Dysregulation of Hedonic Processing in Chronic Pain: Insights from Preclinical Data
Abstract
1. Introduction
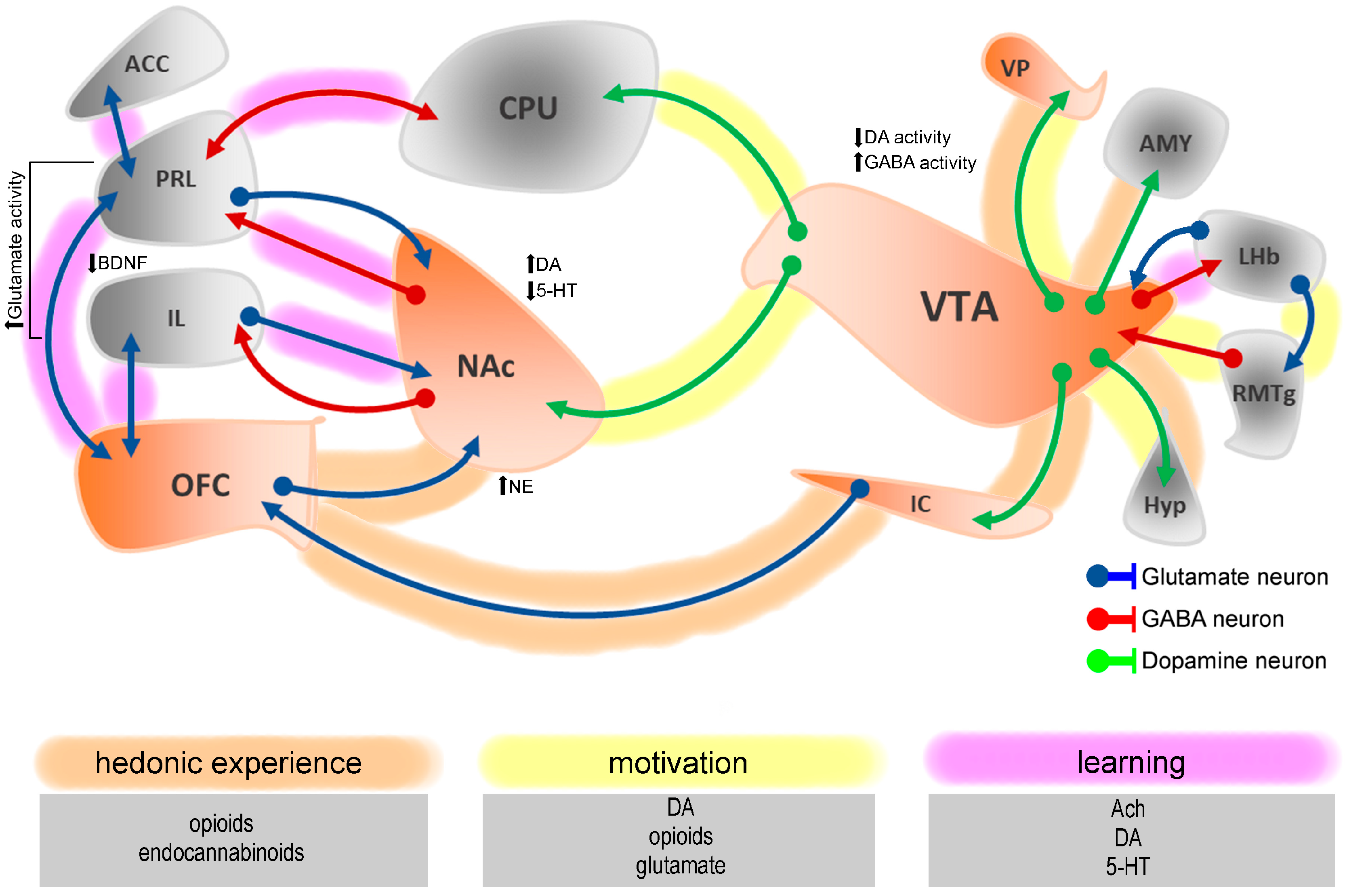
2. Neural Basis of Pain-Induced Neurochemical and Neuroanatomical Changes in Hedonic Processing
3. Temporal Dynamics of Hedonic System Dysregulation in Chronic Pain
4. Sex Differences in Pain-Induced Hedonic Dysregulation
5. Chronic Pain and Maladaptive Reward-Seeking
6. Behavioral Testing of Hedonic Processing in Chronic Pain
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-HT | Serotonin |
| 5-HTergic | Serotoninergic |
| ACC | Anterior cingulate cortex |
| BDNF | Brain-derived neurotrophic factor |
| CCI | Chronic constriction injury |
| CFA | Complete Freund’s adjuvant |
| CRF | Corticotropin-releasing factor |
| CP | Chronic pain |
| DA | Dopamine |
| DAergic | Dopaminergic |
| DRN | Dorsal raphe nucleus |
| GABA | Gamma-aminobutyric acid |
| HPA | Hypothalamic-pituitary-adrenal |
| HPLC | High-performance liquid chromatography |
| IL-1α | Interleukin 1 alfa |
| IL-1β | Interleukin 1 beta |
| IL-6 | Interleukin 6 |
| KOR | κ-opioid receptor |
| LHb | Lateral habenula |
| MEF2C | Myocyte enhancer factor 2C |
| MOR | µ-opioid receptor |
| NAc | Nucleus accumbens |
| NAcC | Nucleus accumbens core area |
| Medial NAc | Nucleus accumbens medial shell area |
| NAcSh | Nucleus accumbens shell area |
| NE | Norepinephrine |
| NGF | Nerve growth factor |
| NP | Neuropathic pain |
| OFC | Orbitofrontal cortex |
| PFC | Prefrontal cortex |
| PL | Prelimbic cortex |
| PNI | Peripheral nerve injury |
| PR | Progressive-ratio operant task |
| PSNL | Partial sciatic nerve ligation |
| RAS | Renin-angiotensin systrem |
| σ1r | Sigma-1 receptor |
| SNI | Spared nerve injury |
| SNL | Spinal nerve ligation |
| SPT | Sucrose preference test |
| TGF-β | Transforming growth factor beta |
| TLRs | Toll-like receptors |
| TNF-α | Tumor necrosis factor alfa |
| TNFR1 | Tumor necrosis factor receptor 1 |
| TR | Taste reactivity test |
| WM | Working memory |
| VGlut2 | Vesicular glutamate transporter 2 |
| VP | Ventral pallidum |
| VTA | Ventral tegmental area |
References
- Melzack, R. From the gate to the neuromatrix. Pain 1999, 82, 121–126. [Google Scholar] [CrossRef]
- Apkarian, A.V.; Sosa, Y.; Krauss, B.R.; Thomas, P.S.; Fredrickson, B.E.; Levy, R.E.; Harden, R.N.; Chialvo, D.R. Chronic pain patients are impaired on an emotional decision-making task. Pain 2004, 108, 129–136. [Google Scholar] [CrossRef]
- Apkarian, A.V.; Baliki, M.N.; Farmer, M.A. Predicting transition to chronic pain. Curr. Opin. Neurol. 2013, 26, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Wang, J.; Zhang, W.; Tian, X. Chronic Pain-Related Cognitive Deficits: Preclinical Insights into Molecular, Cellular, and Circuit Mechanisms. Mol. Neurobiol. 2024, 61, 8123–8143. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, J.A.; Baliki, M.N.; Huang, L.; Baria, A.T.; Torbey, S.; Hermann, K.M.; Schnitzer, T.J.; Apkarian, A.V. Shape shifting pain: Chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain 2013, 136, 2751–2768. [Google Scholar] [CrossRef]
- Kuner, R.; Flor, H. Structural plasticity and reorganisation in chronic pain. Nat. Rev. Neurosci. 2016, 18, 20–30. [Google Scholar] [CrossRef]
- De La Rosa, J.S.; Brady, B.R.; Ibrahim, M.M.; Herder, K.E.; Wallace, J.S.; Padilla, A.R.; Vanderah, T.W. Co-occurrence of chronic pain and anxiety/depression symptoms in U.S. adults: Prevalence, functional impacts, and opportunities. Pain 2024, 165, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.R.; Alemi, M.; Cerqueira-Nunes, M.; Monteiro, C.; Galhardo, V.; Cardoso-Cruz, H. Dynamics of Lateral Habenula-Ventral Tegmental Area Microcircuit on Pain-Related Cognitive Dysfunctions. Neurol. Int. 2023, 15, 1303–1319. [Google Scholar] [CrossRef]
- Bair, M.J.; Robinson, R.L.; Katon, W.; Kroenke, K. Depression and pain comorbidity: A literature review. Arch. Intern. Med. 2003, 163, 2433–2445. [Google Scholar] [CrossRef]
- Smith, M.T.; Edwards, R.R.; Robinson, R.C.; Dworkin, R.H. Suicidal ideation, plans, and attempts in chronic pain patients: Factors associated with increased risk. Pain 2004, 111, 201–208. [Google Scholar] [CrossRef]
- Ambrosi, E.; Arciniegas, D.B.; Curtis, K.N.; Patriquin, M.A.; Spalletta, G.; Sani, G.; Frueh, B.C.; Fowler, J.C.; Madan, A.; Salas, R. Resting-State Functional Connectivity of the Habenula in Mood Disorder Patients With and Without Suicide-Related Behaviors. J. Neuropsychiatry Clin. Neurosci. 2019, 31, 49–56. [Google Scholar] [CrossRef]
- Belujon, P.; Grace, A.A. Dopamine System Dysregulation in Major Depressive Disorders. Int. J. Neuropsychopharmacol. 2017, 20, 1036–1046. [Google Scholar] [CrossRef]
- de la Puente, B.; Zamanillo, D.; Romero, L.; Carceller, A.; Vela, J.M.; Merlos, M.; Portillo-Salido, E. Comprehensive Preclinical Assessment of Sensory, Functional, Motivational-Affective, and Neurochemical Outcomes in Neuropathic Pain: The Case of the Sigma-1 Receptor. ACS Pharmacol. Transl. Sci. 2022, 5, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Navratilova, E.; Xie, J.Y.; Okun, A.; Qu, C.; Eyde, N.; Ci, S.; Ossipov, M.H.; King, T.; Fields, H.L.; Porreca, F. Pain relief produces negative reinforcement through activation of mesolimbic reward-valuation circuitry. Proc. Natl. Acad. Sci. USA 2012, 109, 20709–20713. [Google Scholar] [CrossRef]
- Baliki, M.N.; Petre, B.; Torbey, S.; Herrmann, K.M.; Huang, L.; Schnitzer, T.J.; Fields, H.L.; Apkarian, A.V. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat. Neurosci. 2012, 15, 1117–1119. [Google Scholar] [CrossRef] [PubMed]
