavlou, I.A.; Spandidos, D.A.; Zoumpourlis, V.; Papakosta, V.K. Neurobiology of Bruxism: The Impact of Stress (Review). Biomed. Rep. 2024, 20, 59. [Google Scholar] [CrossRef]
- Chaudhuri, P.N.K. Bruxism in Patients of Moderate to Severe Traumatic Brain Injury: Management Results Suggesting an Etiological Mechanism. Indian J. Neurotrauma 2014, 11, 17–26. [Google Scholar] [CrossRef]
- Volcheck, M.M.; Graham, S.M.; Fleming, K.C.; Mohabbat, A.B.; Luedtke, C.A. Central Sensitization, Chronic Pain, and Other Symptoms: Better Understanding, Better Management. Cleve. Clin. J. Med. 2023, 90, 245–254. [Google Scholar] [CrossRef]
- Herrero Babiloni, A.; Exposto, F.G.; Bouferguene, Y.; Costa, Y.; Lavigne, G.J.; Arbour, C. Temporomandibular Disorders in Traumatic Brain Injury Patients: A Chronic Pain Condition Requiring Further Attention. Pain Med. 2020, 21, 3260–3262. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.M.; Jozsa, F.; Das, J.M. Neuroanatomy, Spinal Trigeminal Nucleus. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Tsai, Y.-C.; Rau, C.-S.; Huang, J.-F.; Chang, Y.-M.; Chia, K.-J.; Hsieh, T.-M.; Chou, S.-E.; Su, W.-T.; Hsu, S.-Y.; Hsieh, C.-H. The Association between Skull Bone Fractures and the Mortality Outcomes of Patients with Traumatic Brain Injury. Emerg. Med. Int. 2022, 2022, 1296590. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, G.; Pająk-Zielińska, B. Association Between Estrogen Levels and Temporomandibular Disorders: An Updated Systematic Review. Int. J. Mol. Sci. 2024, 25, 9867. [Google Scholar] [CrossRef]
- Zieliński, G. Traumatic Brain Injury as a Cause of Bruxism and Temporomandibular Disorders: A Systematic Review. 2024. Available online: https://osf.io/n83fa (accessed on 25 October 2024). [CrossRef]
- Brown, D. A Review of the PubMed PICO Tool: Using Evidence-Based Practice in Health Education. Health Promot. Pract. 2020, 21, 496–498. [Google Scholar] [CrossRef]
- Kothari, S.F.; Devendran, A.; Sørensen, A.B.; Nielsen, J.F.; Svensson, P.; Kothari, M. Occurrence, Presence and Severity of Bruxism and Its Association with Altered State of Consciousness in Individuals with Severe Acquired Brain Injury. J. Oral Rehabil. 2024, 51, 143–149. [Google Scholar] [CrossRef]
- Kothari, M.; Madsen, V.L.F.; Castrillon, E.E.; Nielsen, J.F.; Svensson, P. Spontaneous Jaw Muscle Activity in Patients with Acquired Brain Injuries—Preliminary Findings. J. Prosthodont. Res. 2018, 62, 268–272. [Google Scholar] [CrossRef]
- Suzuki, Y.; Arbour, C.; Khoury, S.; Giguère, J.-F.; Denis, R.; De Beaumont, L.; Lavigne, G.J. Does Sleep Bruxism Contribute to Headache-Related Disability After Mild Traumatic Brain Injury? A Case-Control Study. J. Oral Facial Pain Headache 2017, 31, 306–312. [Google Scholar] [CrossRef]
- Karpuz, S.; Yılmaz, R.; Yılmaz, H. Evaluation of Temporomandibular Joint Dysfunction in Traumatic Brain Injury Patients. J. Oral Rehabil. 2023, 50, 476–481. [Google Scholar] [CrossRef]
- Aguilar-Fuentes, V.; Orozco-Puga, P.; Jiménez-Ruiz, A. The Glasgow Coma Scale: 50-Year Anniversary. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2024, 45, 2899–2901. [Google Scholar] [CrossRef]
- Hankemeier, A.; Rollnik, J.D. The Early Functional Abilities (EFA) Scale to Assess Neurological and Neurosurgical Early Rehabilitation Patients. BMC Neurol. 2015, 15, 207. [Google Scholar] [CrossRef] [PubMed]
- Hagen, C.; Malkmus, D.; Durham, P. Levels of Cognitive Functioning; Rancho Los Amigos Hospital: Downey, CA, USA, 1972; Volume 6. [Google Scholar]
- Raphael, K.G.; Santiago, V.; Lobbezoo, F. Is Bruxism a Disorder or a Behaviour? Rethinking the International Consensus on Defining and Grading of Bruxism. J. Oral Rehabil. 2016, 43, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, G.; Gawda, P. Surface Electromyography in Dentistry—Past, Present and Future. J. Clin. Med. 2024, 13, 1328. [Google Scholar] [CrossRef]
- Carroll, L.J.; Cassidy, J.D.; Holm, L.; Kraus, J.; Coronado, V.G. Methodological Issues and Research Recommendations for Mild Traumatic Brain Injury: The WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. Suppl. 2004, 36, 113–125. [Google Scholar] [CrossRef]
- Rechtschaffen, A.; Kales, A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects; Public Health Service, US Government Printing Office: Washington, DC, USA, 1968.
