Effect of Neck Muscle Vibration Prior to Motor Learning on Short-Latency SEP Peak Amplitudes and Motor Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Somatosensory Evoked Potentials (SEPs) Stimulation Parameters
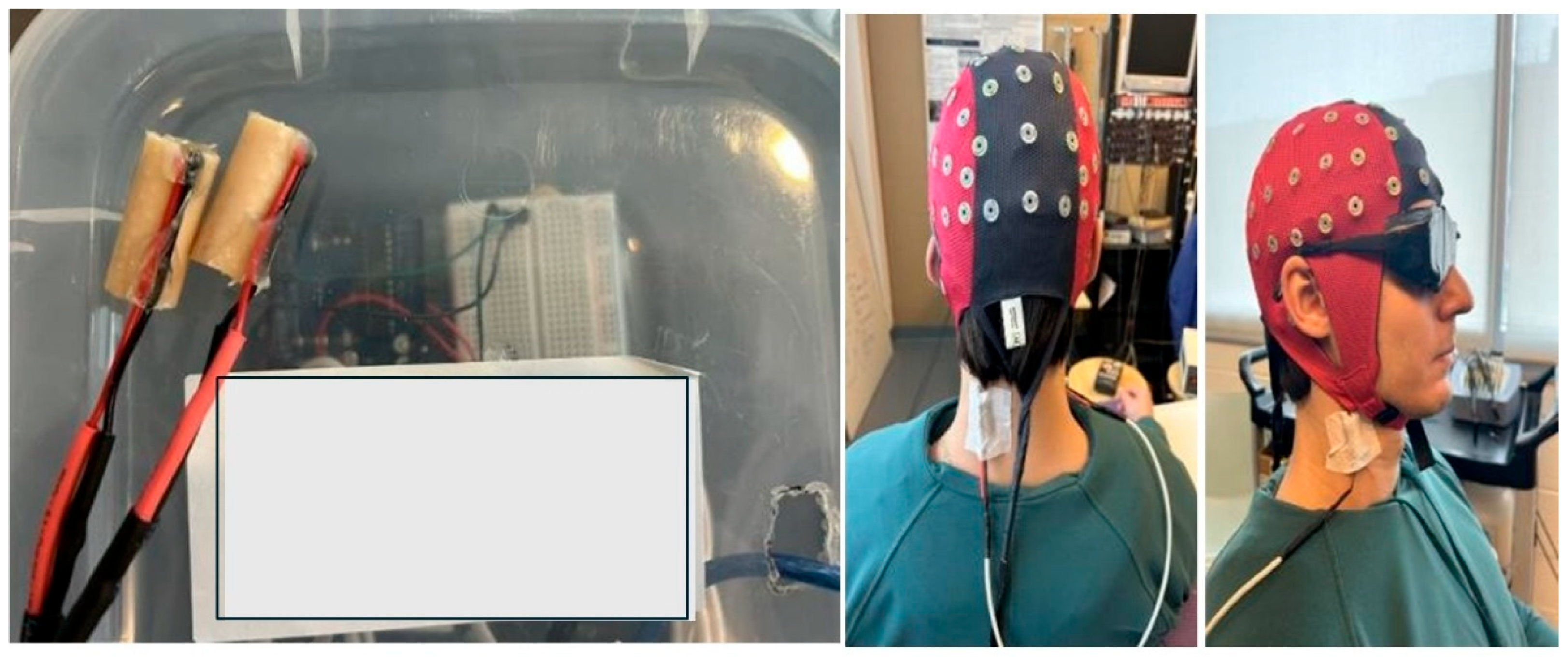
2.3. Recording Parameters
2.4. Neck Muscle Vibration Protocol
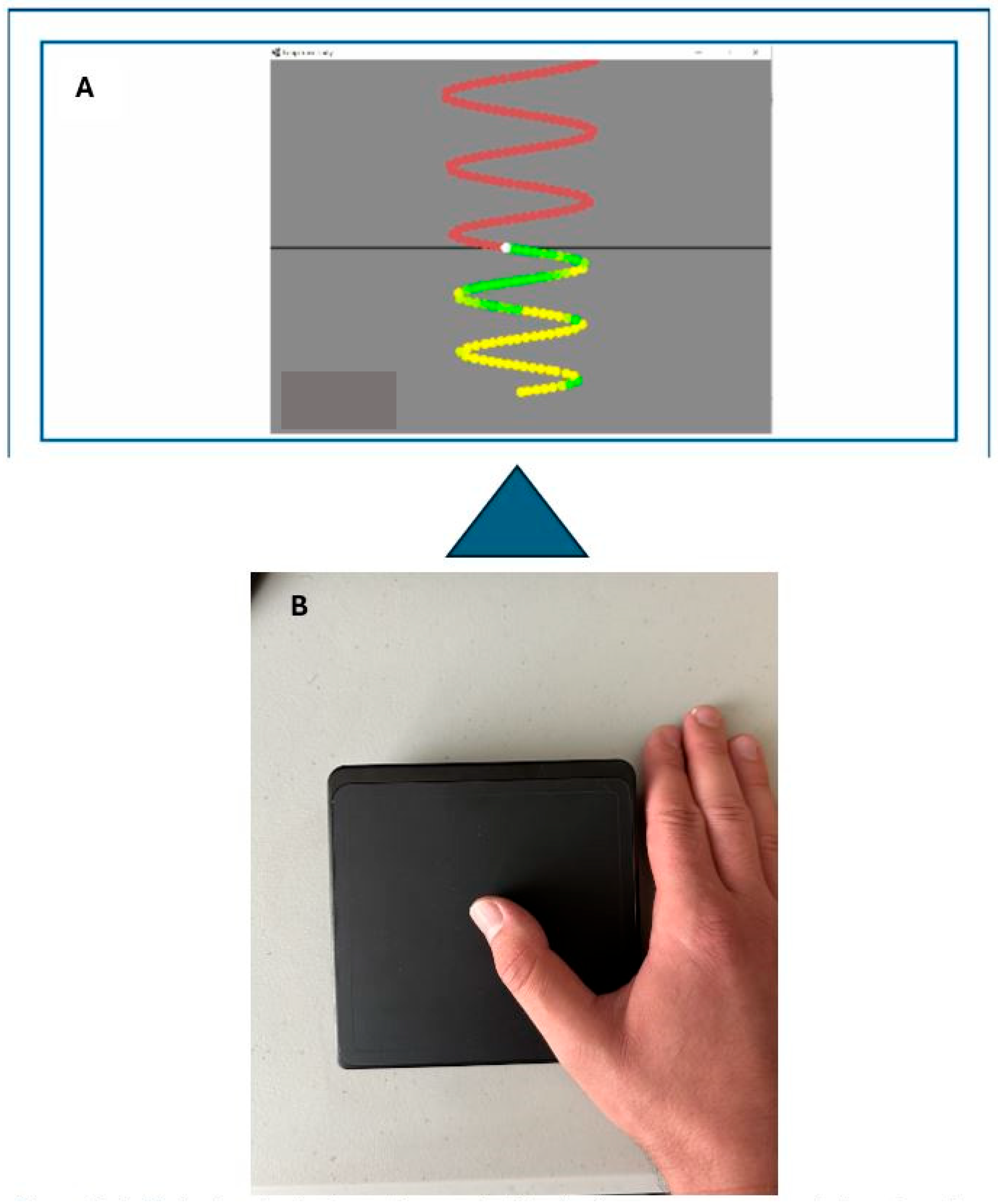
2.5. Motor-Tracing Task
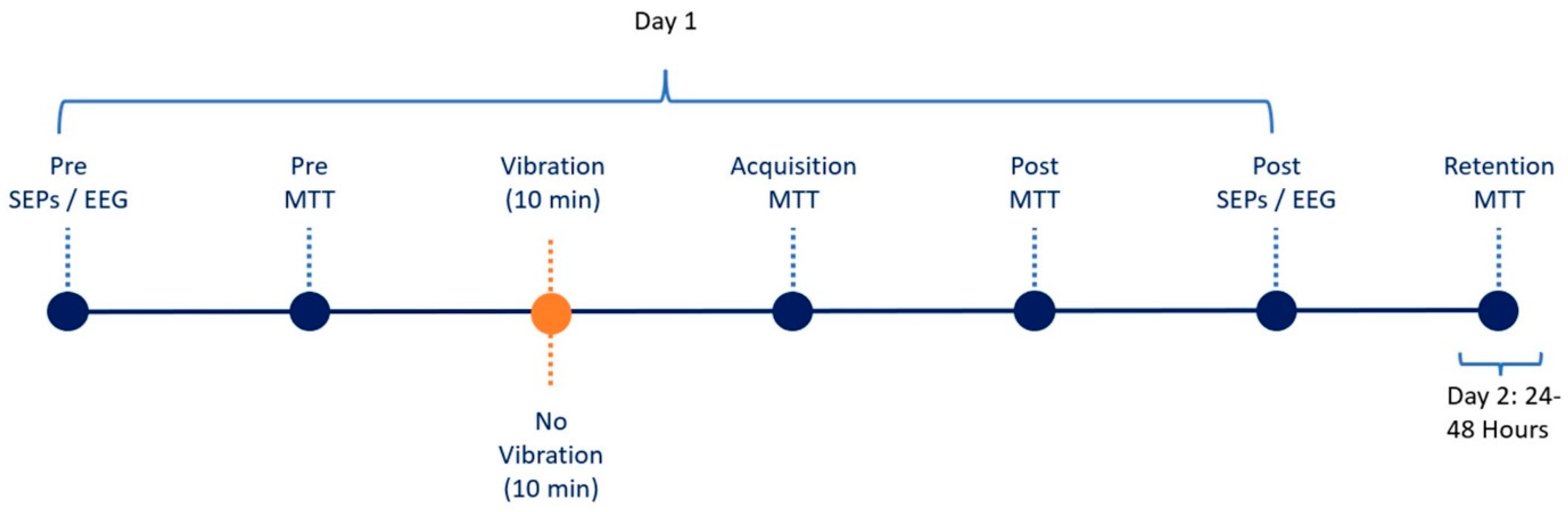
2.6. Experimental Protocol
3. Data Processing
3.1. SEP Analysis
3.2. Motor-Learning Analysis
3.3. Statistical Analyses
4. Results
4.1. Time–Group Interactions
N18 SEP Peak
4.2. Time Effects
P25 SEP Peak
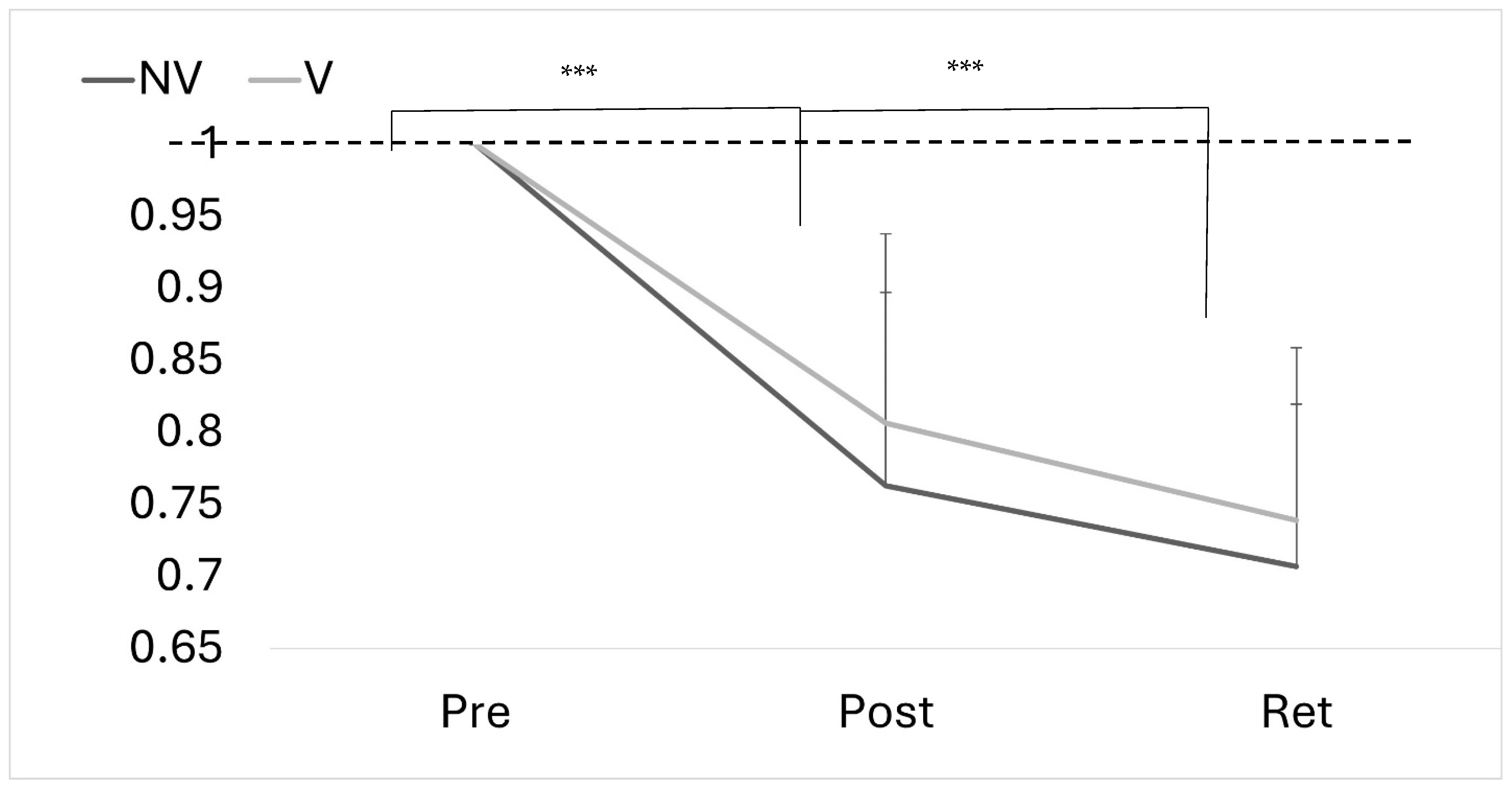
4.3. Motor Performance Accuracy
5. Discussion
5.1. N18 SEP Peak
5.2. N24 SEP Peak
5.3. P25 SEP Peak
5.4. Motor Performance
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APB | Abductor Pollicis Brevis |
| ASA | Advanced Source Analysis |
| CED | Cambridge Electronic Design |
| CEM | Cervical Extensor Muscles |
| DCML | Dorsal Column Medial Lemniscus |
| DCN | Deep-Cerebellar Nuclei |
| EEG | Electroencephalography |
| EHI | Edinburgh Handedness Inventory |
| FMTT | Force-Matching Tracking Task |
| fMRI | Functional Magnetic Resonance Imaging |
| GABA | Gamma-Aminobutyric Acid |
| IFCN | International Federation of Clinical Neurophysiology |
| MTT | Motor Tracing Task |
| NMV | Neck Muscle Vibration |
| NV | No Vibration |
| PET | Positron Emission Tomography |
| SCM | Sternocleidomastoid |
| SCNP | Subclinical Neck Pain |
| SEPs | Somatosensory Evoked Potentials |
| SMI | Sensorimotor Integration |
| S1 | Primary Somatosensory Cortex |
| TMS | Transcranial Magnetic Stimulation |
| UFT | Upper-Fibre Traps |
| V | Vibration |
| VPL | Ventro-Posterior Thalamus |
References
- Machado, S.; Cunha, M.; Velasques, B.; Minc, D.; Teixeira, S.; A Domingues, C.; Silva, J.G.; Bastos, V.H.; Budde, H.; Cagy, M.; et al. Sensorimotor integration: Basic concepts, abnormalities related to movement disorders and sensorimotor training-induced cortical reorganization. Rev. Neurol. 2010, 51, 427–436. [Google Scholar] [PubMed]
- Matur, Z.; Öge, A.E. Sensorimotor Integration During Motor Learning: Transcranial Magnetic Stimulation Studies. Noro Psikiyatr. Ars. 2017, 54, 358–363. [Google Scholar] [CrossRef]
- Goodwin, G.M.; DIMcCloskey Matthews, P.B. Proprioceptive Illusions Induced by Muscle Vibration: Contribution by Muscle Spindles to Perception? Science 1972, 175, 1382–1384. [Google Scholar] [CrossRef] [PubMed]