- Baliki, M.N.; Mansour, A.; Baria, A.T.; Huang, L.; Berger, S.E.; Fields, H.L.; Apkarian, A.V. Parceling human accumbens into putative core and shell dissociates encoding of values for reward and pain. J. Neurosci. 2013, 33, 16383–16393. [Google Scholar] [CrossRef]
- Cardoso-Cruz, H.; Laranjeira, I.; Monteiro, C.; Galhardo, V. Altered prefrontal-striatal theta-band oscillatory dynamics underlie working memory deficits in neuropathic pain rats. Eur. J. Pain 2022, 26, 1546–1568. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Cruz, H.; Monteiro, C.; Galhardo, V. Reorganization of lateral habenula neuronal connectivity underlies pain-related impairment in spatial memory encoding. Pain 2024, 166, 1532–1548. [Google Scholar] [CrossRef]
- Cerqueira-Nunes, M.; Monteiro, C.; Galhardo, V.; Cardoso-Cruz, H. Inflammatory pain modifies reward preferences from larger delayed to smaller immediate rewards in male rats. Neurosci. Lett. 2025, 852, 138183. [Google Scholar] [CrossRef]
- Cerqueira-Nunes, M.; Monteiro, C.; Galhardo, V.; Cardoso-Cruz, H. Orbitostriatal encoding of reward delayed gratification and impulsivity in chronic pain. Brain Res. 2024, 1839, 149044. [Google Scholar] [CrossRef]
- Berridge, K.C.; Kringelbach, M.L. Affective neuroscience of pleasure: Reward in humans and animals. Psychopharmacology 2008, 199, 457–480. [Google Scholar] [CrossRef]
- Berridge, K.C.; Robinson, T.E. Liking, wanting, and the incentive-sensitization theory of addiction. Am. Psychol. 2016, 71, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira-Nunes, M.; Monteiro, C.; Galhardo, V.; Cardoso-Cruz, H. Deciphering the role of the nucleus accumbens shell area on spatial memory deficits induced by neuropathic pain in rats. Appl. Biosci. 2024, 3, 12. [Google Scholar] [CrossRef]
- Primo, M.J.; Fonseca-Rodrigues, D.; Almeida, A.; Teixeira, P.M.; Pinto-Ribeiro, F. Sucrose preference test: A systematic review of protocols for the assessment of anhedonia in rodents. Eur. Neuropsychopharmacol. 2023, 77, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Dourado, M.; Cardoso-Cruz, H.; Monteiro, C.; Galhardo, V. Neuromodulation of Dopamine D2 Receptors Alters Orbitofrontal Neuronal Activity and Reduces Risk-Prone Behavior in Male Rats with Inflammatory Pain. Mol. Neurobiol. 2025, 62, 8187–8203. [Google Scholar] [CrossRef]
- Cardoso-Cruz, H.; Dourado, M.; Monteiro, C.; Matos, M.R.; Galhardo, V. Activation of dopaminergic D2/D3 receptors modulates dorsoventral connectivity in the hippocampus and reverses the impairment of working memory after nerve injury. J. Neurosci. 2014, 34, 5861–5873. [Google Scholar] [CrossRef]
- Mogil, J.S. Sex differences in pain and pain inhibition: Multiple explanations of a controversial phenomenon. Nat. Rev. Neurosci. 2012, 13, 859–866. [Google Scholar] [CrossRef]
- Massaly, N.; Moron, J.A.; Al-Hasani, R. A Trigger for Opioid Misuse: Chronic Pain and Stress Dysregulate the Mesolimbic Pathway and Kappa Opioid System. Front. Neurosci. 2016, 10, 480. [Google Scholar] [CrossRef]
- Al-Hasani, R.; McCall, J.G.; Shin, G.; Gomez, A.M.; Schmitz, G.P.; Bernardi, J.M.; Pyo, C.O.; Park, S.I.; Marcinkiewcz, C.M.; Crowley, N.A.; et al. Distinct Subpopulations of Nucleus Accumbens Dynorphin Neurons Drive Aversion and Reward. Neuron 2015, 87, 1063–1077. [Google Scholar] [CrossRef]
- Wang, X.Y.; Jia, W.B.; Xu, X.; Chen, R.; Wang, L.B.; Su, X.J.; Xu, P.F.; Liu, X.Q.; Wen, J.; Song, X.Y.; et al. A glutamatergic DRN-VTA pathway modulates neuropathic pain and comorbid anhedonia-like behavior in mice. Nat. Commun. 2023, 14, 5124. [Google Scholar] [CrossRef]
- Cappon, D.; Ryterska, A.; Lagrata, S.; Miller, S.; Akram, H.; Hyam, J.; Zrinzo, L.; Matharu, M.; Jahanshahi, M. Ventral tegmental area deep brain stimulation for chronic cluster headache: Effects on cognition, mood, pain report behaviour and quality of life. Cephalalgia 2019, 39, 1099–1110. [Google Scholar] [CrossRef]
- Markovic, T.; Pedersen, C.E.; Massaly, N.; Vachez, Y.M.; Ruyle, B.; Murphy, C.A.; Abiraman, K.; Shin, J.H.; Garcia, J.J.; Yoon, H.J.; et al. Pain induces adaptations in ventral tegmental area dopamine neurons to drive anhedonia-like behavior. Nat. Neurosci. 2021, 24, 1601–1613. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.S.; Berridge, K.C. Opioid limbic circuit for reward: Interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J. Neurosci. 2007, 27, 1594–1605. [Google Scholar] [CrossRef]
- Robinson, T.E.; Berridge, K.C. Incentive-sensitization and addiction. Addiction 2001, 96, 103–114. [Google Scholar] [CrossRef]
- Kringelbach, M.L. The human orbitofrontal cortex: Linking reward to hedonic experience. Nat. Rev. Neurosci. 2005, 6, 691–702. [Google Scholar] [CrossRef]
- Izquierdo, A. Functional Heterogeneity within Rat Orbitofrontal Cortex in Reward Learning and Decision Making. J. Neurosci. 2017, 37, 10529–10540. [Google Scholar] [CrossRef]
- Jo, Y.S.; Lee, J.; Mizumori, S.J. Effects of prefrontal cortical inactivation on neural activity in the ventral tegmental area. J. Neurosci. 2013, 33, 8159–8171. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, N.; Miller, C.; Fields, H.L. Cortico-Accumbens Regulation of Approach-Avoidance Behavior Is Modified by Experience and Chronic Pain. Cell Rep. 2017, 19, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- Boekhoudt, L.; Voets, E.S.; Flores-Dourojeanni, J.P.; Luijendijk, M.C.; Vanderschuren, L.J.; Adan, R.A. Chemogenetic Activation of Midbrain Dopamine Neurons Affects Attention, but not Impulsivity, in the Five-Choice Serial Reaction Time Task in Rats. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2017, 42, 1315–1325. [Google Scholar] [CrossRef]
