Negr1 Deficiency Modulates Sex-Specific Neurobehavioral Adaptations to Social Isolation
Abstract
1. Introduction
2. Materials and Methods
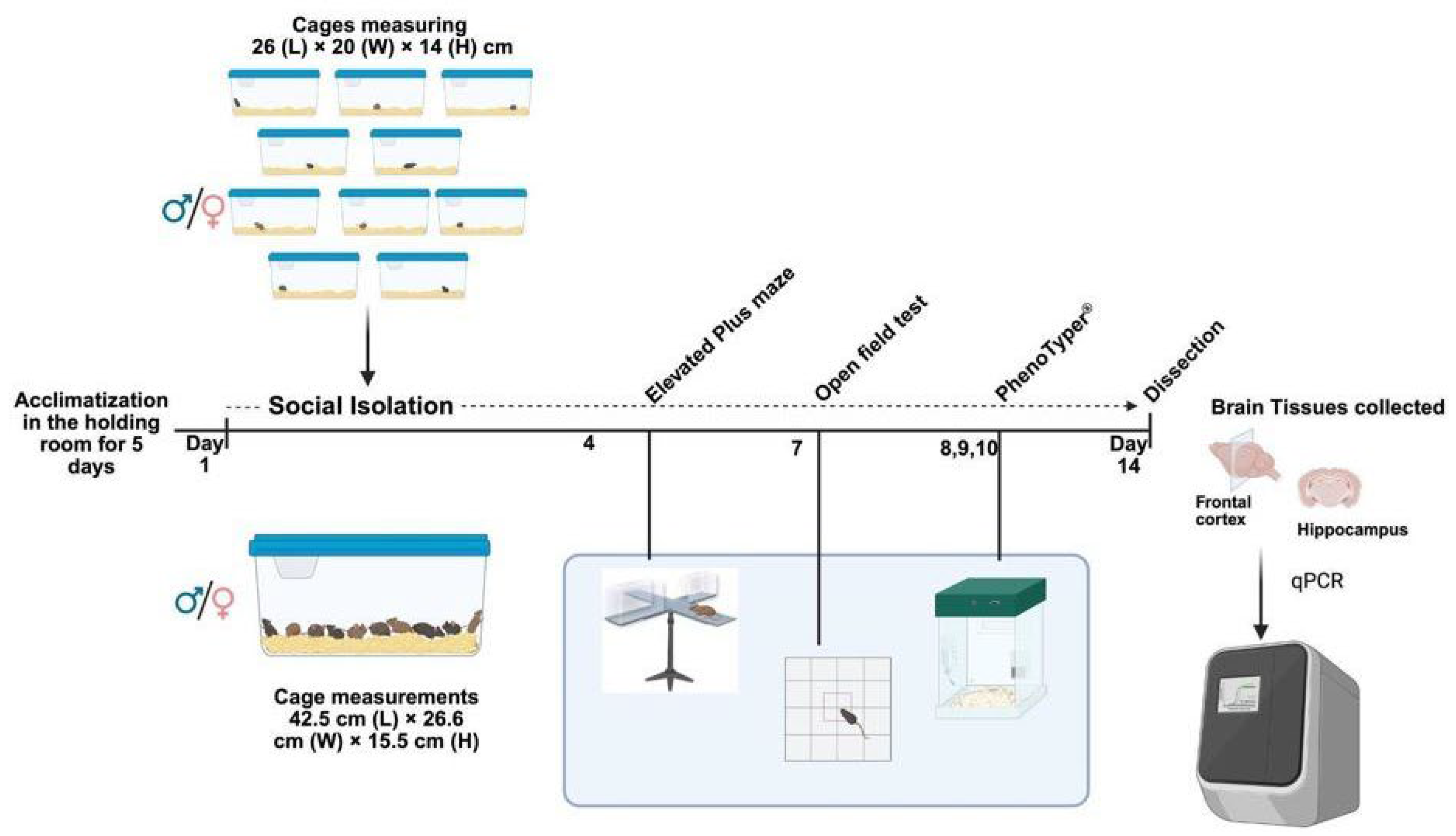
2.1. Animals and Ethics
2.2. Social Isolation
2.3. Elevated Plus Maze (EPM)
2.4. Open Field Test (OFT)
2.5. 24 h Monitoring in PhenoTyper®
2.6. Brain Tissue Dissection and qPCR
2.7. Statistical Analysis
3. Results
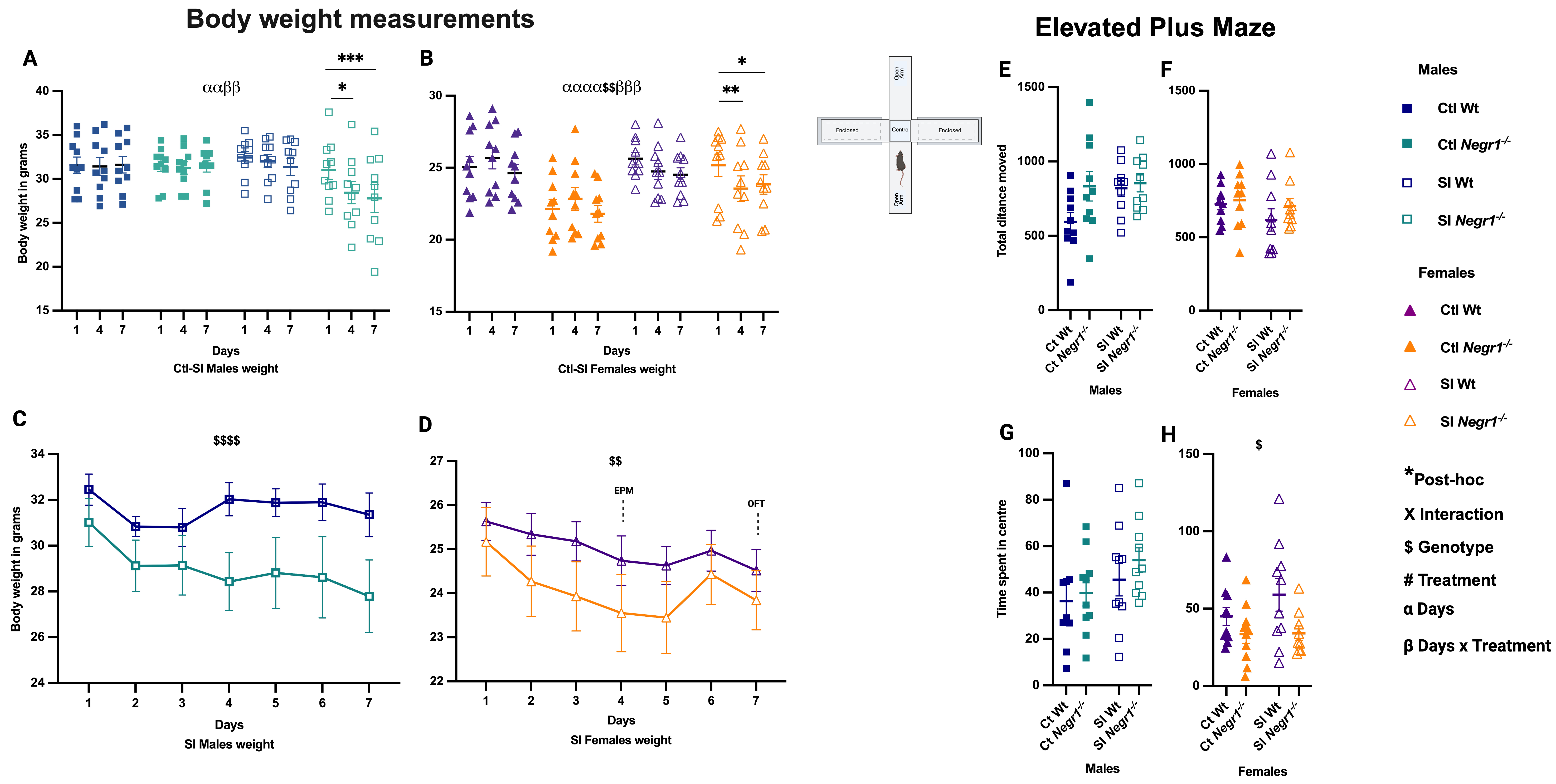
3.1. Effect of Treatment and Genotype on Body Weight and Anxiety-like Behavior
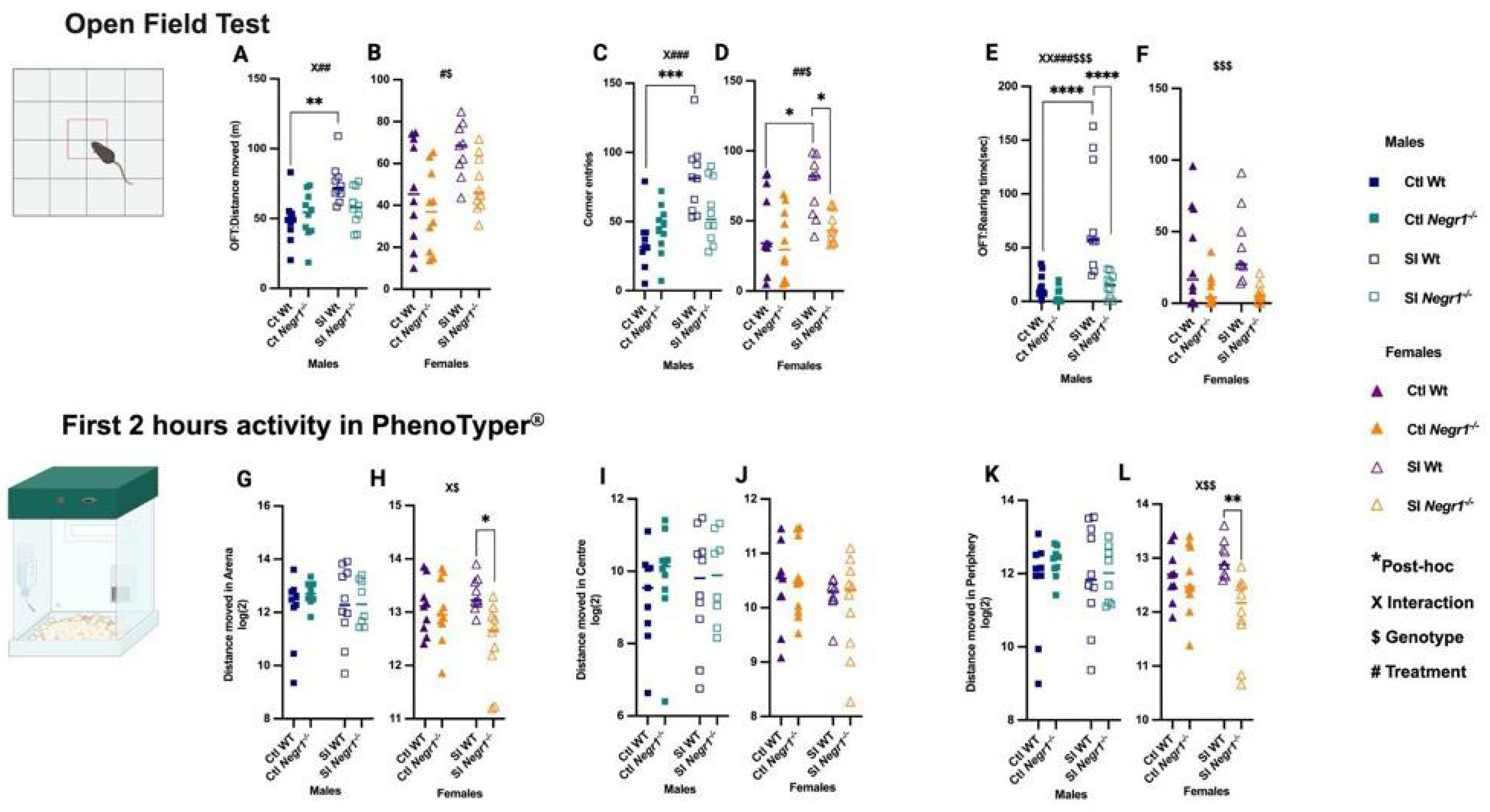
3.2. Changes in Behavior During Social Isolation: Effect of Social Isolation on Locomotion, Exploratory Activity, and Anxiety-like Behavior
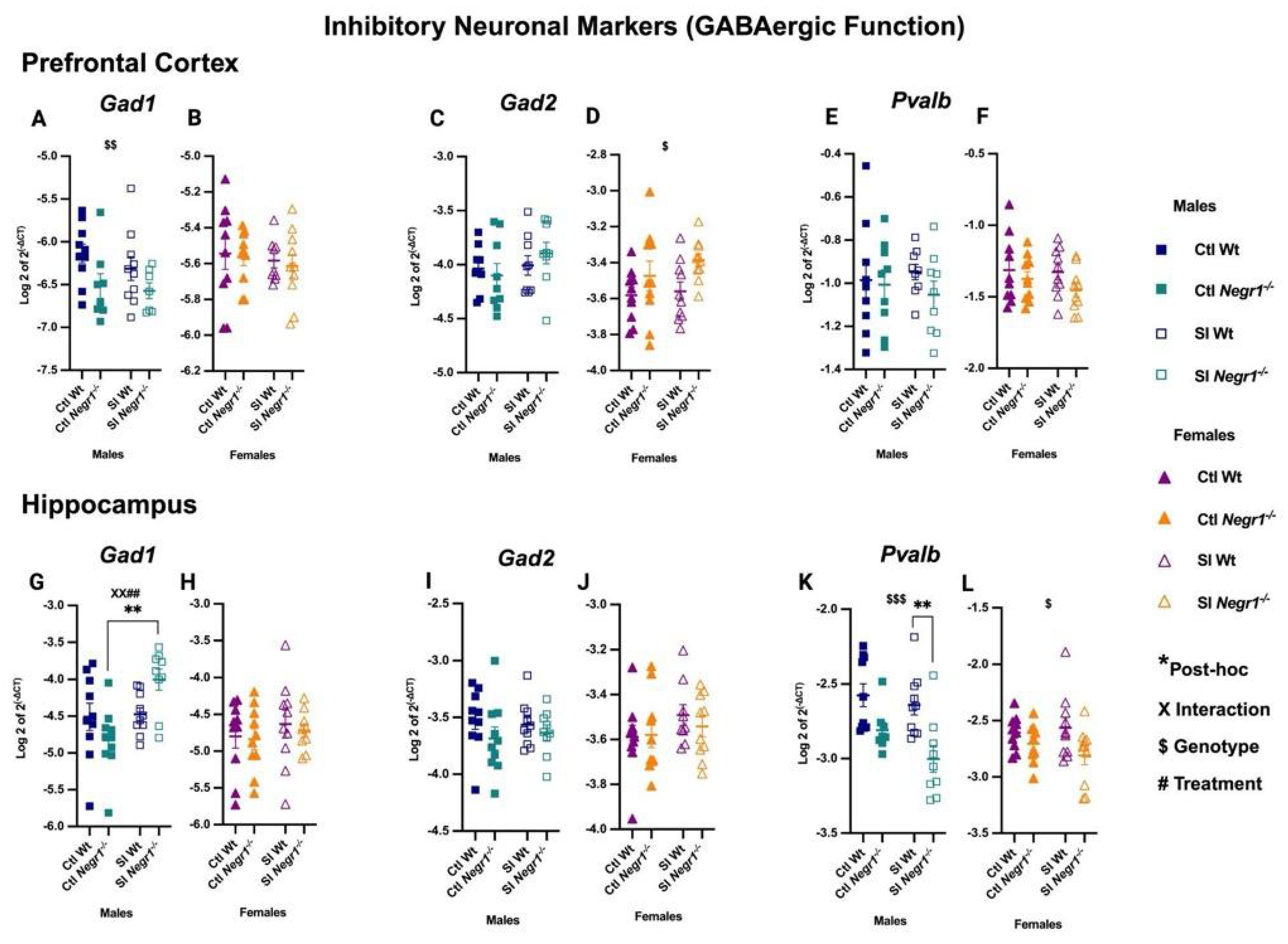
3.3. Dysregulation in the GABAergic Markers Following Social Isolation
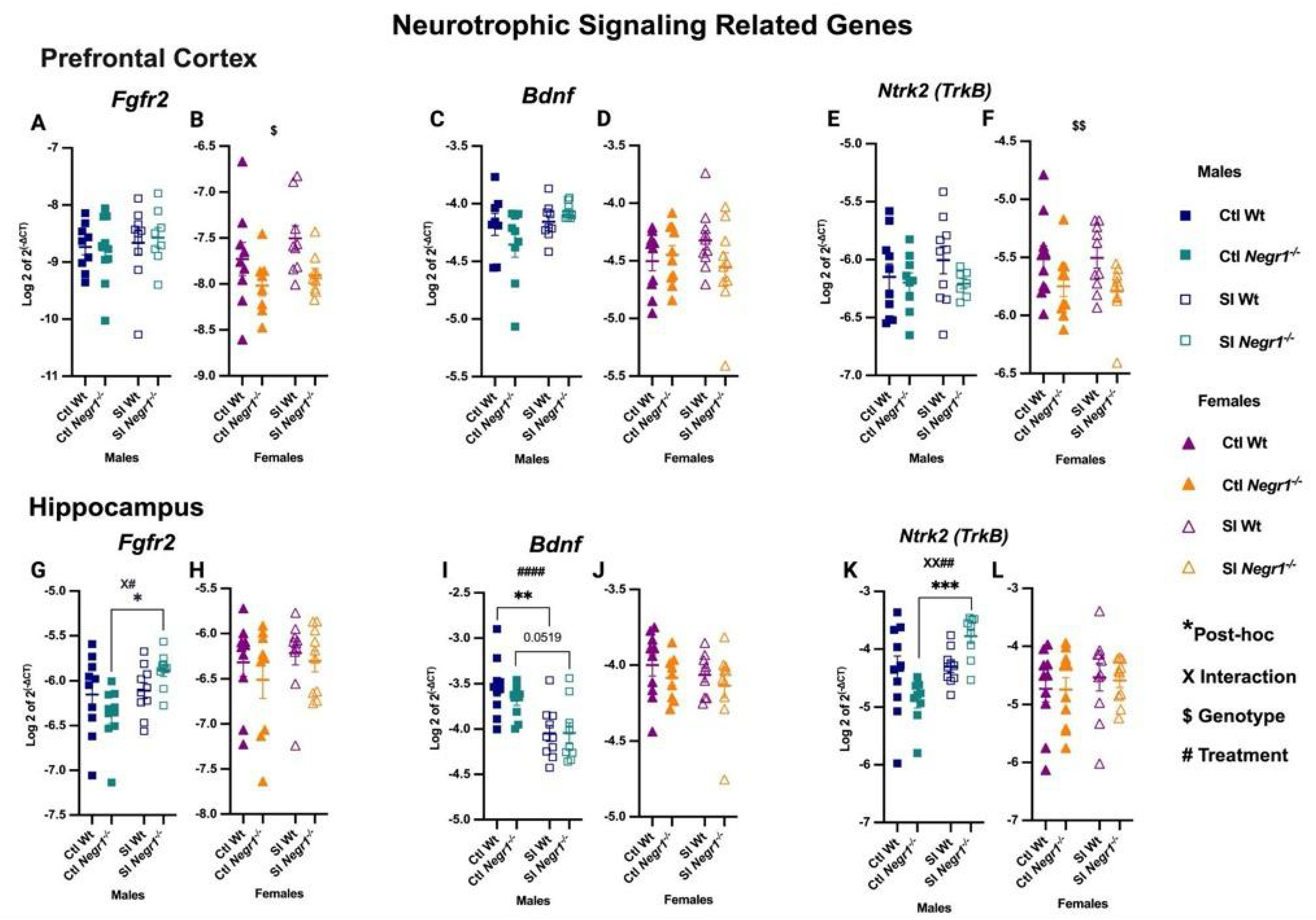
3.4. Change in Neurotrophic Signalling-Related Genes Post Social Isolation
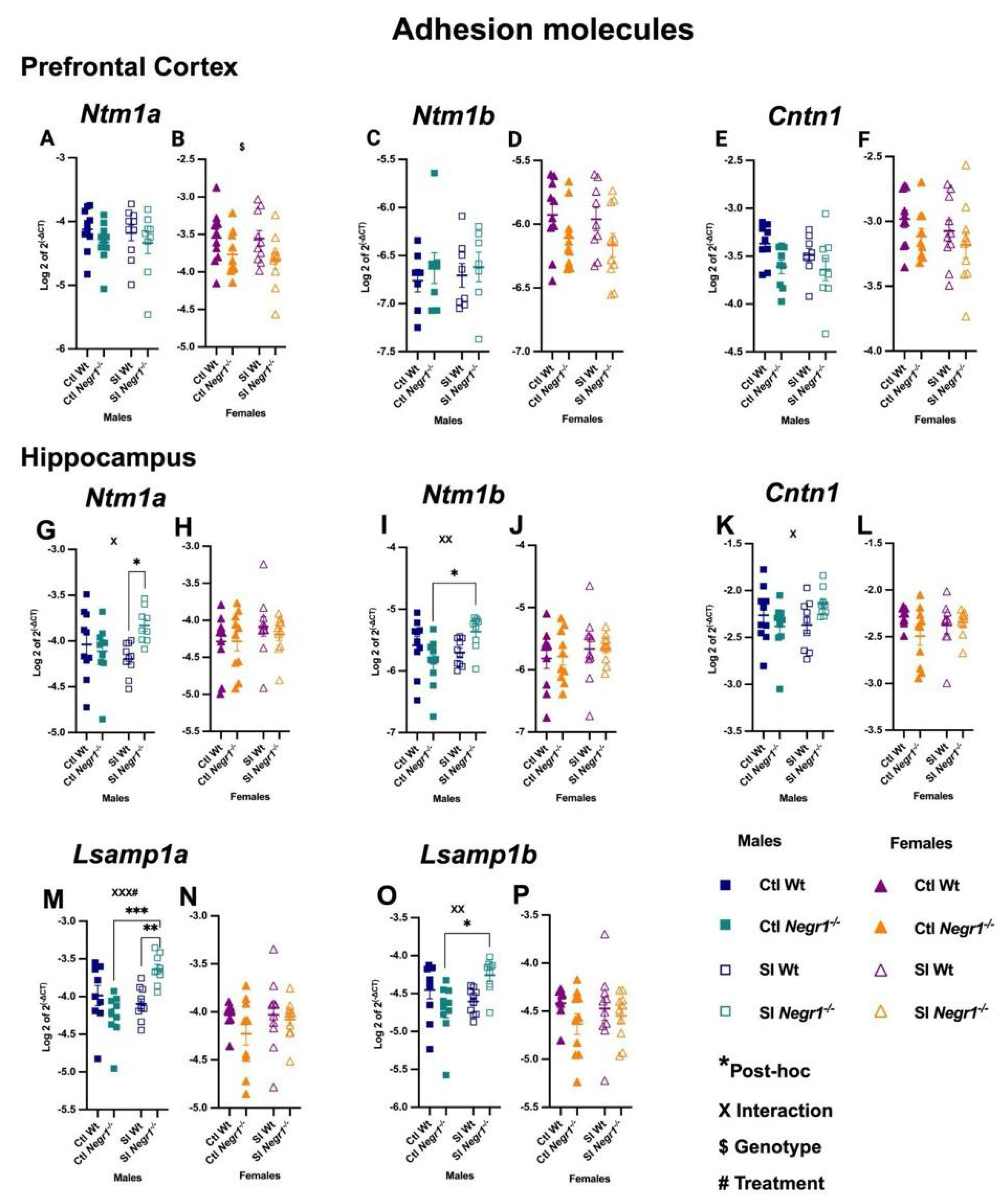
3.5. Expression of Cell Adhesion Molecule Transcripts and Related Interactors
4. Discussion
4.1. Behavioral and Body Weight Effects of Social Isolation Stress
4.2. GABAergic Regulation and Parvalbumin Interneurons
4.3. Gene Expression of Neurotrophic Signalling
4.4. Gene Expression of Neural Adhesion Molecules
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SI | Social isolation |
| Wt | Wild-type |
| IgLONs | Immunoglobulin superfamily of cell adhesion molecules |
| NCAM | Neural cell adhesion molecule |
| Negr1 | Neuronal growth regulator 1 |
| Negr1−/− | Negr1-deficient/knockout |
| Gad1 | Glutamic acid decarboxylase 1 |
| Gad2 | Glutamic acid decarboxylase 2 |
| Pvalb | Parvalbumin |
| Fgfr2 | Fibroblast growth factor receptor 2 |
| Bdnf | Brain-derived neurotrophic factor |
| Ntrk2 | Neurotrophic receptor tyrosine kinase 2 |
| TrkB | Tropomyosin receptor kinase B |
| Ntm 1a/1b | Neurotrimin 1a/1b |
| Lsamp 1a/1b | Limbic system associated protein 1a/1b |
| Cntn1 | Contactin 1 |
| ActB | Actin beta |
| Gapdh | Glyceraldehyde-3-phosphate dehydrogenase |
| Hprt | Hypoxanthine-guanine phosphoribosyltransferase 1 |
| Pgk1 | Phosphoglycerate kinase 1 |
References
- Struyk, A.; Canoll, P.; Wolfgang, M.; Rosen, C.; D’Eustachio, P.; Salzer, J. Cloning of neurotrimin defines a new subfamily of differentially expressed neural cell adhesion molecules. J. Neurosci. 1995, 15, 2141–2156. [Google Scholar] [CrossRef]
- Mann, F.; Zhukareva, V.; Pimenta, A.; Levitt, P.; Bolz, J. Membrane-Associated Molecules Guide Limbic and Nonlimbic Thalamocortical Projections. J. Neurosci. 1998, 18, 9409–9419. [Google Scholar] [CrossRef]
- Chen, S.; Gil, O.; Ren, Y.Q.; Zanazzi, G.; Salzer, J.L.; Hillman, D.E. Neurotrimin expression during cerebellar development suggests roles in axon fasciculation and synaptogenesis. J. Neurocytol. 2001, 30, 927–937. [Google Scholar] [CrossRef]
- Jagomäe, T.; Singh, K.; Philips, M.-A.; Jayaram, M.; Seppa, K.; Tekko, T.; Gilbert, S.F.; Vasar, E.; Lilleväli, K. Alternative Promoter Use Governs the Expression of IgLON Cell Adhesion Molecules in Histogenetic Fields of the Embryonic Mouse Brain. Int. J. Mol. Sci. 2021, 22, 6955. [Google Scholar] [CrossRef]
