Smart Digital Solutions for EARLY Treatment of COGNitive Disability (EARLY-COGN^3): A Study Protocol
Abstract
1. Introduction
2. Materials and Methods
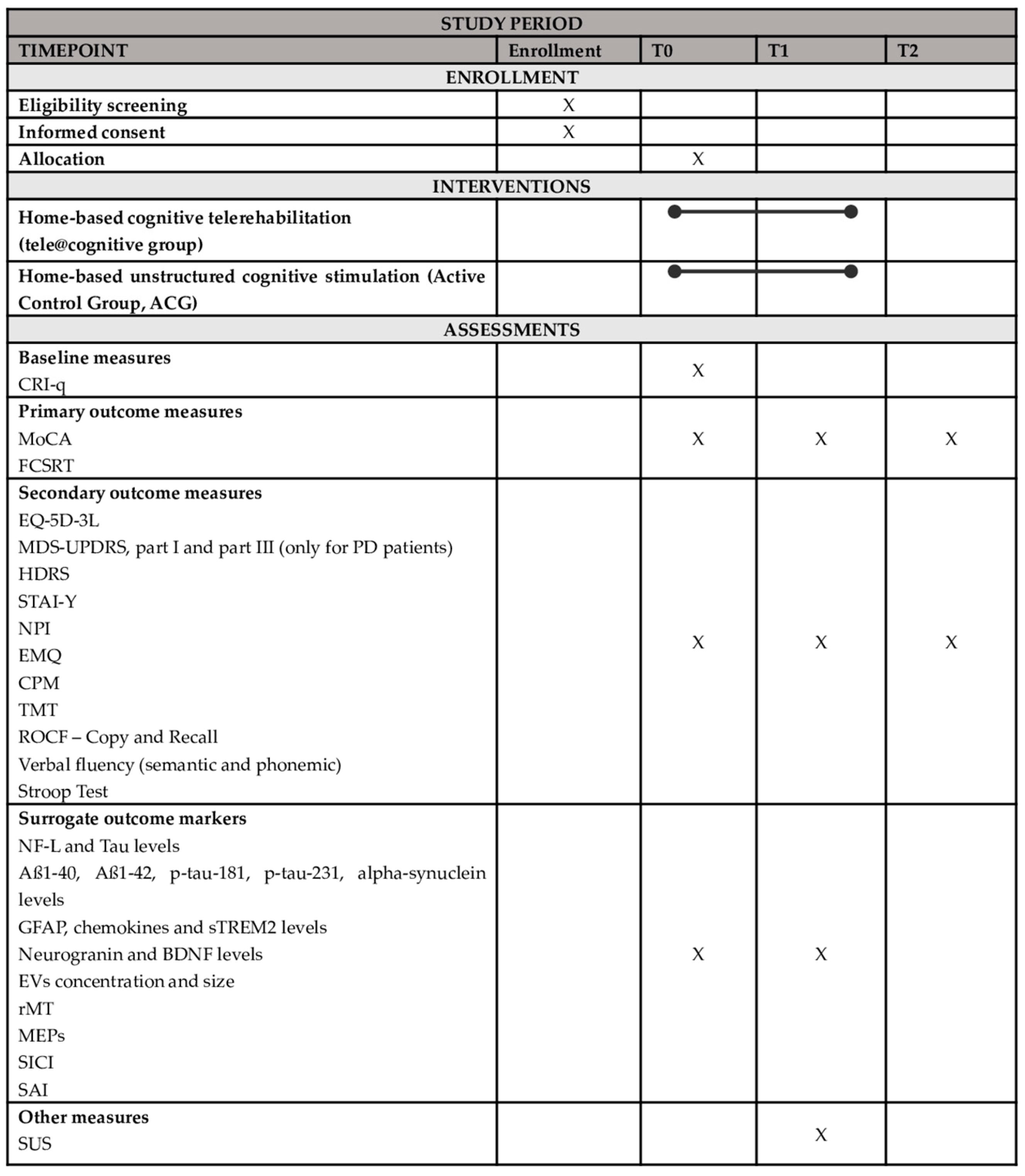
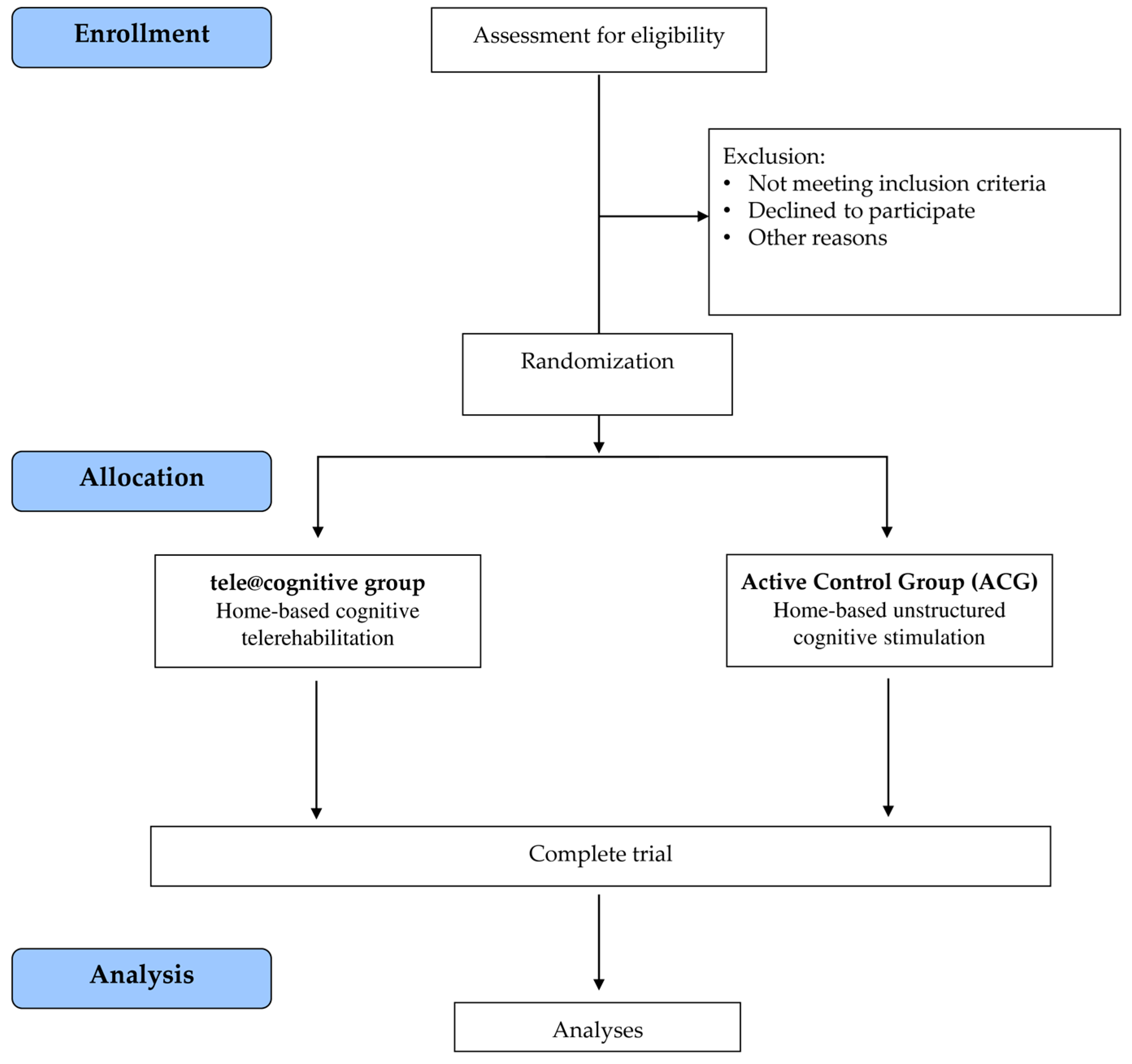
2.1. Trial Design
2.2. Randomization and Blinding
2.3. Sample Size
2.4. Participants
Inclusion and Exclusion Criteria
2.5. Intervention Procedures
- -
- 30 CNDs patients will receive home-based cognitive rehabilitation activities with an innovative digital solution for remote rehabilitation of cognitive difficulties (tele@cognitive group);
- -
- 30 CNDs patients will receive home-based unstructured cognitive rehabilitation treatment (Active Control Group—ACG).
