Cell-Free Therapies for Chronic Pain: The Rise of the Mesenchymal Stem Cell Secretome
Abstract
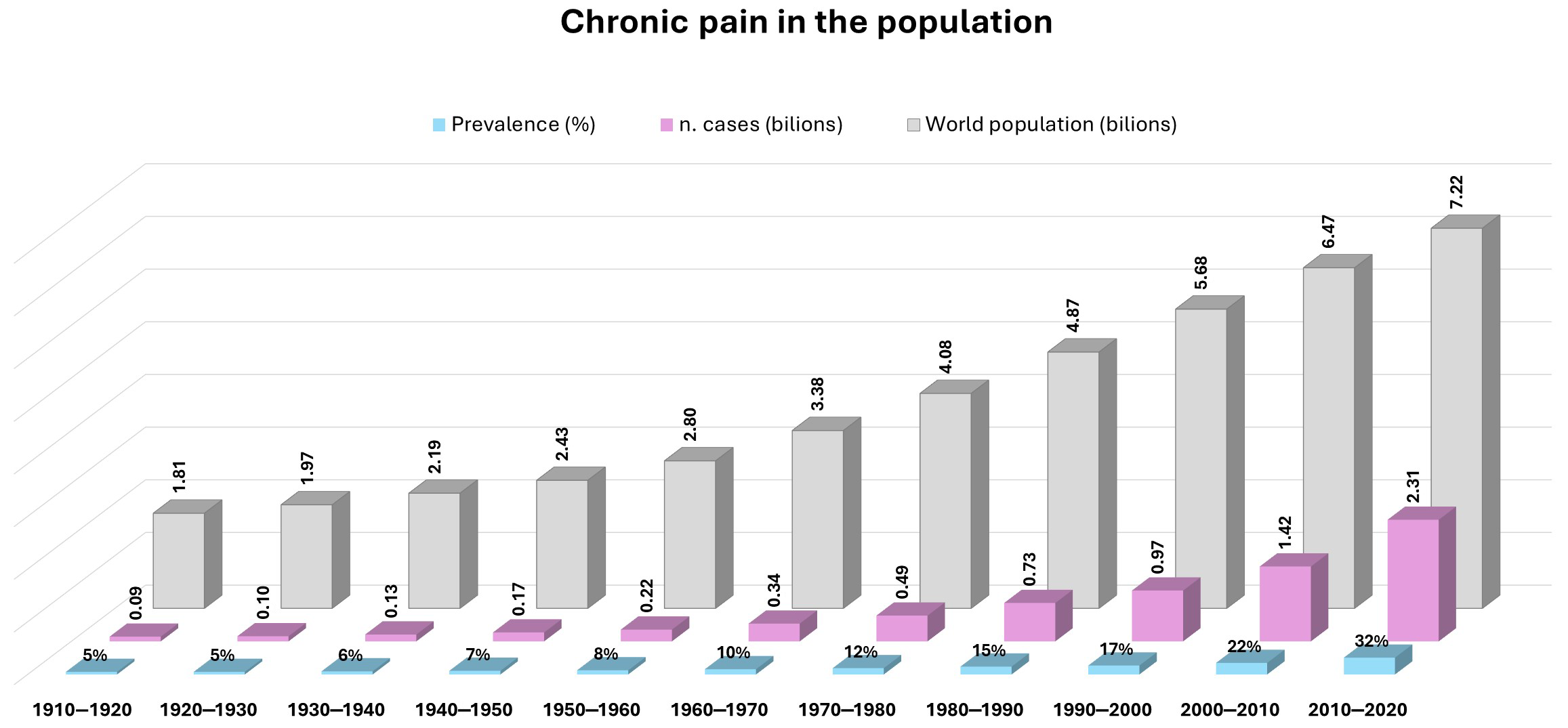
1. Introduction
2. Novel Therapeutic Strategy
3. Secretome
Mesenchymal Stem Cell (MSC)-Derived Secretome
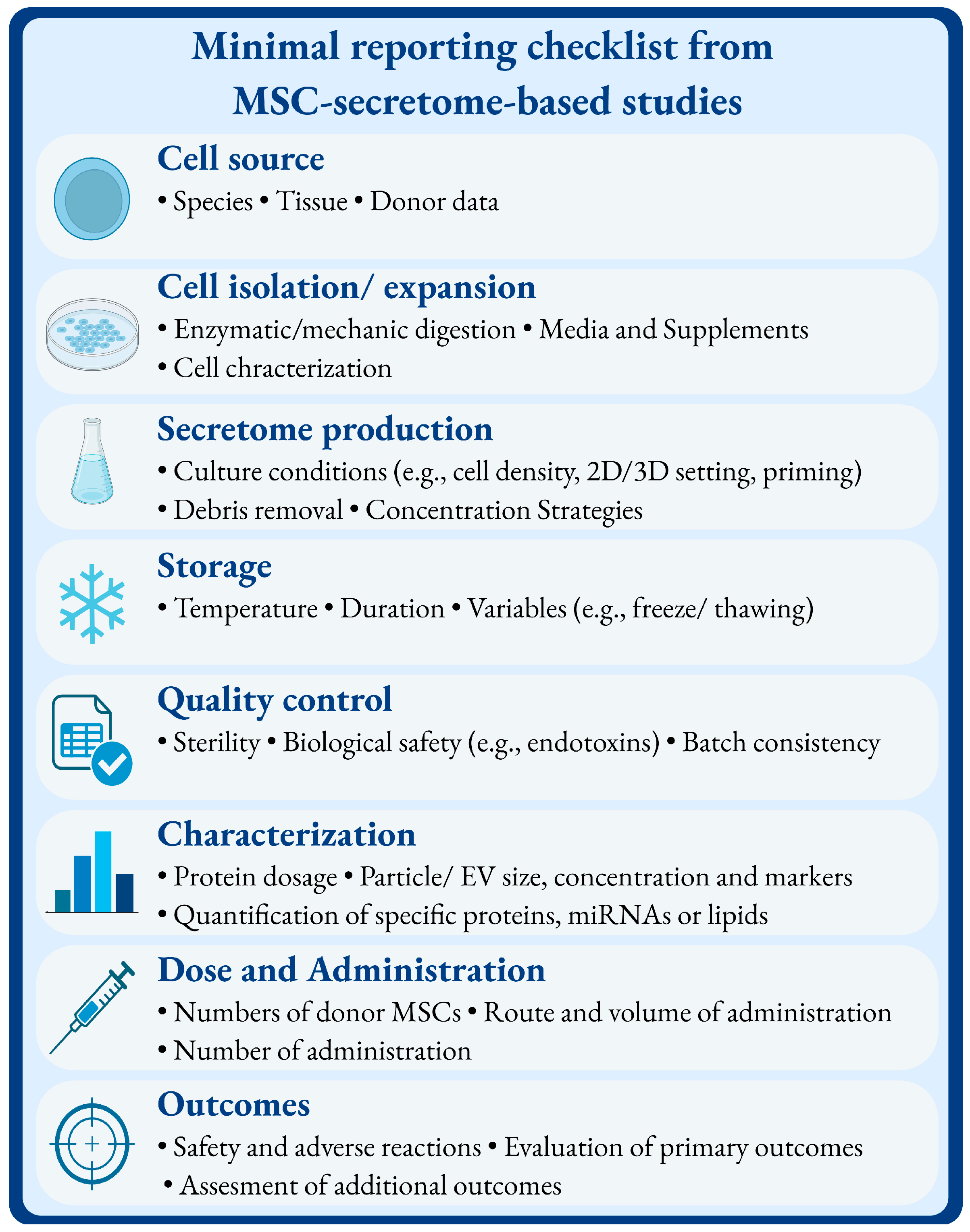
4. Methodology
- (a)
- Filter time: none.
- (b)
- Database: PubMed, Cochrane Library and Embase.
- (c)
- Language: English.
- (d)
- Keywords: terms used were “chronic pain” AND “mesenchymal cells” AND “secretome”, “conditioned medium”, “acellular therapy”, “extracellular vesicles” or “exosomes”.
- (e)
- Key terms were searched in the title, keywords and abstract. The search was open to all parameters in order to avoid information loss. MeSH (Medical Subject Headings) terms were not used.
- (f)
- The papers’ selection was based on critical reading.