- Venkannagari, H.; Kasper, J.M.; Misra, A.; Rush, S.A.; Fan, S.; Lee, H.; Sun, H.; Seshadrinathan, S.; Machius, M.; Hommel, J.D.; et al. Highly Conserved Molecular Features in IgLONs Contrast Their Distinct Structural and Biological Outcomes. J. Mol. Biol. 2020, 432, 5287–5303. [Google Scholar] [CrossRef]
- Merrion, H.G.; Barber, C.N.; Renuse, S.S.; Cutler, J.; Kreimer, S.; Bygrave, A.M.; Meyers, D.J.; Hale, W.D.; Pandey, A.; Huganir, R.L. Dynamic extracellular interactions with AMPA receptors. Neuroscience 2025, 122, e2517436122. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Jayaram, M.; Hanumantharaju, A.; Tõnissoo, T.; Jagomäe, T.; Mikheim, K.; Muthuraman, S.; Gilbert, S.F.; Plaas, M.; Schäfer, M.K.E.; et al. The IgLON family of cell adhesion molecules expressed in developing neural circuits ensure the proper functioning of the sensory system in mice. Sci. Rep. 2024, 14, 22593. [Google Scholar] [CrossRef] [PubMed]
- Maigoro, A.Y.; Kim, J.; Cho, S.; Yoo, A.; Lee, S. Peripheral gene dysregulation in Negr1-deficient mice: Insights into possible links with affective behavior. Front. Mol. Neurosci. 2025, 18, 1602201. [Google Scholar] [CrossRef]
- Noh, K.; Lee, H.; Choi, T.-Y.; Joo, Y.; Kim, S.-J.; Kim, H.; Kim, J.Y.; Jahng, J.W.; Lee, S.; Choi, S.-Y.; et al. Negr1 controls adult hippocampal neurogenesis and affective behaviors. Mol. Psychiatry 2019, 24, 1189–1205. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ni, H.; Wang, Y.; Wu, X.; Bi, J.; Ou, H.; Li, Z.; Ping, J.; Wang, Z.; Chen, R.; et al. Gain of bipolar disorder-related lncRNA AP1AR-DT in mice induces depressive and anxiety-like behaviors by reducing Negr1-mediated excitatory synaptic transmission. BMC Med. 2024, 22, 543. [Google Scholar] [CrossRef]
- Kim, K.H.; Noh, K.; Lee, J.; Lee, S.; Lee, S.J. NEGR1 Modulates Mouse Affective Discrimination by Regulating Adult Olfactory Neurogenesis. Biol. Psychiatry Glob. Open Sci. 2024, 4, 100355. [Google Scholar] [CrossRef]
- Howard, D.M.; Adams, M.J.; Shirali, M.; Clarke, T.-K.; Marioni, R.E.; Davies, G.; Coleman, J.R.I.; Alloza, C.; Shen, X.; Barbu, M.C.; et al. Genome-wide association study of depression phenotypes in UK Biobank identifies variants in excitatory synaptic pathways. Nat. Commun. 2018, 9, 1470. [Google Scholar] [CrossRef]
- The LifeLines Cohort Study; The ADIPOGen Consortium; The AGEN-BMI Working Group; The CARDIOGRAMplusC4D Consortium; The CKDGen Consortium; The GLGC; The ICBP; The MAGIC Investigators; The MuTHER Consortium; The MIGen Consortium; et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206. [Google Scholar] [CrossRef]
- Hyde, C.L.; Nagle, M.W.; Tian, C.; Chen, X.; Paciga, S.A.; Wendland, J.R.; Tung, J.Y.; Hinds, D.A.; Perlis, R.H.; Winslow, A.R. Identification of 15 genetic loci associated with risk of major depression in individuals of European descent. Nat. Genet. 2016, 48, 1031–1036. [Google Scholar] [CrossRef]
- Trubetskoy, V.; Pardiñas, A.F.; Qi, T.; Panagiotaropoulou, G.; Awasthi, S.; Bigdeli, T.B.; Bryois, J.; Chen, C.-Y.; Dennison, C.A.; Hall, L.S.; et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature 2022, 604, 502–508. [Google Scholar] [CrossRef]