2.6. Outcome Measures
2.6.1. Primary Outcome Measures
- Montreal Cognitive Assessment (MoCA). MoCA [96] is a screening test for global cognitive functioning. It includes tasks involving several cognitive domains: visuospatial, executive function, naming, selective and sustained attention, language, abstraction, memory, and orientation (score range min = 0, max = 30, higher score = better outcome).
- Long-term episodic verbal memory as assessed by Free and Cued Selective Reminding Test (FCSRT). FCSRT [103] is a measure of long-term episodic verbal memory. It provides five scores: Immediate Free Recall (IFR, spontaneous recall across three trials; score range min = 0, max = 36), Immediate Total Recall (ITR, total recall across three trials; score range min = 0, max = 36), Delayed Free Recall (DFR, score range min = 0, max = 12), Delayed Total Recall (DTR, score range min = 0, max = 12). Higher scores indicate better performance. Finally, the Index of Sensitivity to Cueing (ISC, score range min = 0, max = 1) reflects the difference between the number of items recalled spontaneously and the number of items recalled with the help of cues. A higher ISC indicates a greater sensitivity to cues.
2.6.2. Secondary Outcome Measures
- Quality of life as assessed by EQ-5D-3L. EQ-5D-3L [104] is a measure of health status consisting of five dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression). Each dimension has three response levels (no problems, some problems, unable to/extreme problems). In addition, the questionnaire includes a visual analog scale that records the patient’s perception of his or her overall health (score range min = 0, max = 100, higher score = better outcome).
- Non-motor experiences of daily living as assessed by MDS-UPDRS scale, part I. MDS-UPDRS scale [105], part I (only for the PD group) is a measure of non-motor experiences of daily living consisting of a total of 13 questions (score range min = 0, max = 52, higher score = worse outcome).
- Motor abilities as assessed by MDS-UPDRS scale, part III. MDS-UPDRS scale [105], part III (only for the PD group) is a measure of motor abilities consisting of a total of 18 questions with 33 individual items. Each item has a 0–4 rating, where 0 = normal, 1 = slight, 2 = mild, 3 = moderate, and 4 = severe (score range min = 0, max = 132, higher score = worse outcome).
- Depressive symptoms as assessed by Hamilton Depression Rating Scale (HDRS). HDRS [106] is a measure of severity of the depressive symptoms consisting of a total of 21 items (score range min = 0, max = 64, higher score = worse outcome).
- Anxiety symptoms as assessed by State-Trait Anxiety Inventory (STAI-Y). The STAI-Y [107] consists of two 20-item scales providing separate measures of state and trait anxiety (S-Anxiety and T-Anxiety). Each scale has a score range from a minimum of 20 to a maximum of 80, with a higher score on the scale indicating a worse outcome.
- Behavior and personality as assessed by Neuropsychiatric Inventory (NPI). NPI [108] is used for assessing various neuropsychiatric symptoms. The inventory consists of 12 core domains, each reflecting specific neuropsychiatric symptoms. Each symptom is rated for both frequency and severity (score range min = 0, max = 144, higher score = worse outcome).
- Memory complaints as assessed by Everyday Memory Questionnaire (EMQ). EMQ [109] is a 20-item questionnaire that evaluate the frequency and impact of memory problems in daily life (score range min = 20, max = 180, higher score = worse outcome).
- Non-verbal abstract reasoning as assessed by Raven’s Colored Progressive Matrices (CPM). CPM [110] is a measure of non-verbal abstract reasoning (score range min = 0, max = 36, higher score = better outcome).
- Attentional abilities as assessed by Trial Making Test (TMT). TMT (Part-A and Part-B) [111] is a measure of attentional abilities, visuo-conceptual and visual–motor tracking. TMT-Part A involves visual scanning, number recognition, number sequencing, and motor speed. TMT-Part B assesses mental flexibility in managing more than one stimulus at a time and in shifting the course of an ongoing activity. High execution times indicate poor performance (score range min = n/a, max = no limits).
- Executive abilities as assessed by Stroop Test. Stroop Test [112] is a measure of executive abilities, including visual attention, selective attention, cognitive flexibility, and inhibitory control of behavior. Two scores are calculated with consideration of the number of errors and the time taken to complete all parts. High execution times and high numbers of errors indicate poor performance.
- Constructional praxia as assessed by Rey–Osterrieth Complex Figure-Copy (ROCF). ROCF [113] is a measure of constructional praxia (score range min = 0, max = 36, higher score = better outcome).
- Fluency abilities as assessed by Verbal Fluency (semantic and phonemic). Verbal Fluency (semantic and phonemic) [114] is a measure of verbal and semantic fluency abilities, executive functions abilities, lexical store size, lexicon access, and lexical organization (score range min = 0, max = no limits, higher score = better outcome).
- Nonverbal long-term memory as assessed by ROCF-Recall. ROCF-Recall [113] is a measure of nonverbal long-term memory (score range min = 0, max = 36, higher score = better outcome).
2.6.3. Surrogate Outcome Measures
- Neurofilament light chain (NF-L) and Tau levels.
- Aß1-40, Aß1-42, p-tau-181, p-tau-231, and alpha-synuclein levels.
- Glial Fibrillary Acidic Protein (GFAP), chemokines, and sTREM2 levels.
- Neurogranin and Brain-Derived Neurotrophic Factor (BDNF) levels.
- Concentration and size of plasma Extracellular Vescicles (EVs).
- Resting Motor Threshold (rMT). rMT reflects membrane excitability of corticospinal neurons. Lower values indicate increased excitability.
- Motor Evoked Potentials (MEPs). MEPs are muscle twitches resulting from complex descending corticospinal volleys occurring after TMS pulses. MEP amplitude and latency reflect the integrity of the corticospinal tract and corticospinal excitability.
- Short-interval intracortical inhibition (SICI). TMS-SICI reflects inhibitory interneuronal circuits acting via GABA A receptors [115].
- Short-latency afferent inhibition (SAI). TMS-SAI reflects cholinergic transmission [116].
2.7. Data Collection
- -
- Aß1-40, Aß1-42, p-tau-181, p-tau-231, NF-L, tau, GFAP, BDNF, Eotaxin-1, Eotaxin-3, alpha-synuclein, neurogranin, and sTREM2 will be measured in plasma or serum by commercially available kits. Coefficients of Variation (CVs) within-run will be accepted when in agreement with information from kit vendors. The concentration and size distribution of EVs will be assessed in light scattering mode with NanoSight NS300 instrument (Malvern, Worcestershire, United Kingdom). Samples will be diluted to obtain an optimal range of 20–150 particles/frame. For each sample, 5 videos of 60″ will be recorded and data will be processed using NanoSight NTA 3.2 software. Optimized post-acquisition settings will be kept constant during the analysis of all samples. Data obtained will be analyzed by comparing concentration (particles/mL), size distribution (nm), and their ratio (conc./size).
- -
- In each TMS recording session, i.e., at T0 and at T1, CNDs participants will be comfortably seated in a dimly lit room and neurophysiological measures will be collected while they are at rest: (a) the resting motor threshold (rMT), defined as the lowest transcranial stimulus intensity at which TMS of motor cortex produces an EMG response in half of the trials, will be estimated with a probability density function; (b) motor-evoked potentials (MEPs) will be collected at stimulation intensity equal to 120% of the resting motor threshold; (c) SICI will be obtained by delivering a TMS pulse at 70% of rMT at 1, 2, 3, 5 ms before the test stimulus; (d) SAI will be measured as change in MEPs when TMS is delivered after the electrical stimulation of the median nerve at different intervals (−28, −24, −20, −16 ms). These measures will be collected by targeting the cortical motor hotspot of the first dorsal interosseous muscle (FDI) on the left hemisphere while measuring responses from muscles of the hand. The position of the coil will be monitored throughout the duration of the experiment, thanks to a neuronavigation system that allows the coil to remain in correspondence with the target area.
2.8. Statistical Analysis
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Action Plan on the Public Health Response to Dementia 2017–2025; WHO: Geneva, Switzerland, 2017.
