Prenatal Magnesium Sulfate Exposure Is Not Associated with Different Neurodevelopmental Outcomes by Sex in Extremely Preterm Infants
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Relationship Between Maternal Characteristics and Prenatal MgSO4 Exposure
3.2. In Unadjusted Analyses, MgSO4 Is Not Associated with Neurodevelopmental Outcomes in Either Sex
3.3. MgSO4 Is Not Associated with Neurodevelopment in Either Sex After Accounting for Indication
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BSID | Bayley Scales for Infant Development 3rd Edition |
| CI | Confidence interval |
| CP | Cerebral palsy |
| EP | Extremely preterm |
| Epo | Erythropoietin |
| GA | Gestational age |
| GEE | Generalized estimating equations |
| GMFCS | Gross Motor Function Classification System |
| HI | Hypoxic–ischemic |
| IL-6 | Interleukin-6 |
| MgSO4 | Magnesium sulfate |
| NICU | Neonatal intensive care unit |
| NMDA | N-methyl-D-aspartate |
| OR | Odds ratio |
| PENUT | Preterm Erythropoietin Neuroprotection Trial |
| PROM | Premature rupture of membranes |
| RR | Risk ratio |
| SGA | Small for gestational age |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| TNF | Tumor necrosis factor |
References
- Chollat, C.; Sentilhes, L.; Marret, S. Fetal Neuroprotection by Magnesium Sulfate: From Translational Research to Clinical Application. Front. Neurol. 2018, 9, 247. [Google Scholar] [CrossRef]
- Mayer, C.; Apodaca-Ramos, I. Tocolysis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Euser, A.G.; Cipolla, M.J. Magnesium Sulfate for the Treatment of Eclampsia. Stroke 2009, 40, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.A.; Brandon, D.H. Hypoxic Ischemic Encephalopathy: Pathophysiology and Experimental Treatments. Newborn Infant Nurs. Rev. 2011, 11, 125–133. [Google Scholar] [CrossRef]
- Vacanti, F.X.; Ames, A. Mild hypothermia and Mg++ protect against irreversible damage during CNS ischemia. Stroke 1984, 15, 695–698. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.W.; Silverstein, F.S.; Johnston, M.V. Magnesium reduces N-(NMDA)-mediated brain injury in perinatal rats. Neurosci. Lett. 1990, 109, 234–238. [Google Scholar] [CrossRef]
- Sun, Y.-J.; Zhang, Z.-Y.; Fan, B.; Li, G.-Y. Neuroprotection by Therapeutic Hypothermia. Front. Neurosci. 2019, 13, 586. [Google Scholar] [CrossRef]
- Galinsky, R.; Bennet, L.; Groenendaal, F.; Lear, C.A.; Tan, S.; van Bel, F.; Juul, S.E.; Robertson, N.J.; Mallard, C.; Gunn, A.J. Magnesium Is Not Consistently Neuroprotective for Perinatal Hypoxia-Ischemia in Term-Equivalent Models in Preclinical Studies: A Systematic Review. Dev. Neurosci. 2014, 36, 73–82. [Google Scholar] [CrossRef]
- Galinsky, R.; Draghi, V.; Wassink, G.; O Davidson, J.; Drury, P.P.; A Lear, C.; Gunn, A.J.; Bennet, L. Magnesium sulfate reduces EEG activity but is not neuroprotective after asphyxia in preterm fetal sheep. J. Cereb. Blood Flow Metab. 2016, 37, 1362–1373. [Google Scholar] [CrossRef] [PubMed]