- Dennis, E.L.; Jahanshad, N.; Braskie, M.N.; Warstadt, N.M.; Hibar, D.P.; Kohannim, O.; Nir, T.M.; McMahon, K.L.; de Zubicaray, G.I.; Montgomery, G.W. Obesity gene NEGR1 associated with white matter integrity in healthy young adults. Neuroimage 2014, 102, 548–557. [Google Scholar] [CrossRef]
- Gamero-Villarroel, C.; González, L.M.; Gordillo, I.; Carrillo, J.A.; García-Herráiz, A.; Flores, I.; Rodríguez-López, R.; Gervasini, G. Impact of NEGR1 genetic variability on psychological traits of patients with eating disorders. Pharmacogenomics J. 2015, 15, 278–283. [Google Scholar] [CrossRef]
- Lee, A.W.S.; Hengstler, H.; Schwald, K.; Berriel-Diaz, M.; Loreth, D.; Kirsch, M.; Kretz, O.; Haas, C.A.; De Angelis, M.H.; Herzig, S.; et al. Functional Inactivation of the Genome-Wide Association Study Obesity Gene Neuronal Growth Regulator 1 in Mice Causes a Body Mass Phenotype. PLoS ONE 2012, 7, e41537. [Google Scholar] [CrossRef] [PubMed]
- Kaare, M.; Mikheim, K.; Lilleväli, K.; Kilk, K.; Jagomäe, T.; Leidmaa, E.; Piirsalu, M.; Porosk, R.; Singh, K.; Reimets, R.; et al. High-Fat Diet Induces Pre-Diabetes and Distinct Sex-Specific Metabolic Alterations in Negr1-Deficient Mice. Biomedicines 2021, 9, 1148. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Pfundstein, G.; Sah, S.; Zhang, S.; Keable, R.; Hagan, D.W.; Sharpe, L.J.; Clemens, K.J.; Begg, D.; Phelps, E.A.; et al. Neuronal growth regulator 1 (NEGR1) promotes the synaptic targeting of glutamic acid decarboxylase 65 (GAD65). J. Neurochem. 2025, 169, e16279. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Loreth, D.; Pöttker, B.; Hefti, K.; Innos, J.; Schwald, K.; Hengstler, H.; Menzel, L.; Sommer, C.J.; Radyushkin, K.; et al. Neuronal Growth and Behavioral Alterations in Mice Deficient for the Psychiatric Disease-Associated Negr1 Gene. Front. Mol. Neurosci. 2018, 11, 30. [Google Scholar] [CrossRef]
- Singh, K.; Jayaram, M.; Kaare, M.; Leidmaa, E.; Jagomäe, T.; Heinla, I.; Hickey, M.A.; Kaasik, A.; Schäfer, M.K.; Innos, J.; et al. Neural cell adhesion molecule Negr1 deficiency in mouse results in structural brain endophenotypes and behavioral deviations related to psychiatric disorders. Sci. Rep. 2019, 9, 5457. [Google Scholar] [CrossRef]
- Noh, K.; Lee, H.; Choi, T.-Y.; Joo, Y.; Kim, S.-J.; Kim, H.; Kim, J.Y.; Jahng, J.W.; Lee, S.; Choi, S.-Y. Negr1 regulates hippocampal Lcn2 expression and affective behaviour via interaction with LIF receptor. Mol. Psychiatry 2019, 24, 1095. Available online: https://www.nature.com/articles/s41380-019-0455-8 (accessed on 7 May 2024). [CrossRef]
- Nahar, L.; Delacroix, B.M.; Nam, H.W. The Role of Parvalbumin Interneurons in Neurotransmitter Balance and Neurological Disease. Front. Psychiatry 2021, 12, 679960. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Zhang, Q.; Yang, Y.; Yu, L.-L.; Fan, N.-L.; Wu, Y.; Wang, J.-Y.; Dang, X.-L.; Guo, Y.-Q.; Li, C.; et al. Elevated NEGR1 in brain induces anxiety or depression-like phenotypes and synaptic dysfunction. Mol. Psychiatry 2025, 30, 4627–4640. [Google Scholar] [CrossRef] [PubMed]
