Comparative Study on Multimodal Imaging Applications in Dementia with Lewy Bodies: From Imaging Features to Clinical Practice Implications
Abstract
1. Introduction
2. Subjects and Methods
2.1. Methods
2.1.1. Clinical and Laboratory Data Collection
2.1.2. Preparation, Injection, Image Acquisition, and Precautions for PET/CT and SPECT Tracers
2.1.3. Analysis of 18F-FDG PET/CT Images
- (1)
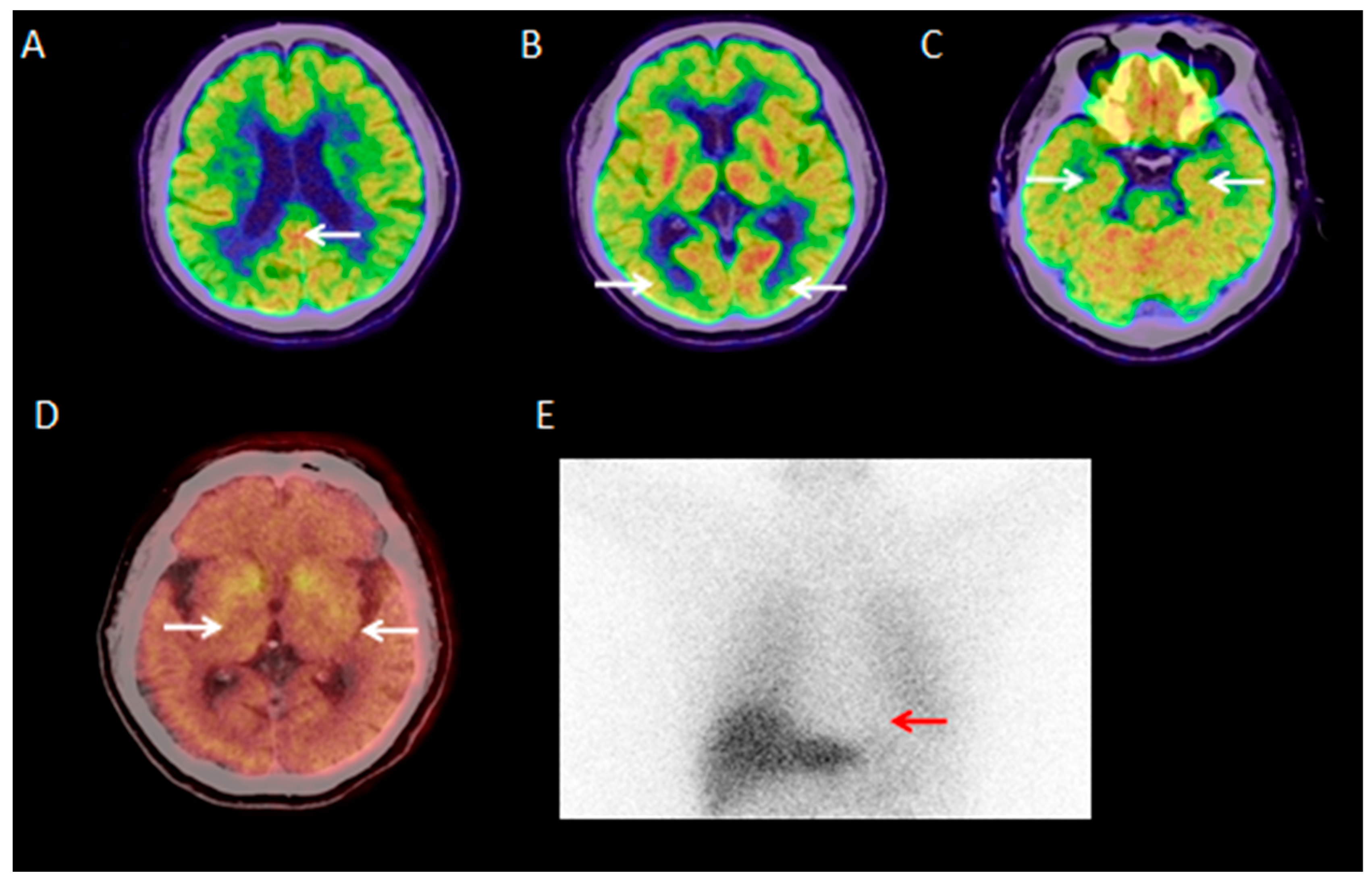
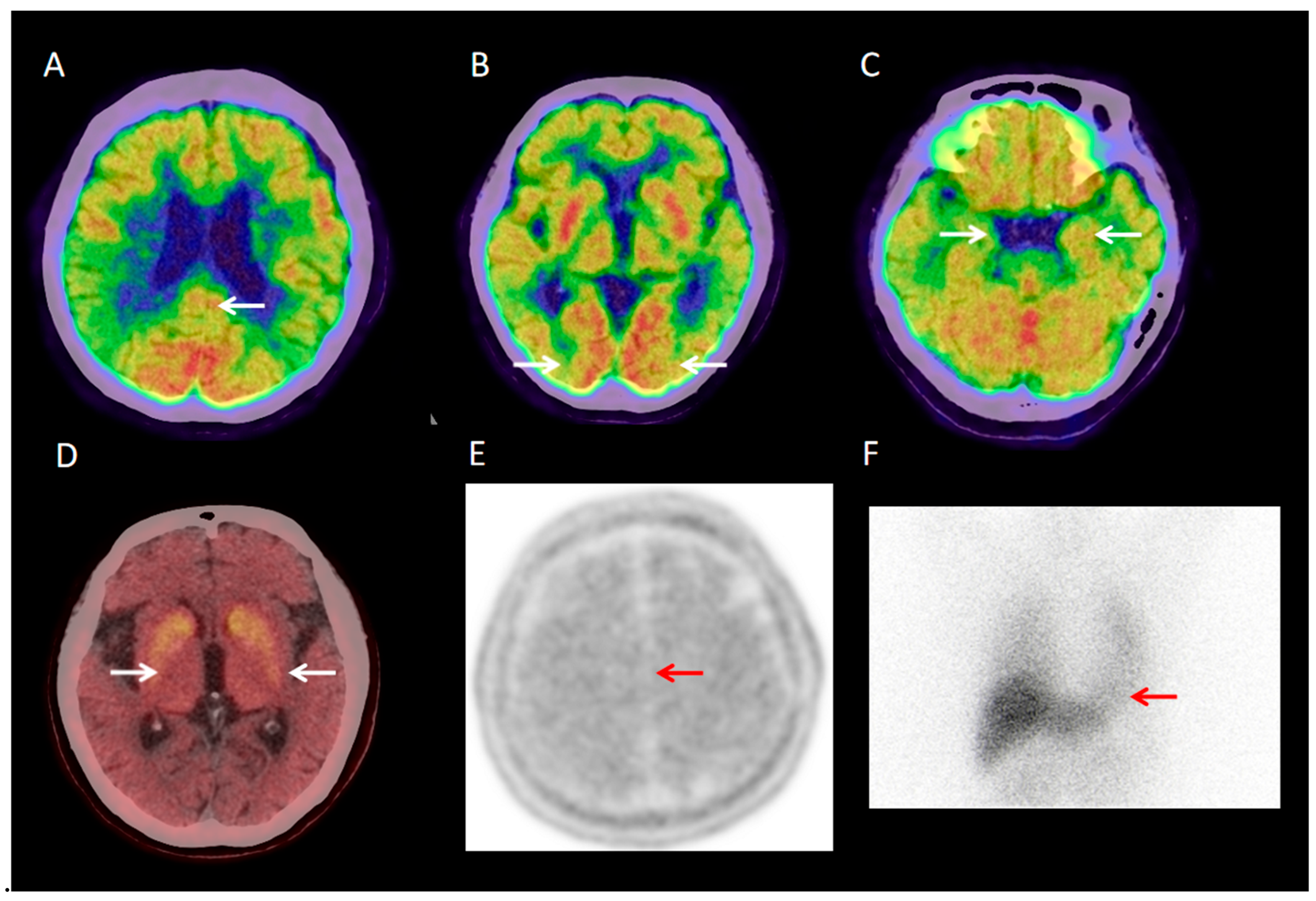
- Visual analysis: Two experienced nuclear medicine physicians from Peking Union Medical College Hospital independently interpreted 18F-FDG PET/CT images without reference to clinical data, assessing 18F-FDG metabolism in cortical and subcortical nuclei, with a focus on the occipital lobe and posterior cingulate gyrus, to diagnose and differentiate diseases. Discrepancies were resolved by a senior physician. DLB patients with atypical 18F-FDG metabolism patterns (e.g., bilateral medial temporal hypometabolism, posterior cingulate hypometabolism, or global hypometabolism) were classified into the “atypical DLB subgroup” (n = 10).