- Wolf, H.T.; Hegaard, H.K.; Greisen, G.; Huusom, L.; Hedegaard, M. Treatment with magnesium sulphate in pre-term birth: A systematic review and meta-analysis of observational studies. J. Obstet. Gynaecol. 2012, 32, 135–140. [Google Scholar] [CrossRef]
- Doyle, L.W.; A Crowther, C.; Middleton, P.; Marret, S. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. In Cochrane Database of Systematic Reviews; Doyle, L.W., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2007. [Google Scholar] [CrossRef]
- Conde-Agudelo, A.; Romero, R. Antenatal magnesium sulfate for the prevention of cerebral palsy in preterm infants less than 34 weeks’ gestation: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2009, 200, 595–609. [Google Scholar] [CrossRef]
- Costantine, M.M.; Weiner, S.J. Effects of Antenatal Exposure to Magnesium Sulfate on Neuroprotection and Mortality in Preterm Infants. Obstet. Gynecol. 2009, 114, 354–364. [Google Scholar] [CrossRef]
- Zeng, X.; Xue, Y.; Tian, Q.; Sun, R.; An, R. Effects and Safety of Magnesium Sulfate on Neuroprotection. Medicine 2016, 95, e2451. [Google Scholar] [CrossRef]
- McLeod, R.M.; Rosenkrantz, T.S.; Fitch, R.H. Antenatal Magnesium Sulfate Benefits Female Preterm Infants but Results in Poor Male Outcomes. Pharmaceuticals 2024, 17, 218. [Google Scholar] [CrossRef]
- Juul, S.E.; Mayock, D.E.; Comstock, B.A.; Heagerty, P.J. Neuroprotective potential of erythropoietin in neonates; design of a randomized trial. Matern. Health Neonatol. Perinatol. 2015, 1, 27. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. The Strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, P.L.; Walter, S.D.; Hanna, S.E.; Palisano, R.J.; Russell, D.J.; Raina, P.; Wood, E.; Bartlett, D.J.; Galuppi, B.E. Prognosis for Gross Motor Function in Cerebral Palsy. JAMA 2002, 288, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Schlaudecker, E.P.; Munoz, F.M.; Bardají, A.; Boghossian, N.S.; Khalil, A.; Mousa, H.; Nesin, M.; Nisar, M.I.; Pool, V.; Spiegel, H.M.; et al. Small for gestational age: Case definition & guidelines for data collection, analysis, and presentation of maternal immunisation safety data. Vaccine 2017, 35, 6518–6528. [Google Scholar] [CrossRef]
- Shennan, A.; Suff, N.; Jacobsson, B. Abstracts of the XXIII FIGO World Congress of Gynecology & Obstetrics. Int. J. Gynaecol. Obstet. 2021, 155 (Suppl. S2), 31–532. [Google Scholar] [CrossRef]
- Nowak, L.; Bregestovski, P.; Ascher, P.; Herbet, A.; Prochiantz, A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature 1984, 307, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.W.; Choi, S.-K.; Park, E.; Chae, S.-J.; Choi, S.; Joo, H.J.; Lee, G.-J.; Park, H.-K. Neuroprotective effects of magnesium-sulfate on ischemic injury mediated by modulating the release of glutamate and reduced of hyperreperfusion. Brain Res. 2011, 1371, 121–128. [Google Scholar] [CrossRef]