- Marín, O. Interneuron dysfunction in psychiatric disorders. Nat. Rev. Neurosci. 2012, 13, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Sohal, V.S.; Rubenstein, J.L.R. Excitation-inhibition balance as a framework for investigating mechanisms in neuropsychiatric disorders. Mol. Psychiatry 2019, 24, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Kaare, M.; Jayaram, M.; Jagomäe, T.; Singh, K.; Kilk, K.; Mikheim, K.; Leevik, M.; Leidmaa, E.; Varul, J.; Nõmm, H.; et al. Depression-Associated Negr1 Gene-Deficiency Induces Alterations in the Monoaminergic Neurotransmission Enhancing Time-Dependent Sensitization to Amphetamine in Male Mice. Brain Sci. 2022, 12, 1696. [Google Scholar] [CrossRef] [PubMed]
- Takashi, H.; Hashimoto, T.; Mayumi, Y.; Yamada, M.; Shohei, M.; Maekawa, S.; Toshihiro, N.; Nakashima, T.; Seiji, M.; Miyata, S. IgLON cell adhesion molecule Kilon is a crucial modulator for synapse number in hippocampal neurons. Brain Res. 2008, 1224, 1–11. [Google Scholar] [CrossRef]
- Pischedda, F.; Szczurkowska, J.; Cirnaru, M.D.; Giesert, F.; Vezzoli, E.; Ueffing, M.; Sala, C.; Francolini, M.; Hauck, S.M.; Cancedda, L.; et al. A Cell Surface Biotinylation Assay to Reveal Membrane-associated Neuronal Cues: Negr1 Regulates Dendritic Arborization. Mol. Cell. Proteom. 2014, 13, 733–748. [Google Scholar] [CrossRef]
- Pourhaghighi, R.; Ash, P.E.A.; Phanse, S.; Goebels, F.; Hu, L.Z.M.; Chen, S.; Zhang, Y.; Wierbowski, S.D.; Boudeau, S.; Moutaoufik, M.T.; et al. BraInMap Elucidates the Macromolecular Connectivity Landscape of Mammalian Brain. Cell Syst. 2020, 11, 208. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, Z.; Hu, G.; Hu, S.; Wang, Y.; Li, N.; Chen, S.; Liu, Q.; Zeng, L.; Tang, T.; et al. Transcription factor 4 controls positioning of cortical projection neurons through regulation of cell adhesion. Mol. Psychiatry 2021, 26, 6562–6577. [Google Scholar] [CrossRef]
- Kennedy, A.J.; Rahn, E.J.; Paulukaitis, B.S.; Savell, K.E.; Kordasiewicz, H.B.; Wang, J.; Lewis, J.W.; Posey, J.; Strange, S.K.; Guzman-Karlsson, M.C.; et al. Tcf4 Regulates Synaptic Plasticity, DNA Methylation, and Memory Function. Cell Rep. 2016, 16, 2666–2685. [Google Scholar] [CrossRef] [PubMed]
- Pischedda, F.; Piccoli, G. The IgLON Family Member Negr1 Promotes Neuronal Arborization Acting as Soluble Factor via FGFR2. Front. Mol. Neurosci. 2016, 8, 89. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Lee, B.; Kim, Y.-B.; Kim, K.H.; Chung, G.; Lee, S.J.; Lee, S.; Sun, W.; Park, H.-K.; et al. Major depression-related factor NEGR1 controls salivary secretion in mouse submandibular glands. iScience 2023, 26, 106773. [Google Scholar] [CrossRef]
- Miao, Z.; Wang, Y.; Sun, Z. The Relationships Between Stress, Mental Disorders, and Epigenetic Regulation of BDNF. Int. J. Mol. Sci. 2020, 21, 1375. [Google Scholar] [CrossRef]
- Fang, X.; Jiang, S.; Wang, J.; Bai, Y.; Kim, C.S.; Blake, D.; Weintraub, N.L.; Lei, Y.; Lu, X.-Y. Chronic unpredictable stress induces depression-related behaviors by suppressing AgRP neuron activity. Mol. Psychiatry 2021, 26, 2299–2315. [Google Scholar] [CrossRef]