- (2)
- Voxel-wise statistical analysis of 18F-FDG PET/CT brain functional imaging data was performed using Statistical Parametric Mapping 12 (SPM12), including preprocessing and statistical analysis. Preprocessing steps were:
- ①
- Spatial normalization: To eliminate inter-individual differences, all data were normalized to the Montreal Neurological Institute (MNI) space using the DARTEL algorithm and resampled to a resolution of 2 × 2 × 2 mm3.
- ②
- Gaussian smoothing: Normalized data were spatially smoothed with an 8 mm full-width at half-maximum Gaussian kernel to approximate a normal distribution.
- ③
- Calculation of standardized uptake value ratio (SUVR): To correct for variations in injection dose, imaging time, and basal metabolic rate, the cerebellar cortex was used as the reference region, and SUVR was calculated voxel-wise as the ratio of each voxel’s uptake to the mean uptake in the reference region.
2.1.4. Analysis of 18F-AV45, 18F-FP-CIT, and 18F-PM-PBB3 PET/CT Images
2.1.5. Analysis of 131I-MIBG Scintigraphy
2.1.6. Statistical Analysis
3. Results
3.1. Clinical and Laboratory Data
3.1.1. General Data of the DLB Group and Control Group
3.1.2. Analysis of Clinical Manifestations
3.2. Imaging Results
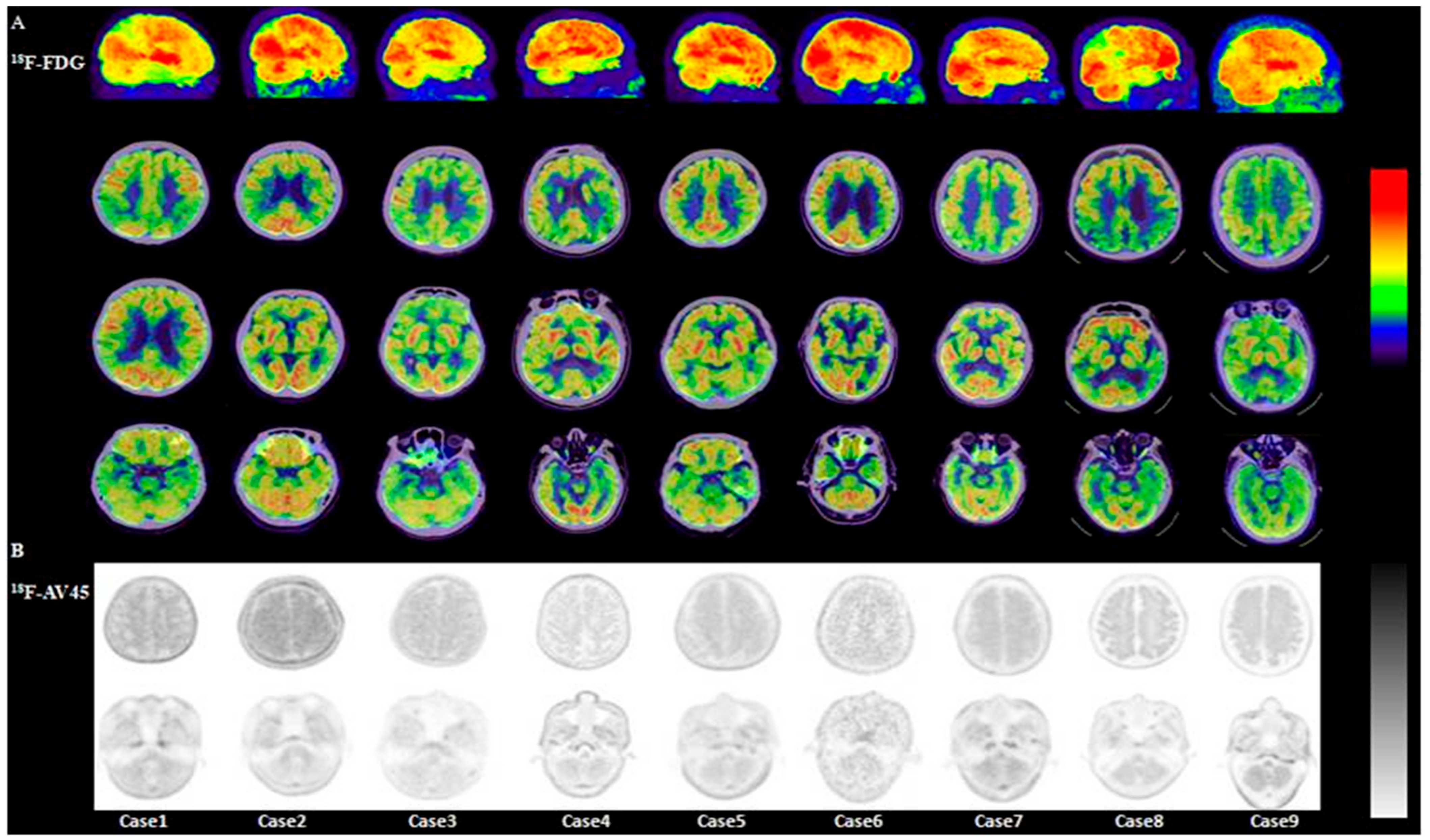
3.2.1. Visual Analysis of 18F-FDG PET/CT
3.2.2. ROI-Based Semi-Quantitative Analysis
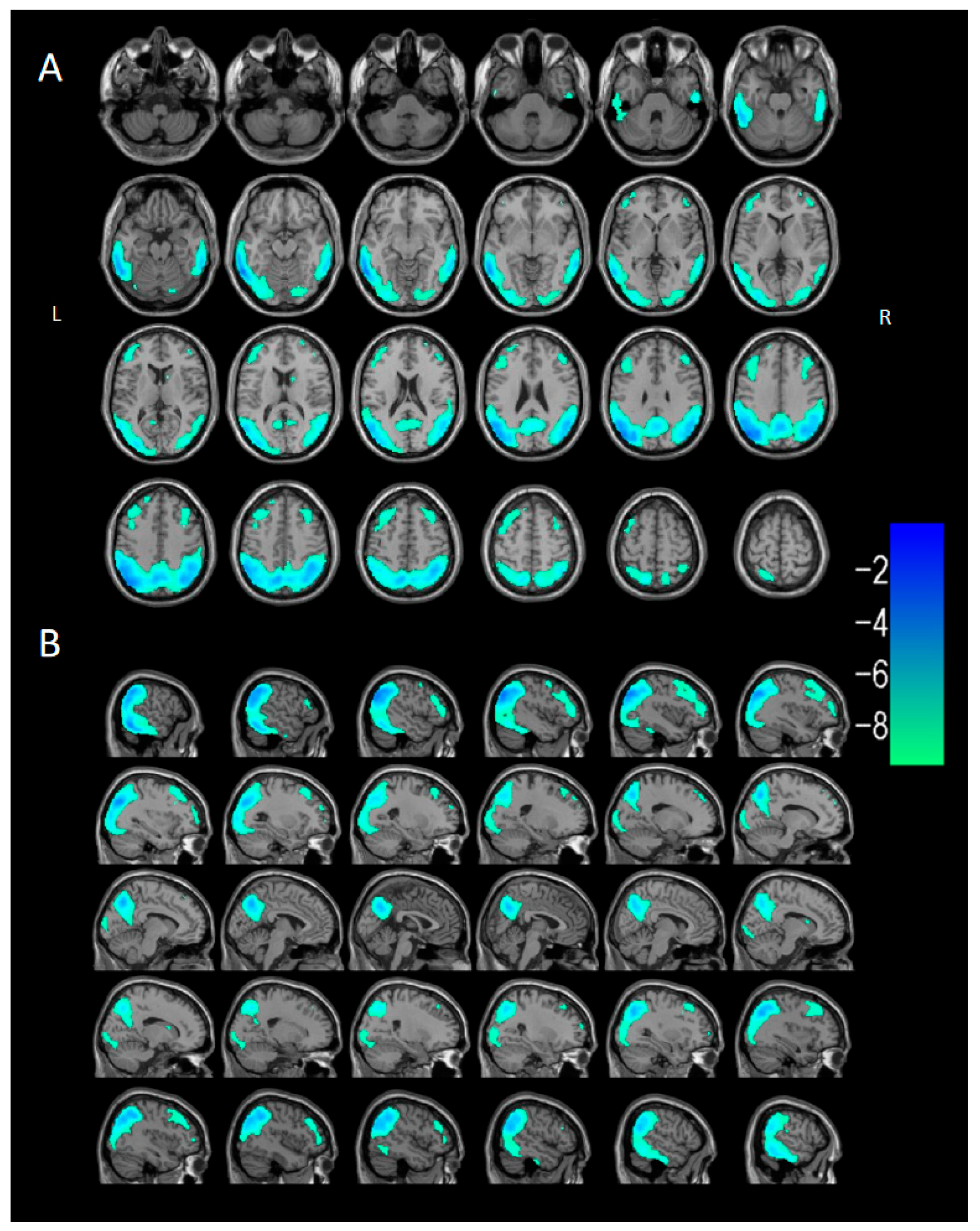
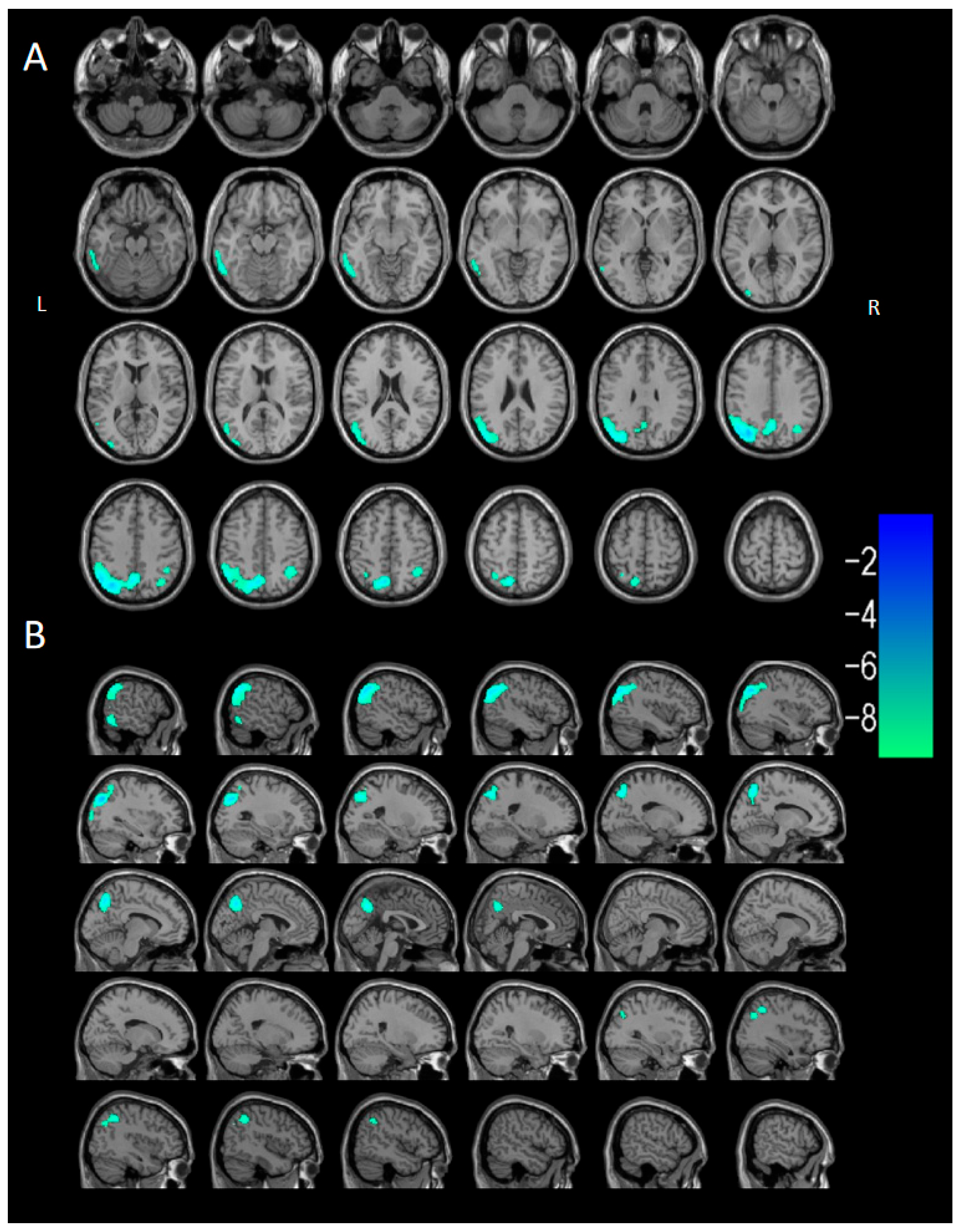
3.2.3. Voxel-Based Semi-Quantitative Analysis
3.2.4. Visual Analysis of 18F-AV45 PET/CT
3.2.5. Semi-Quantitative Analysis of 18F-AV45 PET/CT
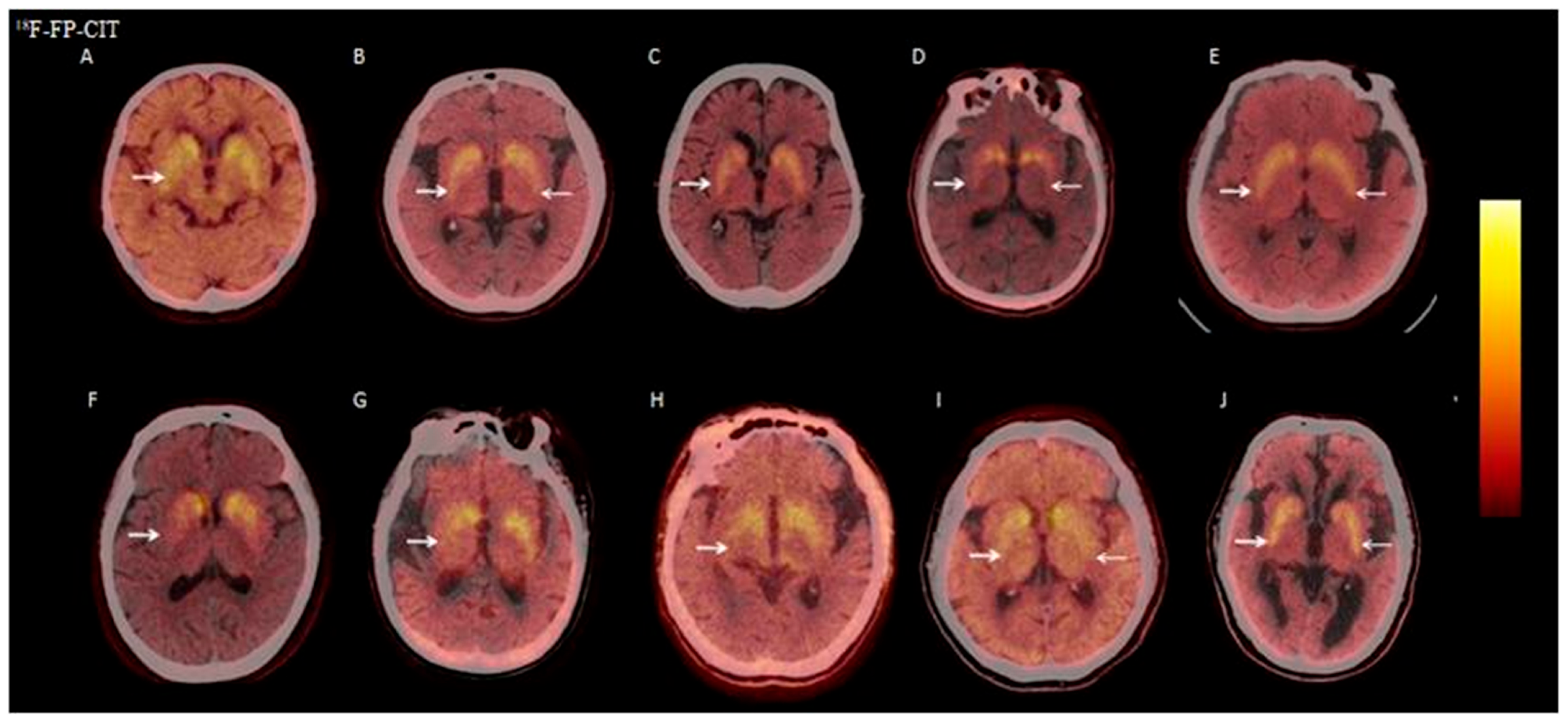
3.2.6. Visual Analysis of 18F-FP-CIT PET/CT
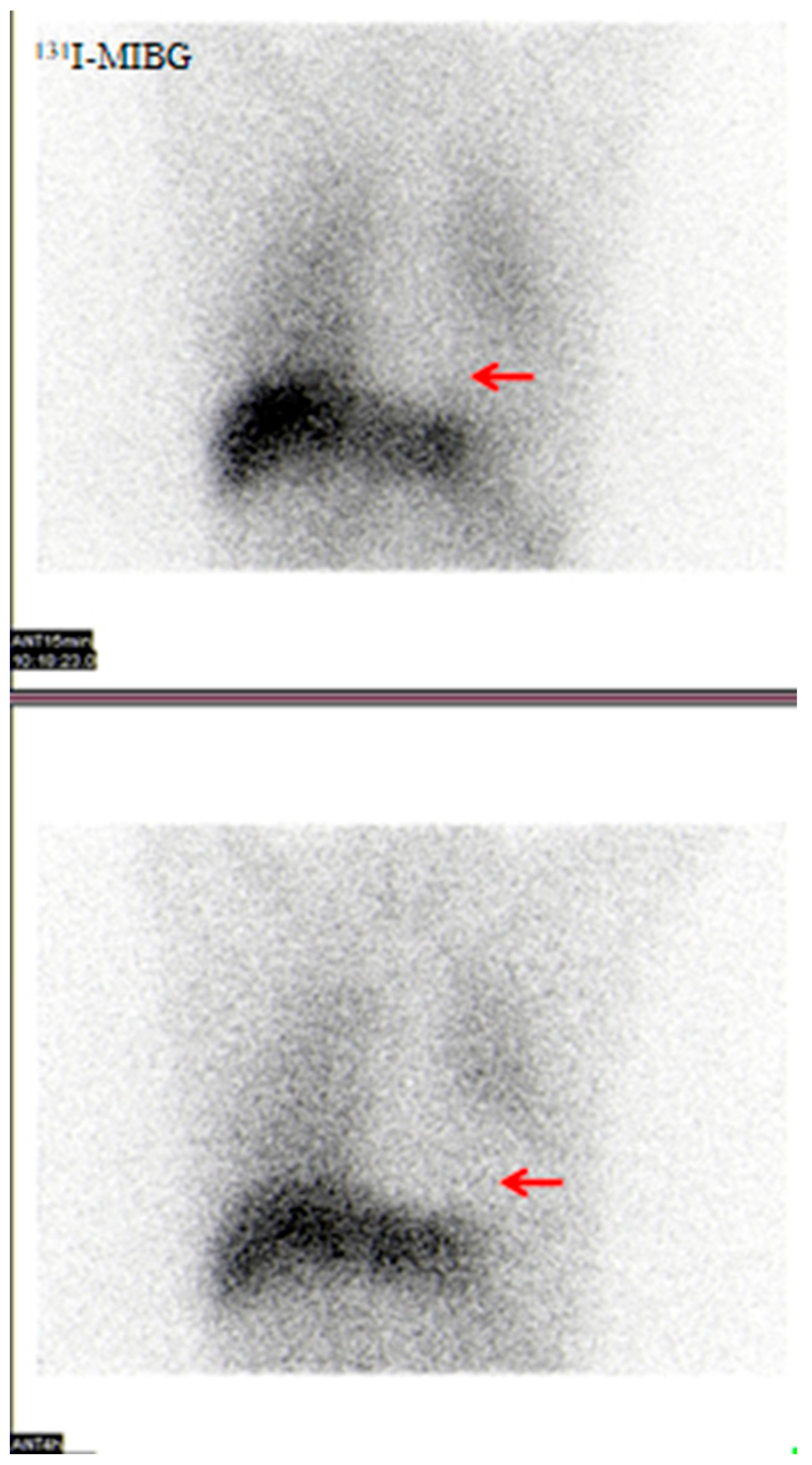
3.2.7. Visual Analysis of 131I-MIBG SPECT
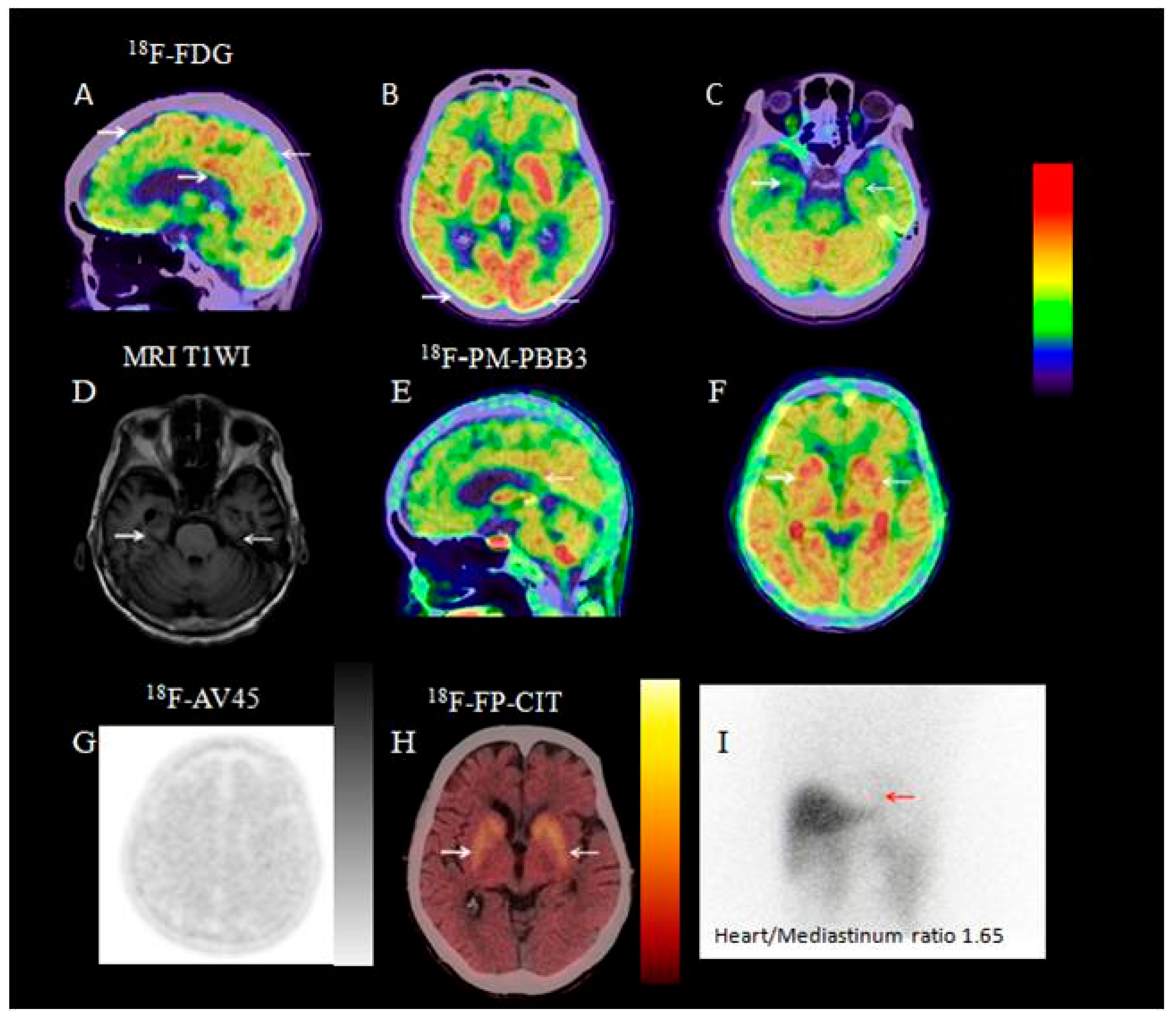
3.2.8. Visual Analysis of 18F-PM-PBB3 PET/CT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Zaccai, J.; Mccracken, C.; Brayne, C. A systematic review of prevalence and incidence studies of dementia with Lewy bodies. Age Ageing 2005, 34, 561–566. [Google Scholar] [CrossRef]
- Bousiges, O.; Blanc, F. Biomarkers of Dementia with Lewy Bodies: Differential Diagnostic with Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 6371. [Google Scholar] [CrossRef]
- Kane, J.P.M.; Surendranathan, A.; Bentley, A.; Barker, S.A.H.; Taylor, J.P.; Thomas, A.J.; Allan, L.M.; McNally, R.J.; James, P.W.; McKeith, I.G.; et al. Clinical prevalence of Lewy body dementia. Alzheimers Res. Ther. 2018, 10, 19. [Google Scholar] [CrossRef]
- Simard, M.V.R.R.; Cohen, T. A review of the cognitive and behavioral symptoms in dementia with Lewy bodies. J. Neuropsychiatry Clin. Neurosci. 2000, 12, 425–450. [Google Scholar] [CrossRef]