- Türkyilmaz, C.; Türkyilmaz, Z.; Atalay, Y.; Söylemezoglu, F.; Celasun, B. Magnesium pre-treatment reduces neuronal apoptosis in newborn rats in hypoxia–ischemia. Brain Res. 2002, 955, 133–137. [Google Scholar] [CrossRef]
- Drommelschmidt, K.; Mayrhofer, T.; Hüning, B.; Stein, A.; Foldyna, B.; Schweiger, B.; Felderhoff-Müser, U.; Sirin, S. Incidence of brain injuries in a large cohort of very preterm and extremely preterm infants at term-equivalent age: Results of a single tertiary neonatal care center over 10 years. Eur. Radiol. 2024, 34, 5239–5249. [Google Scholar] [CrossRef]
- Zaghloul, N.; Ahmed, M. Pathophysiology of periventricular leukomalacia: What we learned from animal models. Neural Regen. Res. 2017, 12, 1795–1796. [Google Scholar] [CrossRef]
- Babcock, M.A.; Kostova, F.V.; Ferriero, D.M.; Johnston, M.V.; Brunstrom, J.E.; Hagberg, H.; Maria, B.L. Injury to the preterm brain and cerebral palsy: Clinical aspects, molecular mechanisms, unanswered questions, and future research directions. J. Child Neurol. 2009, 24, 1064–1084. [Google Scholar] [CrossRef]
- Nabetani, M.; Mukai, T.; Shintaku, H. Preventing Brain Damage from Hypoxic–Ischemic Encephalopathy in Neonates: Update on Mesenchymal Stromal Cells and Umbilical Cord Blood Cells. Am. J. Perinatol. 2021, 39, 1754–1763. [Google Scholar] [CrossRef] [PubMed]
- Fellman, V.; O Raivio, K. Reperfusion Injury as the Mechanism of Brain Damage after Perinatal Asphyxia. Pediatr. Res. 1997, 41, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, Q.; Meng, H.; Duan, H.; Liu, X.; Wu, J.; Gao, F.; Wang, S.; Tan, R.; Yuan, J. Ischemia-reperfusion injury: Molecular mechanisms and therapeutic targets. Signal Transduct. Target. Ther. 2024, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Mazur, A.; Maier, J.A.; Rock, E.; Gueux, E.; Nowacki, W.; Rayssiguier, Y. Magnesium and the inflammatory response: Potential physiopathological implications. Arch. Biochem. Biophys. 2007, 458, 48–56. [Google Scholar] [CrossRef]
- Aryana, P.; Rajaei, S.; Bagheri, A.; Karimi, F.; Dabbagh, A. Acute Effect of Intravenous Administration of Magnesium Sulfate on Serum Levels of Interleukin-6 and Tumor Necrosis Factor-α in Patients Undergoing Elective Coronary Bypass Graft with Cardiopulmonary Bypass. Anesthesiol. Pain Med. 2014, 4, e16316. [Google Scholar] [CrossRef]
- Rayssiguier, Y.; Libako, P.; Nowacki, W.; Rock, E. Magnesium deficiency and metabolic syndrome: Stress and inflammation may reflect calcium activation. Magnes. Res. 2010, 23, 73–80. [Google Scholar] [CrossRef]
- Burd, I.; Breen, K.; Friedman, A.; Chai, J.; Elovitz, M.A. Magnesium sulfate reduces inflammation-associated brain injury in fetal mice. Am. J. Obstet. Gynecol. 2010, 202, 292.e1–292.e9. [Google Scholar] [CrossRef] [PubMed]