- Paulus, I.; Brumagne, S. Altered Interpretation of Neck Proprioceptive Signals in Persons with Subclinical Recurrent Neck Pain. J. Rehabil. Med. 2008, 40, 426–432. [Google Scholar] [CrossRef]
- Proske, U.; Gandevia, S.C. The kinaesthetic senses. J. Physiol. 2009, 587, 4139–4146. [Google Scholar] [CrossRef]
- Taylor, J.L.; McCloskey, D.I. Illusions of head and visual target displacement induced by vibration of neck muscles. Brain 1991, 114 Pt 2, 755–759. [Google Scholar] [CrossRef]
- Farid, B.; Yielder, P.; Holmes, M.; Haavik, H.; Murphy, B.A. Association of Subclinical Neck Pain with Altered Multisensory Integration at Baseline and 4-Week Follow-Up Relative to Asymptomatic Controls. J. Manip. Physiol. Ther. 2018, 41, 81–91. [Google Scholar] [CrossRef]
- Karellas, A.M.; Yielder, P.; Burkitt, J.J.; McCracken, H.S.; Murphy, B.A. The Influence of Subclinical Neck Pain on Neurophysiological and Behavioral Measures of Multisensory Integration. Brain Sci. 2019, 9, 362. [Google Scholar] [CrossRef]
- Guerraz, M.; Caudron, S.; Thomassin, N.; Blouin, J. Influence of head orientation on visually and memory-guided arm movements. Acta Psychol. 2011, 136, 390–398. [Google Scholar] [CrossRef]
- Haavik, H. Subclinical neck pain and the effects of cervical manipulation on elbow joint position sense. J. Manip. Physiol. Ther. JMPT 2011, 34, 88. [Google Scholar] [CrossRef] [PubMed]
- Knox, J.J.; Hodges, P.W. Changes in head and neck position affect elbow joint position sense. Exp. Brain Res. 2005, 165, 107–113. [Google Scholar] [CrossRef]
- Ambalavanar, U.; Yielder, P.; McCracken, H.S.; Tabbert, H.; Murphy, B. Subclinical Neck Pain Leads to Differential Changes in Early Somatosensory Evoked Potentials in Response to a Novel Force Matching Tracking Task. J. Integr. Neurosci. 2024, 23, 10. [Google Scholar] [CrossRef]
- Andrew, D.; Yielder, P.; Haavik, H.; Murphy, B. The effects of subclinical neck pain on sensorimotor integration following a complex motor pursuit task. Exp. Brain Res. 2018, 236, 1–11. [Google Scholar] [CrossRef]
- Brown, M.R. Participants with mildly-disabling chronic neck pain perform differently during explicit compared to implicit motor learning of a reaching task. PLoS ONE 2022, 7, e0266508. [Google Scholar] [CrossRef] [PubMed]
- Tabbert, H.; Ambalavanar, U.; Murphy, B. Neck Muscle Vibration Alters Cerebellar Processing Associated with Motor Skill Acquisition of a Proprioceptive-Based Task. Brain Sci. 2023, 13, 1412. [Google Scholar] [CrossRef] [PubMed]
- Zabihhosseinian, M.; Holmes, M.W.R.; Murphy, B. Neck muscle fatigue alters upper limb proprioception. Exp. Brain Res. 2015, 233, 1663–1675. [Google Scholar] [CrossRef]