- Breitve, M.H.; Chwiszczuk, L.J.; Hynninen, M.J.; Rongve, A.; Brønnick, K.; Janvin, C.; Aarsland, D. A systematic review of cognitive decline in dementia with Lewy bodies versus Alzheimer’s disease. Alzheimers Res. Ther. 2014, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Sugawara Kikuchi, Y.; Shimizu, T. Aripiprazole for the treatment of psychotic symptoms in patients with dementia with Lewy bodies: A case series. Neuropsychiatr. Dis. Treat. 2019, 15, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Manabe, S.; Yanagi, H.; Ozawa, H.; Takagi, A. Neuroleptic malignant syndrome as part of an akinetic crisis associated with sepsis in a patient with Lewy body disease. BMJ Case Rep. 2019, 12, e227216. [Google Scholar] [CrossRef]
- Hershey, L.A.; Coleman-Jackson, R. Pharmacological Management of Dementia with Lewy Bodies. Drugs Aging 2019, 36, 309–319. [Google Scholar] [CrossRef]
- Beach, T.G.; Serrano, G.E.; Zhang, N.; Driver-Dunckley, E.D.; Sue, L.I.; Shill, H.A.; Mehta, S.H.; Belden, C.; Tremblay, C.; Choudhury, P.; et al. Clinicopathological Heterogeneity of Lewy Body Diseases: The Profound Influence of Comorbid Alzheimer’s Disease. medRxiv 2024. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Goodheart, A.E.; Locascio, J.J.; Peterec, E.; Properzi, M.; Thibault, E.G.; Chuba, E.; Johnson, K.A.; Brickhouse, M.J.; Touroutoglou, A.; et al. Differential Vulnerability of Hippocampal Subfields to Amyloid and Tau Deposition in the Lewy Body Diseases. Neurology 2024, 102, e209460. [Google Scholar] [CrossRef] [PubMed]
- Hiraga, K.; Hattori, M.; Satake, Y.; Tamakoshi, D.; Fukushima, T.; Uematsu, T.; Tsuboi, T.; Sato, M.; Yokoi, K.; Suzuki, K.; et al. Plasma biomarkers of neurodegeneration in patients and high risk subjects with Lewy body disease. NPJ Park. Dis. 2024, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- Fereshtehnejad, S.M.D.S.; Cermakova, P. Comorbidity profile in dementia with Lewy bodies versus Alzheimer’s disease: A linkage study between the Swedish Dementia Registry and the Swedish National Patient Registry. Alzheimers Res. Ther. 2014, 6, 65. [Google Scholar] [CrossRef]
- Li, Q.; Wang, J.; Cui, R.; Yuan, J. Identifying Mixed Dementia With Lewy Bodies and Alzheimer Disease Using Multitracer PET Imaging: A Case Study. Clin. Nucl. Med. 2024, 49, 364–365. [Google Scholar] [CrossRef]
- Sousa-Santos, P.E.M.; Pozzobon, P.M.; Teixeira, I.L.E. Frederic Lewy: How the two World Wars changed his life, work, and name. Arq. Neuropsiquiatr. 2024, 82, 1–2. [Google Scholar] [CrossRef]
- Musgrove, R.E.; King, A.E.; Dickson, T.C. Neuroprotective upregulation of endogenous alpha-synuclein precedes ubiquitination in cultured dopaminergic neurons. Neurotox. Res. 2011, 19, 592–602. [Google Scholar] [CrossRef]