- Takatsu-Coleman, A.L.; Patti, C.L.; Zanin, K.A.; Zager, A.; Carvalho, R.C.; Borçoi, A.R.; Ceccon, L.M.B.; Berro, L.F.; Tufik, S.; Andersen, M.L.; et al. Short-term social isolation induces depressive-like behaviour and reinstates the retrieval of an aversive task: Mood-congruent memory in male mice? J. Psychiatry Neurosci. 2013, 38, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Ieraci, A.; Mallei, A.; Popoli, M. Social Isolation Stress Induces Anxious-Depressive-Like Behavior and Alterations of Neuroplasticity-Related Genes in Adult Male Mice. Neural Plast. 2016, 2016, 6212983. [Google Scholar] [CrossRef]
- Paxinos, G.; Franklin, K.B.J. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates, 5th ed.; Academic Press: London, UK, 2019. [Google Scholar]
- Vanaveski, T.; Singh, K.; Narvik, J.; Eskla, K.-L.; Visnapuu, T.; Heinla, I.; Jayaram, M.; Innos, J.; Lilleväli, K.; Philips, M.-A.; et al. Promoter-Specific Expression and Genomic Structure of IgLON Family Genes in Mouse. Front. Neurosci. 2017, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Karis, K.; Eskla, K.-L.; Kaare, M.; Täht, K.; Tuusov, J.; Visnapuu, T.; Innos, J.; Jayaram, M.; Timmusk, T.; Weickert, C.S.; et al. Altered Expression Profile of IgLON Family of Neural Cell Adhesion Molecules in the Dorsolateral Prefrontal Cortex of Schizophrenic Patients. Front. Mol. Neurosci. 2018, 11, 8. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef]
- Li, X.; Sun, H.; Zhu, Y.; Wang, F.; Wang, X.; Han, L.; Cui, D.; Luo, D.; Zhai, Y.; Zhuo, L.; et al. Dysregulation of prefrontal parvalbumin interneurons leads to adult aggression induced by social isolation stress during adolescence. Front. Mol. Neurosci. 2022, 15, 1010152. [Google Scholar] [CrossRef]
- Li, S.; Cao, W.; Zhou, S.; Ma, M.; Zhang, W.; Li, F.; Li, C. Expression of Cntn1 is regulated by stress and associated with anxiety and depression phenotypes. Brain. Behav. Immun. 2021, 95, 142–153. [Google Scholar] [CrossRef]
- Labouesse, M.A.; Dong, E.; Grayson, D.R.; Guidotti, A.; Meyer, U. Maternal immune activation induces GAD1 and GAD2 promoter remodeling in the offspring prefrontal cortex. Epigenetics 2015, 10, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Trifonov, S.; Yamashita, Y.; Kase, M.; Maruyama, M.; Sugimoto, T. Glutamic acid decarboxylase 1 alternative splicing isoforms: Characterization, expression and quantification in the mouse brain. BMC Neurosci. 2014, 15, 114. [Google Scholar] [CrossRef] [PubMed]
- Bennett, S.N.; Chang, A.B.; Rogers, F.D.; Jones, P.; Peña, C.J. Thyroid hormones mediate the impact of early-life stress on ventral tegmental area gene expression and behavior. Horm. Behav. 2024, 159, 105472. [Google Scholar] [CrossRef]
- Tian, W.; Wang, J.; Zhang, K.; Teng, H.; Li, C.; Szyf, M.; Sun, Z.S.; Zhao, M. Demethylation of c-MYB binding site mediates upregulation of Bdnf IV in cocaine-conditioned place preference. Sci. Rep. 2016, 6, 22087. [Google Scholar] [CrossRef]
- Huang, T.; Krimm, R.F. Developmental expression of Bdnf, Ntf4/5, and TrkB in the mouse peripheral taste system. Dev. Dyn. 2010, 239, 2637–2646. [Google Scholar] [CrossRef] [PubMed]
- Sepp, M.; Kannike, K.; Eesmaa, A.; Urb, M.; Timmusk, T. Functional Diversity of Human Basic Helix-Loop-Helix Transcription Factor TCF4 Isoforms Generated by Alternative 5′ Exon Usage and Splicing. PLoS ONE 2011, 6, e22138. [Google Scholar] [CrossRef]
