Naringenin’s Neuroprotective Effect on Diazino-Induced Cerebellar Damage in Male Albino Rats, with Modulation of Acetylcholinesterase
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
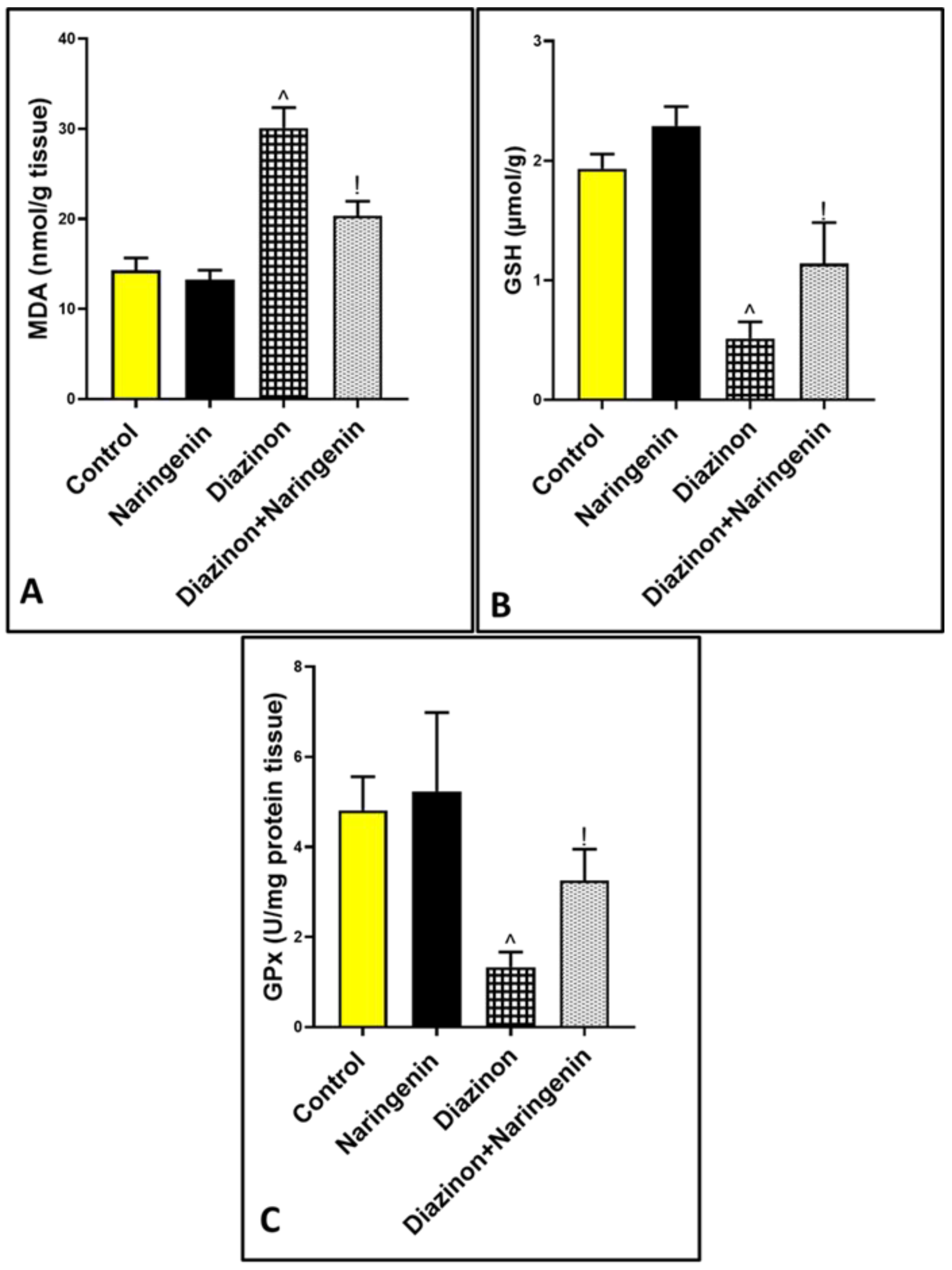
2.3. Oxidative Stress Markers Evaluation
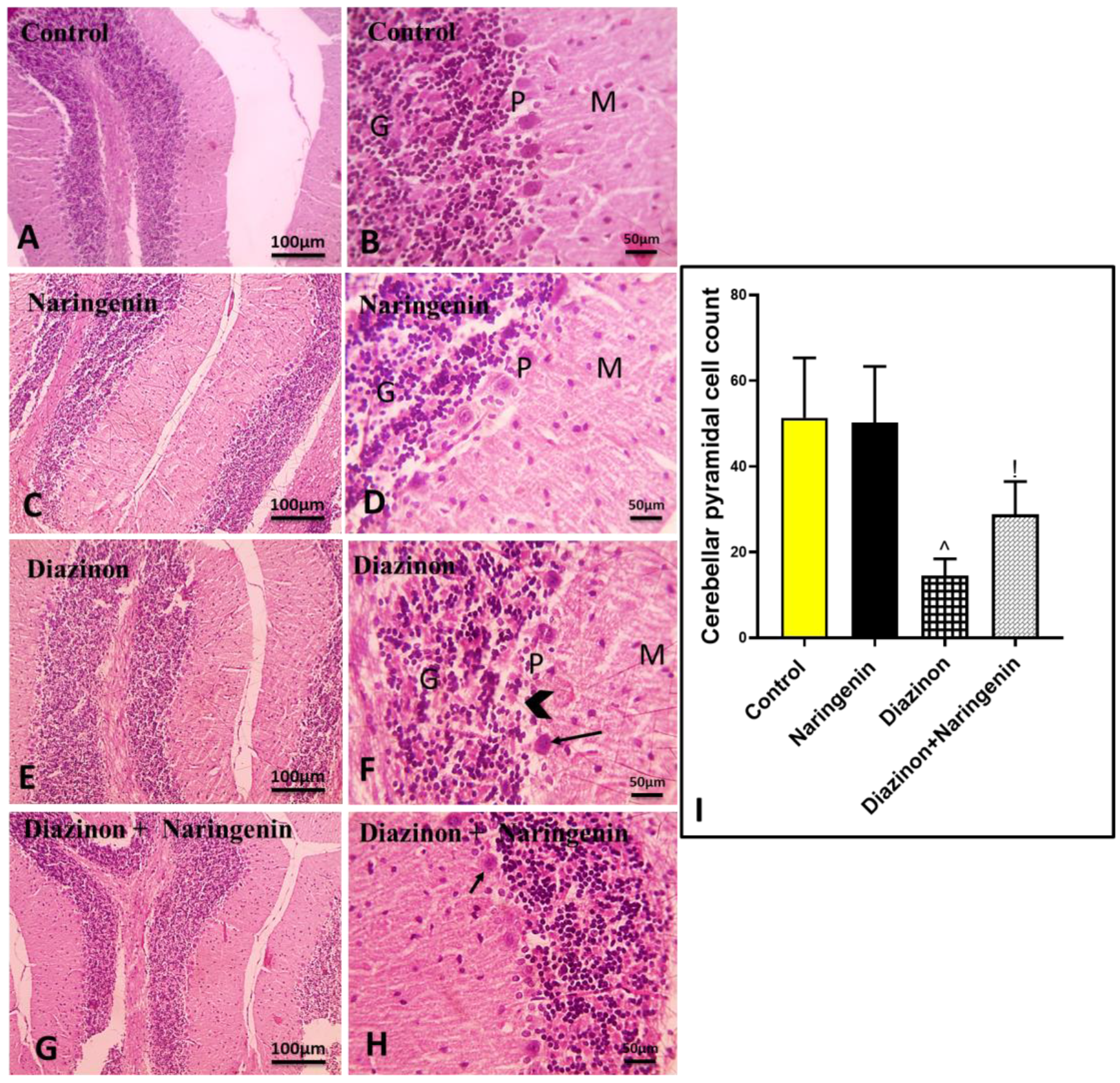
2.4. Histopathological Examination
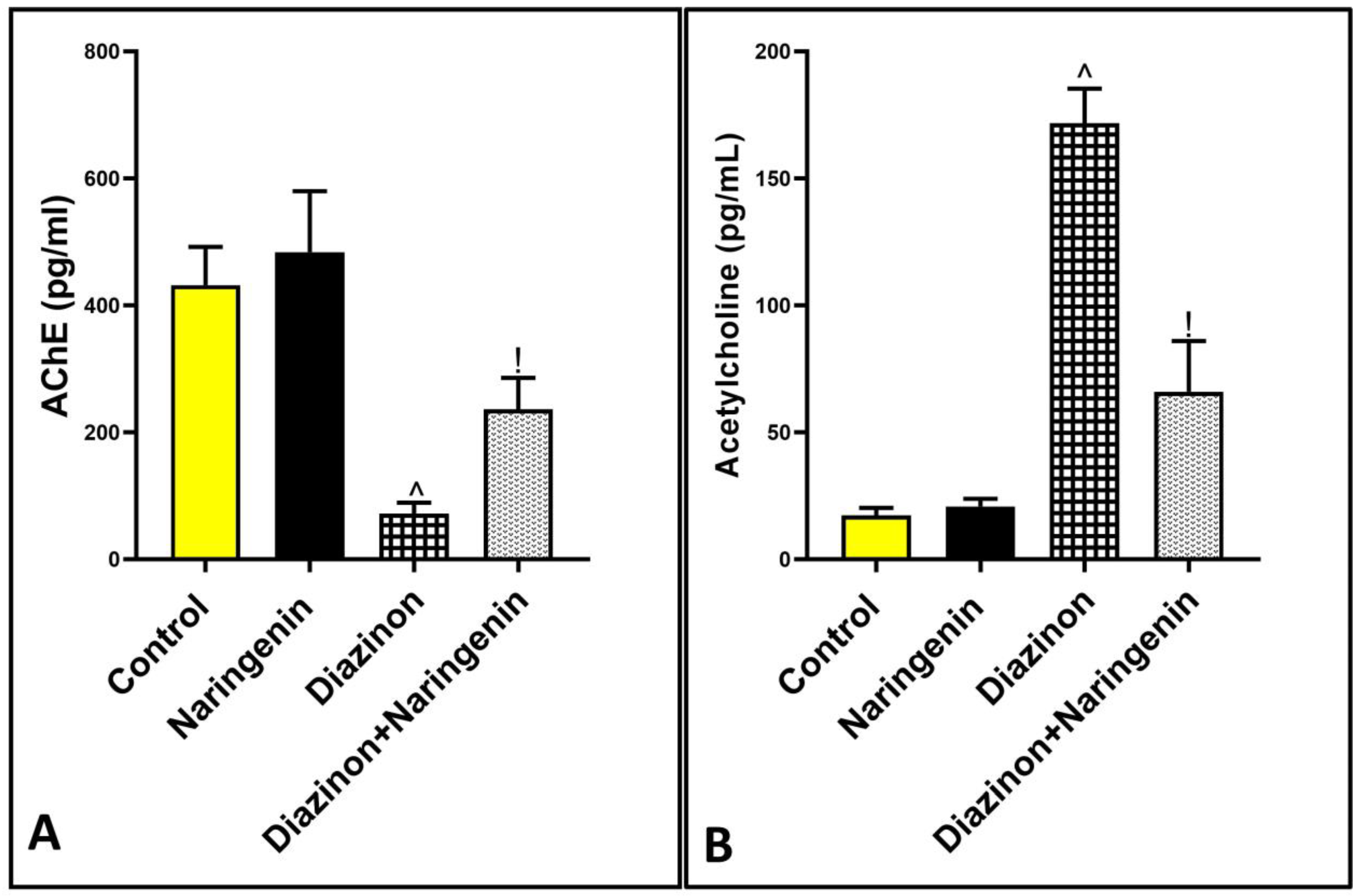
2.5. Detection of AchE Activity, Acetylcholine Neurotransmitters Inflammatory, and Apoptotic Markers in the Cerebellar Supernatant
2.6. Statistical Analysis
3. Results
3.1. Ameliorative Effect of Naringenin Against Diazinon-Induced Cerebellar Damage
3.2. Antioxidant Effects of Naringenin Against Diazinon-Induced Cerebellar Oxidative Damage
3.3. Anti-Inflammatory Role of Naringenin Against Diazinon-Induced Cerebellar Inflammation
3.4. Anti-Apoptotic Effect of Naringenin Against Organophosphorus Diazinon-Induced Purkinje Apoptosis
3.5. Excitatory Effect of Naringenin on AchE Activity in Rats Cerebellum
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamanyire, R.; Karalliedde, L. Organophosphate toxicity and occupational exposure. Occup. Med. 2004, 54, 69–75. [Google Scholar] [CrossRef]
- Roegge, C.S.; Timofeeva, O.A.; Seidler, F.J.; Slotkin, T.A.; Levin, E.D. Developmental diazinon neurotoxicity in rats: Later effects on emotional response. Brain Res. Bull. 2008, 75, 166–172. [Google Scholar] [CrossRef]
- Cakici, O.; Akat, E. Effects of oral exposure to diazinon on mice liver and kidney tissues: Biometric analyses of histopathologic changes. Anal. Quant. Cytopathol. Histpathol 2013, 35, 7–16. [Google Scholar]
- Gokcimen, A.; Gulle, K.; Demirin, H.; Bayram, D.; Kocak, A.; Altuntas, I. Effects of diazinon at different doses on rat liver and pancreas tissues. Pestic. Biochem. Physiol. 2007, 87, 103–108. [Google Scholar] [CrossRef]
- Okamura, A.; Kamijima, M.; Ohtani, K.; Yamanoshita, O.; Nakamura, D.; Ito, Y.; Miyata, M.; Ueyama, J.; Suzuki, T.; Imai, R.; et al. Broken sperm, cytoplasmic droplets and reduced sperm motility are principal markers of decreased sperm quality due to organophosphorus pesticides in rats. J. Occup. Health 2009, 51, 478–487. [Google Scholar] [CrossRef]