- Baba, M.; Nakajo, S.; Tu, P.H.; Tomita, T.; Nakaya, K.; Lee, V.M.; Trojanowski, J.Q.; Iwatsubo, T. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am. J. Pathol. 1998, 152, 879–884. [Google Scholar]
- Gonzalez, M.C.; Oftedal, L.; Lange, J.; Tovar-Rios, D.A.; Tysnes, O.B.; Paquet, C.; Marquié, M.; Boada, M.; Alcolea, D.; Rejdak, K.; et al. Relationship of cognitive decline with glucocerebrosidase activity and amyloid-beta 42 in DLB and PD. Ann. Clin. Transl. Neurol. 2025, 12, 915–924. [Google Scholar] [CrossRef]
- Prasad, S.; Katta, M.R.; Abhishek, S.; Sridhar, R.; Valisekka, S.S.; Hameed, M.; Kaur, J.; Walia, N. Recent advances in Lewy body dementia: A comprehensive review. Dis. Mon. 2023, 69, 101441. [Google Scholar] [CrossRef] [PubMed]
- Mckeith, I.G.; Boeve, B.F.; Dickson, D.W.; Halliday, G.; Taylor, J.P.; Weintraub, D.; Aarsland, D.; Galvin, J.; Attems, J.; Ballard, C.G.; et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 2017, 89, 88–100. [Google Scholar] [CrossRef]
- Neuronal Degeneration Professional Committee of Chinese Society of Microcirculation. Guidelines for diagnosis and treatment of dementia with Lewy bodies in China. Chin. J. Geriatr. 2021, 40, 1473–1484. [Google Scholar]
- Harper, L.; Fumagalli, G.G.; Barkhof, F.; Scheltens, P.; O’Brien, J.T.; Bouwman, F.; Burton, E.J.; Rohrer, J.D.; Fox, N.C.; Ridgway, G.R.; et al. MRI visual rating scales in the diagnosis of dementia: Evaluation in 184 post-mortem confirmed cases. Brain 2016, 139 Pt 4, 1211–1225. [Google Scholar] [CrossRef]
- Lim, S.M.; Katsifis, A.; Villemagne, V.L.; Best, R.; Jones, G.; Saling, M.; Bradshaw, J.; Merory, J.; Woodward, M.; Hopwood, M.; et al. The 18F-FDG PET cingulate island sign and comparison to 123I-beta-CIT SPECT for diagnosis of dementia with Lewy bodies. J. Nucl. Med. 2009, 50, 1638–1645. [Google Scholar] [CrossRef]
- Ala, T.; Bakir, D.; Goel, S.; Feller, N.; Botchway, A.; Womack, C. A Mini-Mental State Examination Formula May Help to Distinguish Alzheimer’s Disease from Dementia with Lewy Bodies. J. Alzheimers Dis. 2022, 89, 1119–1129. [Google Scholar] [CrossRef]
- Miyamoto, M.; Miyamoto, T. Montreal Cognitive Assessment Predicts the Short-Term Risk of Lewy Body Disease in Isolated REM Sleep Behavior Disorder with Reduced MIBG Scintigraphy. Mov. Disord. Clin. Pr. 2023, 10, 32–41. [Google Scholar] [CrossRef]
- Liampas, I.; Kyriakoulopoulou, P.; Siokas, V.; Tsiamaki, E.; Stamati, P.; Kefalopoulou, Z.; Chroni, E.; Dardiotis, E. Apolipoprotein E Gene in alpha-Synucleinopathies: A Narrative Review. Int. J. Mol. Sci. 2024, 25, 1795. [Google Scholar] [CrossRef] [PubMed]