- Philips, M.-A.; Lilleväli, K.; Heinla, I.; Luuk, H.; Hundahl, C.A.; Kongi, K.; Vanaveski, T.; Tekko, T.; Innos, J.; Vasar, E. Lsamp is implicated in the regulation of emotional and social behavior by use of alternative promoters in the brain. Brain Struct. Funct. 2015, 220, 1381–1393. [Google Scholar] [CrossRef]
- Schlaudraff, J.; Paul, M.H.; Deller, T.; Del Turco, D. Precise measurement of gene expression changes in mouse brain areas denervated by injury. Sci. Rep. 2022, 12, 22530. [Google Scholar] [CrossRef] [PubMed]
- Grippo, A.J.; Gerena, D.; Huang, J.; Kumar, N.; Shah, M.; Ughreja, R.; Sue Carter, C. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology 2007, 32, 966–980. [Google Scholar] [CrossRef]
- Fone, K.C.F.; Porkess, M.V. Behavioural and neurochemical effects of post-weaning social isolation in rodents—Relevance to developmental neuropsychiatric disorders. Neurosci. Biobehav. Rev. 2008, 32, 1087–1102. [Google Scholar] [CrossRef]
- eQTLGen; 23andMe; Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium; Wray, N.R.; Ripke, S.; Mattheisen, M.; Trzaskowski, M.; Byrne, E.M.; Abdellaoui, A.; Adams, M.J.; et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat. Genet. 2018, 50, 668–681. [Google Scholar] [CrossRef]
- Carboni, L.; Pischedda, F.; Piccoli, G.; Lauria, M.; Musazzi, L.; Popoli, M.; Mathé, A.A.; Domenici, E. Depression-Associated Gene Negr1-Fgfr2 Pathway Is Altered by Antidepressant Treatment. Cells 2020, 9, 1818. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cheng, W.; Zhu, J.; Yin, H.; Chang, S.; Yue, W.; Yu, H. Integrating genome-wide association study and expression quantitative trait loci data identifies NEGR1 as a causal risk gene of major depression disorder. J. Affect. Disord. 2020, 265, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Suemitsu, S.; Murakami, S.; Kitamura, N.; Wani, K.; Okamoto, M.; Matsumoto, Y.; Ishihara, T. Region-specific impairments in parvalbumin interneurons in social isolation-reared mice. Neuroscience 2017, 359, 196–208. [Google Scholar] [CrossRef]
- Nullmeier, S.; Elmers, C.; D’Hanis, W.; Sandhu, K.V.K.; Stork, O.; Yanagawa, Y.; Panther, P.; Schwegler, H. Glutamic acid decarboxylase 67 haplodeficiency in mice: Consequences of postweaning social isolation on behavior and changes in brain neurochemical systems. Brain Struct. Funct. 2020, 225, 1719–1742. [Google Scholar] [CrossRef]
- Harding, S.M.; Van Dyke, A.R.; Little, M.; LaClair, M.G. Sex differences in behavior and glutamic acid decarboxylase in Long Evans rats after prolonged social isolation beginning in adolescence. Behav. Neurosci. 2024, 138, 321–330. [Google Scholar] [CrossRef]
- Murínová, J.; Hlaváčová, N.; Chmelová, M.; Riečanský, I. The Evidence for Altered BDNF Expression in the Brain of Rats Reared or Housed in Social Isolation: A Systematic Review. Front. Behav. Neurosci. 2017, 11, 101. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, S.; Moradi, K.; Ehghaghi, E.; Badripour, A.; Keykhaei, M.; Ashraf-Ganjouei, A.; Moassefi, M.; Faghani, S.; Dehpour, A.R. Melatonin improves learning and memory of mice with chronic social isolation stress via an interaction between microglia polarization and BDNF/TrkB/CREB signaling pathway. Eur. J. Pharmacol. 2021, 908, 174358. [Google Scholar] [CrossRef] [PubMed]