- Cavari, Y.; Landau, D.; Sofer, S.; Leibson, T.; Lazar, I. Organophosphate poisoning-induced acute renal failure. Pediatr. Emerg. Care 2013, 29, 646–647. [Google Scholar] [CrossRef]
- Devinsky, O.; Kernan, J.; Bear, D.M. Aggressive behavior following exposure to cholinesterase inhibitors. J. Neuropsychiatry Clin. Neurosci. 1992, 4, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, S.S. Effects of acute organophosphate ingestion on cognitive function, assessed with the mini mental state examination. J. Postgrad. Med. 2012, 58, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Win-Shwe, T.T.; Nakajima, D.; Fujimaki, H. Involvement of TLR4 in diazinon-induced neurotoxicity in mice. J. UOEH 2012, 34, 1–13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ezabadi, A.; Peeri, M.; Azarbayjani, M.A.; Hosseini, S.A. The effects of resistance training and berberine chloride supplementation on oxidative stress markers in the cerebellum tissue of diazinon-poisoned rats. Middle East. J. Rehabil. Health Stud. 2019, 6, e92870. [Google Scholar] [CrossRef]
- Adigun, A.A.; Wrench, N.; Seidler, F.J.; Slotkin, T.A. Neonatal organophosphorus pesticide exposure alters the developmental trajectory of cell-signaling cascades controlling metabolism: Differential effects of diazinon and parathion. Environ. Health Perspect. 2010, 118, 210–215. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Afshari, S.; Sarailoo, M.; Asghariazar, V.; Safarzadeh, E.; Dadkhah, M. Persistent diazinon induced neurotoxicity: The effect on inhibitory avoidance memory performance, amyloid precursor proteins, and TNF-α levels in the prefrontal cortex of rats. Hum. Exp. Toxicol. 2024, 43, 9603271241235408. [Google Scholar] [CrossRef] [PubMed]
- Sule, R.O.; Condon, L.; Gomes, A.V. A Common Feature of Pesticides: Oxidative Stress-The Role of Oxidative Stress in Pesticide-Induced Toxicity. Oxid. Med. Cell Longev. 2022, 2022, 5563759. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Duan, L.; Ou, Y.; Ling, Q.; Cao, L.; Qian, H.; Zhang, J.; Wang, J.; Yuan, X. The cerebellum and cognitive neural networks. Front. Hum. Neurosci. 2023, 17, 1197459. [Google Scholar] [CrossRef] [PubMed]