- Cieza, A.; Causey, K.; Kamenov, K.; Hanson, S.W.; Chatterji, S.; Vos, T. Global estimates of the need for rehabilitation based on the global burden of disease study 2019: A systematic analysis for the global burden of disease study 2019. Lancet 2020, 396, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Rossetto, F.; Isernia, S.; Realdon, O.; Borgnis, F.; Blasi, V.; Pagliari, C.; Cabinio, M.; Alberoni, M.; Mantovani, F.; Clerici, M. A digital health home intervention for people within the alzheimer’s disease continuum: Results from the ability-telerehabilitation pilot randomized controlled trial. Ann. Med. 2023, 55, 1080–1091. [Google Scholar] [CrossRef] [PubMed]
- Kyu, H.; Abate, D.; Abate, K.; Abay, S.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A. Gbd 2017 dalys and hale collaborators. Global, regional, and national disability-adjusted life-years (dalys) for 359 diseases and injuries and healthy life expectancy (hale) for 195 countries and territories, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet 2018, 392, 1859–1922. [Google Scholar]
- Salomon, J.A.; Wang, H.; Freeman, M.K.; Vos, T.; Flaxman, A.D.; Lopez, A.D.; Murray, C.J. Healthy life expectancy for 187 countries, 1990–2010: A systematic analysis for the global burden disease study 2010. Lancet 2012, 380, 2144–2162. [Google Scholar] [CrossRef]
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the global burden of disease study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Perrotin, A.; La Joie, R.; de La Sayette, V.; Barré, L.; Mézenge, F.; Mutlu, J.; Guilloteau, D.; Egret, S.; Eustache, F.; Chételat, G. Subjective cognitive decline in cognitively normal elders from the community or from a memory clinic: Differential affective and imaging correlates. Alzheimers Dement. 2017, 13, 550–560. [Google Scholar] [CrossRef]
- Svenningsson, P.; Westman, E.; Ballard, C.; Aarsland, D. Cognitive impairment in patients with parkinson’s disease: Diagnosis, biomarkers, and treatment. Lancet Neurol. 2012, 11, 697–707. [Google Scholar] [CrossRef]
- Jessen, F. Subjective and objective cognitive decline at the pre-dementia stage of alzheimer’s disease. Eur. Arch. Psychiatry Clin. Neurosci. 2014, 264, 3–7. [Google Scholar] [CrossRef]
- Cappa, S.F.; Ribaldi, F.; Chicherio, C.; Frisoni, G.B. Subjective cognitive decline: Memory complaints, cognitive awareness, and metacognition. Alzheimers Dement. 2024, 20, 6622–6631. [Google Scholar] [CrossRef]
- Jessen, F.; Amariglio, R.E.; van Boxtel, M.; Breteler, M.; Ceccaldi, M.; Chételat, G.; Dubois, B.; Dufouil, C.; Ellis, K.A.; van der Flier, W.M.; et al. A conceptual framework for research on subjective cognitive decline in preclinical alzheimer’s disease. Alzheimers Dement. 2014, 10, 844–852. [Google Scholar] [CrossRef]
- Glodzik-Sobanska, L.; Reisberg, B.; De Santi, S.; Babb, J.S.; Pirraglia, E.; Rich, K.E.; Brys, M.; De Leon, M.J. Subjective memory complaints: Presence, severity and future outcome in normal older subjects. Dement. Geriatr. Cogn. Disord. 2007, 24, 177–184. [Google Scholar] [CrossRef]
- Jessen, F.; Wiese, B.; Bachmann, C.; Eifflaender-Gorfer, S.; Haller, F.; Kölsch, H.; Luck, T.; Mösch, E.; van den Bussche, H.; Wagner, M. Prediction of dementia by subjective memory impairment: Effects of severity and temporal association with cognitive impairment. Arch. Gen. Psychiatry 2010, 67, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Vannini, P.; Hanseeuw, B.; Munro, C.E.; Amariglio, R.E.; Marshall, G.A.; Rentz, D.M.; Pascual-Leone, A.; Johnson, K.A.; Sperling, R.A. Hippocampal hypometabolism in older adults with memory complaints and increased amyloid burden. Neurology 2017, 88, 1759–1767. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Hampel, H.; Molinuevo, J.L.; Blennow, K.; DeKosky, S.T.; Gauthier, S.; Selkoe, D.; Bateman, R. Advancing research diagnostic criteria for alzheimer’s disease: The iwg-2 criteria. Lancet Neurol. 2014, 13, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to alzheimer’s disease: Recommendations from the national institute on aging-alzheimer’s association workgroups on diagnostic guidelines for alzheimer’s disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef]
- Petersen, R.C.; Smith, G.E.; Waring, S.C.; Ivnik, R.J.; Tangalos, E.G.; Kokmen, E. Mild cognitive impairment: Clinical characterization and outcome. Arch. Neurol. 1999, 56, 303–308. [Google Scholar] [CrossRef]
- Aarsland, D.; Påhlhagen, S.; Ballard, C.G.; Ehrt, U.; Svenningsson, P. Depression in parkinson disease-epidemiology, mechanisms and management. Nat. Rev. Neurol. 2011, 8, 35–47. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. Mds clinical diagnostic criteria for parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef]
- Biundo, R.; Fiorenzato, E.; Antonini, A. Nonmotor symptoms and natural history of parkinson’s disease: Evidence from cognitive dysfunction and role of noninvasive interventions. Int. Rev. Neurobiol. 2017, 133, 389–415. [Google Scholar]
- Leung, I.H.; Walton, C.C.; Hallock, H.; Lewis, S.J.; Valenzuela, M.; Lampit, A. Cognitive training in parkinson disease: A systematic review and meta-analysis. Neurology 2015, 85, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Buschert, V.C.; Friese, U.; Teipel, S.J.; Schneider, P.; Merensky, W.; Rujescu, D.; Möller, H.J.; Hampel, H.; Buerger, K. Effects of a newly developed cognitive intervention in amnestic mild cognitive impairment and mild alzheimer’s disease: A pilot study. J. Alzheimers Dis. 2011, 25, 679–694. [Google Scholar] [CrossRef] [PubMed]
- Förster, S.; Buschert, V.C.; Buchholz, H.G.; Teipel, S.J.; Friese, U.; Zach, C.; la Fougere, C.; Rominger, A.; Drzezga, A.; Hampel, H.; et al. Effects of a 6-month cognitive intervention program on brain metabolism in amnestic mild cognitive impairment and mild alzheimer’s disease. J. Alzheimers Dis. 2011, 25, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Huckans, M.; Hutson, L.; Twamley, E.; Jak, A.; Kaye, J.; Storzbach, D. Efficacy of cognitive rehabilitation therapies for mild cognitive impairment (mci) in older adults: Working toward a theoretical model and evidence-based interventions. Neuropsychol. Rev. 2013, 23, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Reijnders, J.; van Heugten, C.; van Boxtel, M. Cognitive interventions in healthy older adults and people with mild cognitive impairment: A systematic review. Ageing Res. Rev. 2013, 12, 263–275. [Google Scholar] [CrossRef]
- Bahar-Fuchs, A.; Clare, L.; Woods, B. Cognitive training and cognitive rehabilitation for mild to moderate alzheimer’s disease and vascular dementia. Cochrane 2013, 6, CD003260. [Google Scholar] [CrossRef]
- Cappa, S.F.; Benke, T.; Clarke, S.; Rossi, B.; Stemmer, B.; Van Heugten, C.M. Efns guidelines on cognitive rehabilitation: Report of an efns task force. Eur. J. Neurol. 2003, 10, 11–23. [Google Scholar] [CrossRef]
- Cotelli, M.; Calabria, M.; Zanetti, O. Cognitive rehabilitation in alzheimer’s disease. Aging Clin. Exp. Res. 2006, 18, 141–143. [Google Scholar] [CrossRef]