5. Dissecting the Evidence: From Petri Dish to Complex Organisms
5.1. In Vivo Preclinical Studies
5.2. In Vitro Studies
5.3. Clinical Reports
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | Annulus Fibrosus |
| AKT | Protein Kinase B |
| ASCs | Adipose-derived Stromal/Stem Cells |
| ATMPs | Advanced Therapy Medicinal Products |
| BDNF | Brain-Derived Neurotrophic Factor |
| CD55 | Cluster of Differentiation 55 |
| CeOs | Cerebral Organoids |
| CIPN | Chemotherapy-Induced Peripheral Neuropathy |
| CM | Conditioned Medium |
| DASH | Disabilities of the Arm, Shoulder and Hand |
| DOR | Delta (δ) Opioid Receptor |
| KOR | Kappa (κ) Opioid Receptor |
| DRG | Dorsal Root Ganglion |
| EMA | European Medicines Agency |
| ESCs | Embryonic Stem Cells |
| EU | European Union |
| EVs | Extracellular Vesicles |
| FDA | Food and Drug Administration |
| FGF2 | Fibroblast Growth Factor 2 |
| GDNF | Glial Cell Line-Derived Neurotrophic Factor |
| GMP | Good Manufacturing Practice |
| HA | Hyaluronic Acid |
| IGF | Insulin-like Growth Factor |
| IL-10 | Interleukin 10 |
| IL-1β | Interleukin 1 beta |
| IL-6 | Interleukin 6 |
| IL-8 | Interleukin 8 |
| iPSCs | Induced Pluripotent Stem Cells |
| KL | Kellgren and Lawrence Scale |
| L2-L4 | Lumbar Vertebrae L2 to L4 |
| LPS | Lipopolysaccharide |
| miRNAs | microRNAs |
| MMP1 | Matrix Metalloproteinase-1 |
| MMP3 | Matrix Metalloproteinase-3 |
| MOR | Mu (µ) Opioid Receptor |
| MSCs | Mesenchymal Stem/Stromal Cells |
| mTOR | Mammalian Target of Rapamycin |
| MyD88 | Myeloid Differentiation Primary Response 88 |
| Nav1.7 or Nav1.8 | Voltage-gated Sodium Channel subtypes 1.7 and 1.8 |
| NF-κB | Nuclear Factor kappa B |
| NGF | Nerve Growth Factor |
| NLRP3 | NOD-like receptor family pyrin domain-containing 3 |
| NOD | Nucleotide-binding Oligomerization Domain |
| NRS | Numeric Rating Scale |
| NSAIDs | Non-Steroidal Anti-Inflammatory Drugs |
| NSCs | Neural Stem Cells |
| OA | Osteoarthritis |
| P2X4/P2X7 | Purinergic Receptor P2X4 and P2X7 |
| PBMCs | Peripheral Blood Mononuclear Cells |
| PET | Positron Emission Tomography |
| PGE2 | Prostaglandin E2 |
| PI3K | Phosphoinositide 3-Kinase |
| PLDD | Percutaneous Laser Disc Decompression |
| RNA | Ribonucleic Acid |
| Rsad2 | Radical S-Adenosyl Methionine Domain-containing 2 |
| SNRIs | Serotonin-Norepinephrine Reuptake Inhibitors |
| STZ | Streptozotocin |
| tDCS | Transcranial Direct Current Stimulation |
| TGF-β | Transforming Growth Factor beta |
| TIMP1 | Tissue Inhibitor of Metalloproteinases 1 |
| TIMP2 | Tissue Inhibitor of Metalloproteinases 2 |
| TLR2 | Toll-like Receptor 2 |
| TLRs | Toll-like Receptors |
| TMS | Transcranial Magnetic Stimulation |
| TNF-α | Tumor Necrosis Factor alpha |
| TRPV1 | Transient Receptor Potential Vanilloid 1 |
| U.S. | United States |
| UC-MSC | Umbilical Cord-derived Mesenchymal Stem Cells |
| VAS | Visual Analog Scale |
| VEGF | Vascular Endothelial Growth Factor |
| Wnt | Wnt signaling pathway |
| WOMAC | Western Ontario and McMaster Universities Osteoarthritis Index |
References
- Stretanski, M.F.; Kopitnik, N.L.; Matha, A.; Conermann, T. Chronic Pain. In StatPearls; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Szewczyk, A.K.; Jamroz-Wisniewska, A.; Haratym, N.; Rejdak, K. Neuropathic pain and chronic pain as an underestimated interdisciplinary problem. Int. J. Occup. Med. Environ. Health 2022, 35, 249–264. [Google Scholar] [CrossRef]
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B.; et al. Neuropathic pain. Nat. Rev. Dis. Primers 2017, 3, 17002. [Google Scholar] [CrossRef]
- Scholz, J.; Finnerup, N.B.; Attal, N.; Aziz, Q.; Baron, R.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Cruccu, G.; Davis, K.D.; et al. The IASP classification of chronic pain for ICD-11: Chronic neuropathic pain. Pain 2019, 160, 53–59. [Google Scholar] [CrossRef]
- Rosner, J.; de Andrade, D.C.; Davis, K.D.; Gustin, S.M.; Kramer, J.L.K.; Seal, R.P.; Finnerup, N.B. Central neuropathic pain. Nat. Rev. Dis. Primers 2023, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; Binder, A.; Wasner, G. Neuropathic pain: Diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010, 9, 807–819. [Google Scholar] [CrossRef]
- Ji, R.R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Liu, S.; Wang, Y.; Cui, R.; Zhang, X. The Link between Depression and Chronic Pain: Neural Mechanisms in the Brain. Neural Plast. 2017, 2017, 9724371. [Google Scholar] [CrossRef]
- GBD 2021 Low Back Pain Collaborators. Global, regional, and national burden of low back pain, 1990-2020, its attributable risk factors, and projections to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e316–e329. [Google Scholar] [CrossRef]
- Zhu, M.; Huang, Z.; Liu, T.; Wu, C.; Shang, Z.; Zhang, L. Global burden and trends of norovirus-associated diseases from 1990 to 2021 an observational trend study. Front. Public Health 2024, 12, 1483149. [Google Scholar] [CrossRef] [PubMed]
- Baskozos, G.; Hebert, H.L.; Pascal, M.M.; Themistocleous, A.C.; Macfarlane, G.J.; Wynick, D.; Bennett, D.L.; Smith, B.H. Epidemiology of neuropathic pain: An analysis of prevalence and associated factors in UK Biobank. Pain Rep. 2023, 8, e1066. [Google Scholar] [CrossRef]
- Dash, S.S.; Khatri, N.K.; Divyateja, S.; Bhate, J.; Rao, G.K.S. Advances in neuropathic pain management: A review of real-world studies. J. Curr. Res. Sci. Med. 2024, 10, 15–24. [Google Scholar] [CrossRef]
- Vieira, W.F.; Coelho, D.R.A.; Litwiler, S.T.; McEachern, K.M.; Clancy, J.A.; Morales-Quezada, L.; Cassano, P. Neuropathic pain, mood, and stress-related disorders: A literature review of comorbidity and co-pathogenesis. Neurosci. Biobehav. Rev. 2024, 161, 105673. [Google Scholar] [CrossRef]
- Mills, S.E.E.; Nicolson, K.P.; Smith, B.H. Chronic pain: A review of its epidemiology and associated factors in population-based studies. Br. J. Anaesth. 2019, 123, e273–e283. [Google Scholar] [CrossRef] [PubMed]
- Department of Economic and Social Affairs, United Nations. Population Division. World Population Prospects 2024. Available online: https://population.un.org/wpp/ (accessed on 8 July 2025).