- Uner, N.; Sevgiler, Y.; Durmaz, H.; Piner, P.; Cinkiloğlu, E. N-Acetylcysteine provides dose-dependent protection against fenthion toxicity in the brain of Cyprinus carpio L. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2009, 150, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Amara, I.B.; Soudani, N.; Troudi, A.; Hakim, A.; Zeghal, K.M.; Boudawara, T.; Zeghal, N. Dimethoate induced oxidative damage and histopathological changes in lung of adult rats: Modulatory effects of selenium and/or vitamin E. Biomed. Environ. Sci. 2012, 25, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Hariri, A.T.; Moallem, S.A.; Mahmoudi, M.; Memar, B.; Hosseinzadeh, H. Sub-acute effects of diazinon on biochemical indices and specific biomarkers in rats: Protective effects of crocin and safranal. Food Chem. Toxicol. 2010, 48, 2803–2808. [Google Scholar] [CrossRef]
- Alhazzani, K.; Alqarni, N.N.; Aljerian, K.; Raish, M.; Aljuffali, L.; Alshehri, S.; Alanazi, A.Z. Naringenin Mitigates Dasatinib-Induced Kidney Damage by Modulating Antioxidant Defense, Inflammation, and Apoptosis Pathways. Int. J. Med. Sci. 2025, 22, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.M.; Elnagar, M.R.; Abdelrazik, E.; Mahdi, M.R.; Hamza, E.; Elattar, E.M.; ElNashar, E.M.; Alghamdi, M.A.; Al-Qahtani, Z.; Al-Khater, K.M.; et al. Neuroprotective effect of naringin against cerebellar changes in Alzheimer’s disease through modulation of autophagy, oxidative stress and tau expression: An experimental study. Front. Neuroanat. 2022, 16, 1012422. [Google Scholar] [CrossRef] [PubMed]
- Nouri, Z.; Fakhri, S.; El-Senduny, F.F.; Sanadgol, N.; Abd-ElGhani, G.E.; Farzaei, M.H.; Chen, J.T. On the Neuroprotective Effects of Naringenin: Pharmacological Targets, Signaling Pathways, Molecular Mechanisms, and Clinical Perspective. Biomolecules 2019, 9, 690. [Google Scholar] [CrossRef] [PubMed]