- Zabihhosseinian, M.; Yielder, P.; Berkers, V.; Ambalavanar, U.; Holmes, M.; Murphy, B.A. Neck muscle fatigue impacts plasticity and sensorimotor integration in cerebellum and motor cortex in response to novel motor skill acquisition. J. Neurophysiol. 2020, 124, 844–855. [Google Scholar] [CrossRef]
- Pettorossi, V.E.; Schieppati, M. Neck proprioception shapes body orientation and perception of motion. Front. Hum. Neurosci. 2014, 8, 895. [Google Scholar] [CrossRef]
- Huang, M.; Pang, M.Y.C. Muscle activity and vibration transmissibility during whole-body vibration in chronic stroke. Scand. J. Med. Sci. Sports 2019, 29, 816–825. [Google Scholar] [CrossRef]
- Perchthaler, D.; Hauser, S.; Heitkamp, H.-C.; Hein, T.; Grau, S. Acute effects of whole-body vibration on trunk and neck muscle activity in consideration of different vibration loads. J. Sports Sci. Med. 2015, 14, 155–162. [Google Scholar] [PubMed]
- Ye, J.; Ng, G.; Yuen, K. Acute Effects of Whole-Body Vibration on Trunk Muscle Functioning in Young Healthy Adults. J. Strength Cond. Res. 2014, 28, 2872–2879. [Google Scholar] [CrossRef]
- Beinert, K.; Englert, V.; Taube, W. After-effects of neck muscle vibration on sensorimotor function and pain in neck pain patients and healthy controls–a case-control study. Disabil. Rehabil. 2019, 41, 1906–1913. [Google Scholar] [CrossRef]
- Beinert, K.; Keller, M.; Taube, W. Neck muscle vibration can improve sensorimotor function in patients with neck pain. Spine J. 2015, 15, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Wannaprom, N.; Treleaven, J.; Jull, G.; Uthaikhup, S. Neck muscle vibration produces diverse responses in balance and gait speed between individuals with and without neck pain. Musculoskelet. Sci. Pract. 2018, 35, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Knox, J.; Cordo, P.; Skoss, R.; Durrant, S.; Hodges, P. Illusory Changes in Head Position Induced by Neck Muscle Vibration Can Alter the Perception of Elbow Position. Behav. Neurosci. 2006, 120, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Pettorossi, V.E.; Panichi, R.; Botti, F.M.; Biscarini, A.; Filippi, G.M.; Schieppati, M. Long-lasting effects of neck muscle vibration and contraction on self-motion perception of vestibular origin. Clin. Neurophysiol. 2015, 126, 1886–1900. [Google Scholar] [CrossRef]
- Proske, U. What is the role of muscle receptors in proprioception? Muscle Nerve 2005, 31, 780–787. [Google Scholar] [CrossRef]