- Guido, D.; Leonardi, M.; Mellor-Marsa, B.; Moneta, M.V.; Sanchez-Niubo, A.; Tyrovolas, S.; Gine-Vazquez, I.; Haro, J.M.; Chatterji, S.; Bobak, M.; et al. Pain rates in general population for the period 1991–2015 and 10-years prediction: Results from a multi-continent age-period-cohort analysis. J. Headache Pain 2020, 21, 52. [Google Scholar] [CrossRef]
- Bernard, S.A.; Chelminski, P.R.; Ives, T.J.; Ranapurwala, S.I. Management of Pain in the United States-A Brief History and Implications for the Opioid Epidemic. Health Serv. Insights 2018, 11, 1178632918819440. [Google Scholar] [CrossRef]
- Rugnath, R.; Orzechowicz, C.; Newell, C.; Carullo, V.; Rugnath, A. A Literature Review: The Mechanisms and Treatment of Neuropathic Pain-A Brief Discussion. Biomedicines 2024, 12, 204. [Google Scholar] [CrossRef]
- van Hecke, O.; Austin, S.K.; Khan, R.A.; Smith, B.H.; Torrance, N. Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain 2014, 155, 654–662. [Google Scholar] [CrossRef]
- Cruccu, G.; Truini, A. A review of Neuropathic Pain: From Guidelines to Clinical Practice. Pain Ther. 2017, 6, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, E.; Mammana, S.; Nicoletti, F.; Bramanti, P.; Mazzon, E. The neuropathic pain: An overview of the current treatment and future therapeutic approaches. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419838383. [Google Scholar] [CrossRef] [PubMed]
- Serrano Afonso, A.; Carnaval, T.; Videla Ces, S. Combination Therapy for Neuropathic Pain: A Review of Recent Evidence. J. Clin. Med. 2021, 10, 3533. [Google Scholar] [CrossRef]
- Binder, A.; Baron, R. The Pharmacological Therapy of Chronic Neuropathic Pain. Dtsch. Arztebl. Int. 2016, 113, 616–625. [Google Scholar] [CrossRef]
- Hendler, N.H.; Kozikowski, J.G. Overlooked physical diagnoses in chronic pain patients involved in litigation. Psychosomatics 1993, 34, 494–501. [Google Scholar] [CrossRef]
- van Velzen, M.; Dahan, A.; Niesters, M. Neuropathic Pain: Challenges and Opportunities. Front. Pain Res. 2020, 1, 1. [Google Scholar] [CrossRef]
- Bannister, K.; Dickenson, A.H. The plasticity of descending controls in pain: Translational probing. J. Physiol. 2017, 595, 4159–4166. [Google Scholar] [CrossRef] [PubMed]
- Bannwarth, B.; Kostine, M. Targeting nerve growth factor (NGF) for pain management: What does the future hold for NGF antagonists? Drugs 2014, 74, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpaa, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; Di Lazzaro, V.; Filipovic, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018). Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar] [CrossRef]
- Darnall, B.D.; Ziadni, M.S.; Stieg, R.L.; Mackey, I.G.; Kao, M.C.; Flood, P. Patient-Centered Prescription Opioid Tapering in Community Outpatients With Chronic Pain. JAMA Intern. Med. 2018, 178, 707–708. [Google Scholar] [CrossRef]
- Makridakis, M.; Roubelakis, M.G.; Vlahou, A. Stem cells: Insights into the secretome. Biochim. Biophys. Acta 2013, 1834, 2380–2384. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef] [PubMed]
- Tavasolian, F.; Inman, R.D. Biology and therapeutic potential of mesenchymal stem cell extracellular vesicles in axial spondyloarthritis. Commun. Biol. 2023, 6, 413. [Google Scholar] [CrossRef]
- Bruno, S.; Deregibus, M.C.; Camussi, G. The secretome of mesenchymal stromal cells: Role of extracellular vesicles in immunomodulation. Immunol. Lett. 2015, 168, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Baglio, S.R.; Pegtel, D.M.; Baldini, N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front. Physiol. 2012, 3, 359. [Google Scholar] [CrossRef]
- Lener, T.; Gimona, M.; Aigner, L.; Borger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef]
- Tögel, F.E.; Westenfelder, C. Mesenchymal stem cells: A new therapeutic tool for AKI. Nat. Rev. Nephrol. 2010, 6, 179–183. [Google Scholar] [CrossRef]
- Mancuso, P.; Raman, S.; Glynn, A.; Barry, F.; Murphy, J.M. Mesenchymal Stem Cell Therapy for Osteoarthritis: The Critical Role of the Cell Secretome. Front. Bioeng. Biotechnol. 2019, 7, 9. [Google Scholar] [CrossRef]
- Klingemann, H.; Matzilevich, D.; Marchand, J. Mesenchymal Stem Cells—Sources and Clinical Applications. Transfus. Med. Hemother. 2008, 35, 272–277. [Google Scholar] [CrossRef]
- Nancarrow-Lei, R.; Mafi, P.; Mafi, R.; Khan, W. A Systemic Review of Adult Mesenchymal Stem Cell Sources and their Multilineage Differentiation Potential Relevant to Musculoskeletal Tissue Repair and Regeneration. Curr. Stem Cell Res. Ther. 2017, 12, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, V.V.; Patel, N.H.; Smith, E.E.; Barnes, C.L.; Gustafson, M.P.; Rao, R.R.; Samsonraj, R.M. Clinical utility of mesenchymal stem/stromal cells in regenerative medicine and cellular therapy. J. Biol. Eng. 2023, 17, 44. [Google Scholar] [CrossRef]
- Bozorgmehr, M.; Gurung, S.; Darzi, S.; Nikoo, S.; Kazemnejad, S.; Zarnani, A.H.; Gargett, C.E. Endometrial and Menstrual Blood Mesenchymal Stem/Stromal Cells: Biological Properties and Clinical Application. Front. Cell Dev. Biol. 2020, 8, 497. [Google Scholar] [CrossRef]
- Chen, L.; Qu, J.; Cheng, T.; Chen, X.; Xiang, C. Menstrual blood-derived stem cells: Toward therapeutic mechanisms, novel strategies, and future perspectives in the treatment of diseases. Stem Cell Res. Ther. 2019, 10, 406. [Google Scholar] [CrossRef]
- Zhou, Q.; Cheng, Y.; Sun, F.; Shen, J.; Nasser, M.I.; Zhu, P.; Zhang, X.; Li, Y.; Yin, G.; Wang, Y.; et al. A Comprehensive Review of the Therapeutic Value of Urine-Derived Stem Cells. Front. Genet. 2021, 12, 781597. [Google Scholar] [CrossRef] [PubMed]
- Mane, S.; Taneja, S.; Madala, J.S.; Agarkhedkar, S.; Khetan, M. Study of Stem Cells in Human Milk. Cureus 2022, 14, e23701. [Google Scholar] [CrossRef]
- Rahmani-Moghadam, E.; Zarrin, V.; Mahmoodzadeh, A.; Owrang, M.; Talaei-Khozani, T. Comparison of the Characteristics of Breast Milk-derived Stem Cells with the Stem Cells Derived from the Other Sources: A Comparative Review. Curr. Stem Cell Res. Ther. 2022, 17, 71–90. [Google Scholar] [CrossRef] [PubMed]
- Semita, I.N.; Utomo, D.N.; Suroto, H.; Sudiana, I.K.; Gandi, P. The mechanism of human neural stem cell secretomes improves neuropathic pain and locomotor function in spinal cord injury rat models: Through antioxidant, anti-inflammatory, anti-matrix degradation, and neurotrophic activities. Korean J. Pain 2023, 36, 72–83. [Google Scholar] [CrossRef]
- Drago, D.; Cossetti, C.; Iraci, N.; Gaude, E.; Musco, G.; Bachi, A.; Pluchino, S. The stem cell secretome and its role in brain repair. Biochimie 2013, 95, 2271–2285. [Google Scholar] [CrossRef]
- Deng, H.; Zhao, J.; Li, J.; Chen, C.; Hu, Z.; Wu, X.; Ge, L. Therapeutic Efficacy of Extracellular Vesicles Derived from Stem Cell for Alzheimer’s Disease: A Meta-Analysis Study. Front. Biosci. 2024, 29, 340. [Google Scholar] [CrossRef]