- Maggio, M.G.; De Bartolo, D.; Calabrò, R.S.; Ciancarelli, I.; Cerasa, A.; Tonin, P.; Di Iulio, F.; Paolucci, S.; Antonucci, G.; Morone, G.; et al. Computer-assisted cognitive rehabilitation in neurological patients: State-of-art and future perspectives. Front. Neurol. 2023, 14, 1255319. [Google Scholar] [CrossRef]
- Parsons, T.D. Neuropsychological rehabilitation 3.0: State of the science. In Clinical Neuropsychology Technology: What’s New How We Can Use It; Springer: Cham, Switzerland, 2016; pp. 113–132. [Google Scholar]
- Stuss, D.T.; Winocur, G.; Robertson, I.H. Cognitive Neurorehabilitation: Evidence and Application, 2nd ed.; Cambridge University Press: New York, NY, USA, 2008. [Google Scholar]
- Taub, E.; Uswatte, G.; Elbert, T. New treatments in neurorehabilitation founded on basic research. Nat. Rev. Neurosci. 2002, 3, 228–236. [Google Scholar] [CrossRef]
- Corbetta, D.; Imeri, F.; Gatti, R. Rehabilitation that incorporates virtual reality is more effective than standard rehabilitation for improving walking speed, balance and mobility after stroke: A systematic review. J. Physiother. 2015, 61, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Realdon, O.; Rossetto, F.; Nalin, M.; Baroni, I.; Cabinio, M.; Fioravanti, R.; Saibene, F.L.; Alberoni, M.; Mantovani, F.; Romano, M.; et al. Technology-enhanced multi-domain at home continuum of care program with respect to usual care for people with cognitive impairment: The ability-telerehabilitation study protocol for a randomized controlled trial. BMC Psychiatry 2016, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Brennan, D.M.; Lum, P.S.; Uswatte, G.; Taub, E.; Gilmore, B.M.; Barman, J. A telerehabilitation platform for home-based automated therapy of arm function. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2011, 2011, 1819–1822. [Google Scholar] [PubMed]
- Peretti, A.; Amenta, F.; Tayebati, S.K.; Nittari, G.; Mahdi, S.S. Telerehabilitation: Review of the state-of-the-art and areas of application. JMIR 2017, 4, e7. [Google Scholar] [CrossRef]
- Maresca, G.; Maggio, M.G.; De Luca, R.; Manuli, A.; Tonin, P.; Pignolo, L.; Calabrò, R.S. Tele-neuro-rehabilitation in italy: State of the art and future perspectives. Front. Neurol. 2020, 11, 563375. [Google Scholar] [CrossRef]
- De Cola, M.C.; Maresca, G.; D’Aleo, G.; Carnazza, L.; Giliberto, S.; Maggio, M.G.; Bramanti, A.; Calabrò, R.S. Teleassistance for frail elderly people: A usability and customer satisfaction study. Geriatr. Nurs. 2020, 41, 463–467. [Google Scholar] [CrossRef]
- Isernia, S.; Di Tella, S.; Pagliari, C.; Jonsdottir, J.; Castiglioni, C.; Gindri, P.; Salza, M.; Gramigna, C.; Palumbo, G.; Molteni, F. Effects of an innovative telerehabilitation intervention for people with parkinson’s disease on quality of life, motor, and non-motor abilities. Front. Neurol. 2020, 11, 846. [Google Scholar] [CrossRef]
- Pitt, R.; Theodoros, D.; Hill, A.J.; Russell, T. The impact of the telerehabilitation group aphasia intervention and networking programme on communication, participation, and quality of life in people with aphasia. Int. J. Speech-Lang. Pathol. 2019, 21, 513–523. [Google Scholar] [CrossRef]
- Brennan, D.; Georgeadis, A.; Baron, C. Telerehabilitation tools for the provision of remote speech-language treatment. Top. Stroke Rehabil. 2002, 8, 71–78. [Google Scholar] [CrossRef]
- Rosen, M.J. Telerehabilitation. Telemed. J. e-Health 2004, 10, 115–117. [Google Scholar] [CrossRef]
- Kairy, D.; Lehoux, P.; Vincent, C.; Visintin, M. A systematic review of clinical outcomes, clinical process, healthcare utilization and costs associated with telerehabilitation. Disabil. Rehabil. 2009, 31, 427–447. [Google Scholar] [CrossRef] [PubMed]
- Mashima, P.A.; Doarn, C.R. Overview of telehealth activities in speech-language pathology. Telemed. J. e-Health 2008, 14, 1101–1117. [Google Scholar] [CrossRef] [PubMed]
- Poon, P.; Hui, E.; Dai, D.; Kwok, T.; Woo, J. Cognitive intervention for community-dwelling older persons with memory problems: Telemedicine versus face-to-face treatment. Int. J. Geriatr. Psychiatry 2005, 20, 285–286. [Google Scholar] [CrossRef] [PubMed]
- Jelcic, N.; Agostini, M.; Meneghello, F.; Bussè, C.; Parise, S.; Galano, A.; Tonin, P.; Dam, M.; Cagnin, A. Feasibility and efficacy of cognitive telerehabilitation in early alzheimer’s disease: A pilot study. Clin. Interv. Aging 2014, 9, 1605–1611. [Google Scholar] [PubMed]
- Vermeij, A.; Claassen, J.A.; Dautzenberg, P.L.; Kessels, R.P. Transfer and maintenance effects of online working-memory training in normal ageing and mild cognitive impairment. Neuropsychol. Rehabil. 2016, 26, 783–809. [Google Scholar] [CrossRef]
- Antonietti, A.; Gandolla, M.; Rossini, M.; Molteni, F.; Pedrocchi, A.; Consortium, A. Interference between cognitive and motor recovery in elderly dementia patients through a holistic tele-rehabilitation platform. In Wireless Mobile Communication and Healthcare: 6th International Conference, MobiHealth 2016, Milan, Italy, 14–16 November 2016, Proceedings; Springer: Cham, Switzerland, 2017; pp. 359–366. [Google Scholar]
- Boutron, I.; Altman, D.G.; Moher, D.; Schulz, K.F.; Ravaud, P. Consort statement for randomized trials of nonpharmacologic treatments: A 2017 update and a consort extension for nonpharmacologic trial abstracts. Ann. Intern. Med. 2017, 167, 40–47. [Google Scholar] [CrossRef]
- Cotelli, M.; Manenti, R.; Brambilla, M.; Gobbi, E.; Ferrari, C.; Binetti, G.; Cappa, S.F. Cognitive telerehabilitation in mild cognitive impairment, alzheimer’s disease and frontotemporal dementia: A systematic review. J. Telemed. Telecare 2019, 25, 67–79. [Google Scholar] [CrossRef]
- Isernia, S.; Pagliari, C.; Jonsdottir, J.; Castiglioni, C.; Gindri, P.; Gramigna, C.; Palumbo, G.; Salza, M.; Molteni, F.; Baglio, F. Efficiency and patient-reported outcome measures from clinic to home: The human empowerment aging and disability program for digital-health rehabilitation. Front. Neurol. 2019, 10, 1206. [Google Scholar] [CrossRef]
- Alaimo, C.; Campana, E.; Stoppelli, M.R.; Gobbi, E.; Baglio, F.; Rossetto, F.; Binetti, G.; Zanetti, O.; Manenti, R.; Cotelli, M. Cognitive tele-enhancement in healthy older adults and subjects with subjective memory complaints: A review. Front. Neurol. 2021, 12, 650553. [Google Scholar] [CrossRef]
- Cherney, L.R.; Van Vuuren, S. Telerehabilitation, virtual therapists, and acquired neurologic speech and language disorders. Sem. Speech Lang. 2012, 33, 243–258. [Google Scholar] [CrossRef]
- McCue, M.; Fairman, A.; Pramuka, M. Enhancing quality of life through telerehabilitation. Phys. Med. Rehabil. Clin. 2010, 21, 195–205. [Google Scholar] [CrossRef]
- Bahar-Fuchs, A.; Clare, L.; Woods, B. Cognitive training and cognitive rehabilitation for persons with mild to moderate dementia of the alzheimer’s or vascular type: A review. J. Alzheimers Res. Ther. 2013, 5, 1–14. [Google Scholar] [CrossRef]
- Bahar-Fuchs, A.; Martyr, A.; Goh, A.M.; Sabates, J.; Clare, L. Cognitive training for people with mild to moderate dementia. Cochrane Database Syst. Rev. 2019, 3, CD013069. [Google Scholar] [CrossRef]