- Cho, G.J.; Hong, H.-R.; Hong, S.-C.; Oh, M.-J.; Kim, H.-J. The neuroprotective effect of magnesium sulfate in preterm fetal mice. J. Perinat. Med. 2015, 43, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Shen, F.; He, S.; Chen, M.; Han, Q.; Fang, M.; Zeng, H.; Chen, C.; Deng, Y. IL-1β induces hypomyelination in the periventricular white matter through inhibition of oligodendrocyte progenitor cell maturation via FYN/MEK/ERK signaling pathway in septic neonatal rats. Glia 2015, 64, 583–602. [Google Scholar] [CrossRef]
- Favrais, G.; van de Looij, Y.; Fleiss, B.; Ramanantsoa, N.; Bonnin, P.; Stoltenburg-Didinger, G.; Lacaud, A.; Saliba, E.; Dammann, O.; Gallego, J.; et al. Systemic inflammation disrupts the developmental program of white matter. Ann. Neurol. 2011, 70, 550–565. [Google Scholar] [CrossRef]
- Pang, Y.; Campbell, L.; Zheng, B.; Fan, L.; Cai, Z.; Rhodes, P. Lipopolysaccharide-activated microglia induce death of oligodendrocyte progenitor cells and impede their development. Neuroscience 2010, 166, 464–475. [Google Scholar] [CrossRef]
- Levison, S.W.; Rocha-Ferreira, E.; Kim, B.H.; Hagberg, H.; Fleiss, B.; Gressens, P.; Dobrowolski, R. Mechanisms of Tertiary Neurodegeneration after Neonatal Hypoxic-Ischemic Brain Damage. Pediatr. Med. 2022, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Frost, P.S.; Barros-Aragão, F.; da Silva, R.T.; Venancio, A.; Matias, I.; e Silva, N.M.L.; Kincheski, G.C.; Pimentel-Coelho, P.M.; De Felice, F.G.; Gomes, F.C.A.; et al. Neonatal infection leads to increased susceptibility to Aβ oligomer-induced brain inflammation, synapse loss and cognitive impairment in mice. Cell Death Dis. 2019, 10, 323. [Google Scholar] [CrossRef]
- Loane, D.J.; Kumar, A.; Stoica, B.A.; Cabatbat, R.; Faden, A.I. Progressive Neurodegeneration After Experimental Brain Trauma. J. Neuropathol. Exp. Neurol. 2014, 73, 14–29. [Google Scholar] [CrossRef]
- Cayam-Rand, D.; Guo, T.; Grunau, R.E.; Benavente-Fernández, I.; Synnes, A.; Chau, V.; Branson, H.; Latal, B.; McQuillen, P.; Miller, S.P. Predicting developmental outcomes in preterm infants. Neurology 2019, 93, e1231–e1240. [Google Scholar] [CrossRef]
- Martinez-Biarge, M.; Groenendaal, F.; Kersbergen, K.J.; Benders, M.J.; Foti, F.; van Haastert, I.C.; Cowan, F.M.; de Vries, L.S. Neurodevelopmental Outcomes in Preterm Infants with White Matter Injury Using a New MRI Classification. Neonatology 2019, 116, 227–235. [Google Scholar] [CrossRef]
- Woodward, L.J.; Clark, C.A.C.; Bora, S.; Inder, T.E. Neonatal white matter abnormalities an important predictor of neurocognitive outcome for very preterm children. PLoS ONE 2012, 7, e51879. [Google Scholar] [CrossRef]