- Doyon, J.; Owen, A.M.; Petrides, M.; Sziklas, V.; Evans, A.C. Functional Anatomy of Visuomotor Skill Learning in Human Subjects Examined with Positron Emission Tomography. Eur. J. Neurosci. 1996, 8, 637–648. [Google Scholar] [CrossRef]
- Doyon, J.; Penhune, V.; Ungerleider, L.G. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia 2003, 41, 252–262. [Google Scholar] [CrossRef]
- Doyon, J.; Song, A.W.; Karni, A.; Lalonde, F.; Adams, M.M.; Ungerleider, L.G. Experience-dependent changes in cerebellar contributions to motor sequence learning. Proc. Natl. Acad. Sci. USA 2002, 99, 1017–1022. [Google Scholar] [CrossRef]
- Ambalavanar, U.; La Delfa, N.; McCracken, H.S.; Zabihhosseinian, M.; Yielder, P.C.; Murphy, B. Differential Changes in Somatosensory Evoked Potentials and Motor Performance: Pursuit Movement Task versus Force Matching Tracking Task. J. Neurophysiol. 2022, 128, 1453–1465. [Google Scholar] [CrossRef]
- Andrew, D.; Haavik, H.; Dancey, E.; Yielder, P.; Murphy, B. Somatosensory evoked potentials show plastic changes following a novel motor training task with the thumb. Clin. Neurophysiol. 2015, 126, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.J.; Wei, K.; Minciacchi, D. Visuomotor perturbation in a continuous circle tracing task: Novel approach for quantifying motor adaptation. Sci. Rep. 2019, 9, 18679. [Google Scholar] [CrossRef] [PubMed]
- Zabihhosseinian, M.; Yielder, P.; Wise, R.; Holmes, M.; Murphy, B. Effect of Neck Muscle Fatigue on Hand Muscle Motor Performance and Early Somatosensory Evoked Potentials. Brain Sci. 2021, 11, 1481. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, V.; Chandy, M.J.; Babu, K.S. Quantitative study of muscle spindles in suboccipital muscles of human foetuses. Neurol. India 2001, 49, 355–359. [Google Scholar]
- Peng, B.; Yang, L.; Li, Y.; Liu, T.; Liu, Y. Cervical Proprioception Impairment in Neck Pain-Pathophysiology, Clinical Evaluation, and Management: A Narrative Review. Pain Ther. 2021, 10, 143–164. [Google Scholar] [CrossRef]
- Knox, J.J. Changes in head and neck position have a greater effect on elbow joint position sense in people with whiplash-associated disorders. Clin. J. Pain 2006, 22, 512. [Google Scholar] [CrossRef]
- Brown, M.C.; Engberg, I.; Matthews, P.B.C. The relative sensitivity to vibration of muscle receptors of the cat. J. Physiol. 1967, 192, 773–800. [Google Scholar] [CrossRef]
- Cordo, P.; Bevan, L.; Gurfinkel, V.; Carlton, L.; Carlton, M.; Kerr, G. Proprioceptive coordination of discrete movement sequences: Mechanism and generality. Can. J. Physiol. Pharmacol. 1995, 73, 305–315. [Google Scholar] [CrossRef]