- Philips, M.-A.; Abramov, U.; Lilleväli, K.; Luuk, H.; Kurrikoff, K.; Raud, S.; Plaas, M.; Innos, J.; Puussaar, T.; Kõks, S.; et al. Myg1-deficient mice display alterations in stress-induced responses and reduction of sex-dependent behavioural differences. Behav. Brain Res. 2010, 207, 182–195. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Aldridge, J.W.; LaPorte, J.L.; Murphy, D.L.; Tuohimaa, P. Analyzing grooming microstructure in neurobehavioral experiments. Nat. Protoc. 2007, 2, 2538–2544. [Google Scholar] [CrossRef]
- Innos, J.; Philips, M.-A.; Raud, S.; Lilleväli, K.; Kõks, S.; Vasar, E. Deletion of the Lsamp gene lowers sensitivity to stressful environmental manipulations in mice. Behav. Brain Res. 2012, 228, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Dalla, C.; Pitychoutis, P.M.; Kokras, N.; Papadopoulou-Daifoti, Z. Sex Differences in Response to Stress and Expression of Depressive-Like Behaviours in the Rat. In Biological Basis of Sex Differences in Psychopharmacology; Neill, J.C., Kulkarni, J., Eds.; Current Topics in Behavioral Neurosciences; Springer: Berlin/Heidelberg, Germany, 2010; Volume 8, pp. 97–118. [Google Scholar] [CrossRef]
- Bangasser, D.A.; Valentino, R.J. Sex differences in stress-related psychiatric disorders: Neurobiological perspectives. Front. Neuroendocrinol. 2014, 35, 303–319. [Google Scholar] [CrossRef]
- Ghosal, S.; Hare, B.D.; Duman, R.S. Prefrontal cortex GABAergic deficits and circuit dysfunction in the pathophysiology and treatment of chronic stress and depression. Curr. Opin. Behav. Sci. 2017, 14, 1–8. [Google Scholar] [CrossRef]
- Lewis, D.A.; Hashimoto, T.; Volk, D.W. Cortical inhibitory neurons and schizophrenia. Nat. Rev. Neurosci. 2005, 6, 312–324. [Google Scholar] [CrossRef]
- Enwright, J.F.; Sanapala, S.; Foglio, A.; Berry, R.; Fish, K.N.; Lewis, D.A. Reduced Labeling of Parvalbumin Neurons and Perineuronal Nets in the Dorsolateral Prefrontal Cortex of Subjects with Schizophrenia. Neuropsychopharmacology 2016, 41, 2206–2214. [Google Scholar] [CrossRef]
- Duman, R.S.; Monteggia, L.M. A Neurotrophic Model for Stress-Related Mood Disorders. Biol. Psychiatry 2006, 59, 1116–1127. [Google Scholar] [CrossRef]
- Badowska, D.M.; Brzózka, M.M.; Kannaiyan, N.; Thomas, C.; Dibaj, P.; Chowdhury, A.; Steffens, H.; Turck, C.W.; Falkai, P.; Schmitt, A.; et al. Modulation of cognition and neuronal plasticity in gain- and loss-of-function mouse models of the schizophrenia risk gene Tcf4. Transl. Psychiatry 2020, 10, 343. [Google Scholar] [CrossRef]