- Poppe, T.; Thompson, B.; Boardman, J.P.; Bastin, M.E.; Alsweiler, J.; Deib, G.; Harding, J.E.; Crowther, C.A. Effect of antenatal magnesium sulphate on MRI biomarkers of white matter development at term equivalent age: The MagNUM Study. eBioMedicine 2022, 78, 103923. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, D.; Gan, Y.; Peng, Z.; Wu, Y.; Xiang, W. Identification of potential biomarkers for cerebral palsy and the development of prediction models. Exp. Biol. Med. 2024, 249, 10101. [Google Scholar] [CrossRef]
- Chen, B.; Wang, L.M.; Xie, D.M.; Wang, Y. Bioinformatics-based discovery of biomarkers and immunoinflammatory targets in children with cerebral palsy: An observational study. Medicine 2024, 103, e37828. [Google Scholar] [CrossRef]
- Kaukola, T.; Kallankari, H.; Tuimala, J.; Olsén, P.; Tammela, O.; Kingsmore, S.F.; Hallman, M. Perinatal immunoproteins predict the risk of cerebral palsy in preterm children. Ann. Med. 2011, 45, 57–65. [Google Scholar] [CrossRef]
- Salomon, I. Neurobiological Insights Into Cerebral Palsy: A Review of the Mechanisms and Therapeutic Strategies. Brain Behav. 2024, 14, e70065. [Google Scholar] [CrossRef] [PubMed]
- Kuban, K.C.K.; O’sHea, T.M.; Allred, E.N.; Paneth, N.; Hirtz, D.; Fichorova, R.N.; Leviton, A. Systemic Inflammation and Cerebral Palsy Risk in Extremely Preterm Infants. J. Child Neurol. 2014, 29, 1692–1698. [Google Scholar] [CrossRef] [PubMed]
- Horbar, J.D.; Greenberg, L.T.; Buzas, J.S.; Ehret, D.E.; Soll, R.F.; Edwards, E.M. Trends in Mortality and Morbidities for Infants Born 24 to 28 Weeks in the US: 1997–2021. Pediatrics 2023, 153, e2023064153. [Google Scholar] [CrossRef] [PubMed]
- Zivaljevic, J.; Jovandaric, M.Z.; Babic, S.; Raus, M. Complications of Preterm Birth—The Importance of Care for the Outcome: A Narrative Review. Medicina 2024, 60, 1014. [Google Scholar] [CrossRef]
- Qazi, K.R.; Jensen, G.B.; van der Heiden, M.; Björkander, S.; Holmlund, U.; Haileselassie, Y.; Kokkinou, E.; Marchini, G.; Jenmalm, M.C.; Abrahamsson, T.; et al. Extremely Preterm Infants Have Significant Alterations in Their Conventional T Cell Compartment during the First Weeks of Life. J. Immunol. 2020, 204, 68–77. [Google Scholar] [CrossRef]
- Walsh, M.C.; Bell, E.F.; Kandefer, S.; Saha, S.; A Carlo, W.; D’Angio, C.T.; Laptook, A.R.; Sanchez, P.J.; Stoll, B.J.; Shankaran, S.; et al. Neonatal outcomes of moderately preterm infants compared to extremely preterm infants. Pediatr. Res. 2017, 82, 297–304. [Google Scholar] [CrossRef]
- Tau, G.Z.; Peterson, B.S. Normal Development of Brain Circuits. Neuropsychopharmacology 2010, 35, 147–168. [Google Scholar] [CrossRef]
- Malik, S.; Vinukonda, G.; Vose, L.R.; Diamond, D.; Bhimavarapu, B.B.R.; Hu, F.; Zia, M.T.; Hevner, R.; Zecevic, N.; Ballabh, P. Neurogenesis continues in the third trimester of pregnancy and is suppressed by premature birth. J. Neurosci. 2013, 33, 411–423. [Google Scholar] [CrossRef]
- Lax, I.D.; Duerden, E.G.; Lin, S.Y.; Chakravarty, M.M.; Donner, E.J.; Lerch, J.P.; Taylor, M.J. Neuroanatomical consequences of very preterm birth in middle childhood. Anat. Embryol. 2012, 218, 575–585. [Google Scholar] [CrossRef]