- Mansour, L.A.H.; Elshopakey, G.E.; Abdelhamid, F.M.; Albukhari, T.A.; Almehmadi, S.J.; Refaat, B.; El-Boshy, M.; Risha, E.F. Hepatoprotective and Neuroprotective Effects of Naringenin against Lead-Induced Oxidative Stress, Inflammation, and Apoptosis in Rats. Biomedicines 2023, 11, 1080. [Google Scholar] [CrossRef] [PubMed]
- Şahinoğulları, Z.U.; Güzel, S.; Canacankatan, N.; Yalaza, C.; Kibar, D.; Bayrak, G. Naringenin reduces hepatic inflammation and apoptosis induced by vancomycin in rats. Clin. Exp. Health Sci. 2021, 11, 191–198. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Fu, M.; Wang, S.; Chen, W.; Wang, J.; Zhang, N. Naringin ameliorates memory deficits and exerts neuroprotective effects in a mouse model of Alzheimer’s disease by regulating multiple metabolic pathways. Mol. Med. Rep. 2021, 23, 332. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.A.; Ahmed, H.I.; El-Morsy, E.M. Melatonin protects against diazinon-induced neurobehavioral changes in rats. Neurochem. Res. 2013, 38, 2227–2236. [Google Scholar] [CrossRef]
- Kei, S. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin. Chim. Acta 1978, 90, 37–43. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar] [PubMed]
- Gamble, M. 9—The Hematoxylins and Eosin. In Theory and Practice of Histological Techniques, 6th ed.; Bancroft, J.D., Gamble, M., Eds.; Churchill Livingstone: London, UK, 2008. [Google Scholar]
- Mavroudis, I.; Petridis, F.; Kazis, D.; Njau, S.N.; Costa, V.; Baloyannis, S.J. Purkinje cells pathology in Alzheimer’s disease. Am. J. Alzheimers Dis. Other Dement. 2019, 34, 439–449. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Garfitt, S.J.; Jones, K.; Mason, H.J.; Cocker, J. Exposure to the organophosphate diazinon: Data from a human volunteer study with oral and dermal doses. Toxicol. Lett. 2002, 134, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Messarah, M.; Amamra, W.; Boumendjel, A.; Barkat, L.; Bouasla, I.; Abdennour, C.; Boulakoud, M.S.; El Feki, A. Ameliorating effects of curcumin and vitamin E on dia-zinon-induced oxidative damage in rat liver and erythrocytes. Toxicol. Ind. Health 2013, 29, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Altuntas, I.; Kilinc, I.; Orhan, H.; Demirel, R.; Koylu, H.; Delibas, N. The effects of dia-zinon on lipid peroxidation and antioxidant enzymes in erythrocytes in vitro. Hum. Exp. Toxicol. 