- Uban, K.A.; Rummel, J.; Floresco, S.B.; Galea, L.A. Estradiol modulates effort-based decision making in female rats. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2012, 37, 390–401. [Google Scholar] [CrossRef]
- Almey, A.; Milner, T.A.; Brake, W.G. Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females. Horm. Behav. 2015, 74, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Bendis, P.C.; Zimmerman, S.; Onisiforou, A.; Zanos, P.; Georgiou, P. The impact of estradiol on serotonin, glutamate, and dopamine systems. Front. Neurosci. 2024, 18, 1348551. [Google Scholar] [CrossRef] [PubMed]
- Almey, A.; Filardo, E.J.; Milner, T.A.; Brake, W.G. Estrogen receptors are found in glia and at extranuclear neuronal sites in the dorsal striatum of female rats: Evidence for cholinergic but not dopaminergic colocalization. Endocrinology 2012, 153, 5373–5383. [Google Scholar] [CrossRef]
- Bush, G.; Luu, P.; Posner, M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000, 4, 215–222. [Google Scholar] [CrossRef]
- Gu, X.; Hof, P.R.; Friston, K.J.; Fan, J. Anterior insular cortex and emotional awareness. J. Comp. Neurol. 2013, 521, 3371–3388. [Google Scholar] [CrossRef]
- Craig, A.D. How do you feel--now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009, 10, 59–70. [Google Scholar] [CrossRef]
- Janak, P.H.; Tye, K.M. From circuits to behaviour in the amygdala. Nature 2015, 517, 284–292. [Google Scholar] [CrossRef]
- Yang, S.; Boudier-Revéret, M.; Choo, Y.J.; Chang, M.C. Association between chronic pain and alterations in the mesolimbic dopaminergic system. Brain Sci. 2020, 10, 701. [Google Scholar] [CrossRef]
- Monteiro, C.; Cardoso-Cruz, H.; Matos, M.; Dourado, M.; Lima, D.; Galhardo, V. Increased fronto-hippocampal connectivity in the Prrxl1 knockout mouse model of congenital hypoalgesia. Pain 2016, 157, 2045–2056. [Google Scholar] [CrossRef] [PubMed]
- Marciano, G.; Siniscalchi, A.; Di Gennaro, G.; Rania, V.; Vocca, C.; Palleria, C.; Catarisano, L.; Muraca, L.; Citraro, R.; Evangelista, M.; et al. Assessing Gender Differences in Neuropathic Pain Management: Findings from a Real-Life Clinical Cross-Sectional Observational Study. J. Clin. Med. 2024, 13, 5682. [Google Scholar] [CrossRef]
- Berridge, K.C.; Kringelbach, M.L. Pleasure systems in the brain. Neuron 2015, 86, 646–664. [Google Scholar] [CrossRef]
- Dossat, A.M.; Diaz, R.; Gallo, L.; Panagos, A.; Kay, K.; Williams, D.L. Nucleus accumbens GLP-1 receptors influence meal size and palatability. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E1314–E1320. [Google Scholar] [CrossRef]
- Katsuura, Y.; Heckmann, J.A.; Taha, S.A. mu-Opioid receptor stimulation in the nucleus accumbens elevates fatty tastant intake by increasing palatability and suppressing satiety signals. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R244–R254. [Google Scholar] [CrossRef]
- Castro, D.C.; Berridge, K.C. Opioid hedonic hotspot in nucleus accumbens shell: Mu, delta, and kappa maps for enhancement of sweetness “liking” and “wanting”. J. Neurosci. 2014, 34, 4239–4250. [Google Scholar] [CrossRef]
- Mahler, S.V.; Smith, K.S.; Berridge, K.C. Endocannabinoid hedonic hotspot for sensory pleasure: Anandamide in nucleus accumbens shell enhances ‘liking’ of a sweet reward. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2007, 32, 2267–2278. [Google Scholar] [CrossRef]
- Reynolds, S.M.; Berridge, K.C. Positive and negative motivation in nucleus accumbens shell: Bivalent rostrocaudal gradients for GABA-elicited eating, taste “liking”/”disliking” reactions, place preference/avoidance, and fear. J. Neurosci. 2002, 22, 7308–7320. [Google Scholar] [CrossRef]
- Tejeda, H.A.; Wu, J.; Kornspun, A.R.; Pignatelli, M.; Kashtelyan, V.; Krashes, M.J.; Lowell, B.B.; Carlezon, W.A., Jr.; Bonci, A. Pathway- and Cell-Specific Kappa-Opioid Receptor Modulation of Excitation-Inhibition Balance Differentially Gates D1 and D2 Accumbens Neuron Activity. Neuron 2017, 93, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Mateo, Y.; Johnson, K.A.; Covey, D.P.; Atwood, B.K.; Wang, H.L.; Zhang, S.; Gildish, I.; Cachope, R.; Bellocchio, L.; Guzman, M.; et al. Endocannabinoid Actions on Cortical Terminals Orchestrate Local Modulation of Dopamine Release in the Nucleus Accumbens. Neuron 2017, 96, 1112–1126.e5. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, E.C.; Kremer, Y.; Lefort, S.; Harada, M.; Pascoli, V.; Rohner, C.; Luscher, C. Accumbal D1R Neurons Projecting to Lateral Hypothalamus Authorize Feeding. Neuron 2015, 88, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Boschen, S.L.; Wietzikoski, E.C.; Winn, P.; Da Cunha, C. The role of nucleus accumbens and dorsolateral striatal D2 receptors in active avoidance conditioning. Neurobiol. Learn. Mem. 2011, 96, 254–262. [Google Scholar] [CrossRef]
- Francis, T.C.; Lobo, M.K. Emerging Role for Nucleus Accumbens Medium Spiny Neuron Subtypes in Depression. Biol. Psychiatry 2017, 81, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Pekarskaya, E.A.; Galiza Soares, J.A.; Hough, F.G.; Langreck, C.B.; Tucciarone, J.M.; Javitch, J.A. Habenular mu-opioid receptor knockout and chronic systemic receptor blockade promote negative affect and heighten nociceptive sensitivity. bioRxiv 2025. [Google Scholar] [CrossRef]
- Liu, D.; Xu, F.X.; Yu, Z.; Huang, X.J.; Zhu, Y.B.; Wang, L.J.; Wu, C.W.; Zhang, X.; Cao, J.L.; Li, J. Distinct nucleus accumbens neural pathways underlie separate behavioral features of chronic pain and comorbid depression. J. Clin. Investig. 2025, 135, e191270. [Google Scholar] [CrossRef]