- Migliori, C.; Braga, M.; Siragusa, V.; Villa, M.C.; Luzi, L. The impact of gender medicine on neonatology: The disadvantage of being male: A narrative review. Ital. J. Pediatr. 2023, 49, 65. [Google Scholar] [CrossRef] [PubMed]
- Peacock, J.L.; Marston, L.; Marlow, N.; Calvert, S.A.; Greenough, A. Neonatal and infant outcome in boys and girls born very prematurely. Pediatr. Res. 2012, 71, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Demarest, T.G.; McCarthy, M.M. Sex differences in mitochondrial (dys)function: Implications for neuroprotection. J. Bioenerg. Biomembr. 2014, 47, 173–188. [Google Scholar] [CrossRef]
- Pansiot, J.; Pham, H.; Dalous, J.; Chevenne, D.; Colella, M.; Schwendimann, L.; Fafouri, A.; Mairesse, J.; Moretti, R.; Schang, A.-L.; et al. Glial response to 17β-estradiol in neonatal rats with excitotoxic brain injury. Exp. Neurol. 2016, 282, 56–65. [Google Scholar] [CrossRef]
- Peterson, B.L.; Won, S.; Geddes, R.I.; Sayeed, I.; Stein, D.G. Sex-related differences in effects of progesterone following neonatal hypoxic brain injury. Behav. Brain Res. 2015, 286, 152–165. [Google Scholar] [CrossRef]
- Kim-Fine, S.; Regnault, T.R.H.; Lee, J.S.; Gimbel, S.A.; Greenspoon, J.A.; Fairbairn, J.; Summers, K.; de Vrijer, B. Male gender promotes an increased inflammatory response to lipopolysaccharide in umbilical vein blood. J. Matern. Neonatal Med. 2012, 25, 2470–2474. [Google Scholar] [CrossRef] [PubMed]
- Romeo, D.M.; Venezia, I.; Pede, E.; Brogna, C. Cerebral palsy and sex differences in children: A narrative review of the literature. J. Neurosci. Res. 2022, 101, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Kastrati, L.; Farina, S.; Gris, A.V.; Raeisi-Dehkordi, H.; Llanaj, E.; Quezada-Pinedo, H.G.; Bally, L.; Muka, T.; A Ioannidis, J.P. Evaluation of reported claims of sex-based differences in treatment effects across meta-analyses: A meta-research study. BMJ Evid.-Based Med. 2025. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
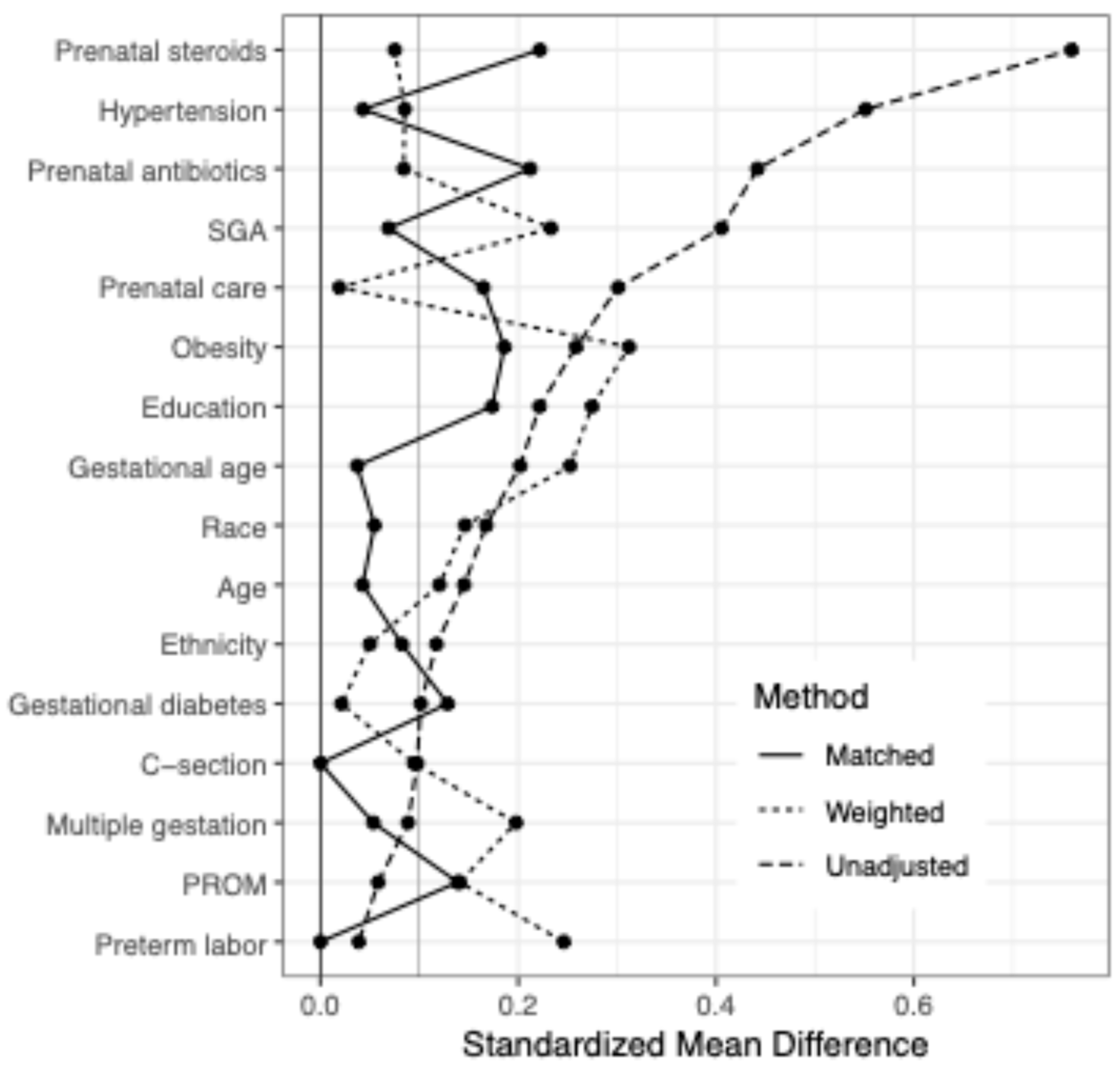
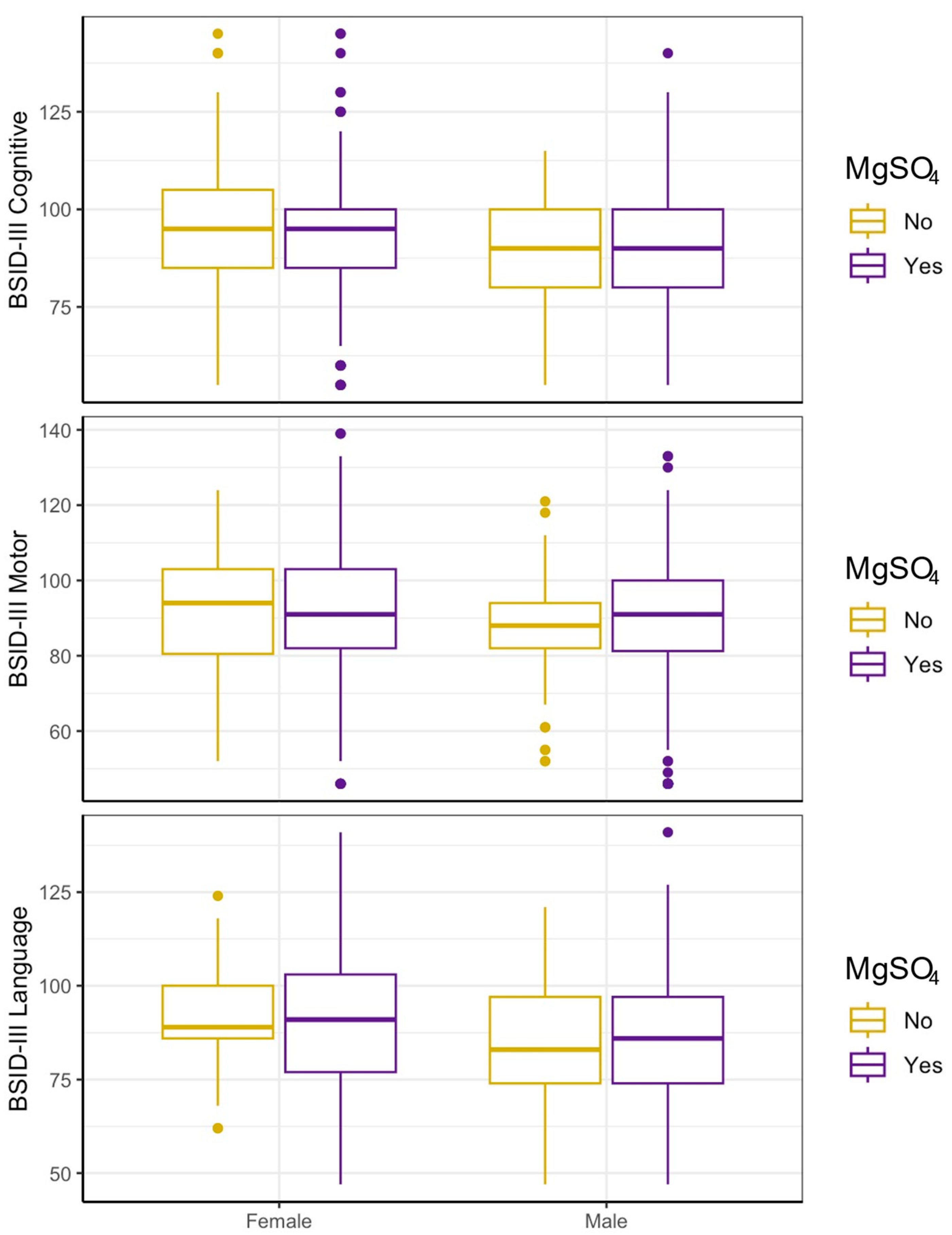
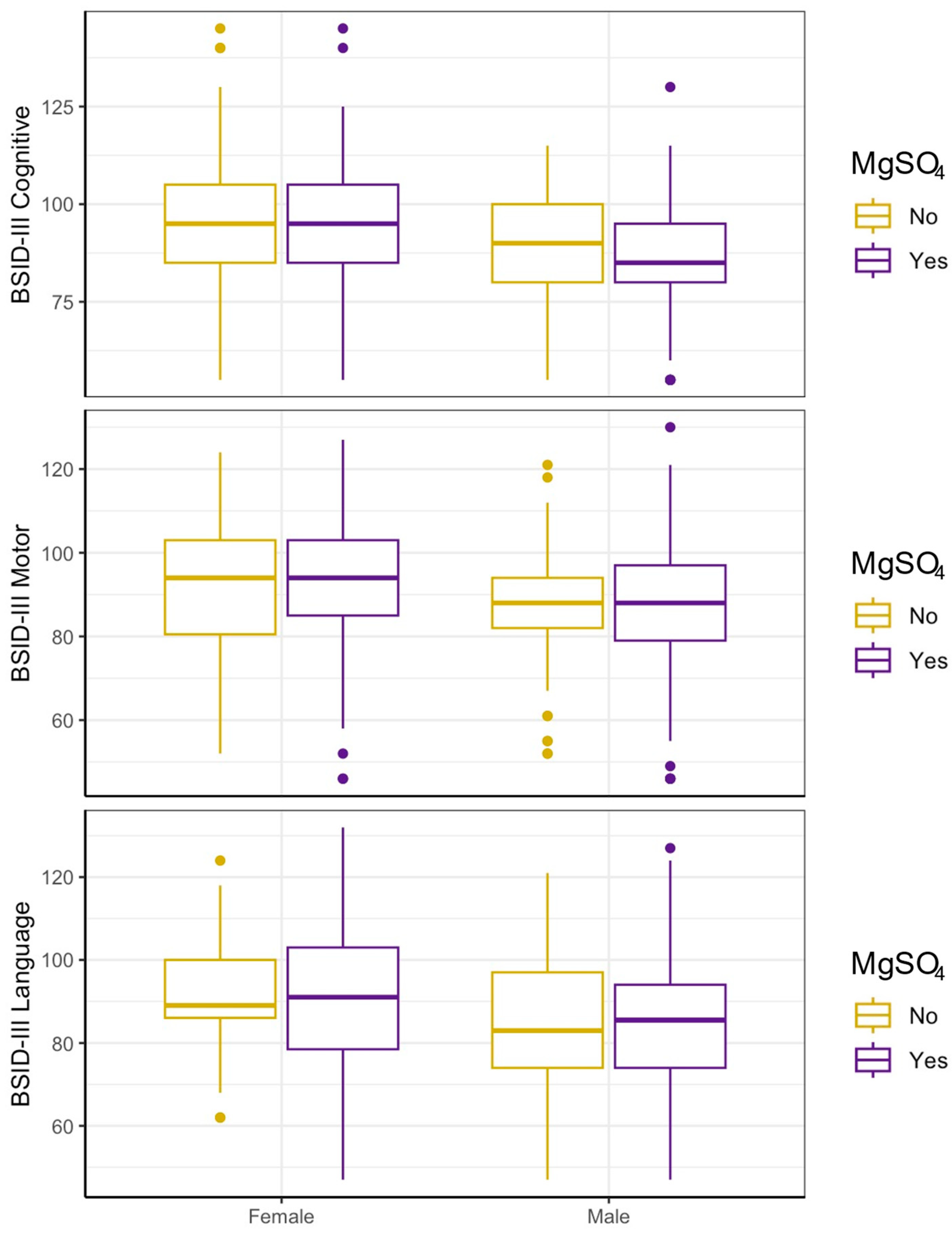
| Unadjusted (A) | MgSO4 Exposure | Matched (B) | MgSO4 Exposure | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Yes | All | p-Value | No | Yes | All | p-Value | ||
| N | 104 | 562 | 666 | N | 104 | 208 | 312 | ||
| Maternal age | 28.4 (6.6) | 29.3 (6) | 29.2 (6.1) | 0.22 | Maternal age | 28.4 (6.6) | 28.1 (6) | 28.2 (6.2) | 0.74 |
| Gestational age | 25.8 (1.1) | 26 (1.2) | 26 (1.2) | 0.08 | Gestational age | 25.8 (1.1) | 25.8 (1.2) | 25.8 (1.2) | 0.78 |
| Sex | Sex | ||||||||
| Female | 55 (53) | 273 (49) | 328 (49) | 0.46 | Female | 55 (53) | 96 (46) | 151 (48) | 0.30 |
| Male | 49 (47) | 289 (51) | 338 (51) | Male | 49 (47) | 112 (54) | 161 (52) | ||
| Maternal ethnicity | Maternal ethnicity | ||||||||