2004, 23, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Milatovic, D.; Gupta, R.C.; Aschner, M. Anticholinesterase toxicity and oxidative stress. Sci. World J. 2006, 6, 295–310. [Google Scholar] [CrossRef]
- Akhgari, M.; Abdollahi, M.; Kebryaeezadeh, A.; Hosseini, R.; Sabzevari, O. Biochemical evidence for free radical induced lipid peroxidation as a mechanism for sub-chronic toxicity of malathion in blood and liver of rats. Hum. Exp. Toxicol. 2003, 22, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Md, S.; Alhakamy, N.A.; Aldawsari, H.M.; Asfour, H.Z. Neuroprotective and Antioxidant Effect of Naringenin-Loaded Nanoparticles for Nose-to-Brain Delivery. Brain Sci. 2019, 9, 275. [Google Scholar] [CrossRef]
- Rai, R.; Kalar, P.L.; Jat, D.; Mishra, S.K. Naringenin mitigates nanoparticulate-aluminium induced neuronal degeneration in brain cortex and hippocampus through downregulation of oxidative stress and neuroinflammation. Neurochem. Int. 2024, 178, 105799. [Google Scholar] [CrossRef]
- Mishra, S.; Palanivelu, K. The effect of curcumin (turmeric) on Alzheimer’s disease: An overview. Ann. Indian. Acad. Neurol. 2008, 11, 13–19. [Google Scholar] [CrossRef]
- Paduraru, E.; Flocea, E.I.; Lazado, C.C.; Simionov, I.A.; Nicoara, M.; Ciobica, A.; Faggio, C.; Jijie, R. Vitamin C Mitigates Oxidative Stress and Behavioral Impairments Induced by Deltamethrin and Lead Toxicity in Zebrafish. Int. J. Mol. Sci. 2021, 22, 12714. [Google Scholar] [CrossRef]
- Bradlow, R.C.J.; Berk, M.; Kalivas, P.W.; Back, S.E.; Kanaan, R.A. The Potential of N-Acetyl-L-Cysteine (NAC) in the Treatment of Psychiatric Disorders. CNS Drugs 2022, 36, 451–482. [Google Scholar] [CrossRef] [PubMed]
- Guignet, M.; Lein, P.J. Neuroinflammation in organophosphate-induced neurotoxicity. In Advances in Neurotoxicology; Academic Press: Cambridge, MA, USA, 2019; Volume 3, pp. 35–79. [Google Scholar]