- Tabbert, H.; Ambalavanar, U.; Murphy, B. Neck Muscle Vibration Alters Upper Limb Proprioception as Demonstrated by Changes in Accuracy and Precision during an Elbow Repositioning Task. Brain Sci. 2022, 12, 1532. [Google Scholar] [CrossRef]
- Chalimourdas, A.; Gilles, A.; De Hertogh, W.; Michiels, S. Does vibration frequency and location influence the effect of neck muscle vibration on postural sway? A cross-sectional study in asymptomatic participants. Exp. Brain Res. 2023, 241, 2261–2273. [Google Scholar] [CrossRef]
- Pearce, A.J.; Kidgell, D.J. Comparison of corticomotor excitability during visuomotor dynamic and static tasks. J. Sci. Med. Sport 2009, 13, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.G.; Caligiuri, M.; Meloy, M.; Eberson, S.C.; Kindermann, S.S.; Frank, L.R.; Zorrilla, L.T.E.; Lohr, J.B. Functional Brain Asymmetries During Visuomotor Tracking. J. Clin. Exp. Neuropsychol. 2004, 26, 356–368. [Google Scholar] [CrossRef]
- Tomasi, D.; Volkow, N.D. Associations between handedness and brain functional connectivity patterns in children. Nat. Commun. 2024, 15, 2355. [Google Scholar] [CrossRef]
- Rossi, S.; della Volpe, R.; Ginanneschi, F.; Ulivelli, M.; Bartalini, S.; Spidalieri, R.; Rossi, A. Early somatosensory processing during tonic muscle pain in humans: Relation to loss of proprioception and motor ‘defensive’ strategies. Clin. Neurophysiol. 2003, 114, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Cruccu, G.; Aminoff, M.; Curio, G.; Guerit, J.; Kakigi, R.; Mauguiere, F.; Rossini, P.; Treede, R.-D.; Garcia-Larrea, L. Recommendations for the clinical use of somatosensory-evoked potentials. Clin. Neurophysiol. 2008, 119, 1705–1719. [Google Scholar] [CrossRef]
- Nuwer, M.R.; Aminoff, M.; Desmedt, J.; Eisen, A.A.; Goodin, D.; Matsuoka, S.; Mauguière, F.; Shibasaki, H.; Sutherling, W.; Vibert, J.-F. IFCN recommended standards for short latency somatosensory evoked potentials. Report of an IFCN committee. International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. 1994, 91, 6–11. [Google Scholar] [CrossRef]
- Haavik, H.; Murphy, B.A. Selective changes in cerebellar-cortical processing following motor training. Exp. Brain Res. 2013, 231, 397–403. [Google Scholar] [CrossRef]