- Guimaraes, M.R.; Anjo, S.I.; Cunha, A.M.; Esteves, M.; Sousa, N.; Almeida, A.; Manadas, B.; Leite-Almeida, H. Chronic pain susceptibility is associated with anhedonic behavior and alterations in the accumbal ubiquitin-proteasome system. Pain 2021, 162, 1722–1731. [Google Scholar] [CrossRef] [PubMed]
- Serafini, R.A.; Farzinpour, Z.; Patel, V.; Kelley, A.M.; Estill, M.; Pryce, K.D.; Sakloth, F.; Teague, C.D.; Torres-Berrio, A.; Nestler, E.J.; et al. Nucleus accumbens myocyte enhancer factor 2C mediates the maintenance of peripheral nerve injury-induced physiological and behavioral maladaptations. Pain 2024, 165, 2733–2748. [Google Scholar] [CrossRef]
- Creed, M.C.; Ntamati, N.R.; Tan, K.R. VTA GABA neurons modulate specific learning behaviors through the control of dopamine and cholinergic systems. Front. Behav. Neurosci. 2014, 8, 8. [Google Scholar] [CrossRef]
- van Zessen, R.; Phillips, J.L.; Budygin, E.A.; Stuber, G.D. Activation of VTA GABA neurons disrupts reward consumption. Neuron 2012, 73, 1184–1194. [Google Scholar] [CrossRef]
- Zhu, Z.; Gong, R.; Rodriguez, V.; Quach, K.T.; Chen, X.; Sternson, S.M. Hedonic eating is controlled by dopamine neurons that oppose GLP-1R satiety. Science 2025, 387, eadt0773. [Google Scholar] [CrossRef]
- Lecourtier, L.; Defrancesco, A.; Moghaddam, B. Differential tonic influence of lateral habenula on prefrontal cortex and nucleus accumbens dopamine release. Eur. J. Neurosci. 2008, 27, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Omelchenko, N.; Bell, R.; Sesack, S.R. Lateral habenula projections to dopamine and GABA neurons in the rat ventral tegmental area. Eur. J. Neurosci. 2009, 30, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Jhou, T.C.; Geisler, S.; Marinelli, M.; Degarmo, B.A.; Zahm, D.S. The mesopontine rostromedial tegmental nucleus: A structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J. Comp. Neurol. 2009, 513, 566–596. [Google Scholar] [CrossRef]
- Alemi, M.; Pereira, A.R.; Cerqueira-Nunes, M.; Monteiro, C.; Galhardo, V.; Cardoso-Cruz, H. Role of Glutamatergic Projections from Lateral Habenula to Ventral Tegmental Area in Inflammatory Pain-Related Spatial Working Memory Deficits. Biomedicines 2023, 11, 820. [Google Scholar] [CrossRef] [PubMed]
- Li, A.L.; Sibi, J.E.; Yang, X.; Chiao, J.C.; Peng, Y.B. Stimulation of the ventral tegmental area increased nociceptive thresholds and decreased spinal dorsal horn neuronal activity in rat. Exp. Brain Res. 2016, 234, 1505–1514. [Google Scholar] [CrossRef]
- Shelton, L.; Pendse, G.; Maleki, N.; Moulton, E.A.; Lebel, A.; Becerra, L.; Borsook, D. Mapping pain activation and connectivity of the human habenula. J. Neurophysiol. 2012, 107, 2633–2648. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.L.; Palacorolla, H.; Brady, D.; Riegger, K.; Elmer, G.I.; Shepard, P.D. Habenula-Induced Inhibition of Midbrain Dopamine Neurons Is Diminished by Lesions of the Rostromedial Tegmental Nucleus. J. Neurosci. 2017, 37, 217–225. [Google Scholar] [CrossRef]
- Breton, J.M.; Charbit, A.R.; Snyder, B.J.; Fong, P.T.K.; Dias, E.V.; Himmels, P.; Lock, H.; Margolis, E.B. Relative contributions and mapping of ventral tegmental area dopamine and GABA neurons by projection target in the rat. J. Comp. Neurol. 2019, 527, 916–941. [Google Scholar] [CrossRef] [PubMed]
- Hauser, T.U.; Eldar, E.; Dolan, R.J. Separate mesocortical and mesolimbic pathways encode effort and reward learning signals. Proc. Natl. Acad. Sci. USA 2017, 114, E7395–E7404. [Google Scholar] [CrossRef]
- Shen, X.; Ruan, X.; Zhao, H. Stimulation of midbrain dopaminergic structures modifies firing rates of rat lateral habenula neurons. PLoS ONE 2012, 7, e34323. [Google Scholar] [CrossRef]
- Shumake, J.; Ilango, A.; Scheich, H.; Wetzel, W.; Ohl, F.W. Differential neuromodulation of acquisition and retrieval of avoidance learning by the lateral habenula and ventral tegmental area. J. Neurosci. 2010, 30, 5876–5883. [Google Scholar] [CrossRef]
- Yue, L.; Ma, L.Y.; Cui, S.; Liu, F.Y.; Yi, M.; Wan, Y. Brain-derived neurotrophic factor in the infralimbic cortex alleviates inflammatory pain. Neurosci. Lett. 2017, 655, 7–13. [Google Scholar] [CrossRef]
- Woodburn, S.C.; Asrat, H.S.; Flurer, J.K.; Schwierling, H.C.; Bollinger, J.L.; Vollmer, L.L.; Wohleb, E.S. Depletion of microglial BDNF increases susceptibility to the behavioral and synaptic effects of chronic unpredictable stress. Brain Behav. Immun. 2023, 109, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, N.; Temkin, P.; Jurado, S.; Lim, B.K.; Heifets, B.D.; Polepalli, J.S.; Malenka, R.C. Decreased motivation during chronic pain requires long-term depression in the nucleus accumbens. Science 2014, 345, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Dellarole, A.; Morton, P.; Brambilla, R.; Walters, W.; Summers, S.; Bernardes, D.; Grilli, M.; Bethea, J.R. Neuropathic pain-induced depressive-like behavior and hippocampal neurogenesis and plasticity are dependent on TNFR1 signaling. Brain Behav. Immun. 2014, 41, 65–81. [Google Scholar] [CrossRef]
- Okun, A.; McKinzie, D.L.; Witkin, J.M.; Remeniuk, B.; Husein, O.; Gleason, S.D.; Oyarzo, J.; Navratilova, E.; McElroy, B.; Cowen, S.; et al. Hedonic and motivational responses to food reward are unchanged in rats with neuropathic pain. Pain 2016, 157, 2731–2738. [Google Scholar] [CrossRef]
- Taylor, A.M.; Roberts, K.W.; Pradhan, A.A.; Akbari, H.A.; Walwyn, W.; Lutfy, K.; Carroll, F.I.; Cahill, C.M.; Evans, C.J. Anti-nociception mediated by a kappa opioid receptor agonist is blocked by a delta receptor agonist. Br. J. Pharmacol. 2015, 172, 691–703. [Google Scholar] [CrossRef]