- Cheon, M.; Kim, S.M.; Ha, S.W.; Kang, M.J.; Yang, H.E.; Yoo, J. Diagnostic Performance for Differential Diagnosis of Atypical Parkinsonian Syndromes from Parkinson’s Disease Using Quantitative Indices of (18)F-FP-CIT PET/CT. Diagnostics 2022, 12, 1402. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Kinney, J.W.; Cordes, D.; Alzheimer’s Disease Neuroimaging Initiative. Uptake of 18F-AV45 in the Putamen Provides Additional Insights into Alzheimer’s Disease beyond the Cortex. Biomolecules 2024, 14, 157. [Google Scholar] [CrossRef] [PubMed]
- Leuzy, A.; Chiotis, K.; Lemoine, L.; Gillberg, P.G.; Almkvist, O.; Rodriguez-Vieitez, E.; Nordberg, A. Tau PET imaging in neurodegenerative tauopathies-still a challenge. Mol. Psychiatry 2019, 24, 1112–1134. [Google Scholar] [CrossRef]
- Klunk, W.E.; Koeppe, R.A.; Price, J.C.; Benzinger, T.L.; Devous, M.D.; Sr Jagust, W.J.; Johnson, K.A.; Mathis, C.A.; Minhas, D.; Pontecorvo, M.J. The Centiloid Project: Standardizing quantitative amyloid plaque estimation by PET. Alzheimers Dement. 2015, 11, 1–15.e4. [Google Scholar] [CrossRef]
- Walker, Z.; Jaros, E.; Walker, R.W.; Lee, L.; Costa, D.C.; Livingston, G.; Ince, P.G.; Perry, R.; McKeith, I.; Katona, C.L. Dementia with Lewy bodies: A comparison of clinical diagnosis, FP-CIT single photon emission computed tomography imaging and autopsy. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1176–1181. [Google Scholar] [CrossRef]
- Kaneko, Y.; Hirao, K.; Serisawa, S.; Kanetaka, H.; Shimizu, S.; Hirai, H.; Shishido-Hara, Y.; Umahara, T.; Sakurai, H.; Hanyu, H. Association between clinical symptoms and post-mortem neuropathology in dementia with Lewy bodies. Geriatr. Gerontol. Int. 2020, 20, 261–262. [Google Scholar] [CrossRef]
- Jellinger, K.A.; Korczyn, A.D. Are dementia with Lewy bodies and Parkinson’s disease dementia the same disease? BMC Med. 2018, 16, 34. [Google Scholar] [CrossRef]
- Abadir, A.; Dalton, R.; Zheng, W.; Pincavitch, J.; Tripathi, R. Neuroleptic Sensitivity in Dementia with Lewy Body and Use of Pimavanserin in an Inpatient Setting: A Case Report. Am. J. Case Rep. 2022, 23, e937397. [Google Scholar] [CrossRef]
- Burke, W.J.P.R.; Mccomb, R.D. Neuroleptic sensitivity to clozapine in dementia with Lewy bodies. J. Neuropsychiatry Clin. Neurosci. 1998, 10, 227–229. [Google Scholar] [CrossRef]
- Delli Pizzi, S.; Franciotti, R.; Tartaro, A.; Caulo, M.; Thomas, A.; Onofrj, M.; Bonanni, L. Structural alteration of the dorsal visual network in DLB patients with visual hallucinations: A cortical thickness MRI study. PLoS ONE 2014, 9, e86624. [Google Scholar] [CrossRef] [PubMed]
- Vignando, M.; Ffytche, D.; Lewis, S.J.G.; Lee, P.H.; Chung, S.J.; Weil, R.S.; Hu, M.T.; Mackay, C.E.; Griffanti, L.; Pins, D.; et al. Mapping brain structural differences and neuroreceptor correlates in Parkinson’s disease visual hallucinations. Nat. Commun. 2022, 13, 519. [Google Scholar] [CrossRef]