- Nuwer, M.R.; Daube, J.; Fischer, C.; Schramm, J.; Yingling, C.D. Neuromonitoring during surgery. Report of an IFCN Committee. Electroencephalogr. Clin. Neurophysiol. 1993, 87, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Mauguière, F.; Allison, T.; Babiloni, C.; Buchner, H.; A Eisen, A.; Goodin, D.S.; Jones, S.J.; Kakigi, R.; Matsuoka, S.; Nuwer, M.; et al. Somatosensory evoked potentials. The International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999, 52, 79–90. [Google Scholar]
- Bakeman, R. Recommended effect size statistics for repeated measures designs. Behav. Res. Methods 2005, 37, 379–384. [Google Scholar] [CrossRef]
- Richardson, J.T.E. Eta squared and partial eta squared as measures of effect size in educational research. Educ. Res. Rev. 2011, 6, 135–147. [Google Scholar] [CrossRef]
- Abdulbaki, A.; Wöhrle, J.C.; Blahak, C.; Weigel, R.; Kollewe, K.; Capelle, H.H.; Bäzner, H.; Krauss, J.K. Somatosensory evoked potentials recorded from DBS electrodes: The origin of subcortical N18. J. Neural Transm. 2024, 131, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Light, G.A.; Williams, L.E.; Minow, F.; Sprock, J.; Rissling, A.; Sharp, R.; Swerdlow, N.R.; Braff, D.L. Electroencephalography (EEG) and event-related potentials (ERPs) with human participants. Curr. Protoc. Neurosci. 2010, 52, 25. [Google Scholar] [CrossRef] [PubMed]
- Manzano, G.M.; Negrão, N.; Nóbrega, J.A. The N18 component of the median nerve SEP is not reduced by vibration. Electroencephalogr. Clin. Neurophysiol. 1998, 108, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Sonoo, M. Anatomic origin and clinical application of the widespread N18 potential in median nerve somatosensory evoked potentials. J. Clin. Neurophysiol. 2000, 17, 258–268. [Google Scholar] [CrossRef]
- García-Gomar, M.G.; Soto-Abraham, J.; Velasco-Campos, F.; Concha, L. Anatomic characterization of prelemniscal radiations by probabilistic tractography: Implications in Parkinson’s disease. Brain Struct. Funct. 2017, 222, 71–81. [Google Scholar] [CrossRef]
- Noël, P.; Ozaki, I.; Desmedt, J.E. Origin of N18 and P14 far-fields of median nerve somatosensory evoked potentials studied in patients with a brain-stem lesion. Electroencephalogr. Clin. Neurophysiol. 1996, 98, 167–170. [Google Scholar] [CrossRef]
- Rezaei, A.; Lahtinen, J.; Neugebauer, F.; Antonakakis, M.; Piastra, M.C.; Koulouri, A.; Wolters, C.H.; Pursiainen, S. Reconstructing subcortical and cortical somatosensory activity via the RAMUS inverse source analysis technique using median nerve SEP data. NeuroImage 2021, 245, 118726. [Google Scholar] [CrossRef]
- American Clinical Neurophysiology Society. Guideline 9D: Guidelines on Short-Latency Somatosensory Evoked Potentials. Am. J. Electroneurodiagn. Technol. 2006, 23, 168–179.