- Lorente, J.D.; Cuitavi, J.; Rullo, L.; Candeletti, S.; Romualdi, P.; Hipolito, L. Sex-dependent effect of inflammatory pain on negative affective states is prevented by kappa opioid receptors blockade in the nucleus accumbens shell. Neuropharmacology 2024, 242, 109764. [Google Scholar] [CrossRef] [PubMed]
- Abdul, M.; Yan, H.Q.; Zhao, W.N.; Lyu, X.B.; Xu, Z.; Yu, X.L.; Gao, Y.H.; Cao, J.L. VTA-NAc glutaminergic projection involves in the regulation of pain and pain-related anxiety. Front. Mol. Neurosci. 2022, 15, 1083671. [Google Scholar] [CrossRef]
- Taylor, A.M.; Castonguay, A.; Taylor, A.J.; Murphy, N.P.; Ghogha, A.; Cook, C.; Xue, L.; Olmstead, M.C.; De Koninck, Y.; Evans, C.J.; et al. Microglia disrupt mesolimbic reward circuitry in chronic pain. J. Neurosci. 2015, 35, 8442–8450. [Google Scholar] [CrossRef]
- Walker, A.K.; Kavelaars, A.; Heijnen, C.J.; Dantzer, R. Neuroinflammation and comorbidity of pain and depression. Pharmacol. Rev. 2014, 66, 80–101. [Google Scholar] [CrossRef]
- Miller, A.H.; Haroon, E.; Felger, J.C. Therapeutic Implications of Brain-Immune Interactions: Treatment in Translation. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2017, 42, 334–359. [Google Scholar] [CrossRef]
- Felger, J.C.; Mun, J.; Kimmel, H.L.; Nye, J.A.; Drake, D.F.; Hernandez, C.R.; Freeman, A.A.; Rye, D.B.; Goodman, M.M.; Howell, L.L.; et al. Chronic interferon-alpha decreases dopamine 2 receptor binding and striatal dopamine release in association with anhedonia-like behavior in nonhuman primates. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2013, 38, 2179–2187. [Google Scholar] [CrossRef]
- Ji, R.R.; Donnelly, C.R.; Nedergaard, M. Astrocytes in chronic pain and itch. Nat. Rev. Neurosci. 2019, 20, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Balogh, M.; Aguilar, C.; Nguyen, N.T.; Shepherd, A.J. Angiotensin receptors and neuropathic pain. Pain Rep. 2021, 6, e869. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Meijide, A.; Rodriguez-Perez, A.I.; Diaz-Ruiz, C.; Guerra, M.J.; Labandeira-Garcia, J.L. Dopamine modulates astroglial and microglial activity via glial renin-angiotensin system in cultures. Brain Behav. Immun. 2017, 62, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Castro-Hernandez, J.; Afonso-Oramas, D.; Cruz-Muros, I.; Salas-Hernandez, J.; Barroso-Chinea, P.; Moratalla, R.; Millan, M.J.; Gonzalez-Hernandez, T. Prolonged treatment with pramipexole promotes physical interaction of striatal dopamine D3 autoreceptors with dopamine transporters to reduce dopamine uptake. Neurobiol. Dis. 2015, 74, 325–335. [Google Scholar] [CrossRef]
- Fornari Laurindo, L.; Aparecido Dias, J.; Cressoni Araujo, A.; Torres Pomini, K.; Machado Galhardi, C.; Rucco Penteado Detregiachi, C.; Santos de Argollo Haber, L.; Donizeti Roque, D.; Dib Bechara, M.; Vialogo Marques de Castro, M.; et al. Immunological dimensions of neuroinflammation and microglial activation: Exploring innovative immunomodulatory approaches to mitigate neuroinflammatory progression. Front. Immunol. 2024, 14, 1305933. [Google Scholar] [CrossRef]
- Yirmiya, R.; Rimmerman, N.; Reshef, R. Depression as a microglial disease. Trends Neurosci. 2015, 38, 637–658. [Google Scholar] [CrossRef]
- Xu, H.; Gelyana, E.; Rajsombath, M.; Yang, T.; Li, S.; Selkoe, D. Environmental Enrichment Potently Prevents Microglia-Mediated Neuroinflammation by Human Amyloid beta-Protein Oligomers. J. Neurosci. 2016, 36, 9041–9056. [Google Scholar] [CrossRef]
- Gong, X.; Chen, Y.; Chang, J.; Huang, Y.; Cai, M.; Zhang, M. Environmental enrichment reduces adolescent anxiety- and depression-like behaviors of rats subjected to infant nerve injury. J. Neuroinflam. 2018, 15, 262. [Google Scholar] [CrossRef]
- Crine, V.; Papenberg, G.; Johansson, J.; Boraxbekk, C.J.; Wahlin, A.; Lindenberger, U.; Lovden, M.; Riklund, K.; Backman, L.; Nyberg, L.; et al. Associations between inflammation and striatal dopamine D2-receptor availability in aging. J. Neuroinflam. 2025, 22, 24. [Google Scholar] [CrossRef]
- Li, D.; Yang, K.; Li, J.; Xu, X.; Gong, L.; Yue, S.; Wei, H.; Yue, Z.; Wu, Y.; Yin, S. Single-cell sequencing reveals glial cell involvement in development of neuropathic pain via myelin sheath lesion formation in the spinal cord. J. Neuroinflam. 2024, 21, 213. [Google Scholar] [CrossRef]
- Hu, S.; Tang, Z.; Sun, S.; Liu, L.; Wang, Y.; Xu, L.; Yuan, J.; Chen, Y.; Sun, M.; Zhao, L. Single-Nucleus Transcriptomics Reveals Glial Metabolic-Immune Rewiring and Intercellular Signaling Disruption in Chronic Migraine. Biomolecules 2025, 15, 942. [Google Scholar] [CrossRef]
- Massaly, N.; Moron, J.A. Pain And Opioid Systems, Implications In The Opioid Epidemic. Curr. Opin. Behav. Sci. 2019, 26, 69–74. [Google Scholar] [CrossRef]
- Sorge, R.E.; Mapplebeck, J.C.; Rosen, S.; Beggs, S.; Taves, S.; Alexander, J.K.; Martin, L.J.; Austin, J.S.; Sotocinal, S.G.; Chen, D.; et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 2015, 18, 1081–1083. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiao, X.; Zhang, X.M.; Zhao, Z.Q.; Zhang, Y.Q. Estrogen facilitates spinal cord synaptic transmission via membrane-bound estrogen receptors: Implications for pain hypersensitivity. J. Biol. Chem. 2012, 287, 33268–33281. [Google Scholar] [CrossRef]
- Amandusson, A.; Blomqvist, A. Estrogenic influences in pain processing. Front. Neuroendocr. 2013, 34, 329–349. [Google Scholar] [CrossRef] [PubMed]
- Craft, R.M. Modulation of pain by estrogens. Pain 2007, 132 (Suppl. S1), S3–S12. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, W.; Sadana, N.; Chen, X. Estrogen receptors in pain modulation: Cellular signaling. Biol. Sex. Differ. 2021, 12, 22. [Google Scholar] [CrossRef]