| Hispanic | 25 (24) | 125 (22) | 150 (23) | 0.52 | Hispanic | 25 (24) | 52 (25) | 77 (25) | 0.84 |
| Not Hispanic | 77 (74) | 433 (77) | 510 (77) | Not Hispanic | 77 (74) | 154 (74) | 231 (74) | ||
| Unknown | 2 (2) | 4 (1) | 6 (1) | Unknown | 2 (2) | 2 (1) | 4 (1) | ||
| Maternal race | Maternal race | ||||||||
| White | 64 (62) | 389 (69) | 453 (68) | 0.54 | White | 64 (62) | 129 (62) | 193 (62) | 0.98 |
| Black | 27 (26) | 116 (21) | 143 (21) | Black | 27 (26) | 51 (25) | 78 (25) | ||
| Other | 9 (9) | 36 (6) | 45 (7) | Other | 9 (9) | 18 (9) | 27 (9) | ||
| Unknown | 4 (4) | 21 (4) | 25 (4) | Unknown | 4 (4) | 10 (5) | 14 (4) | ||
| Maternal education | Maternal education | ||||||||
| HS or less | 31 (30) | 181 (32) | 212 (32) | 0.25 | High school or less | 31 (30) | 63 (30) | 94 (30) | 0.65 |
| Some college | 29 (28) | 177 (31) | 206 (31) | Some college | 29 (28) | 71 (34) | 100 (32) | ||
| BS or greater | 27 (26) | 153 (27) | 180 (27) | BS or greater | 27 (26) | 41 (20) | 68 (22) | ||
| Unknown | 17 (16) | 51 (9) | 68 (10) | Unknown | 17 (16) | 33 (16) | 50 (16) | ||
| Maternal obesity | 5 (5) | 67 (12) | 72 (11) | 0.04 | Maternal obesity | 5 (5) | 20 (10) | 25 (8) | 0.16 |
| Gestational diabetes | 4 (4) | 34 (6) | 38 (6) | 0.39 | Gestational diabetes | 4 (4) | 14 (7) | 18 (6) | 0.34 |
| Maternal hypertension | 5 (5) | 131 (23) | 136 (20) | <0.001 | Maternal hypertension | 5 (5) | 12 (6) | 17 (5) | 0.73 |
| Prenatal care | 94 (90) | 548 (98) | 642 (96) | 0.002 | Prenatal care | 94 (90) | 197 (95) | 291 (93) | 0.18 |
| Multiple gestation | 30 (29) | 140 (25) | 170 (26) | 0.50 | Multiple gestation | 30 (29) | 55 (26) | 85 (27) | 0.72 |
| Preterm labor | 68 (65) | 357 (64) | 425 (64) | 0.74 | Preterm labor | 68 (65) | 136 (65) | 204 (65) | 1.00 |
| PROM | 26 (25) | 155 (28) | 181 (27) | 0.63 | PROM | 26 (25) | 65 (31) | 91 (29) | 0.31 |
| Prenatal antibiotics | 21 (20) | 225 (40) | 246 (37) | 0.001 | Prenatal antibiotics | 21 (20) | 61 (29) | 82 (26) | 0.12 |
| Prenatal steroids | 44 (42) | 434 (77) | 478 (72) | <0.001 | Prenatal steroids | 44 (42) | 111 (53) | 155 (50) | 0.10 |
| C-section | 66 (63) | 384 (68) | 450 (68) | 0.37 | C-section | 66 (63) | 134 (64) | 200 (64) | 0.88 |
| Small for Gestational Age | 4 (4) | 83 (15) | 87 (13) | 0.01 | Small for Gestational Age | 4 (4) | 9 (4) | 13 (4) | 0.83 |
| Unadjusted (n = 666) | p-Value | Weighted (n = 666) | p-Value | Matched (n = 312) | p-Value | |
|---|---|---|---|---|---|---|
| BSID—Cognitive | ||||||
| Female | −1.67 (−6.9, 3.57) | 0.53 | 0.57 (−8.92, 10.06) | 0.91 | −0.91 (−7.22, 5.41) | 0.78 |
| Male | −0.83 (−6.52, 4.86) | 0.78 | −1.72 (−7.63, 4.19) | 0.57 | −2.53 (−8.59, 3.54) | 0.41 |
| Interaction | 0.84 (−6.84, 8.53) | 0.83 | −2.29 (−13.44, 8.87) | 0.69 | −1.62 (−10.1, 6.86) | 0.71 |
| BSID—Motor | ||||||
| Female | −0.18 (−4.79, 4.42) | 0.94 | 3.51 (−3.62, 10.64) | 0.33 | 1.73 (−3.66, 7.13) | 0.53 |