- Restuccia, D.; Valeriani, M.; Barba, C.; Le Pera, D.; Capecci, M.; Filippini, V.; Molinari, M. Functional changes of the primary somatosensory cortex in patients with unilateral cerebellar lesions. Brain 2001, 124, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Chemnad, K.; Alshakhsi, S.; Almourad, M.B.; Altuwairiqi, M.; Phalp, K.; Ali, R. Smartphone Usage before and during COVID-19: A Comparative Study Based on Objective Recording of Usage Data. Informatics 2022, 9, 98. [Google Scholar] [CrossRef]
- Haavik-Taylor, H.; Murphy, B. Cervical spine manipulation alters sensorimotor integration: A somatosensory evoked potential study. Clin. Neurophysiol. 2007, 118, 391–402. [Google Scholar] [CrossRef]
- Valeriani, M.; Restuccia, D.; Di Lazzaro, V.; Le Pera, D.; Barba, C.; Tonali, P. The scalp to earlobe montage as standard in routine SEP recording. Comparison with the non-cephalic reference in patients with lesions of the upper cervical cord. Electroencephalogr. Clin. Neurophysiol. 1998, 108, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Di Russo, F.; Committeri, G.; Pitzalis, S.; Spitoni, G.; Piccardi, L.; Galati, G.; Catagni, M.; Nico, D.; Guariglia, C.; Pizzamiglio, L. Cortical plasticity following surgical extension of lower limbs. NeuroImage 2006, 30, 172–183. [Google Scholar] [CrossRef]
- Iodice, P.; Scuderi, N.; Saggini, R.; Pezzulo, G. Multiple timescales of body schema reorganization due to plastic surgery. Hum. Mov. Sci. 2015, 42, 54–70. [Google Scholar] [CrossRef]
- Nisticò, V.; Ilia, N.; Conte, F.; Broglia, G.; Sanguineti, C.; Lombardi, F.; Scaravaggi, S.; Mangiaterra, L.; Tedesco, R.; Gambini, O.; et al. Forearm bisection task suggests an alteration in body schema in patients with functional movement disorders (motor conversion disorders). J. Psychosom. Res. 2024, 178, 111610. [Google Scholar] [CrossRef]
- Sposito, A.V.; Bolognini, N.; Vallar, G.; Posteraro, L.; Maravita, A. The spatial encoding of body parts in patients with neglect and neurologically unimpaired participants. Neuropsychologia 2010, 48, 334–340. [Google Scholar] [CrossRef]