- Clare, L. Rehabilitation for people living with dementia: A practical framework of positive support. PLoS Med. 2017, 14, e1002245. [Google Scholar] [CrossRef] [PubMed]
- Clare, L.; Linden, D.E.; Woods, R.T.; Whitaker, R.; Evans, S.J.; Parkinson, C.H.; van Paasschen, J.; Nelis, S.M.; Hoare, Z.; Yuen, K.S.; et al. Goal-oriented cognitive rehabilitation for people with early-stage alzheimer disease: A single-blind randomized controlled trial of clinical efficacy. Am. J. Geriatr. Psychiatry 2010, 18, 928–939. [Google Scholar] [CrossRef]
- Clare, L.; Woods, R.T. Cognitive training and cognitive rehabilitation for people with early-stage alzheimer’s disease: A review. J. Neuropsychol. Rehabil. 2004, 14, 385–401. [Google Scholar] [CrossRef]
- Gates, N.J.; Sachdev, P. Is cognitive training an effective treatment for preclinical and early alzheimer’s disease? J. Alzheimers Dis. 2014, 42 (Suppl. 4), S551–S559. [Google Scholar] [CrossRef]
- Hong, Y.J.; Jang, E.H.; Hwang, J.; Roh, J.H.; Lee, J.H. The efficacy of cognitive intervention programs for mild cognitive impairment: A systematic review. Curr. Alzheimer Res. 2015, 12, 527–542. [Google Scholar] [CrossRef]
- Kortte, K.B.; Rogalski, E.J. Behavioural interventions for enhancing life participation in behavioural variant frontotemporal dementia and primary progressive aphasia. Int. Rev. Psychiatry 2013, 25, 237–245. [Google Scholar] [CrossRef]
- Kudlicka, A.; Martyr, A.; Bahar-Fuchs, A.; Sabates, J.; Woods, B.; Clare, L. Cognitive rehabilitation for people with mild to moderate dementia. Cochrane Database Syst. Rev. 2023, 6, CD013388. [Google Scholar] [CrossRef]
- Rai, H.; Yates, L.; Orrell, M. Cognitive stimulation therapy for dementia. Clin. Geriatr. Med. 2018, 34, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Woods, R.T.; Britton, P.G. Psychological approaches to the treatment of the elderly. Age Ageing 1977, 6, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Orgeta, V.; McDonald, K.R.; Poliakoff, E.; Hindle, J.V.; Clare, L.; Leroi, I. Cognitive training interventions for dementia and mild cognitive impairment in parkinson’s disease. Cochrane Database Syst. Rev. 2020, 2, CD011961. [Google Scholar] [CrossRef] [PubMed]
- Walton, C.C.; Naismith, S.L.; Lampit, A.; Mowszowski, L.; Lewis, S.J. Cognitive training in parkinson’s disease. Neurorehabilit. Neural Repair 2017, 31, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Lisko, I.; Kulmala, J.; Annetorp, M.; Ngandu, T.; Mangialasche, F.; Kivipelto, M. How can dementia and disability be prevented in older adults: Where are we today and where are we going? J. Intern. Med. 2021, 289, 807–830. [Google Scholar] [CrossRef]
- Bello-Haas, V.D. A framework for rehabilitation of neurodegenerative diseases: Planning care and maximizing quality of life. J. Neurol. Phys. Ther. 2002, 26, 115–129. [Google Scholar] [CrossRef]
- Alloni, A.; Sinforiani, E.; Zucchella, C.; Sandrini, G.; Bernini, S.; Cattani, B.; Pardell, D.T.; Quaglini, S.; Pistarini, C. Computer-based cognitive rehabilitation: The core system. Disabil. Rehabil. 2017, 39, 407–417. [Google Scholar] [CrossRef]
- Manenti, R.; Gobbi, E.; Baglio, F.; Macis, A.; Ferrari, C.; Pagnoni, I.; Rossetto, F.; Di Tella, S.; Alemanno, F.; Cimino, V. Effectiveness of an innovative cognitive treatment and telerehabilitation on subjects with mild cognitive impairment: A multicenter, randomized, active-controlled study. Front. Aging Neurosci. 2020, 12, 585988. [Google Scholar] [CrossRef]
- Sokolov, A.A.; Collignon, A.; Bieler-Aeschlimann, M. Serious video games and virtual reality for prevention and neurorehabilitation of cognitive decline because of aging and neurodegeneration. Curr. Opin. Neurol. 2020, 33, 239–248. [Google Scholar] [CrossRef]
- Janoutová, J.; Serý, O.; Hosák, L.; Janout, V. Is mild cognitive impairment a precursor of alzheimer’s disease? Short review. Cent. Eur. J. Public Health 2015, 23, 365–367. [Google Scholar] [CrossRef]
- Pagliari, C.; Di Tella, S.; Jonsdottir, J.; Mendozzi, L.; Rovaris, M.; De Icco, R.; Milanesi, T.; Federico, S.; Agostini, M.; Goffredo, M.; et al. Effects of home-based virtual reality telerehabilitation system in people with multiple sclerosis: A randomized controlled trial. J. Telemed. Telecare 2024, 30, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Burton, R.L.; O’Connell, M.E. Telehealth rehabilitation for cognitive impairment: Randomized controlled feasibility trial. JMIR Res. Protoc. 2018, 7, e43. [Google Scholar] [CrossRef] [PubMed]
- Vellata, C.; Belli, S.; Balsamo, F.; Giordano, A.; Colombo, R.; Maggioni, G. Effectiveness of telerehabilitation on motor impairments, non-motor symptoms and compliance in patients with parkinson’s disease: A systematic review. Front. Neurol. 2021, 12, 627999. [Google Scholar] [CrossRef]
- Cacciante, L.; Pietà, C.D.; Rutkowski, S.; Cieślik, B.; Szczepańska-Gieracha, J.; Agostini, M.; Kiper, P. Cognitive telerehabilitation in neurological patients: Systematic review and meta-analysis. Neurol. Sci. 2022, 43, 847–862. [Google Scholar] [CrossRef]
- Bianchini, E.; Onelli, C.; Morabito, C.; Alborghetti, M.; Rinaldi, D.; Anibaldi, P.; Marcolongo, A.; Salvetti, M.; Pontieri, F.E. Feasibility, safety, and effectiveness of telerehabilitation in mild-to-moderate parkinson’s disease. Front. Neurol. 2022, 13, 909197. [Google Scholar] [CrossRef]
- Manenti, R.; Baglio, F.; Pagnoni, I.; Gobbi, E.; Campana, E.; Alaimo, C.; Rossetto, F.; Di Tella, S.; Pagliari, C.; Geviti, A.; et al. Long-lasting improvements in episodic memory among subjects with mild cognitive impairment who received transcranial direct current stimulation combined with cognitive treatment and telerehabilitation: A multicentre, randomized, active-controlled study. Front. Aging Neurosci. 2024, 16, 1414593. [Google Scholar] [CrossRef]
- Bernini, S.; Panzarasa, S.; Quaglini, S.; Costa, A.; Picascia, M.; Cappa, S.F.; Cerami, C.; Tassorelli, C.; Vecchi, T.; Bottiroli, S. Homecore system for telerehabilitation in individuals at risk of dementia: A usability and user experience study. Front. Med. 2023, 10, 1129914. [Google Scholar] [CrossRef]
- Chan, A.T.C.; Ip, R.T.F.; Tran, J.Y.S.; Chan, J.Y.C.; Tsoi, K.K.F. Computerized cognitive training for memory functions in mild cognitive impairment or dementia: A systematic review and meta-analysis. NPJ Digit. Med. 2024, 7, 1–11. [Google Scholar] [CrossRef]
- Mosca, I.E.; Salvadori, E.; Gerli, F.; Fabbri, L.; Pancani, S.; Lucidi, G.; Lombardi, G.; Bocchi, L.; Pazzi, S.; Baglio, F.; et al. Analysis of feasibility, adherence, and appreciation of a newly developed tele-rehabilitation program for people with mci and vci. Front. Neurol. 2020, 11, 583368. [Google Scholar] [CrossRef]
- Nousia, A.; Martzoukou, M.; Siokas, V.; Aretouli, E.; Aloizou, A.M.; Folia, V.; Peristeri, E.; Messinis, L.; Nasios, G.; Dardiotis, E. Beneficial effect of computer-based multidomain cognitive training in patients with mild cognitive impairment. Appl. Neuropsychol. Adult 2021, 28, 717–726. [Google Scholar] [CrossRef]