| Male | 0.95 (−4.79, 6.69) | 0.75 | −0.05 (−6.16, 6.06) | 0.99 | −0.38 (−6.48, 5.72) | 0.90 |
| Interaction | 1.13 (−6.17, 8.43) | 0.76 | −3.56 (−12.91, 5.79) | 0.46 | −2.12 (−10.08, 5.84) | 0.60 |
| BSID—Language | ||||||
| Female | −0.15 (−4.37, 4.07) | 0.95 | 0.4 (−4.74, 5.53) | 0.88 | −0.36 (−5.71, 5) | 0.90 |
| Male | 1.38 (−4.86, 7.63) | 0.66 | 0.34 (−7.08, 7.77) | 0.93 | 0.15 (−6.53, 6.84) | 0.96 |
| Interaction | 1.53 (−5.94, 8.99) | 0.69 | −0.05 (−9.03, 8.92) | 0.99 | 0.51 (−7.72, 8.74) | 0.90 |
| Unmatched (A) | No MgSO4 | MgSO4 | GMFCS Level | p-Value | Matched (B) | No MgSO4 | MgSO4 | GMFCS Level | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Female | n = 54 | n = 273 | Female | n = 54 | n = 96 | ||||
| 47 (87) | 240 (88) | 0 | 0.65 | 47 (87) | 84 (88) | 0 | 0.54 | ||
| 3 (6) | 14 (5) | 0.5 | 3 (6) | 4 (4) | 0.5 | ||||
| 2 (4) | 14 (5) | 1 | 2 (4) | 7 (7) | 1 | ||||
| 2 (4) | 3 (1) | 2 | 2 (4) | 1 (1) | 2 | ||||
| 0 (0) | 0 (0) | 3 | 0 (0) | 0 (0) | 3 | ||||
| 0 (0) | 2 (0.7) | 4 | 0 (0) | 0 (0) | 4 | ||||
| 0 (0) | 0 (0) | 5 | 0 (0) | 0 (0) | 5 | ||||
| Male | n = 49 | n = 287 | Male | n = 49 | n = 111 | ||||
| 41 (84) | 239 (83) | 0 | 0.74 | 41 (84) | 89 (80) | 0 | 0.74 | ||
| 2 (4) | 18 (6) | 0.5 | 2 (4) | 9 (8) | 0.5 | ||||
| 5 (10) | 13 (5) | 1 | 5 (10) | 6 (5) | 1 | ||||
| 1 (2) | 8 (3) | 2 | 1 (2) | 4 (4) | 2 | ||||
| 0 (0) | 4 (1) | 3 | 0 (0) | 2 (2) | 3 | ||||
| 0 (0) | 3 (1) | 4 | 0 (0) | 1 (1) | 4 | ||||
| 0 (0) | 2 (1) | 5 | 0 (0) | 0 (0) | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
DiNucci, K.F.; Rue, T.C.; Brandon, O.C.; Corry, K.A.; Mayock, D.E.; Heagerty, P.J.; Juul, S.E.; Wood, T.R. Prenatal Magnesium Sulfate Exposure Is Not Associated with Different Neurodevelopmental Outcomes by Sex in Extremely Preterm Infants. Brain Sci. 2025, 15, 1273. https://doi.org/10.3390/brainsci15121273
DiNucci KF, Rue TC, Brandon OC, Corry KA, Mayock DE, Heagerty PJ, Juul SE, Wood TR. Prenatal Magnesium Sulfate Exposure Is Not Associated with Different Neurodevelopmental Outcomes by Sex in Extremely Preterm Infants. Brain Sciences. 2025; 15(12):1273. https://doi.org/10.3390/brainsci15121273
Chicago/Turabian StyleDiNucci, Kate F., Tessa C. Rue, Olivia C. Brandon, Kylie A. Corry, Dennis E. Mayock, Patrick J. Heagerty, Sandra E. Juul, and Thomas R. Wood. 2025. "Prenatal Magnesium Sulfate Exposure Is Not Associated with Different Neurodevelopmental Outcomes by Sex in Extremely Preterm Infants" Brain Sciences 15, no. 12: 1273. https://doi.org/10.3390/brainsci15121273
APA StyleDiNucci, K. F., Rue, T. C., Brandon, O. C., Corry, K. A., Mayock, D. E., Heagerty, P. J., Juul, S. E., & Wood, T. R. (2025). Prenatal Magnesium Sulfate Exposure Is Not Associated with Different Neurodevelopmental Outcomes by Sex in Extremely Preterm Infants. Brain Sciences, 15(12), 1273. https://doi.org/10.3390/brainsci15121273