- Ohashi, H.; Gribble, P.L.; Ostry, D.J. Somatosensory cortical excitability changes precede those in motor cortex during human motor learning. J. Neurophysiol. 2019, 122, 1397–1405. [Google Scholar] [CrossRef]
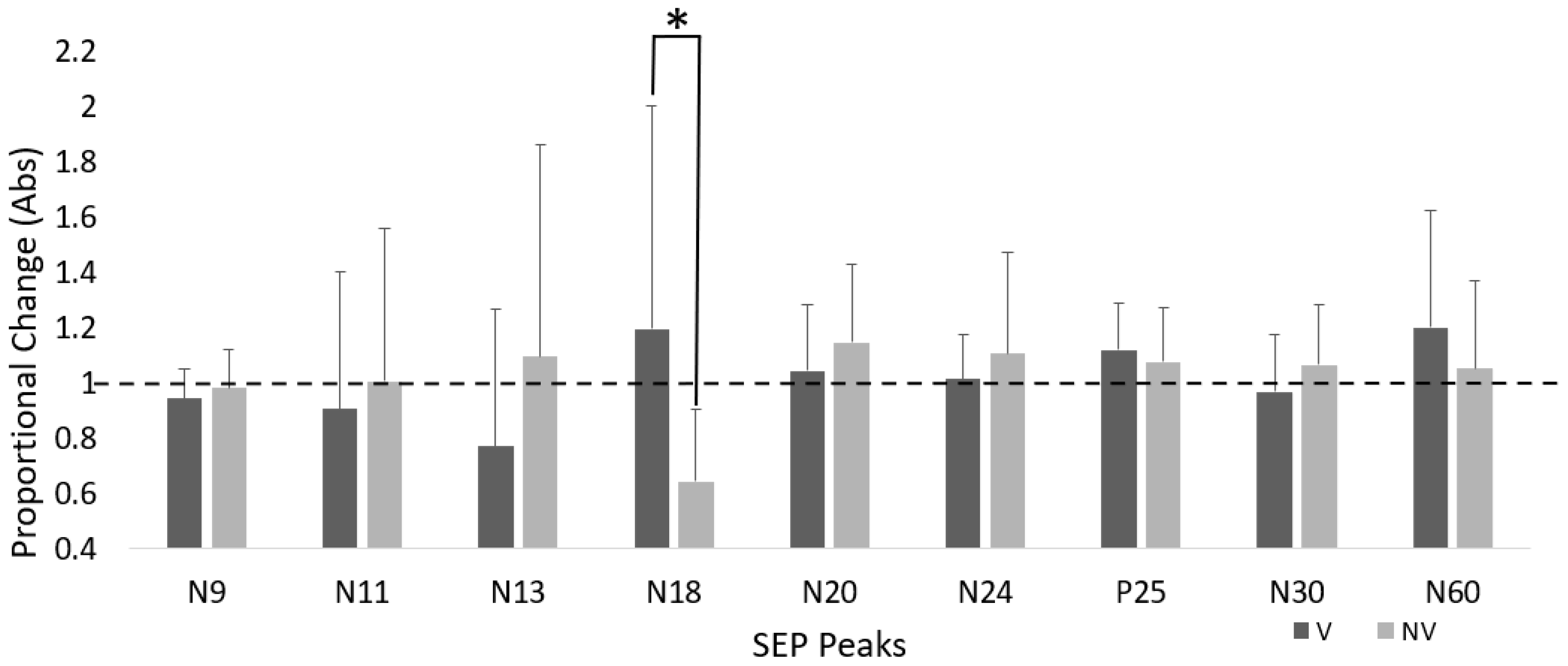
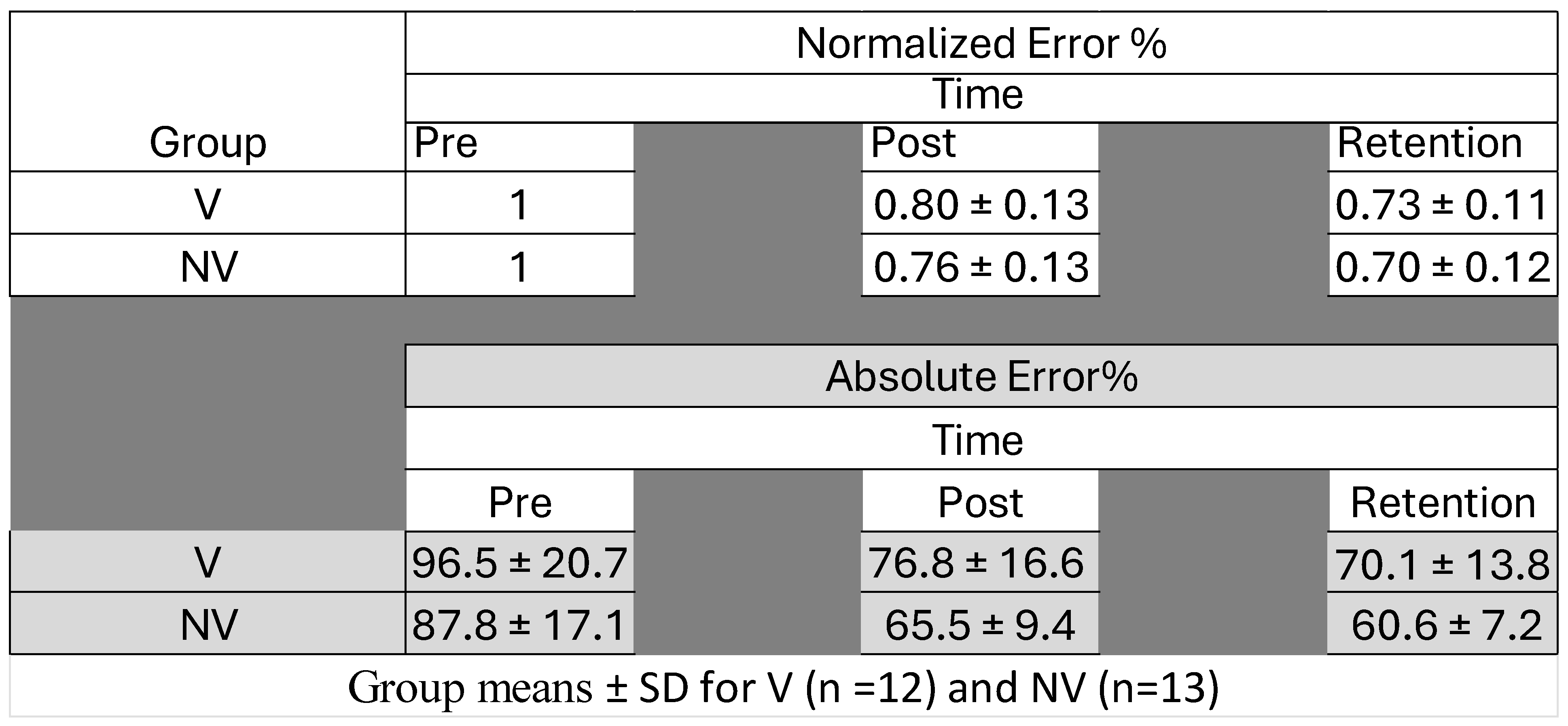
| Proportional Changes in SEP Peak Amplitude | Group | Time–Group p-Values | Effect of Time p-Values | |
|---|---|---|---|---|
| SEP Peaks | V | NV | ||
| N9 | 0.94 ± 0.11 | 0.98 ± 0.13 | 0.361 | 0.033 |
| N11 | 0.90 ± 0.49 | 1.00 ± 0.55 | 0.425 | 0.522 |
| N13 | 0.77 ± 0.49 | 1.09 ± 0.76 | 0.203 | 0.564 |
| N18 | 1.19 ± 0.80 | 0.64 ± 0.25 | 0.035 | 0.513 |
| N20 | 1.04 ± 0.23 | 1.14 ± 0.28 | 0.429 | 0.120 |
| N24 | 1.01 ± 0.16 | 1.10 ± 0.36 | 0.453 | 0.338 |
| P25 | 1.11 ± 0.17 | 1.07 ± 0.19 | 0.572 | 0.014 |
| N30 | 0.96 ± 0.20 | 1.06 ± 0.21 | 0.256 | 0.708 |
| N60 | 1.19 ± 0.42 | 1.05 ± 0.31 | 0.341 | 0.110 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalogerakis, A.; Yielder, P.; Tabbert, H.; Murphy, B. Effect of Neck Muscle Vibration Prior to Motor Learning on Short-Latency SEP Peak Amplitudes and Motor Performance. Brain Sci. 2025, 15, 1311. https://doi.org/10.3390/brainsci15121311
Kalogerakis A, Yielder P, Tabbert H, Murphy B. Effect of Neck Muscle Vibration Prior to Motor Learning on Short-Latency SEP Peak Amplitudes and Motor Performance. Brain Sciences. 2025; 15(12):1311. https://doi.org/10.3390/brainsci15121311
Chicago/Turabian StyleKalogerakis, Alexandre, Paul Yielder, Hailey Tabbert, and Bernadette Murphy. 2025. "Effect of Neck Muscle Vibration Prior to Motor Learning on Short-Latency SEP Peak Amplitudes and Motor Performance" Brain Sciences 15, no. 12: 1311. https://doi.org/10.3390/brainsci15121311
APA StyleKalogerakis, A., Yielder, P., Tabbert, H., & Murphy, B. (2025). Effect of Neck Muscle Vibration Prior to Motor Learning on Short-Latency SEP Peak Amplitudes and Motor Performance. Brain Sciences, 15(12), 1311. https://doi.org/10.3390/brainsci15121311