- Sharma, B.; Sehrawat, H.; Gupta, V. Chapter 14—Advances in regenerative medicines based on mesenchymal stem cell secretome. In Computational Biology for Stem Cell Research; Raghav, P.K., Kumar, R., Lathwal, A., Sharma, N., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 175–185. [Google Scholar]
- Ratajczak, J.; Wysoczynski, M.; Hayek, F.; Janowska-Wieczorek, A.; Ratajczak, M.Z. Membrane-derived microvesicles: Important and underappreciated mediators of cell-to-cell communication. Leukemia 2006, 20, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, J.; Miekus, K.; Kucia, M.; Zhang, J.; Reca, R.; Dvorak, P.; Ratajczak, M.Z. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: Evidence for horizontal transfer of mRNA and protein delivery. Leukemia 2006, 20, 847–856. [Google Scholar] [CrossRef]
- Willis, C.M.; Nicaise, A.M.; Hamel, R.; Pappa, V.; Peruzzotti-Jametti, L.; Pluchino, S. Harnessing the Neural Stem Cell Secretome for Regenerative Neuroimmunology. Front. Cell Neurosci. 2020, 14, 590960. [Google Scholar] [CrossRef] [PubMed]
- Cervenka, J.; Tyleckova, J.; Kupcova Skalnikova, H.; Vodickova Kepkova, K.; Poliakh, I.; Valekova, I.; Pfeiferova, L.; Kolar, M.; Vaskovicova, M.; Pankova, T.; et al. Proteomic Characterization of Human Neural Stem Cells and Their Secretome During in vitro Differentiation. Front. Cell Neurosci. 2020, 14, 612560. [Google Scholar] [CrossRef]
- Muzes, G.; Sipos, F. Mesenchymal Stem Cell-Derived Secretome: A Potential Therapeutic Option for Autoimmune and Immune-Mediated Inflammatory Diseases. Cells 2022, 11, 2300. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Lee, J.; Kwon, Y.; Park, K.S.; Jeong, J.H.; Choi, S.J.; Bang, S.I.; Chang, J.W.; Lee, C. Comparative Proteomic Analysis of the Mesenchymal Stem Cells Secretome from Adipose, Bone Marrow, Placenta and Wharton’s Jelly. Int. J. Mol. Sci. 2021, 22, 845. [Google Scholar] [CrossRef] [PubMed]
- El Omar, R.; Beroud, J.; Stoltz, J.F.; Menu, P.; Velot, E.; Decot, V. Umbilical cord mesenchymal stem cells: The new gold standard for mesenchymal stem cell-based therapies? Tissue Eng. Part. B Rev. 2014, 20, 523–544. [Google Scholar] [CrossRef]
- Um, S.; Ha, J.; Choi, S.J.; Oh, W.; Jin, H.J. Prospects for the therapeutic development of umbilical cord blood-derived mesenchymal stem cells. World J. Stem Cells 2020, 12, 1511–1528. [Google Scholar] [CrossRef]
- Naeem, A.; Gupta, N.; Naeem, U.; Khan, M.J.; Elrayess, M.A.; Cui, W.; Albanese, C. A comparison of isolation and culture protocols for human amniotic mesenchymal stem cells. Cell Cycle 2022, 21, 1543–1556. [Google Scholar] [CrossRef]
- Loukogeorgakis, S.P.; De Coppi, P. Stem cells from amniotic fluid--Potential for regenerative medicine. Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 31, 45–57. [Google Scholar] [CrossRef]
- Biswas, A.; Rajasekaran, R.; Saha, B.; Dixit, K.; Vaidya, P.V.; Ojha, A.K.; Dhara, S. Human placenta/umbilical cord derivatives in regenerative medicine—Prospects and challenges. Biomater. Sci. 2023, 11, 4789–4821. [Google Scholar] [CrossRef]
- Sardesai, V.S.; Shafiee, A.; Fisk, N.M.; Pelekanos, R.A. Avoidance of Maternal Cell Contamination and Overgrowth in Isolating Fetal Chorionic Villi Mesenchymal Stem Cells from Human Term Placenta. Stem Cells Transl. Med. 2017, 6, 1070–1084. [Google Scholar] [CrossRef]
- Chu, D.T.; Phuong, T.N.T.; Tien, N.L.B.; Tran, D.K.; Thanh, V.V.; Quang, T.L.; Truong, D.T.; Pham, V.H.; Ngoc, V.T.N.; Chu-Dinh, T.; et al. An Update on the Progress of Isolation, Culture, Storage, and Clinical Application of Human Bone Marrow Mesenchymal Stem/Stromal Cells. Int. J. Mol. Sci. 2020, 21, 708. [Google Scholar] [CrossRef]
- Melief, S.M.; Zwaginga, J.J.; Fibbe, W.E.; Roelofs, H. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl. Med. 2013, 2, 455–463. [Google Scholar] [CrossRef]
- Usas, A.; Maciulaitis, J.; Maciulaitis, R.; Jakuboniene, N.; Milasius, A.; Huard, J. Skeletal muscle-derived stem cells: Implications for cell-mediated therapies. Medicina 2011, 47, 469–479. [Google Scholar] [CrossRef]
- Ferretti, C.; Mattioli-Belmonte, M. Periosteum derived stem cells for regenerative medicine proposals: Boosting current knowledge. World J. Stem Cells 2014, 6, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Gao, J.; Mi, L.; Zhang, G.; Zhang, L.; Zhang, N.; Huo, R.; Hu, J.; Xu, K. Synovial membrane mesenchymal stem cells: Past life, current situation, and application in bone and joint diseases. Stem Cell Res. Ther. 2020, 11, 381. [Google Scholar] [CrossRef]
- Jones, E.A.; Crawford, A.; English, A.; Henshaw, K.; Mundy, J.; Corscadden, D.; Chapman, T.; Emery, P.; Hatton, P.; McGonagle, D. Synovial fluid mesenchymal stem cells in health and early osteoarthritis: Detection and functional evaluation at the single-cell level. Arthritis Rheum. 2008, 58, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Rikkers, M.; Korpershoek, J.V.; Levato, R.; Malda, J.; Vonk, L.A. The clinical potential of articular cartilage-derived progenitor cells: A systematic review. NPJ Regen. Med. 2022, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Adesida, A.B.; Jomha, N.M. Meniscus repair using mesenchymal stem cells—A comprehensive review. Stem Cell Res. Ther. 2015, 6, 86. [Google Scholar] [CrossRef]
- Bruno, S.; Herrera Sanchez, M.B.; Chiabotto, G.; Fonsato, V.; Navarro-Tableros, V.; Pasquino, C.; Tapparo, M.; Camussi, G. Human Liver Stem Cells: A Liver-Derived Mesenchymal Stromal Cell-Like Population With Pro-regenerative Properties. Front. Cell Dev. Biol. 2021, 9, 644088. [Google Scholar] [CrossRef]
- Doherty, D.F.; Roets, L.; Krasnodembskaya, A.D. The Role of Lung Resident Mesenchymal Stromal Cells in the Pathogenesis and Repair of Chronic Lung Disease. Stem Cells 2023, 41, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Ledesma-Martinez, E.; Mendoza-Nunez, V.M.; Santiago-Osorio, E. Mesenchymal Stem Cells Derived from Dental Pulp: A Review. Stem Cells Int. 2016, 2016, 4709572. [Google Scholar] [CrossRef]
- Peng, Y.; Jaar, J.; Tran, S.D. Gingival mesenchymal stem cells: Biological properties and therapeutic applications. J. Oral Biol. Craniofac. Res. 2024, 14, 547–569. [Google Scholar] [CrossRef]
- Langthasa, J.; Guan, L.; Jinagal, S.L.; Le, Q.T. Salivary gland stem/progenitor cells: Advancing from basic science to clinical applications. Cell Regen. 2025, 14, 4. [Google Scholar] [CrossRef]
- Queckborner, S.; Syk Lundberg, E.; Gemzell-Danielsson, K.; Davies, L.C. Endometrial stromal cells exhibit a distinct phenotypic and immunomodulatory profile. Stem Cell Res. Ther. 2020, 11, 15. [Google Scholar] [CrossRef]
- Ouryazdanpanah, N.; Dabiri, S.; Derakhshani, A.; Vahidi, R.; Farsinejad, A. Peripheral Blood-Derived Mesenchymal Stem Cells: Growth Factor-Free Isolation, Molecular Characterization and Differentiation. Iran. J. Pathol. 2018, 13, 461–466. [Google Scholar]