- Walker, Z.; Possin, K.L.; Boeve, B.F.; Aarsland, D. Lewy body dementias. Lancet 2015, 386, 1683–1697. [Google Scholar] [CrossRef] [PubMed]
- Wennström, M.; Surova, Y.; Hall, S.; Nilsson, C.; Minthon, L.; Boström, F.; Hansson, O.; Nielsen, H.M. Low CSF levels of both alpha-synuclein and the alpha-synuclein cleaving enzyme neurosin in patients with synucleinopathy. PLoS ONE 2013, 8, e53250. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Burre, J. alpha-Synuclein in synaptic function and dysfunction. Trends Neurosci. 2023, 46, 153–166. [Google Scholar] [CrossRef]
- Ruan, D.; Sun, L. Amyloid-beta PET in Alzheimer’s disease: A systematic review and Bayesian meta-analysis. Brain Behav. 2023, 13, e2850. [Google Scholar] [CrossRef]
- Li, Q.; Wang, J.; Yuan, J.; Cui, R. Neuroimaging findings of common types of neurodegenerative dementia. Basic. Clin. Med. 2024, 44, 1741–1745. [Google Scholar]
- Schneider, J.A.; Arvanitakis, Z.; Yu, L.; Boyle, P.A.; Leurgans, S.E.; Bennett, D.A. Cognitive impairment, decline and fluctuations in older community-dwelling subjects with Lewy bodies. Brain 2012, 135 Pt 10, 3005–3014. [Google Scholar] [CrossRef]
- Lee, Y.G.; Jeon, S.; Baik, K.; Kang, S.W.; Ye, B.S. Substantia nigral dopamine transporter uptake in dementia with Lewy bodies. NPJ Park. Dis. 2023, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Shankar, V.; Elkider, M. Comment on: Dopamine Transporter (DaT) Scan Utilization in a Movement Disorder Center. Mov. Disord. Clin. Pr. 2017, 4, 154. [Google Scholar] [CrossRef]
- O’Shea, D.M.; Arkhipenko, A.; Galasko, D.; Goldman, J.G.; Sheikh, Z.H.; Petrides, G.; Toledo, J.B.; Galvin, J.E. Practical use of DAT SPECT imaging in diagnosing dementia with Lewy bodies: A US perspective of current guidelines and future directions. Front. Neurol. 2024, 15, 1395413. [Google Scholar] [CrossRef]
- Gargiulo, P.; Acampa, W.; Asile, G.; Abbate, V.; Nardi, E.; Marzano, F.; Assante, R.; Nappi, C.; Parlati, A.L.M.; Basile, C.; et al. (123)I-MIBG imaging in heart failure: Impact of comorbidities on cardiac sympathetic innervation. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Kashihara, K.; Ohno, M.; Kawada, S.; Okumura, Y. Reduced cardiac uptake and enhanced washout of 123I-MIBG in pure autonomic failure occurs conjointly with Parkinson’s disease and dementia with Lewy bodies. J. Nucl. Med. 2006, 47, 1099–1101. [Google Scholar] [PubMed]
- Pontico, M.; Brunotti, G.; Conte, M.; Corica, F.; Cosma, L.; De Angelis, C.; De Feo, M.S.; Lazri, J.; Matto, A.; Montebello, M. The prognostic value of (123)I-mIBG SPECT cardiac imaging in heart failure patients: A systematic review. J. Nucl. Cardiol. 2022, 29, 1799–1809. [Google Scholar] [CrossRef]