- Mitro, V.; Caso, A.R.; Sacchi, F.; Gilli, M.; Lombardo, G.; Monarchi, G.; Pagano, S.; Tullio, A. Fonseca’s Questionnaire Is a Useful Tool for Carrying Out the Initial Evaluation of Temporomandibular Disorders in Dental Students. Clin. Pract. 2024, 14, 1650–1668. [Google Scholar] [CrossRef] [PubMed]
- Lobbezoo, F.; Naeije, M. Bruxism Is Mainly Regulated Centrally, Not Peripherally. J. Oral Rehabil. 2001, 28, 1085–1091. [Google Scholar] [CrossRef]
- Zieliński, G.; Ginszt, M.; Zawadka, M.; Rutkowska, K.; Podstawka, Z.; Szkutnik, J.; Majcher, P.; Gawda, P. The Relationship between Stress and Masticatory Muscle Activity in Female Students. J. Clin. Med. 2021, 10, 3459. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, D.S.; Pal, U.S.; Jurel, S.K. Etiological Factors of Temporomandibular Joint Disorders. Natl. J. Maxillofac. Surg. 2011, 2, 116–119. [Google Scholar] [CrossRef]
- Ginszt, M.; Zieliński, G.; Szkutnik, J.; Wójcicki, M.; Baszczowski, M.; Litko-Rola, M.; Rózyło-Kalinowska, I.; Majcher, P. The Effects of Wearing a Medical Mask on the Masticatory and Neck Muscle Activity in Healthy Young Women. J. Clin. Med. 2022, 11, 303. [Google Scholar] [CrossRef]
- Zieliński, G.; Matysik-Woźniak, A.; Pankowska, A.; Pietura, R.; Rejdak, R.; Jonak, K. High Myopia and Thickness of Extraocular and Masticatory Muscles-7T MRI, Preliminary Study. J. Clin. Med. 2023, 12, 4166. [Google Scholar] [CrossRef]
- Zieliński, G.; Wójcicki, M.; Rapa, M.; Matysik-Woźniak, A.; Baszczowski, M.; Ginszt, M.; Litko-Rola, M.; Szkutnik, J.; Różyło-Kalinowska, I.; Rejdak, R.; et al. Masticatory Muscle Thickness and Activity Correlates to Eyeball Length, Intraocular Pressure, Retinal and Choroidal Thickness in Healthy Women versus Women with Myopia. J. Pers. Med. 2022, 12, 626. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, C.E.; Amiri, A.; Jaime, J.; Delaney, P. The Relationship of Whiplash Injury and Temporomandibular Disorders: A Narrative Literature Review. J. Chiropr. Med. 2009, 8, 171. [Google Scholar] [CrossRef] [PubMed]
- Häggman-Henrikson, B.; List, T.; Westergren, H.T.; Axelsson, S.H. Temporomandibular Disorder Pain after Whiplash Trauma: A Systematic Review. J. Orofac. Pain 2013, 27, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Kesikburun, S.; Alaca, R.; Aras, B.; Tuğcu, I.; Tan, A.K. Botulinum Toxin Injection for Bruxism Associated with Brain Injury: Case Report. J. Rehabil. Res. Dev. 2014, 51, 661–664. [Google Scholar] [CrossRef]
- Yi, H.S.; Kim, H.S.; Seo, M.R. Trial of Oral Metoclopramide on Diurnal Bruxism of Brain Injury. Ann. Rehabil. Med. 2013, 37, 871–874. [Google Scholar] [CrossRef]
- List, T.; Jensen, R.H. Temporomandibular Disorders: Old Ideas and New Concepts. Cephalalgia Int. J. Headache 2017, 37, 692–704. [Google Scholar] [CrossRef]
| Databases | PubMed | Web of Science | Scopus | Cochrane Collaboration |
|---|---|---|---|---|