- Sun, Y.; Zhao, H.; Yang, S.; Wang, G.; Zhu, L.; Sun, C.; An, Y. Urine-derived stem cells: Promising advancements and applications in regenerative medicine and beyond. Heliyon 2024, 10, e27306. [Google Scholar] [CrossRef]
- Estermann, M.A.; Mariette, M.M.; Moreau, J.L.M.; Combes, A.N.; Smith, C.A. PAX2 (+) Mesenchymal Origin of Gonadal Supporting Cells Is Conserved in Birds. Front. Cell Dev. Biol. 2021, 9, 735203. [Google Scholar] [CrossRef] [PubMed]
- Kohno, Y.; Mizuno, M.; Ozeki, N.; Katano, H.; Komori, K.; Fujii, S.; Otabe, K.; Horie, M.; Koga, H.; Tsuji, K.; et al. Yields and chondrogenic potential of primary synovial mesenchymal stem cells are comparable between rheumatoid arthritis and osteoarthritis patients. Stem Cell Res. Ther. 2017, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Qu, J.; Xiang, C. The multi-functional roles of menstrual blood-derived stem cells in regenerative medicine. Stem Cell Res. Ther. 2019, 10, 1. [Google Scholar] [CrossRef]
- Teixeira, F.G.; Carvalho, M.M.; Sousa, N.; Salgado, A.J. Mesenchymal stem cells secretome: A new paradigm for central nervous system regeneration? Cell Mol. Life Sci. 2013, 70, 3871–3882. [Google Scholar] [CrossRef]
- Spinelli, M.; Fusco, S.; Grassi, C. Therapeutic potential of stem cell-derived extracellular vesicles in neurodegenerative diseases associated with cognitive decline. Stem Cells 2025, 43, sxae074. [Google Scholar] [CrossRef]
- Cofano, F.; Boido, M.; Monticelli, M.; Zenga, F.; Ducati, A.; Vercelli, A.; Garbossa, D. Mesenchymal Stem Cells for Spinal Cord Injury: Current Options, Limitations, and Future of Cell Therapy. Int. J. Mol. Sci. 2019, 20, 2698. [Google Scholar] [CrossRef]
- Caplan, A.I.; Correa, D. The MSC: An injury drugstore. Cell Stem Cell 2011, 9, 11–15. [Google Scholar] [CrossRef]
- Kaka, G.R.; Modarresi, F. Conditioned medium derived from mesenchymal stem cells and spinal cord injury: A review of the current therapeutic capacities. IBRO Neurosci. Rep. 2025, 18, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Ankrum, J.; Karp, J.M. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol. Med. 2010, 16, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Discher, D.E.; Peault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef]
- Pischiutta, F.; Tribuzio, F.; Magatti, M.; De Simone, G.; Moro, F.; Nattino, G.; Signorini, F.; Loose, L.; Caruso, E.; Bertani, C.; et al. Mesenchymal Stromal Cell Secretome and Its Key Bioactive Metabolites Induce Long-Term Neuroprotection After Traumatic Brain Injury in Mice. Adv. Sci. 2025, 12, e15508. [Google Scholar] [CrossRef]
- Hasanein, P.; Emamjomeh, A.; Chenarani, N.; Bohlooli, M. Beneficial effects of rutin in diabetes-induced deficits in acquisition learning, retention memory and pain perception in rats. Nutr. Neurosci. 2020, 23, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Joshi, H.P.; Jo, H.J.; Kim, Y.H.; An, S.B.; Park, C.K.; Han, I. Stem Cell Therapy for Modulating Neuroinflammation in Neuropathic Pain. Int. J. Mol. Sci. 2021, 22, 4853. [Google Scholar] [CrossRef]
- Giannasi, C.; Della Morte, E.; Cadelano, F.; Valenza, A.; Casati, S.; Dei Cas, M.; Niada, S.; Brini, A.T. Boosting the therapeutic potential of cell secretome against osteoarthritis: Comparison of cytokine-based priming strategies. Biomed. Pharmacother. 2024, 170, 115970. [Google Scholar] [CrossRef]
- Ragni, E.; Perucca Orfei, C.; De Luca, P.; Mondadori, C.; Vigano, M.; Colombini, A.; de Girolamo, L. Inflammatory priming enhances mesenchymal stromal cell secretome potential as a clinical product for regenerative medicine approaches through secreted factors and EV-miRNAs: The example of joint disease. Stem Cell Res. Ther. 2020, 11, 165. [Google Scholar] [CrossRef]
- Rossello-Gelabert, M.; Igartua, M.; Santos-Vizcaino, E.; Hernandez, R.M. Fine-tuning licensing strategies to boost MSC-based immunomodulatory secretome. Stem Cell Res. Ther. 2025, 16, 183. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, Y.; Casado-Santos, A.; Gonzalez-Cubero, E.; Gonzalez-Fernandez, M.L.; Selles-Egea, A.; Villar-Suarez, V. Optimizing mesenchymal stromal cells priming strategies for tailored effects on the secretome. Biomed. Pharmacother. 2025, 188, 118218. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Wang, L.; Zhang, Z.; Lu, D.; Lu, M.; Chopp, M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 2001, 32, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.; Ene, J.; Nathani, A.; Singh, M.; Li, Y.; Zeng, C. Effects of Physical Cues on Stem Cell-Derived Extracellular Vesicles toward Neuropathy Applications. Biomedicines 2024, 12, 489. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A. Exosomes for the Management of Low Back Pain: A Review of Current Clinical Evidence. Cureus 2024, 16, e57539. [Google Scholar] [CrossRef] [PubMed]
- Ferrini, F.; Juarez, E.H.; Merighi, A. Does mesenchymal stem cell’s secretome affect spinal sensory circuits? Implication for pain therapies. Neural Regen. Res. 2025, 20, 181–183. [Google Scholar] [CrossRef]
- Brini, A.T.; Amodeo, G.; Ferreira, L.M.; Milani, A.; Niada, S.; Moschetti, G.; Franchi, S.; Borsani, E.; Rodella, L.F.; Panerai, A.E.; et al. Therapeutic effect of human adipose-derived stem cells and their secretome in experimental diabetic pain. Sci. Rep. 2017, 7, 9904. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; Tenci, B.; Micheli, L.; Vona, A.; Corti, F.; Zanardelli, M.; Lapucci, A.; Clemente, A.M.; Failli, P.; Ghelardini, C. Adipose-derived stem cells decrease pain in a rat model of oxaliplatin-induced neuropathy: Role of VEGF-A modulation. Neuropharmacology 2018, 131, 166–175. [Google Scholar] [CrossRef]
- Evangelista, A.F.; Vannier-Santos, M.A.; de Assis Silva, G.S.; Silva, D.N.; Juiz, P.J.L.; Nonaka, C.K.V.; Dos Santos, R.R.; Soares, M.B.P.; Villarreal, C.F. Bone marrow-derived mesenchymal stem/stromal cells reverse the sensorial diabetic neuropathy via modulation of spinal neuroinflammatory cascades. J. Neuroinflamm. 2018, 15, 189. [Google Scholar] [CrossRef] [PubMed]
- Gama, K.B.; Santos, D.S.; Evangelista, A.F.; Silva, D.N.; de Alcantara, A.C.; Dos Santos, R.R.; Soares, M.B.P.; Villarreal, C.F. Conditioned Medium of Bone Marrow-Derived Mesenchymal Stromal Cells as a Therapeutic Approach to Neuropathic Pain: A Preclinical Evaluation. Stem Cells Int. 2018, 2018, 8179013. [Google Scholar] [CrossRef] [PubMed]
- Khatab, S.; van Osch, G.J.; Kops, N.; Bastiaansen-Jenniskens, Y.M.; Bos, P.K.; Verhaar, J.A.; Bernsen, M.R.; van Buul, G.M. Mesenchymal stem cell secretome reduces pain and prevents cartilage damage in a murine osteoarthritis model. Eur. Cell Mater. 2018, 36, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Shiue, S.J.; Rau, R.H.; Shiue, H.S.; Hung, Y.W.; Li, Z.X.; Yang, K.D.; Cheng, J.K. Mesenchymal stem cell exosomes as a cell-free therapy for nerve injury-induced pain in rats. Pain 2019, 160, 210–223. [Google Scholar] [CrossRef]