- Andrew, P.M.; MacMahon, J.A.; Bernardino, P.N.; Tsai, Y.-H.; Hobson, B.A.; Porter, V.A.; Huddleston, S.L.; Luo, A.S.; Bruun, D.A.; Saito, N.H.; et al. Shifts in the spatiotemporal profile of inflammatory phenotypes of innate immune cells in the rat brain following acute intoxication with the organophosphate diisopropylfluorophosphate. J. Neuroinflammation 2024, 21, 285. [Google Scholar] [CrossRef] [PubMed]
- Khajevand-Khazaei, M.-R.; Ziaee, P.; Motevalizadeh, S.-A.; Rohani, M.; Afshin-Majd, S.; Baluchnejadmojarad, T.; Roghani, M. Naringenin ameliorates learning and memory impairment following systemic lipopolysaccharide challenge in the rat. Eur. J. Pharmacol. 2018, 826, 114–122. [Google Scholar] [CrossRef]
- Hutchins, A.P.; Diez, D.; Miranda-Saavedra, D. The IL-10/STAT3-mediated anti-inflammatory response: Recent developments and future challenges. Brief. Funct. Genom. 2013, 12, 489–498. [Google Scholar] [CrossRef]
- Paraoanu, L.E.; Mocko, J.B.; Becker-Roeck, M.; Smidek-Huhn, J.; Layer, P.G. Exposure to diazinon alters in vitro retinogenesis: Retinospheroid morphology, development of chicken retinal cell types, and gene expression. Toxicol. Sci. 2006, 89, 314–324. [Google Scholar] [CrossRef]
- Shakouri, E.; Azarbayjani, M.A.; Jameie, S.B.; Peeri, M.; Farhadi, M. Berberine supplement and resistance training may ameliorate diazinon induced neural toxicity in rat hippocampus via the activation of the TrkB and ERK signaling pathway. Int. Clin. Neurosci. J. 2021, 8, 14–21. [Google Scholar] [CrossRef]
- Fabrizi, L.; Gemma, S.; Testai, E.; Vittozzi, L. Identification of the cytochrome P450 iso-enzymes involved in the metabolism of diazinon in the rat liver. J. Biochem. Mol. Toxicol. 1999, 13, 53–61. [Google Scholar] [CrossRef]
- Kappers, W.A.; Edwards, R.J.; Murray, S.; Boobis, A.R. Diazinon is activated by CYP2C19 in human liver. Toxicol. Appl. Pharmacol. 2001, 177, 68–76. [Google Scholar] [CrossRef]