- Heinla, I.; Leidmaa, E.; Kongi, K.; Pennert, A.; Innos, J.; Nurk, K.; Tekko, T.; Singh, K.; Vanaveski, T.; Reimets, R.; et al. Gene expression patterns and environmental enrichment-induced effects in the hippocampi of mice suggest importance of Lsamp in plasticity. Front. Neurosci. 2015, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Innos, J.; Philips, M.-A.; Leidmaa, E.; Heinla, I.; Raud, S.; Reemann, P.; Plaas, M.; Nurk, K.; Kurrikoff, K.; Matto, V.; et al. Lower anxiety and a decrease in agonistic behaviour in Lsamp-deficient mice. Behav. Brain Res. 2011, 217, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Lilleväli, K.; Gilbert, S.F.; Bregin, A.; Narvik, J.; Jayaram, M.; Rahi, M.; Innos, J.; Kaasik, A.; Vasar, E.; et al. The combined impact of IgLON family proteins Lsamp and Neurotrimin on developing neurons and behavioral profiles in mouse. Brain Res. Bull. 2018, 140, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Pischedda, F.; Szczurkowska, J.; Daniela Cirnaru, M.; Cancedda, L.; Piccoli, G. The role of Negr1 in cortical development via NCAM-FGFR2 signaling. SpringerPlus 2015, 4, P38. [Google Scholar] [CrossRef]
| Target Genes | Primer Sequence | Amplicon Size | Sources |
|---|---|---|---|
| Fgfr2 | F_TGCACGCAGGATGGACCTCTCT R_TGCTCCTCGGGGACACGGTTAA | 131 bp | [45] |
| Cntn1 | F_CGCGTTTCAAGTCAAAGTGA R_TTTGACCCCTACCTCTGTGG | 121 bp | [46] |
| Gad 1 | F_ATGATACTTGGTGTGGCGTAG R_GACTCTTCTCTTCCAGGCTATTG | 99 bp | [47] |
| Gad 2 | F_CATTGATAAGTGTTTGGAGCTAGCA R_GTGCGCAAACTAGGAGGTACAA | 135 bp | [48] |
| Pvalb | F_TTCTGAAGGGCTTCTCCTCA R_TTCTTCAACCCCAATCTTGC | 107 bp | [49] |
| Bdnf | F_TGGCTGACACTTTTGAGCAC R_AAGTGTACAAGTCCGCGTCC | 99 bp | [50] |
| TrkB(Ntrk2) | F_AAGGACTTTCATCGGGAAGCTG R_TCGCCCTCCACACAGACAC | 86 bp | [51] |
| Tcf4-tot | F_GACCACACGAACAACAGCTT R_TCTTCGATTCGGCTTTGCAG | 161 bp | [52] based on human primers |
| Negr1 | F_TGCTCGAACCAGTGGCTGGC R_CCCTTTGATGCTCCATCTTCCA | 161 bp | [41] |
| Ntm1a | F_CTGGCGGCTCTGTGCCTCT R_GGTGACTCGGTTGTCAATTGTG | 135 bp | [41] |
| Ntm 1b | F_CTCTCAGGCTGCTATTCCTTGTA R_GGTGACTCGGTTGTCAATTGTG | 140 bp | [41] |
| Lsamp 1a | F_GCATTTTGGAACCAGCCTCCTG R_TTCTTGTCTTCTACCACACACCTG | 155 bp | [53] |
| Lsamp 1b | F_CGATCGGAAACAGTTGCCGC R_TTCTTGTCTTCTACCACACACCTG | 156 bp | [53] |
| Pgk1 | F_CGTGATGAGGGTGGACTT R_TGGAACAGCAGCCTTGAT | 79 bp | [54] |
| Gapdh | F_ACAATGAATACGGCTACAG R_GGTCCAGGGTTTCTTACT | 78 bp | [54] |
| Hprt | F_GCAGTACAGCCCCAAAATGG R_AACAAAGTCTGGCCTGTATCCAA | 85 bp | [41] |
| Act B | F_ACCATGTACCCAGGCATTGC R_AGCCACCGATCCACACAGAG | 121 bp | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reinsberg, A.; Singh, K.; Jayaram, M.; Mikheim, K.; Philips, M.-A.; Vasar, E. Negr1 Deficiency Modulates Sex-Specific Neurobehavioral Adaptations to Social Isolation. Brain Sci. 2025, 15, 1286. https://doi.org/10.3390/brainsci15121286
Reinsberg A, Singh K, Jayaram M, Mikheim K, Philips M-A, Vasar E. Negr1 Deficiency Modulates Sex-Specific Neurobehavioral Adaptations to Social Isolation. Brain Sciences. 2025; 15(12):1286. https://doi.org/10.3390/brainsci15121286
Chicago/Turabian StyleReinsberg, Arpana, Katyayani Singh, Mohan Jayaram, Kaie Mikheim, Mari-Anne Philips, and Eero Vasar. 2025. "Negr1 Deficiency Modulates Sex-Specific Neurobehavioral Adaptations to Social Isolation" Brain Sciences 15, no. 12: 1286. https://doi.org/10.3390/brainsci15121286
APA StyleReinsberg, A., Singh, K., Jayaram, M., Mikheim, K., Philips, M.-A., & Vasar, E. (2025). Negr1 Deficiency Modulates Sex-Specific Neurobehavioral Adaptations to Social Isolation. Brain Sciences, 15(12), 1286. https://doi.org/10.3390/brainsci15121286