- De Gregorio, C.; Contador, D.; Diaz, D.; Carcamo, C.; Santapau, D.; Lobos-Gonzalez, L.; Acosta, C.; Campero, M.; Carpio, D.; Gabriele, C.; et al. Human adipose-derived mesenchymal stem cell-conditioned medium ameliorates polyneuropathy and foot ulceration in diabetic BKS db/db mice. Stem Cell Res. Ther. 2020, 11, 168. [Google Scholar] [CrossRef]
- Fan, B.; Li, C.; Szalad, A.; Wang, L.; Pan, W.; Zhang, R.; Chopp, M.; Zhang, Z.G.; Liu, X.S. Mesenchymal stromal cell-derived exosomes ameliorate peripheral neuropathy in a mouse model of diabetes. Diabetologia 2020, 63, 431–443. [Google Scholar] [CrossRef]
- Amodeo, G.; Niada, S.; Moschetti, G.; Franchi, S.; Savadori, P.; Brini, A.T.; Sacerdote, P. Secretome of human adipose-derived mesenchymal stem cell relieves pain and neuroinflammation independently of the route of administration in experimental osteoarthritis. Brain Behav. Immun. 2021, 94, 29–40. [Google Scholar] [CrossRef]
- Fan, B.; Chopp, M.; Zhang, Z.G.; Liu, X.S. Treatment of diabetic peripheral neuropathy with engineered mesenchymal stromal cell-derived exosomes enriched with microRNA-146a provide amplified therapeutic efficacy. Exp. Neurol. 2021, 341, 113694. [Google Scholar] [CrossRef]
- Masoodifar, M.; Hajihashemi, S.; Pazhoohan, S.; Nazemi, S.; Mojadadi, M.S. Effect of the conditioned medium of mesenchymal stem cells on the expression levels of P2X4 and P2X7 purinergic receptors in the spinal cord of rats with neuropathic pain. Purinergic Signal. 2021, 17, 143–150. [Google Scholar] [CrossRef]
- Hershfield, M.R.; Strain, M.M.; Chavez, R.; Priess, M.R.; Mares, A.; Gautam, A.; Dimitrov, G.; Yang, R.; Trevino, A.V.; Wells, C.K.; et al. Exploring the Secretome’s Biomarker and Analgesic Potential. Am. J. Psychiatry Neurosci. 2022, 10, 63–76. [Google Scholar] [CrossRef]
- Liu, Y.; Kano, F.; Hashimoto, N.; Xia, L.; Zhou, Q.; Feng, X.; Hibi, H.; Miyazaki, A.; Iwamoto, T.; Matsuka, Y.; et al. Conditioned Medium From the Stem Cells of Human Exfoliated Deciduous Teeth Ameliorates Neuropathic Pain in a Partial Sciatic Nerve Ligation Model. Front. Pharmacol. 2022, 13, 745020. [Google Scholar] [CrossRef]
- Gonzalez-Cubero, E.; Gonzalez-Fernandez, M.L.; Rodriguez-Diaz, M.; Palomo-Irigoyen, M.; Woodhoo, A.; Villar-Suarez, V. Application of adipose-derived mesenchymal stem cells in an in vivo model of peripheral nerve damage. Front. Cell Neurosci. 2022, 16, 992221. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, Y.; Ouyang, F.; Su, M.; Li, W.; Chen, J.; Xiao, H.; Zhou, X.; Liu, B. Extracellular vesicles derived from mesenchymal stem cells alleviate neuroinflammation and mechanical allodynia in interstitial cystitis rats by inhibiting NLRP3 inflammasome activation. J. Neuroinflamm. 2022, 19, 80. [Google Scholar] [CrossRef]
- Bousnaki, M.; Bakopoulou, A.; Grivas, I.; Bekiari, C.; Pich, A.; Rizk, M.; Keklikoglou, K.; Papachristou, E.; Papadopoulos, G.C.; Kritis, A.; et al. Managing Temporomandibular Joint Osteoarthritis by Dental Stem Cell Secretome. Stem Cell Rev. Rep. 2023, 19, 2957–2979. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Gao, L.F.; Zhang, Y.N.; Kong, X.Q.; Jia, S.; Meng, C.Y. Huc-MSCs-derived exosomes attenuate neuropathic pain by inhibiting activation of the TLR2/MyD88/NF-kappaB signaling pathway in the spinal microglia by targeting Rsad2. Int. Immunopharmacol. 2023, 114, 109505. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Gao, L.F.; Kong, X.Q.; Zhang, Y.N.; Jia, S.; Meng, C.Y. Mesenchymal stem cell-derived extracellular vesicles carrying miR-99b-3p restrain microglial activation and neuropathic pain by stimulating autophagy. Int. Immunopharmacol. 2023, 115, 109695. [Google Scholar] [CrossRef]
- Nazemi, S.; Helmi, M.; Kafami, M.; Amin, B.; Mojadadi, M.S. Preemptive administration of mesenchymal stem cells-derived conditioned medium can attenuate the development of neuropathic pain in rats via downregulation of proinflammatory cytokines. Behav. Brain Res. 2024, 461, 114858. [Google Scholar] [CrossRef]
- Quintanilla, M.E.; Quezada, M.; Morales, P.; Berrios-Carcamo, P.; Santapau, D.; Ezquer, M.; Herrera-Marschitz, M.; Israel, Y.; Ezquer, F. Effect of human mesenchymal stem cell secretome administration on morphine self-administration and relapse in two animal models of opioid dependence. Transl. Psychiatry 2022, 12, 462. [Google Scholar] [CrossRef]
- Quintanilla, M.E.; Ezquer, F.; Morales, P.; Santapau, D.; Berrios-Carcamo, P.; Ezquer, M.; Herrera-Marschitz, M.; Israel, Y. Intranasal mesenchymal stem cell secretome administration markedly inhibits alcohol and nicotine self-administration and blocks relapse-intake: Mechanism and translational options. Stem Cell Res. Ther. 2019, 10, 205. [Google Scholar] [CrossRef] [PubMed]
- Ezquer, F.; Quintanilla, M.E.; Morales, P.; Santapau, D.; Ezquer, M.; Kogan, M.J.; Salas-Huenuleo, E.; Herrera-Marschitz, M.; Israel, Y. Intranasal delivery of mesenchymal stem cell-derived exosomes reduces oxidative stress and markedly inhibits ethanol consumption and post-deprivation relapse drinking. Addict. Biol. 2019, 24, 994–1007. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, Y.; Song, Y.; Gao, Y.; Hao, C.; Zhou, Y.; Bao, S.; Guo, J.; Li, X. Human umbilical cord mesenchymal stem cells reverse depression in rats induced by chronic unpredictable mild stress combined with lipopolysaccharide. CNS Neurosci. Ther. 2024, 30, e14644. [Google Scholar] [CrossRef]
- Liu, G.; Miao, L.; Niu, H.; Wang, H.; Yan, L.; Chen, Y.; Zhang, C.; Li, X.; Mi, Y.; Xu, L.; et al. Human Umbilical Cord Mesenchymal Stem Cells Ameliorated Chronic Unpredictable Mild Stress-Induced Depression and Anxiety by Alleviating Neuroinflammation. J. Neuroimmune Pharmacol. 2025, 20, 45. [Google Scholar] [CrossRef]
- Huang, X.; Fei, G.Q.; Liu, W.J.; Ding, J.; Wang, Y.; Wang, H.; Ji, J.L.; Wang, X. Adipose-derived mesenchymal stem cells protect against CMS-induced depression-like behaviors in mice via regulating the Nrf2/HO-1 and TLR4/NF-kappaB signaling pathways. Acta Pharmacol. Sin. 2020, 41, 612–619. [Google Scholar] [CrossRef]
- Santamaria, G.; Brandi, E.; Vitola, P.; Grandi, F.; Ferrara, G.; Pischiutta, F.; Vegliante, G.; Zanier, E.R.; Re, F.; Uccelli, A.; et al. Intranasal delivery of mesenchymal stem cell secretome repairs the brain of Alzheimer’s mice. Cell Death Differ. 2021, 28, 203–218. [Google Scholar] [CrossRef]
- Reza-Zaldivar, E.E.; Hernandez-Sapiens, M.A.; Gutierrez-Mercado, Y.K.; Sandoval-Avila, S.; Gomez-Pinedo, U.; Marquez-Aguirre, A.L.; Vazquez-Mendez, E.; Padilla-Camberos, E.; Canales-Aguirre, A.A. Mesenchymal stem cell-derived exosomes promote neurogenesis and cognitive function recovery in a mouse model of Alzheimer’s disease. Neural Regen. Res. 2019, 14, 1626–1634. [Google Scholar] [CrossRef]