- Nicotra, L.; Loram, L.C.; Watkins, L.R.; Hutchinson, M.R. Toll-like receptors in chronic pain. Exp. Neurol. 2012, 234, 316–329. [Google Scholar] [CrossRef]
- Taves, S.; Berta, T.; Liu, D.L.; Gan, S.; Chen, G.; Kim, Y.H.; Van de Ven, T.; Laufer, S.; Ji, R.R. Spinal inhibition of p38 MAP kinase reduces inflammatory and neuropathic pain in male but not female mice: Sex-dependent microglial signaling in the spinal cord. Brain Behav. Immun. 2016, 55, 70–81. [Google Scholar] [CrossRef]
- Lemos, J.C.; Wanat, M.J.; Smith, J.S.; Reyes, B.A.; Hollon, N.G.; Van Bockstaele, E.J.; Chavkin, C.; Phillips, P.E. Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature 2012, 490, 402–406. [Google Scholar] [CrossRef]
- Gee, T.A.; Weintraub, N.C.; Lu, D.; Phelps, C.E.; Navratilova, E.; Heien, M.L.; Porreca, F. A pain-induced tonic hypodopaminergic state augments phasic dopamine release in the nucleus accumbens. Pain 2020, 161, 2376–2384. [Google Scholar] [CrossRef]
- Hagelberg, N.; Martikainen, I.K.; Mansikka, H.; Hinkka, S.; Någren, K.; Hietala, J.; Scheinin, H.; Pertovaara, A. Dopamine D2 receptor binding in the human brain is associated with the response to painful stimulation and pain modulatory capacity. Pain 2002, 99, 273–279. [Google Scholar] [CrossRef]
- Towers, E.B.; Williams, I.L.; Qillawala, E.I.; Lynch, W.J. Role of nucleus accumbens dopamine 2 receptors in motivating cocaine use in male and female rats prior to and following the development of an addiction-like phenotype. Front. Pharmacol. 2023, 14, 1237990. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yang, S.; Yang, C.; Jin, G.; Zhen, X. Estrogen regulates responses of dopamine neurons in the ventral tegmental area to cocaine. Psychopharmacology 2008, 199, 625–635. [Google Scholar] [CrossRef]
- Vandegrift, B.J.; You, C.; Satta, R.; Brodie, M.S.; Lasek, A.W. Estradiol increases the sensitivity of ventral tegmental area dopamine neurons to dopamine and ethanol. PLoS ONE 2017, 12, e0187698. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Z.; Shi, X.; Han, H.; Li, A.N.; Zhang, B.; Yuan, W.; Sun, Y.H.; Li, X.M.; Lian, H.; et al. Investigating the effect of Arvcf reveals an essential role on regulating the mesolimbic dopamine signaling-mediated nicotine reward. Commun. Biol. 2025, 8, 429. [Google Scholar] [CrossRef]
- Orsini, C.A.; Willis, M.L.; Gilbert, R.J.; Bizon, J.L.; Setlow, B. Sex differences in a rat model of risky decision making. Behav. Neurosci. 2016, 130, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.N.; Karacabeyli, E.S.; Gorzalka, B.B. Estrogen recruits the endocannabinoid system to modulate emotionality. Psychoneuroendocrinology 2007, 32, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Beiko, J.; Lander, R.; Hampson, E.; Boon, F.; Cain, D.P. Contribution of sex differences in the acute stress response to sex differences in water maze performance in the rat. Behav. Brain Res. 2004, 151, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Trainor, B.C. Stress responses and the mesolimbic dopamine system: Social contexts and sex differences. Horm. Behav. 2011, 60, 457–469. [Google Scholar] [CrossRef]
- Sheng, J.A.; Bales, N.J.; Myers, S.A.; Bautista, A.I.; Roueinfar, M.; Hale, T.M.; Handa, R.J. The Hypothalamic-Pituitary-Adrenal Axis: Development, Programming Actions of Hormones, and Maternal-Fetal Interactions. Front. Behav. Neurosci. 2021, 14, 601939. [Google Scholar] [CrossRef]
- Mogil, J.S. Qualitative sex differences in pain processing: Emerging evidence of a biased literature. Nat. Rev. Neurosci. 2020, 21, 353–365. [Google Scholar] [CrossRef]
- Higginbotham, J.A.; Markovic, T.; Massaly, N.; Moron, J.A. Endogenous opioid systems alterations in pain and opioid use disorder. Front. Syst. Neurosci. 2022, 16, 1014768. [Google Scholar] [CrossRef]
- Wawrzczak-Bargiela, A.; Ziolkowska, B.; Piotrowska, A.; Starnowska-Sokol, J.; Rojewska, E.; Mika, J.; Przewlocka, B.; Przewlocki, R. Neuropathic Pain Dysregulates Gene Expression of the Forebrain Opioid and Dopamine Systems. Neurotox. Res. 2020, 37, 800–814. [Google Scholar] [CrossRef]
- Massaly, N.; Copits, B.A.; Wilson-Poe, A.R.; Hipolito, L.; Markovic, T.; Yoon, H.J.; Liu, S.; Walicki, M.C.; Bhatti, D.L.; Sirohi, S.; et al. Pain-Induced Negative Affect Is Mediated via Recruitment of The Nucleus Accumbens Kappa Opioid System. Neuron 2019, 102, 564–573.e6. [Google Scholar] [CrossRef] [PubMed]
- Hipolito, L.; Wilson-Poe, A.; Campos-Jurado, Y.; Zhong, E.; Gonzalez-Romero, J.; Virag, L.; Whittington, R.; Comer, S.D.; Carlton, S.M.; Walker, B.M.; et al. Inflammatory Pain Promotes Increased Opioid Self-Administration: Role of Dysregulated Ventral Tegmental Area mu Opioid Receptors. J. Neurosci. 2015, 35, 12217–12231. [Google Scholar] [CrossRef] [PubMed]
- Garland, E.L.; Bryan, C.J.; Kreighbaum, L.; Nakamura, Y.; Howard, M.O.; Froeliger, B. Prescription opioid misusing chronic pain patients exhibit dysregulated context-dependent associations: Investigating associative learning in addiction with the cue-primed reactivity task. Drug Alcohol. Depend. 2018, 187, 13–21. [Google Scholar] [CrossRef]
- Cuitavi, J.; Torres-Perez, J.V.; Lorente, J.D.; Campos-Jurado, Y.; Andres-Herrera, P.; Polache, A.; Agustin-Pavon, C.; Hipolito, L. Crosstalk between Mu-Opioid receptors and neuroinflammation: Consequences for drug addiction and pain. Neurosci. Biobehav. Rev. 2023, 145, 105011. [Google Scholar] [CrossRef]