- Čolović, M.B.; Vasić, V.M.; Avramović, N.S.; Gajić, M.M.; Djurić, D.M.; Krstić, D.Z. In vitro evaluation of neurotoxicity potential and oxidative stress responses of diazinon and its degradation products in rat brain synaptosomes. Toxicol. Lett. 2015, 233, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Sidiropoulou, E.; Sachana, M.; Flaskos, J.; Harris, W.; Hargreaves, A.J.; Woldehiwet, Z. Diazinon oxon affects the differentiation of mouse N2a neuroblastoma cells. Arch. Toxicol. 2009, 83, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.A.; Beretta, E.M.; Ferreira, L.A.; da Silva, E.S.; Coimbra, B.Z.; Pereira, P.T.; Miranda, R.G.; Dorta, D.J.; Rodrigues, F.T.V.; Mingatto, F.E. Role of biotransformation in the diazinon-induced toxicity in HepG2 cells and antioxidant protection by tetrahydro-curcumin. Toxicol. Rep. 2022, 10, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Heo, H.J.; Kim, M.-J.; Lee, J.-M.; Choi, S.J.; Cho, H.-Y.; Hong, B.; Kim, H.-K.; Kim, E.; Shin, D.-H. Naringenin from Citrus junos has an inhibitory effect on acetylcholinesterase and a mitigating effect on amnesia. Dement. Geriatr. Cogn. Disord. 2004, 17, 151–157. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saati, A.A. Naringenin’s Neuroprotective Effect on Diazino-Induced Cerebellar Damage in Male Albino Rats, with Modulation of Acetylcholinesterase. Brain Sci. 2025, 15, 242. https://doi.org/10.3390/brainsci15030242
Saati AA. Naringenin’s Neuroprotective Effect on Diazino-Induced Cerebellar Damage in Male Albino Rats, with Modulation of Acetylcholinesterase. Brain Sciences. 2025; 15(3):242. https://doi.org/10.3390/brainsci15030242
Chicago/Turabian StyleSaati, Abdullah A. 2025. "Naringenin’s Neuroprotective Effect on Diazino-Induced Cerebellar Damage in Male Albino Rats, with Modulation of Acetylcholinesterase" Brain Sciences 15, no. 3: 242. https://doi.org/10.3390/brainsci15030242
APA StyleSaati, A. A. (2025). Naringenin’s Neuroprotective Effect on Diazino-Induced Cerebellar Damage in Male Albino Rats, with Modulation of Acetylcholinesterase. Brain Sciences, 15(3), 242. https://doi.org/10.3390/brainsci15030242