- Teixeira, F.G.; Carvalho, M.M.; Panchalingam, K.M.; Rodrigues, A.J.; Mendes-Pinheiro, B.; Anjo, S.; Manadas, B.; Behie, L.A.; Sousa, N.; Salgado, A.J. Impact of the Secretome of Human Mesenchymal Stem Cells on Brain Structure and Animal Behavior in a Rat Model of Parkinson’s Disease. Stem Cells Transl. Med. 2017, 6, 634–646. [Google Scholar] [CrossRef]
- Oses, C.; Olivares, B.; Ezquer, M.; Acosta, C.; Bosch, P.; Donoso, M.; Leniz, P.; Ezquer, F. Preconditioning of adipose tissue-derived mesenchymal stem cells with deferoxamine increases the production of pro-angiogenic, neuroprotective and anti-inflammatory factors: Potential application in the treatment of diabetic neuropathy. PLoS ONE 2017, 12, e0178011. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, C.; Ezquer, F. Sensory neuron cultures derived from adult db/db mice as a simplified model to study type-2 diabetes-associated axonal regeneration defects. Dis. Model. Mech. 2021, 14, 046334. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.M.; Campos, J.; Silva, D.; Barata Antunes, S.; Lima, R.; Coelho, C.; Marote, A.M.; Leite-Almeida, H.; Silva, N.; Salgado, A.J. Cerebral Organoids to Study Central Mechanisms of Pain: The Effect of Stem Cell Secretome on Opioid Receptors and Neuroplasticity. Stem Cells Dev. 2022, 31, 641–657. [Google Scholar] [CrossRef]
- Goncalves, R.M.; Saggese, T.; Yong, Z.; Ferreira, J.R.; Ignatius, A.; Wilke, H.J.; Neidlinger-Wilke, C.; Teixeira, G.Q. Interleukin-1beta More Than Mechanical Loading Induces a Degenerative Phenotype in Human Annulus Fibrosus Cells, Partially Impaired by Anti-Proteolytic Activity of Mesenchymal Stem Cell Secretome. Front. Bioeng. Biotechnol. 2021, 9, 802789. [Google Scholar] [CrossRef]
- Gonzalez-Cubero, E.; Gonzalez-Fernandez, M.L.; Olivera, E.R.; Villar-Suarez, V. Extracellular vesicle and soluble fractions of adipose tissue-derived mesenchymal stem cells secretome induce inflammatory cytokines modulation in an in vitro model of discogenic pain. Spine J. 2022, 22, 1222–1234. [Google Scholar] [CrossRef]
- Thompson, S.J.; Pitcher, M.H.; Stone, L.S.; Tarum, F.; Niu, G.; Chen, X.; Kiesewetter, D.O.; Schweinhardt, P.; Bushnell, M.C. Chronic neuropathic pain reduces opioid receptor availability with associated anhedonia in rat. Pain 2018, 159, 1856–1866. [Google Scholar] [CrossRef]
- Kaczmarski, P.; Karuga, F.F.; Szmyd, B.; Sochal, M.; Bialasiewicz, P.; Strzelecki, D.; Gabryelska, A. The Role of Inflammation, Hypoxia, and Opioid Receptor Expression in Pain Modulation in Patients Suffering from Obstructive Sleep Apnea. Int. J. Mol. Sci. 2022, 23, 9080. [Google Scholar] [CrossRef]
- Zhang, X.; Bao, L.; Li, S. Opioid receptor trafficking and interaction in nociceptors. Br. J. Pharmacol. 2015, 172, 364–374. [Google Scholar] [CrossRef]
- Hernandez Baltazar, D.; Nadella, R.; Barrientos Bonilla, A.; Flores Martinez, Y.; Olguin, A.; Heman Bozadas, P.; Rovirosa Hernandez, M.; Cibrian Llanderal, I. Does lipopolysaccharide-based neuroinflammation induce microglia polarization? Folia Neuropathol. 2020, 58, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Skrzypczak-Wiercioch, A.; Salat, K. Lipopolysaccharide-Induced Model of Neuroinflammation: Mechanisms of Action, Research Application and Future Directions for Its Use. Molecules 2022, 27, 5481. [Google Scholar] [CrossRef]
- Sano, M.; Tanabe, A.; Urushihata, N.; Liu, X.L. Effect of human adipose-derived mesenchymal stem cell conditioned medium on musculoskeletal pain. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 1570–1578. [Google Scholar] [CrossRef] [PubMed]
- Partan, R.U.; Putra, K.M.; Kusuma, N.F.; Darma, S.; Reagan, M.; Muthia, P.; Radiandina, A.S.; Saleh, M.I.; Salim, E.M. Umbilical Cord Mesenchymal Stem Cell Secretome Improves Clinical Outcomes and Changes Biomarkers in Knee Osteoarthritis. J. Clin. Med. 2023, 12, 7138. [Google Scholar] [CrossRef]
- Rahyussalim, A.J.; Priyono, A.H.; Budhy, F.; Muntaha, M.; Ramadhani, R.; Canintika, A.F. Percutaneous laser disc decompression combined with secretome of umbilical cord-derived mesenchymal stem cells in a patient with spinal cord injury: A case report. Int. J. Surg. Case Rep. 2024, 114, 109219. [Google Scholar] [CrossRef] [PubMed]
- Widodo, W.; Dilogo, I.H.; Kamal, A.F.; Antarianto, R.D.; Wuyung, P.E.; Siregar, N.C.; Octaviana, F.; Kekalih, A.; Suroto, H.; Latief, W.; et al. Functional outcome and histologic analysis of late onset total type brachial plexus injury treated with intercostal nerve transfer to median nerve with local umbilical cord-derived mesenchymal stem cells or secretome injection: A double-blinded, randomized control study. Eur. J. Orthop. Surg. Traumatol. 2024, 34, 4073–4082. [Google Scholar] [CrossRef]
- Mello, D.B.; Mesquita, F.C.P.; Silva Dos Santos, D.; Asensi, K.D.; Dias, M.L.; Campos de Carvalho, A.C.; Goldenberg, R.; Kasai-Brunswick, T.H. Mesenchymal Stromal Cell-Based Products: Challenges and Clinical Therapeutic Options. Int. J. Mol. Sci. 2024, 25, 6063. [Google Scholar] [CrossRef]
- Teixeira, F.G.; Salgado, A.J. Mesenchymal stem cells secretome: Current trends and future challenges. Neural Regen. Res. 2020, 15, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Kavaldzhieva, K.; Mladenov, N.; Markova, M.; Belemezova, K. Mesenchymal Stem Cell Secretome: Potential Applications in Human Infertility Caused by Hormonal Imbalance, External Damage, or Immune Factors. Biomedicines 2025, 13, 586. [Google Scholar] [CrossRef] [PubMed]
- Chaurawal, N.; Kataria, M.; Kumar, M.V.; Mishra, N.P.; Goni, V.G.; Raza, K. Emerging Advances in Nanocarriers Approaches in the Effective Therapy of Pain Related Disorders: Recent Evidence and Futuristic Needs. AAPS PharmSciTech 2023, 24, 111. [Google Scholar] [CrossRef] [PubMed]
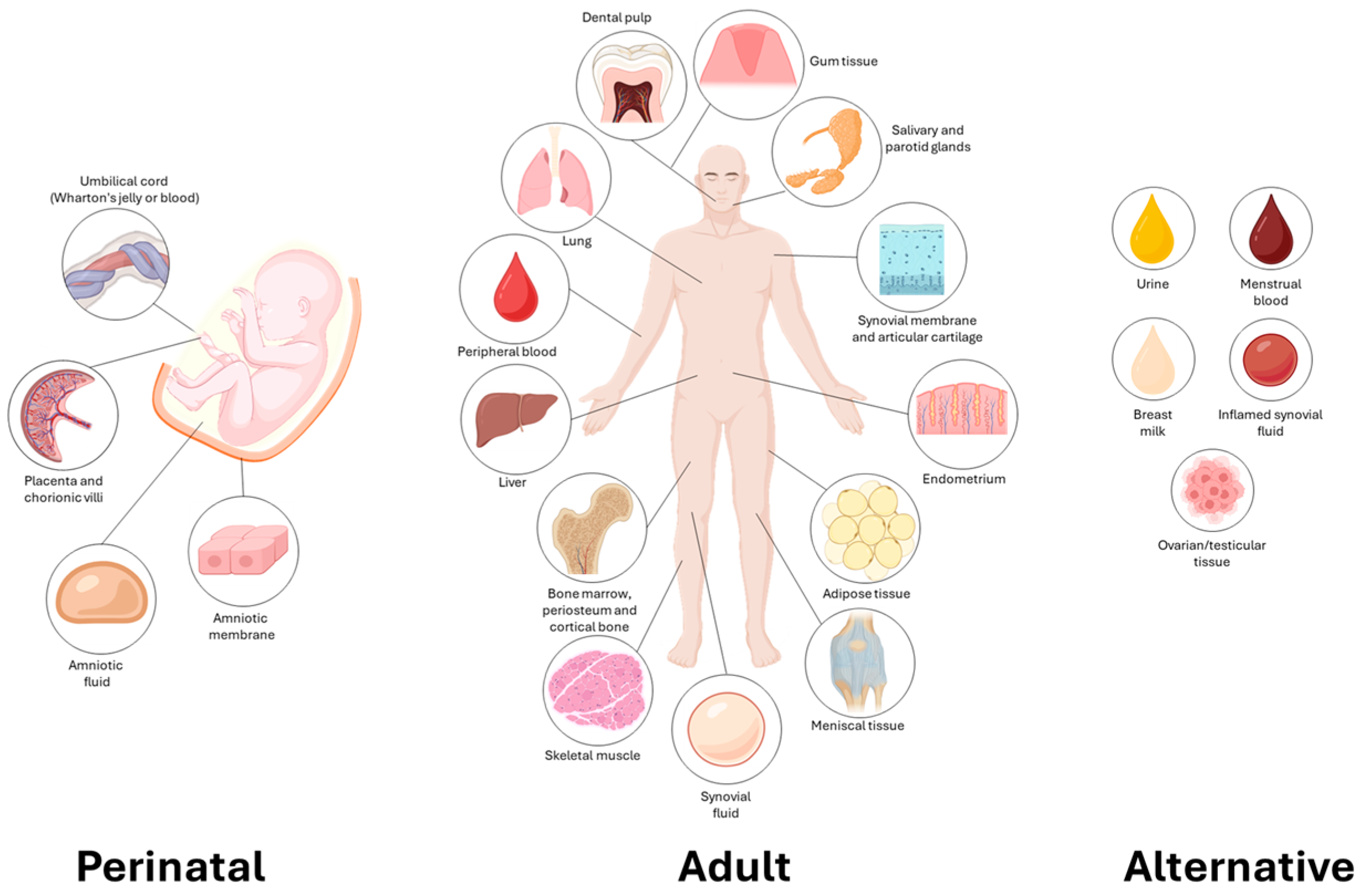
| Source | Tissue | Abbreviation | Proliferation | Immunomodulation | Differentiation Potential | Collection Ease | Clinical/Research Status | Notes | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Perinatal | Umbilical cord (Wharton’s Jelly) | UC-MSC/ WJ-MSC | High | Very high | Good | Non-invasive | In clinical trials | Collected at birth | [59] |
| Umbilical cord blood | UCB-MSC | Low | High | Limited | Non-invasive | Rarely used | Contains fewer MSC than WJ | [60] | |
| Amniotic membrane | AM-MSC | High | Very high | Medium | Surgical discard | Interest in ophthalmology, wound healing | inner fetal membrane, non-tumorigenic | [61] | |
| Amniotic fluid | AF-MSC | High | High | High (pluripotent-like) | Only during pregnancy | Advanced experimental | Isolated in mid- to late pregnancy | [62] | |
| Placenta (chorion and decidua) | PL-MSC | High | High | Good | Postpartum recovery | Under clinical study | Both fetal and maternal origin | [63] | |
| Chorionic villi | CV-MSC | Medium | High | High | Invasive | Mostly in research | Rarely used but high potential | [64] | |
| Adult | Bone marrow | BM-MSC | Medium | High | High | Invasive | Clinically used | Gold standard MSC | [65] |
| Adipose tissue | AD-MSC | High | High | Good | Easy | Clinically used | Abundant; minimally invasive harvesting (lipoaspirate) | [66] | |