- Cuitavi, J.; Andres-Herrera, P.; Meseguer, D.; Campos-Jurado, Y.; Lorente, J.D.; Caruana, H.; Hipolito, L. Focal mu-opioid receptor activation promotes neuroinflammation and microglial activation in the mesocorticolimbic system: Alterations induced by inflammatory pain. Glia 2023, 71, 1906–1920. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.J.; Pitcher, M.H.; Stone, L.S.; Tarum, F.; Niu, G.; Chen, X.; Kiesewetter, D.O.; Schweinhardt, P.; Bushnell, M.C. Chronic neuropathic pain reduces opioid receptor availability with associated anhedonia in rat. Pain 2018, 159, 1856–1866. [Google Scholar] [CrossRef] [PubMed]
- Loggia, M.L.; Berna, C.; Kim, J.; Cahalan, C.M.; Gollub, R.L.; Wasan, A.D.; Harris, R.E.; Edwards, R.R.; Napadow, V. Disrupted brain circuitry for pain-related reward/punishment in fibromyalgia. Arthritis Rheumatol. 2014, 66, 203–212. [Google Scholar] [CrossRef]
- Martucci, K.T.; Borg, N.; MacNiven, K.H.; Knutson, B.; Mackey, S.C. Altered prefrontal correlates of monetary anticipation and outcome in chronic pain. Pain 2018, 159, 1494–1507. [Google Scholar] [CrossRef]
- Baliki, M.N.; Geha, P.Y.; Fields, H.L.; Apkarian, A.V. Predicting value of pain and analgesia: Nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron 2010, 66, 149–160. [Google Scholar] [CrossRef]
- Ren, W.; Centeno, M.V.; Berger, S.; Wu, Y.; Na, X.; Liu, X.; Kondapalli, J.; Apkarian, A.V.; Martina, M.; Surmeier, D.J. The indirect pathway of the nucleus accumbens shell amplifies neuropathic pain. Nat. Neurosci. 2016, 19, 220–222. [Google Scholar] [CrossRef]
- Neugebauer, V.; Li, W.; Bird, G.C.; Han, J.S. The amygdala and persistent pain. Neuroscientist 2004, 10, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Simons, L.E.; Elman, I.; Borsook, D. Psychological processing in chronic pain: A neural systems approach. Neurosci. Biobehav. Rev. 2014, 39, 61–78. [Google Scholar] [CrossRef]
- Smith, K.S.; Tindell, A.J.; Aldridge, J.W.; Berridge, K.C. Ventral pallidum roles in reward and motivation. Behav. Brain Res. 2009, 196, 155–167. [Google Scholar] [CrossRef]
- Zhou, J.; Gardner, M.P.H.; Stalnaker, T.A.; Ramus, S.J.; Wikenheiser, A.M.; Niv, Y.; Schoenbaum, G. Rat Orbitofrontal Ensemble Activity Contains Multiplexed but Dissociable Representations of Value and Task Structure in an Odor Sequence Task. Curr. Biol. 2019, 29, 897–907.e3. [Google Scholar] [CrossRef]
- Dimitrov, E.L.; Tsuda, M.C.; Cameron, H.A.; Usdin, T.B. Anxiety- and depression-like behavior and impaired neurogenesis evoked by peripheral neuropathy persist following resolution of prolonged tactile hypersensitivity. J. Neurosci. 2014, 34, 12304–12312. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E.; Schmeichel, A.M.; Sandroni, P.; Low, P.A.; Parisi, J.E. Differential involvement of hypothalamic vasopressin neurons in multiple system atrophy. Brain 2006, 129, 2688–2696. [Google Scholar] [CrossRef]
- Wang, J.; Goffer, Y.; Xu, D.; Tukey, D.S.; Shamir, D.B.; Eberle, S.E.; Zou, A.H.; Blanck, T.J.; Ziff, E.B. A single subanesthetic dose of ketamine relieves depression-like behaviors induced by neuropathic pain in rats. Anesthesiology 2011, 115, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.T.; Shao, Y.W.; Yen, C.T.; Shaw, F.Z. Acid-induced hyperalgesia and anxio-depressive comorbidity in rats. Physiol. Behav. 2014, 131, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Grill, H.J.; Norgren, R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978, 143, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Grill, H.J.; Norgren, R. The taste reactivity test. II. Mimetic Responses Gustatory Stimuli Chronic Thalamic Chronic Decerebrate rats. Brain Res. 1978, 143, 281–297. [Google Scholar] [CrossRef]
- Taylor, A.M.W.; Becker, S.; Schweinhardt, P.; Cahill, C. Mesolimbic dopamine signaling in acute and chronic pain: Implications for motivation, analgesia, and addiction. Pain 2016, 157, 1194–1198. [Google Scholar] [CrossRef]
- Pais-Vieira, M.; Lima, D.; Galhardo, V. Sustained attention deficits in rats with chronic inflammatory pain. Neurosci. Lett. 2009, 463, 98–102. [Google Scholar] [CrossRef]
- Cardoso-Cruz, H.; Lima, D.; Galhardo, V. Impaired spatial memory performance in a rat model of neuropathic pain is associated with reduced hippocampus-prefrontal cortex connectivity. J. Neurosci. 2013, 33, 2465–2480. [Google Scholar] [CrossRef]

| Ref. | Pain Model | Brain Regions | Methodology | Key Findings | Implications for Hedonic/ Motivational Processing |
|---|---|---|---|---|---|
| de la Puente et al., (2022) [13] | PSNL (mice) | NAc | Microdialysis, HPLC, SPT, operant conditioning, σ1r blockade | Decreased sucrose preference and operant responses to food rewards, indicating anhedonia. Reduced extracellular DA and increased 5-HT in NAc; blunted DAergic and 5-HTergic responses to palatable stimuli; σ1r blockade restored DA and 5-HT levels. | CP induces anhedonia via disrupted DAergic and serotonergic signaling in NAc; σ1r modulates reward signaling. |
| Markovic et al., (2021) [32] | CFA (mice and rat) | VTA, NAc | Optogenetic and chemogenetic stimulation, operant conditioning | Reduced operant performance for sucrose rewards in pain model; restored by VTA DA stimulation. Inhibition of VTA DA neurons post-CFA impaired reward-seeking; optogenetic VTA DA stimulation in SNI mice restored operant performance for sucrose rewards. | VTA DA hypofunction is a causal factor in pain-induced anhedonia; motivational deficits are reversible with targeted stimulation. |