| Keyword compliant | “Traumatic brain injury” AND ‘’bruxism” “Brain injury” AND “bruxism” “TBI” AND “bruxism” “traumatic brain injury” AND “temporomandibular disorders” “Brain injury” AND “temporomandibular disorders” “TBI” AND “temporomandibular disorders” | “Traumatic brain Injury” AND “bruxism” “Brain injury” AND “bruxism” “TBI” AND “bruxism” “traumatic brain injury” AND “temporomandibular disorders” “Brain injury” AND “temporomandibular disorders” “TBI” AND “temporomandibular disorders” | “Traumatic brain injury” AND “bruxism” “Brain injury” AND “bruxism” “TBI” AND “bruxism” “traumatic brain injury” AND “temporomandibular disorders” “Brain injury” AND “temporomandibular disorders” “TBI” AND “temporomandibular disorders” | “Traumatic brain injury” AND “bruxism” “Brain injury” AND “bruxism” “TBI” AND “bruxism” “traumatic brain injury” AND “temporomandibular disorders” “Brain injury” AND “temporomandibular disorders” “TBI” AND “temporomandibular disorders” |
| Inclusion Criteria | |
|---|---|
| Population | |
| Individuals who have suffered a traumatic brain injury (TBI), including both adults and children, regardless of the severity of the injury. This group may include patients in clinical settings, rehabilitation centers, or emergency departments. | |
| Intervention | |
| The evaluation of the incidence and severity of bruxism and temporomandibular disorders (TMDs) in individuals with a history of TBI. This may involve diagnostic assessments, questionnaires, and clinical evaluations to identify and measure the presence of these conditions. | |
| Comparison | |
| The comparison group could consist of individuals without a history of TBI, matched for age, gender, and other relevant factors. This group would serve as a baseline to assess the differences in the prevalence and severity of bruxism and TMDs. | |
| Outcome | |
| The primary outcomes of interest would be the incidence rates of bruxism and TMDs among individuals with TBI compared to those without. Secondary outcomes could include the severity of symptoms, quality-of-life assessments, and any potential correlations between the severity of the brain injury and the severity of bruxism or TMD symptoms. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pająk-Zielińska, B.; Pająk, A.; Drab, A.; Gawda, P.; Zieliński, G. Could Traumatic Brain Injury Be a Risk Factor for Bruxism and Temporomandibular Disorders? A Scoping Review. Brain Sci. 2025, 15, 276. https://doi.org/10.3390/brainsci15030276
Pająk-Zielińska B, Pająk A, Drab A, Gawda P, Zieliński G. Could Traumatic Brain Injury Be a Risk Factor for Bruxism and Temporomandibular Disorders? A Scoping Review. Brain Sciences. 2025; 15(3):276. https://doi.org/10.3390/brainsci15030276
Chicago/Turabian StylePająk-Zielińska, Beata, Agnieszka Pająk, Agnieszka Drab, Piotr Gawda, and Grzegorz Zieliński. 2025. "Could Traumatic Brain Injury Be a Risk Factor for Bruxism and Temporomandibular Disorders? A Scoping Review" Brain Sciences 15, no. 3: 276. https://doi.org/10.3390/brainsci15030276
APA StylePająk-Zielińska, B., Pająk, A., Drab, A., Gawda, P., & Zieliński, G. (2025). Could Traumatic Brain Injury Be a Risk Factor for Bruxism and Temporomandibular Disorders? A Scoping Review. Brain Sciences, 15(3), 276. https://doi.org/10.3390/brainsci15030276