- Stefanelli, A.; Treglia, G.; Bruno, I.; Rufini, V.; Giordano, A. Pharmacological interference with 123I-metaiodobenzylguanidine: A limitation to developing cardiac innervation imaging in clinical practice? Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1326–1333. [Google Scholar]
| Parameter | DLB Group (n = 40) | Control Group (n = 40) |
|---|---|---|
| Sex | ||
| Male | 29 | 29 |
| Female | 11 | 11 |
| Age (years) | 72.1 ± 6.9 | 72.1 ± 8.0 |
| MMSE score | 18.1 ± 5.5 | - |
| Clinical manifestations, n (%) | - | |
| Cognitive impairment | 40 (100%) | - |
| Parkinsonian symptoms | 40 (100%) | - |
| Visual hallucinations | 38 (95%) | - |
| REM sleep behavior disorder | 26 (65%) | - |
| Brain Region | 18F-FDG SUVR | Statistical Values | ||
|---|---|---|---|---|
| DLB Group (n = 40) | Control Group (n = 40) | t-Value | p-Value | |
| Calcarine_R | 1.12 ± 0.13 | 1.23 ± 0.13 | −3.69 | 0.00 |
| Calcarine_L | 1.12 ± 0.15 | 1.22 ± 0.15 | −3.03 | 0.00 |
| Cuneus_R | 1.21 ± 0.14 | 1.34 ± 0.14 | −4.18 | 0.00 |
| Cuneus_L | 1.34 ± 0.18 | 1.48 ± 0.19 | −3.04 | 0.00 |
| Occipital_Sup_R | 0.95 ± 0.12 | 1.08 ± 0.11 | −5.07 | 0.00 |
| Occipital_Sup_L | 1.20 ± 0.15 | 1.32 ± 0.16 | −3.60 | 0.00 |
| Occipital_Mid_R | 0.89 ± 0.11 | 1.04 ± 0.10 | −6.29 | 0.00 |
| Occipital_Mid_L | 0.95 ± 0.11 | 1.08 ± 0.12 | −4.99 | 0.00 |
| Occipital_Inf_R | 1.05 ± 0.11 | 1.21 ± 0.10 | −6.57 | 0.00 |
| Occipital_Inf_L | 1.08 ± 0.12 | 1.21 ± 0.12 | −4.83 | 0.00 |
| Precuneus_R | 1.05 ± 0.10 | 1.21 ± 0.13 | −6.43 | 0.00 |
| Precuneus_L | 1.14 ± 0.12 | 1.33 ± 0.14 | −6.56 | 0.00 |
| Angular_R | 0.87 ± 0.09 | 1.04 ± 0.11 | −7.32 | 0.00 |
| Angular_L | 0.81 ± 0.09 | 0.96 ± 0.11 | −6.62 | 0.00 |
| Temporal_Mid_R | 0.91 ± 0.07 | 1.03 ± 0.09 | −6.53 | 0.00 |
| Temporal_Mid_L | 0.89 ± 0.08 | 0.97 ± 0.08 | −4.46 | 0.00 |
| Temporal_Inf_R | 0.86 ± 0.06 | 0.97 ± 0.06 | −7.60 | 0.00 |
| Temporal_Inf_L | 0.89 ± 0.07 | 0.95 ± 0.06 | −4.32 | 0.00 |
| Hippocampus_R | 0.89 ± 0.07 | 0.86 ± 0.06 | 1.72 | 0.09 |
| Hippocampus_L | 0.83 ± 0.07 | 0.81 ± 0.05 | 1.22 | 0.23 |
| Cingulate_Post_R | 0.62 ± 0.07 | 0.68 ± 0.10 | −3.04 | 0.00 |
| Cingulate_Post_L | 0.73 ± 0.08 | 0.81 ± 0.14 | −2.81 | 0.00 |
| Amygdala_R | 0.83 ± 0.08 | 0.79 ± 0.06 | 2.66 | 0.02 |
| Amygdala_L | 0.81 ± 0.10 | 0.77 ± 0.06 | 2.40 | 0.01 |
| Brain Region of Cluster | Cluster Size (Voxels) | Peak t | Peak MNI Coordinates | ||
|---|---|---|---|---|---|
| x | y | z | |||
| DLB < HC | |||||
| Cluster 1 | 29,280 | −11.5121 | −44 | −74 | 36 |
| Occipital_Mid_L (aal) | 2736 | ||||
| Temporal_Mid_L (aal) | 2409 | ||||
| Parietal_Inf_L (aal) | 1864 | ||||
| Temporal_Mid_R (aal) | 1836 | ||||
| Precuneus_L (aal) | 1653 | ||||