| Wang et al., (2023) [30] | SNI (mice) | DRN, VTA, Medial NAc | Optogenetic activation, behavioral assays (mechanical allodynia, SPT) | Reduced mechanical allodynia and restored sucrose preference in neuropathic pain models. Activation of DRN-VTA excitatory projections alleviated mechanical allodynia and reversed anhedonia-like behaviors; increased DA release in medial NAc via D1/D2 receptors. | DRN-VTA circuit regulates pain-induced motivational deficits; enhanced DA release in medial NAc restores hedonic tone. |
| Schwartz et al., (2014) [82] | SNI, CFA (mice) | NAc | PR operant tasks, immunohisto-chemistry | Decreased motivation for rewards, not explained by physical limitations. Reduced PR breakpoints in operant tasks; linked to neuroinflammatory changes and reduced DA release in NAc. | CP impairs motivation via NAc neuroinflammation and DAergic hypofunction. |
| Cardoso-Cruz et al., (2022) [17] | SNI (rat) | PL, NAcC | Electrophysiology, WM tasks | Altered prefrontal-striatal theta-band oscillatory dynamics; WM deficits in neuropathic pain rats. | Disrupted prefrontal-striatal connectivity contributes to cognitive and motivational impairments in CP. |
| Cardoso-Cruz et al., (2024) [18] | SNI (rat) | LHb | Electrophysiology, spatial memory tasks | Reduced cognitive accuracy in spatial memory tasks. Reorganization of intra-LHb connectivity; impaired spatial memory encoding linked to pain-related cognitive deficits. | LHb hyperactivity suppresses DA signaling, contributing to cognitive and affective dysfunctions in CP. |
| Dellarole et al., (2014) [83] | CCI (mice) | Hippocampus | SPT, TNFR1 signaling analysis | Reduced sucrose preference; depressive-like behaviors linked to TNFR1 signaling and impaired hippocampal neurogenesis. | Hippocampal plasticity changes contribute to anhedonia and depressive phenotypes in CP. |
| Okun et al., (2016) [84] | SNL, CFA (rat) | Not applicable | TR test, operant conditioning | Intact hedonic responses but impaired motivational drive in operant tasks. No change in positive orofacial responses to sucrose in TR tests; reduced lever pressing in operant tasks. | Dissociation between preserved brainstem-mediated hedonic responses and forebrain-dependent motivational deficits in CP. |
| Taylor et al., (2015) [85] | PNI (mice) | NAc, VTA | Microglial analysis, DA release measurement | Reduced reward consumption and motivation in CP. Microglia-mediated disruption of mesolimbic reward circuitry; reduced DA release in NAc linked to motivational deficits. | Neuroinflammation in NAc and VTA impairs reward processing, contributing to motivational deficits. |
| Lorente et al., (2024) [86] | CFA (rat) | NAcSh | Pharmacological modulation, behavioral assays | Reduced sucrose preference and increased negative affect, more pronounced in females. Sex- and time-dependent negative affect; KOR blockade in NAcSh prevented pain-induced affective disturbances in females. | KOR-mediated negative affect in NAcSh drives pain-induced hedonic dysregulation, with sex-specific effects. |
| Abdul et al., (2022) [87] | CCI (mice) | VTA, NAcSh | Optogenetic modulation, Microdialysis, behavioral assays | Reduced palatable food intake and reward-seeking behavior.Impaired NAc DA release within one week after CCI; disrupted reward consumption dynamics. | Early DAergic impairments in NAc contribute to motivational deficits in CP. |
| Behavioral Test | Description | Assessment | Limitations |
|---|---|---|---|
| Sucrose preference test (SPT) | Animal chooses between two solutions, one of them containing sucrose (% of liquid consumed) | Reward sensitivity | Experimental protocols: inconsistency in test duration and preceding conditions of water and food deprivation |
| Taste reactivity test (TR) | Intraoral delivery of palatable or aversive stimuli (assessment of orofacial responses) | Responses to gustatory stimuli | Difficulty to evaluate and interpret affective reactions by experimenters |
| Operant conditioning fixed-ratio (FR) | Animal performs a certain number of responses to receive reward | Motivation | Potential for devaluation of activity due to certainty |
| Operant conditioning progressive-ratio (PR) | Animal performs an incremental number of responses to receive reward | Motivation | Difficulty in separating hedonic value (liking) from motivation (wanting) |
| Conditioned place preference (CPP) | Animal chooses between two chambers with distinct cues and substances/protocols | Motivation | Possibility of confounding effects with exploration; Difficulty in separating hedonic value (liking) from motivation (wanting) |
| Intracranial self-stimulation | Animal delivers brief electrical pulses into his own brain | Motivation | Requires invasive procedures; Difficulty in separating hedonic value (liking) from motivation (wanting) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerqueira-Nunes, M.; Monteiro, C.; Galhardo, V.; Cardoso-Cruz, H. Dysregulation of Hedonic Processing in Chronic Pain: Insights from Preclinical Data. Brain Sci. 2025, 15, 1265. https://doi.org/10.3390/brainsci15121265
Cerqueira-Nunes M, Monteiro C, Galhardo V, Cardoso-Cruz H. Dysregulation of Hedonic Processing in Chronic Pain: Insights from Preclinical Data. Brain Sciences. 2025; 15(12):1265. https://doi.org/10.3390/brainsci15121265
Chicago/Turabian StyleCerqueira-Nunes, Mariana, Clara Monteiro, Vasco Galhardo, and Helder Cardoso-Cruz. 2025. "Dysregulation of Hedonic Processing in Chronic Pain: Insights from Preclinical Data" Brain Sciences 15, no. 12: 1265. https://doi.org/10.3390/brainsci15121265
APA StyleCerqueira-Nunes, M., Monteiro, C., Galhardo, V., & Cardoso-Cruz, H. (2025). Dysregulation of Hedonic Processing in Chronic Pain: Insights from Preclinical Data. Brain Sciences, 15(12), 1265. https://doi.org/10.3390/brainsci15121265