- Maggio, M.G.; Luca, A.; Cicero, C.E.; Calabrò, R.S.; Drago, F.; Zappia, M.; Nicoletti, A. Effectiveness of telerehabilitation plus virtual reality (tele-rv) in cognitive e social functioning: A randomized clinical study on parkinson’s disease. Park. Relat. Disord. 2024, 119. [Google Scholar] [CrossRef]
- Isernia, S.; Di Tella, S.; Rossetto, F.; Borgnis, F.; Realdon, O.; Cabinio, M.; Pagliari, C.; Torchio, A.; Castagna, A.; Blasi, V.; et al. Exploring cognitive reserve’s influence: Unveiling the dynamics of digital telerehabilitation in parkinson’s disease resilience. NPJ Digit. Med. 2024, 7, 1–8. [Google Scholar] [CrossRef]
- Latella, D.; Maresca, G.; Formica, C.; Sorbera, C.; Bringandì, A.; Di Lorenzo, G.; Quartarone, A.; Marino, S. The role of telemedicine in the treatment of cognitive and psychological disorders in parkinson’s disease: An overview. Brain Sci. 2023, 13, 499. [Google Scholar] [CrossRef]
- Matamala-Gomez, M.; Maisto, M.; Montana, J.I.; Mavrodiev, P.A.; Baglio, F.; Rossetto, F.; Mantovani, F.; Riva, G.; Realdon, O. The role of engagement in teleneurorehabilitation: A systematic review. Front. Neurol. 2020, 11, 354. [Google Scholar] [CrossRef]
- Moccia, M.; Brigo, F.; Brennan, S.; Bonavita, S. Digital technology in neurology: From clinical assessment to neurorehabilitation. Front. Neurol. 2021, 11, 614074. [Google Scholar] [CrossRef]
- Berlucchi, G. Brain plasticity and cognitive neurorehabilitation. Neuropsychol. Rehabil. 2011, 21, 560–578. [Google Scholar] [CrossRef]
- Marzola, P.; Melzer, T.; Pavesi, E.; Gil-Mohapel, J.; Brocardo, P.S. Exploring the role of neuroplasticity in development, aging, and neurodegeneration. Brain Sci. 2023, 13, 1610. [Google Scholar] [CrossRef]
- Rodríguez-González, V.; Gómez, C.; Hoshi, H.; Shigihara, Y.; Hornero, R.; Poza, J. Exploring the interactions between neurophysiology and cognitive and behavioral changes induced by a non-pharmacological treatment: A network approach. Front. Aging Neurosci. 2021, 13, 696174. [Google Scholar] [CrossRef]
- Chan, A.W.; Tetzlaff, J.M.; Altman, D.G.; Laupacis, A.; Gøtzsche, P.C.; Krleža-Jerić, K.; Hróbjartsson, A.; Mann, H.; Dickersin, K.; Berlin, J.A.; et al. Spirit 2013 statement: Defining standard protocol items for clinical trials. Ann. Intern. Med. 2013, 158, 200–207. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The montreal cognitive assessment, moca: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Conti, S.; Bonazzi, S.; Laiacona, M.; Masina, M.; Coralli, M.V. Montreal cognitive assessment (moca)-italian version: Regression based norms and equivalent scores. Neurol. Sci. 2015, 36, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Pirrotta, F.; Timpano, F.; Bonanno, L.; Nunnari, D.; Marino, S.; Bramanti, P.; Lanzafame, P. Italian validation of montreal cognitive assessment. Eur. J. Psychol. Assess. 2015, 31, 131–137. [Google Scholar] [CrossRef]
- Goetz, C.G.; Poewe, W.; Rascol, O.; Sampaio, C.; Stebbins, G.T.; Counsell, C.; Giladi, N.; Holloway, R.G.; Moore, C.G.; Wenning, G.K. Movement disorder society task force report on the hoehn and yahr staging scale: Status and recommendations the movement disorder society task force on rating scales for parkinson’s disease. Mov. Disord. 2004, 19, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.C. The clinical dementia rating (cdr) current version and scoring rules. Neurology 1993, 43, 2412–2414. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Robins, L.N.; Helzer, J.E. The mini-mental state examination. Arch. Gen. Psychiatry 1983, 40, 812. [Google Scholar] [CrossRef]
- Magni, E.; Binetti, G.; Bianchetti, A.; Rozzini, R.; Trabucchi, M. Mini-mental state examination: A normative study in italian elderly population. Eur. J. Neurol. 1996, 3, 198–202. [Google Scholar] [CrossRef]
- Measso, G.; Cavarzeran, F.; Zappalà, G.; Lebowitz, B.D.; Crook, T.H.; Pirozzolo, F.J.; Amaducci, L.A.; Massari, D.; Grigoletto, F. The mini-mental state examination: Normative study of an italian random sample. Dev. Neuropsychol. 1993, 9, 77–85. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: Dsm-5; American Psychiatric Association: Washington, DC, USA, 2013; Volume 5. [Google Scholar]
- Frasson, P.; Ghiretti, R.; Catricalà, E.; Pomati, S.; Marcone, A.; Parisi, L.; Rossini, P.M.; Cappa, S.F.; Mariani, C.; Vanacore, N.; et al. Free and cued selective reminding test: An italian normative study. Neurol. Sci. 2011, 32, 1057–1062. [Google Scholar] [CrossRef]
- Scalone, L.; Cortesi, P.A.; Ciampichini, R.; Cesana, G.; Mantovani, L.G. Health related quality of life norm data of the italian general population: Results using the eq-5d-3l and eq-5d-5l instruments. Epidemiol. Biostat. Public Health 2015, 12. [Google Scholar] [CrossRef]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R. Movement disorder society-sponsored revision of the unified parkinson’s disease rating scale (mds-updrs): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Spielberger, C.; Gorsuch, R.; Lushene, R.; Vagg, P.; Jacobs, G. Manual for the State-Trait Anxiety Inventory (form y1–y2); Consulting Psychologists Press: Palo Alto, CA, USA, 1983; Volume 4. [Google Scholar]
- Cummings, J.L.; Mega, M.; Gray, K.; Rosenberg-Thompson, S.; Carusi, D.A.; Gornbein, J. The neuropsychiatric inventory: Comprehensive assessment of psychopathology in dementia. Neurology 1994, 44, 2308. [Google Scholar] [CrossRef] [PubMed]
- Calabria, M.; Manenti, R.; Rosini, S.; Zanetti, O.; Miniussi, C.; Cotelli, M. Objective and subjective memory impairment in elderly adults: A revised version of the everyday memory questionnaire. Aging Clin. Exp. Res. 2011, 23, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Basso, A.; Capitani, E.; Laiacona, M. Raven’s coloured progressive matrices: Normative values on 305 adult normal controls. Funct. Neurol. 1987, 2, 189–194. [Google Scholar]
- Giovagnoli, A.R.; Del Pesce, M.; Mascheroni, S.; Simoncelli, M.; Laiacona, M.; Capitani, E. Trail making test: Normative values from 287 normal adult controls. Ital. J. Neurol. Sci. 1996, 17, 305–309. [Google Scholar] [CrossRef]
- Caffarra, P.; Vezzadini, G.; Dieci, F.; Zonato, F.; Venneri, A. A short version of the stroop test: Normative data in an italian population sample. Nuova Riv. Di Neurol. 2002, 12, 111–115. [Google Scholar]
- Caffarra, P.; Vezzadini, G.; Dieci, F.; Zonato, F.; Venneri, A. Rey-osterrieth complex figure: Normative values in an italian population sample. Neurol. Sci. 2002, 22, 443–447. [Google Scholar] [CrossRef]
- Novelli, G.; Papagno, C.; Capitani, E.; Laiacona, M. Tre test clinici di ricerca e produzione lessicale. Taratura su sogetti normali. Arch. Psicol. Neurol. Psichiatr. 1986, 47, 477–506. [Google Scholar]
- Di Lazzaro, V.; Pilato, F.; Dileone, M.; Profice, P.; Ranieri, F.; Ricci, V.; Bria, P.; Tonali, P.A.; Ziemann, U. Segregating two inhibitory circuits in human motor cortex at the level of gabaa receptor subtypes: A tms study. Clin. Neurophysiol. 2007, 118, 2207–2214. [Google Scholar] [CrossRef]