| Precuneus_R (aal) | 1609 | ||||
| Temporal_Inf_L (aal) | 1536 | ||||
| Occipital_Mid_R (aal) | 1460 | ||||
| Angular_R (aal) | 1460 | ||||
| Temporal_Inf_R (aal) | 1337 | ||||
| Parietal_Sup_L (aal) | 1167 | ||||
| Angular_L (aal) | 1104 | ||||
| Parietal_Inf_R (aal) | 1056 | ||||
| SupraMarginal_R (aal) | 847 | ||||
| Parietal_Sup_R (aal) | 814 | ||||
| Occipital_Inf_L (aal) | 701 | ||||
| Occipital_Sup_L (aal) | 669 | ||||
| SupraMarginal_L (aal) | 656 | ||||
| Occipital_Sup_R (aal) | 491 | ||||
| Fusiform_L (aal) | 479 | ||||
| Cuneus_L (aal) | 402 | ||||
| Cuneus_R (aal) | 279 | ||||
| Occipital_Inf_R (aal) | 234 | ||||
| Temporal_Sup_R (aal) | 216 | ||||
| Calcarine_R (aal) | 194 | ||||
| Lingual_R (aal) | 185 | ||||
| Lingual_L (aal) | 147 | ||||
| Calcarine_L (aal) | 136 | ||||
| Temporal_Sup_L (aal) | 128 | ||||
| Cingulum_Post_L (aal) | 70 | ||||
| Cingulum_Post_R (aal) | 33 | ||||
| Cluster 2 | 1037 | −6.4095 | 42 | 32 | 38 |
| Frontal_Mid_R (aal) | 908 | ||||
| Frontal_Inf_Tri_R (aal) | 57 | ||||
| Cluster 3 | 2379 | −6.5266 | −32 | 24 | 52 |
| Frontal_Mid_L (aal) | 1628 | ||||
| Frontal_Inf_Tri_L (aal) | 473 | ||||
| Frontal_Sup_L (aal) | 104 | ||||
| CL Value | Cerebral SUV (g/mL) | Cerebellar SUV (g/mL) | |
|---|---|---|---|
| Case | |||
| 1 | −17 | 0.55 | 0.54 |
| 2 | 54 | 0.99 | 0.71 |
| 3 | 18 | 0.68 | 0.57 |
| 4 | −34 | 0.68 | 0.73 |
| 5 | −50 | 0.25 | 0.30 |
| 6 | 31 | 0.67 | 0.53 |
| 7 | 31 | 1.04 | 0.81 |
| 8 | 53 | 0.99 | 0.71 |
| 9 | 42 | 1.04 | 0.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Huang, Z.; Wang, J.; Liang, M.; Jia, C.; Yuan, J.; Cui, R. Comparative Study on Multimodal Imaging Applications in Dementia with Lewy Bodies: From Imaging Features to Clinical Practice Implications. Brain Sci. 2025, 15, 1264. https://doi.org/10.3390/brainsci15121264
Li Q, Huang Z, Wang J, Liang M, Jia C, Yuan J, Cui R. Comparative Study on Multimodal Imaging Applications in Dementia with Lewy Bodies: From Imaging Features to Clinical Practice Implications. Brain Sciences. 2025; 15(12):1264. https://doi.org/10.3390/brainsci15121264
Chicago/Turabian StyleLi, Qijun, Zhaoxia Huang, Junshan Wang, Menglin Liang, Chenhao Jia, Jing Yuan, and Ruixue Cui. 2025. "Comparative Study on Multimodal Imaging Applications in Dementia with Lewy Bodies: From Imaging Features to Clinical Practice Implications" Brain Sciences 15, no. 12: 1264. https://doi.org/10.3390/brainsci15121264
APA StyleLi, Q., Huang, Z., Wang, J., Liang, M., Jia, C., Yuan, J., & Cui, R. (2025). Comparative Study on Multimodal Imaging Applications in Dementia with Lewy Bodies: From Imaging Features to Clinical Practice Implications. Brain Sciences, 15(12), 1264. https://doi.org/10.3390/brainsci15121264