- Ni, Z.; Charab, S.; Gunraj, C.; Nelson, A.J.; Udupa, K.; Yeh, I.J.; Chen, R. Transcranial magnetic stimulation in different current directions activates separate cortical circuits. J. Neurophysiol. 2011, 105, 749–756. [Google Scholar] [CrossRef]
- Jeon, H.; Kim, D.Y.; Park, S.W.; Lee, B.S.; Han, H.W.; Jeon, N.; Kim, M.; Kang, M.; Kim, S. A systematic review of cognitive telerehabilitation in patients with cognitive dysfunction. Front. Neurol. 2024, 15, 1450977. [Google Scholar] [CrossRef] [PubMed]
- Howell, D.C. Statistical Methods for Psychology, 8th ed.; Wadsworth Cengage Learning: Belmont, CA, USA, 2019. [Google Scholar]
- Yang, J.; Li, H.; Zhao, H.; Xie, Y.; Li, J.; Wang, M. Effectiveness of telerehabilitation in patients with post-covid-19: A systematic review and meta-analysis of randomised controlled trials. BMJ Open 2024, 14, e074325. [Google Scholar] [CrossRef] [PubMed]
- McGinn, L.C. Nonparametric Statistics for the Behavioral Sciences: By Sidney Siegel. J. Frankl. Inst. 1957, 263, 168–169. [Google Scholar] [CrossRef]
- Guyon, I.; Elisseeff, A. An introduction to variable and feature selection. J. Mach. Learn. Res. 2003, 3, 1157–1182. [Google Scholar]
- Sheskin, D.J. Handbook of Parametric and Nonparametric Statistical Procedures, 5th ed.; Chapman and Hall: New York, NY, USA, 2011. [Google Scholar]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning; Springer: New York, NY, USA, 2001. [Google Scholar]
- Bergstra, J.; Bengio, Y. Random search for hyper-parameter optimization. J. Mach. Learn. Res. 2012, 13, 281–305. [Google Scholar]
- Lundberg, S.; Lee, S. A unified approach to interpreting model predictions. arXiv 2017, arXiv:1705.07874. [Google Scholar]
- Lasaponara, S.; Marson, F.; Doricchi, F.; Cavallo, M. A scoping review of cognitive training in neurodegenerative diseases via computerized and virtual reality tools: What we know so far. Brain Sci. 2021, 11, 528. [Google Scholar] [CrossRef]
- Sanjuán, M.; Navarro, E.; Calero, M.D. Effectiveness of cognitive interventions in older adults: A review. Eur. J. Investig. Health Psychol. Educ. 2020, 10, 876–898. [Google Scholar] [CrossRef]
- Brueggen, K.; Kasper, E.; Ochmann, S.; Pfaff, H.; Webel, S.; Schneider, W.; Teipel, S. Cognitive rehabilitation in alzheimer’s disease: A controlled intervention trial. J. Alzheimers Dis. 2017, 57, 1315–1324. [Google Scholar] [CrossRef]
- Carrion, C.; Folkvord, F.; Anastasiadou, D.; Aymerich, M. Cognitive therapy for dementia patients: A systematic review. Dement. Geriatr. Cogn. Disord. 2018, 46, 1–26. [Google Scholar] [CrossRef]
- Hampstead, B.M.; Sathian, K.; Bikson, M.; Stringer, A.Y. Combined mnemonic strategy training and high-definition transcranial direct current stimulation for memory deficits in mild cognitive impairment. Alzheimers Dement. 2017, 3, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Huntley, J.D.; Gould, R.L.; Liu, K.; Smith, M.; Howard, R.J. Do cognitive interventions improve general cognition in dementia? A meta-analysis and meta-regression. BMJ Open 2015, 5, e005247. [Google Scholar] [CrossRef] [PubMed]
- Kallio, E.L.; Öhman, H.; Kautiainen, H.; Hietanen, M.; Pitkälä, K. Cognitive training interventions for patients with alzheimer’s disease: A systematic review. J. Alzheimers Dis. 2017, 56, 1349–1372. [Google Scholar] [CrossRef] [PubMed]
- Orrell, M.; Yates, L.; Leung, P.; Kang, S.; Hoare, Z.; Whitaker, C.; Burns, A.; Knapp, M.; Leroi, I.; Moniz-Cook, E.; et al. The impact of individual cognitive stimulation therapy (icst) on cognition, quality of life, caregiver health, and family relationships in dementia: A randomised controlled trial. PLoS Med. 2017, 14, e1002269. [Google Scholar] [CrossRef]
- Mendes, L.; Oliveira, J.; Barbosa, F.; Castelo-Branco, M. A conceptual view of cognitive intervention in older adults with and without cognitive decline-a systemic review. Front. Aging 2022, 3, 844725. [Google Scholar] [CrossRef]
- Castro, C.B.; Costa, L.M.; Dias, C.B.; Chen, J.; Hillebrandt, H.; Gardener, S.L.; Brown, B.M.; Loo, R.L.; Garg, M.L.; Rainey-Smith, S.R.; et al. Multi-domain interventions for dementia prevention—A systematic review. J. Nutr. Health Aging 2023, 27, 1271–1280. [Google Scholar] [CrossRef]
- Cheng, S.T. Cognitive reserve and the prevention of dementia: The role of physical and cognitive activities. Curr. Psychiatry Rep. 2016, 18, 1–12. [Google Scholar] [CrossRef]
- Gates, N.; Valenzuela, M. Cognitive exercise and its role in cognitive function in older adults. Curr. Psychiatry Rep. 2010, 12, 20–27. [Google Scholar] [CrossRef]
- Buschert, V.; Bokde, A.L.; Hampel, H. Cognitive intervention in alzheimer disease. Nat. Rev. Neurol. 2010, 6, 508–517. [Google Scholar] [CrossRef]
- Cabeza, R.; Albert, M.; Belleville, S.; Craik, F.I.M.; Duarte, A.; Grady, C.L.; Lindenberger, U.; Nyberg, L.; Park, D.C.; Reuter-Lorenz, P.A.; et al. Maintenance, reserve and compensation: The cognitive neuroscience of healthy ageing. Nat. Rev. Neurosci. 2018, 19, 701–710. [Google Scholar] [CrossRef]
- Kumar, J.; Patel, T.; Sugandh, F.; Dev, J.; Kumar, U.; Adeeb, M.; Kachhadia, M.P.; Puri, P.; Prachi, F.; Zaman, M.U.; et al. Innovative approaches and therapies to enhance neuroplasticity and promote recovery in patients with neurological disorders: A narrative review. Cureus 2023, 15, e4191. [Google Scholar] [CrossRef] [PubMed]
- Dinius, C.J.; Pocknell, C.E.; Caffrey, M.P.; Roche, R.A.P. Cognitive interventions for memory and psychological well-being in aging and dementias. Front. Psychol. 2023, 14, 1070012. [Google Scholar] [CrossRef] [PubMed]
- Black, J.E.; Polinsky, M.; Greenough, W.T. Progressive failure of cerebral angiogenesis supporting neural plasticity in aging rats. Neurobiol. Aging 1989, 10, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G.; Kuhn, H.G.; Winkler, J.; Gage, F.H. New nerve cells for the adult brain. Adult neurogenesis and stem cell concepts in neurologic research. Der Nervenarzt 1998, 69, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Klintsova, A.Y.; Greenough, W.T. Synaptic plasticity in cortical systems. Curr. Opin. Neurobiol. 1999, 9, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Rao, B.S.; Raju, T.R.; Meti, B.L. Increased numerical density of synapses in ca3 region of hippocampus and molecular layer of motor cortex after self-stimulation rewarding experience. Neuroscience 1999, 91, 799–803. [Google Scholar]
- Stern, Y. Cognitive reserve and alzheimer disease. Alzheimer Dis. Assoc. Disord. 2006, 20, S69–S74. [Google Scholar] [CrossRef]
- Valenzuela, M.J.; Sachdev, P. Brain reserve and dementia: A systematic review. Psychol. Med. 2006, 36, 441–454. [Google Scholar] [CrossRef]
- Stern, Y.; Habeck, C.; Moeller, J.; Scarmeas, N.; Anderson, K.E.; Hilton, H.J.; Flynn, J.; Sackeim, H.; van Heertum, R. Brain networks associated with cognitive reserve in healthy young and old adults. Cereb. Cortex 2005, 15, 394–402. [Google Scholar] [CrossRef]
- Pettigrew, C.; Soldan, A. Defining cognitive reserve and implications for cognitive aging. Curr. Neurol. Neurosci. Rep. 2019, 19, 1–12. [Google Scholar] [CrossRef]
- Panico, F.; Sagliano, L.; Magliacano, A.; Santangelo, G.; Trojano, L. The relationship between cognitive reserve and cognition in healthy adults: A systematic review. Curr. Psychol. 2023, 42, 24751–24763. [Google Scholar] [CrossRef]
- Sandrini, M.; Manenti, R.; Gobbi, E.; Pagnoni, I.; Geviti, A.; Alaimo, C.; Campana, E.; Binetti, G.; Cotelli, M. Cognitive reserve predicts episodic memory enhancement induced by transcranial direct current stimulation in healthy older adults. Sci. Rep. 2024, 14, 4879. [Google Scholar] [CrossRef]
- Valenzuela, M.; Sachdev, P. Can cognitive exercise prevent the onset of dementia? Systematic review of randomized clinical trials with longitudinal follow-up. Am. J. Geriatr. Psychiatry 2009, 17, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Esiri, M.M.; Chance, S.A. Cognitive reserve, cortical plasticity and resistance to alzheimer’s disease. Alzheimers Res. Ther. 2012, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, G.; Liu, L.; He, Y.; Gong, W. Cognitive reserve over the life course and risk of dementia: A systematic review and meta-analysis. Front. Aging Neurosci. 2024, 16, 1358992. [Google Scholar] [CrossRef]
- Brennan, D.; Tindall, L.; Theodoros, D.; Brown, J.; Campbell, M.; Christiana, D.; Smith, D.; Cason, J.; Lee, A. A blueprint for telerehabilitation guidelines. Int. J. Telerehabil. 2010, 2, 31–34. [Google Scholar] [CrossRef]
- Brennan, D.M.; Mawson, S.; Brownsell, S. Telerehabilitation: Enabling the remote delivery of healthcare, rehabilitation, and self management. Stud. Health Technol. Inform. 2009, 145, 231–248. [Google Scholar]
- Hailey, D.; Roine, R.; Ohinmaa, A.; Dennett, L. Evidence of benefit from telerehabilitation in routine care: A systematic review. J. Telemed. Telecare 2011, 17, 281–287. [Google Scholar] [CrossRef]
- Zampolini, M.; Todeschini, E.; Bernabeu Guitart, M.; Hermens, H.; Ilsbroukx, S.; Macellari, V.; Magni, R.; Rogante, M.; Scattareggia Marchese, S.; Vollenbroek, M.; et al. Tele-rehabilitation: Present and future. Ann. Ist. Super. Sanita 2008, 44, 125–134. [Google Scholar]
- Rossetto, F.; Mestanza Mattos, F.G.; Gervasoni, E.; Germanotta, M.; Pavan, A.; Cattaneo, D.; Aprile, I.; Baglio, F. Efficacy of telerehabilitation with digital and robotic tools for the continuity of care of people with chronic neurological disorders: The teleneuro@rehab protocol for a randomized controlled trial. Digit. Health 2024, 10, 20552076241228928. [Google Scholar] [CrossRef]
- Goffredo, M.; Pagliari, C.; Turolla, A.; Tassorelli, C.; Di Tella, S.; Federico, S.; Pournajaf, S.; Jonsdottir, J.; De Icco, R.; Pellicciari, L.; et al. Non-immersive virtual reality telerehabilitation system improves postural balance in people with chronic neurological diseases. J. Clin. Med. 2023, 12, 3178. [Google Scholar] [CrossRef] [PubMed]
- Maggio, M.G.; Baglio, F.; Arcuri, F.; Borgnis, F.; Contrada, M.; Diaz, M.D.M.; Leochico, C.F.; Neira, N.J.; Laratta, S.; Suchan, B.; et al. Cognitive telerehabilitation: An expert consensus paper on current evidence and future perspective. Front. Neurol. 2024, 15, 1338873. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, R.S.; Bonanno, M.; Torregrossa, W.; Cacciante, L.; Celesti, A.; Rifici, C.; Tonin, P.; De Luca, R.; Quartarone, A. Benefits of telerehabilitation for patients with severe acquired brain injury: Promising results from a multicenter randomized controlled trial using nonimmersive virtual reality. J. Med. Internet Res. 2023, 25, e45458. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, R.S.; Bramanti, A.; Garzon, M.; Celesti, A.; Russo, M.; Portaro, S.; Naro, A.; Manuli, A.; Tonin, P.; Bramanti, P. Telerehabilitation in individuals with severe acquired brain injury: Rationale, study design, and methodology. Medicine 2018, 97, e13292. [Google Scholar] [CrossRef]
- Rossetto, F.; Borgnis, F.; Blasi, V.; Banfi, P.I.; Tavanelli, M.; Realdon, O.; Mantovani, F.; Foglia, E.; Garagiola, E.; Croce, D. System integrated digital empowerment and rehabilitation to promote patient activation and well-being (sidera^b): Protocol for a randomized crossover trial on effectiveness and implementation. medRxiv 2022. [Google Scholar] [CrossRef]
- Pergantis, P.; Bamicha, V.; Skianis, C.; Drigas, A. Ai chatbots and cognitive control: Enhancing executive functions through chatbot interactions: A systematic review. Brain Sci. 2025, 15, 47. [Google Scholar] [CrossRef]
- Stoltzfus, M.; Kaur, A.; Chawla, A.; Gupta, V.; Anamika, F.N.U.; Jain, R. The role of telemedicine in healthcare: An overview and update. Egypt. J. Intern. Med. 2023, 35, 49. [Google Scholar] [CrossRef]
- Pergantis, P. Developmental coordination disorder and the role of new technologies as intervention tool. World J. Adv. Res. Rev. 2023, 19, 519–528. [Google Scholar] [CrossRef]
- Dávalos, M.E.; French, M.T.; Burdick, A.E.; Simmons, S.C. Economic evaluation of telemedicine: Review of the literature and research guidelines for benefit-cost analysis. Telemed. J. e-Health 2009, 15, 933–948. [Google Scholar] [CrossRef]
- Laugwitz, B.; Held, T.; Schrepp, M. HCI and Usability for Education and Work. In Construction and Evaluation of a User Experience Questionnaire; Holzinger, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 63–76. [Google Scholar]
- Laugwitz, B.; Schrepp, M.; Held, T. Konstruktion eines fragebogens zur messung der user experience von softwareprodukten. In Mensch Und Computer 2006; Heinecke, H.M., Paul, H., Eds.; Oldenbourg Wissenschaftsverlag: München, Germany, 2006; pp. 125–134. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cotelli, M.; Baglio, F.; Gobbi, E.; Campana, E.; Pagnoni, I.; Cannarella, G.; Del Torto, A.; Rossetto, F.; Comanducci, A.; Tartarisco, G.; et al. Smart Digital Solutions for EARLY Treatment of COGNitive Disability (EARLY-COGN^3): A Study Protocol. Brain Sci. 2025, 15, 239. https://doi.org/10.3390/brainsci15030239
Cotelli M, Baglio F, Gobbi E, Campana E, Pagnoni I, Cannarella G, Del Torto A, Rossetto F, Comanducci A, Tartarisco G, et al. Smart Digital Solutions for EARLY Treatment of COGNitive Disability (EARLY-COGN^3): A Study Protocol. Brain Sciences. 2025; 15(3):239. https://doi.org/10.3390/brainsci15030239
Chicago/Turabian StyleCotelli, Maria, Francesca Baglio, Elena Gobbi, Elena Campana, Ilaria Pagnoni, Giovanna Cannarella, Alessandro Del Torto, Federica Rossetto, Angela Comanducci, Gennaro Tartarisco, and et al. 2025. "Smart Digital Solutions for EARLY Treatment of COGNitive Disability (EARLY-COGN^3): A Study Protocol" Brain Sciences 15, no. 3: 239. https://doi.org/10.3390/brainsci15030239
APA StyleCotelli, M., Baglio, F., Gobbi, E., Campana, E., Pagnoni, I., Cannarella, G., Del Torto, A., Rossetto, F., Comanducci, A., Tartarisco, G., Calabrò, R. S., Campisi, S., Maione, R., Saraceno, C., Dognini, E., Bellini, S., Bortoletto, M., Binetti, G., Ghidoni, R., & Manenti, R. (2025). Smart Digital Solutions for EARLY Treatment of COGNitive Disability (EARLY-COGN^3): A Study Protocol. Brain Sciences, 15(3), 239. https://doi.org/10.3390/brainsci15030239