| Skeletal muscle | SM-MSC | Medium | Medium | Osteo-myogenic | Invasive | Experimental | Rarely used; complex isolation | [67] | |
| Periosteum/cortical bone | PO-MSC | Medium | Medium | High (bone) | Invasive | Preclinical | Rich in osteoprogenitors; bone regeneration | [68] | |
| Synovial membrane | SF-MSC (adult) | Medium | High | High chondrogenic | Invasive | Preclinical/early translational research (OA) | Harvested via biopsy or arthroscopy | [69] | |
| Synovial fluid | SF-MSC (early life) | Medium-High | Medium-High | Good (incl. chondrogenic) | Minimally invasive; rare sample | Very limited; early preclinical only | Low in quantity; harvested during joint surgeries | [70] | |
| Articular cartilage | N/A | Low | N/A | High chondrogenic | Not easily accessible | Highly experimental | Difficult to isolate | [71] | |
| Meniscus | N/A | Low | Medium | Fibrocartilaginous | Surgical | Early-stage research | Specific to meniscal repair | [72] | |
| Liver | Hepatic MSC | Low | High | Hepatogenic | Invasive | Preclinical | Harvested via biopsy | [73] | |
| Lung | Pulmonary MSC | Medium | High | Pulmonary | Invasive | In respiratory research models | Present in alveolar tissue, harvested via biopsy | [74] | |
| Dental pulp (including the one of deciduous tooth) | DP-MSC (SHED) | High | High | Good | Minimally invasive (Easy, natural shedding) | Under investigation, in clinical development | From either deciduous or permanent teeth | [75] | |
| Gingival tissue | GMSC | High | High | Good | Easy | Dental studies | Easily accessible | [76] | |
| Salivary and parotid glands | SG-MSC | Medium | High | Good | Surgical biopsy | Experimental | High plasticity | [77] | |
| Endometrium | EM-MSC | High | High | Vascular, mesodermal | Cyclical collection | Under research | Cyclically regenerating MSCs | [78] | |
| Peripheral blood | PB-MSC | Very low | Medium | Limited | Non-invasive | Low yield | Extremely rare | [79] | |
| Alternative | Urine | USCs | High | High | Urologic, muscular | Non-invasive | In development | Promising for urological and nephrological applications | [80] |
| Breast milk | N/A | High | High | Good | Non-invasive | Very experimental | Multilineage potential; currently under investigation | [48] | |
| Ovarian/Testicular tissue | N/A | Medium-High | High | Gonadal support (follicle, sperm development) | Invasive biopsy | Preclinical (ovarian regeneration) | Very preliminary studies | [81] | |
| Inflamed synovial fluid (OA, RA) | N/A | Medium–Low (senescence in RA) | Medium–High (inflammation-enhanced) | High (chondrogenic) | Arthrocentesis, low and variable yield | Research (OA/RA) | Potential therapeutic auto-feedback mechanism | [82] | |
| Menstrual blood | MB-MSCs | High | High | Good | Very easy | Promising | High proliferation rate | [83] |
| Pain Model | Secretome Source | Type |
Processing
and Storage |
Treatment Time After Model
Induction | Dose | Route and Volume | Effect on Pain |
Biochemical Evaluations:
Time and Effect | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| STZ (mouse) | human ASC | whole | Processing: concentrated Storage: −80 °C | W2 | 2 × 106 (single dose) | Intravenous, 200 µL | ↓ Mechanical allodynia (von Frey test) | W3 and W14—PNS and CNS: ↓ Pro-inflammatory cytokines ↑ Anti-inflammatory cytokines | [102] |
| W6 | 2 × 106 (single dose) | ||||||||
| W2 + W6 | 2 × 106 (total dose 4 × 106) | N/A | |||||||
| CIPN-oxaliplatin (rat) | rat ASC | whole | Processing: freeze-dried Storage: frozen | W2 | 1 × 106 (single dose) | Intraperitoneal, 3.5 mL | ¬ Weight-bearing changes (incapacitance test) | N/A | [103] |
| STZ (mouse) | mouse BM-MSC | whole | Processing: concentrated Storage: −80 °C | W4 | 1 × 106 (single dose) | Intravenous, 100 µL | ↓ Mechanical allodynia (von Frey filaments) ↓ Thermal hypoalgesia (Plantar test) | N/A | [104] |
| PNL (mouse) | mouse BM-MSC | whole | Processing: concentrated Storage: −80 °C | W1 | 1 × 106 (single dose) | Intravenous, 100 µL | ↓ Mechanical allodynia (von Frey filaments) ↓ Thermal hyperalgesia (Plantar test) | W3—PNS and CNS: ↓ Pro-inflammatory cytokines ↑ Anti-inflammatory cytokines | [105] |
| K-OA—collagenase type VII (mouse) | human BM-MSC | whole | Processing: concentrated Storage: −80 °C | W1 (3 treatments: one every two days) | 2 × 104 (total dose 6 × 104) | Intra-articular, 6 µL | ↓ Weight-bearing changes (incapacitance test) | W4—OA knee: ¬ modulation on subchondral bone volume, synovial membrane thickness and synovial inflammation | [106] |
| SNL (rat) | human UC-MSC | EVs | Processing: concentrated Storage: −80 °C | W0 (day 3 post SNL) | 0.12 mg/mL (single dose) | Intrathecal catheter, 10 µL | Acute effect: ↓ Dose–response of mechanical allodynia (von Frey filaments) ↓ Dose–response of thermal hyperalgesia (hot plate test) | N/A | [107] |
| 0.6 mg/mL (single dose) | |||||||||
| 1.2 mg/mL (single dose) | |||||||||
| W0 (day 8 post SNL) | 0.12 mg/mL (single dose) | Intrathecal catheter, 10 µL | Acute effect: ↓ Dose–response of mechanical allodynia (von Frey filaments) ↓ Dose–response of thermal hyperalgesia (hot plate test) | N/A | |||||
| 0.6 mg/mL (single dose) | |||||||||
| 1.2 mg/mL (single dose) | |||||||||
| W0 (preventive treatment) (one treatment every day for 8 days from the SNL day) | 1.2 mg/mL (total dose 9.6mg/mL) | Intrathecal catheter, 10 µL | Prevents the onset of: Mechanical allodynia (von Frey filaments) Thermal hyperalgesia (hot plate test) | W1—PNS and/or CNS: ↓ Pro-inflammatory cytokines ↑ Anti-inflammatory cytokines ↓ Glia activation | |||||
| W0 (therapeutic treatment) (one treatment every day for 8 days from day 4 after SNL) | 1.2 mg/mL (total dose 9.6 mg/mL) | Intrathecal catheter, 10 µL | ↓ Mechanical allodynia (von Frey filaments) ↓ Thermal hyperalgesia (hot plate test) | N/A | |||||
| db/db mice | human ASC | whole | Processing: concentrated Storage: N/A | W18 + W20 + W22 + W24 | 1 × 109 (total dose 4 × 109) | Intravenous, 50 µL | ↓ Mechanical allodynia (von Frey test) ↓ Thermal hyperalgesia (plantar test) | W26—PNS: ↑ Intraepidermal nerve fiber density ↓ Pro-inflammatory markers, neurodegeneration and apoptosis | [108] |
| db/db mice | mouse BM-MSC | EVs | Processing: concentrated Storage: −80 °C or freshly used | W20 + W21 + W22 + W23 + W24 + W25 + W26 + W27 (8 treatments: one a week) (see Note 1) | 1 × 109 particles (total dose: 8 × 109 particles) | Intravenous, N/A | ↓ Mechanical allodynia (von Frey filaments) ↓ Thermal hyperalgesia (plantar test) | W28—PNS: ↓ Pro-inflammatory cytokines, ↓ Neurovascular dysfunction and axonal demyelination, ↑ neurological outcomes | [109] |
| K-OA—MIA (mouse) | human ASC | whole | Processing: concentrated Storage: −80 °C | W1 | 2 × 106 (single dose) | Intravenous, 200 µL | ↓ Mechanical allodynia (von Frey test) ↓ Thermal hyperalgesia (Plantar test). Note: IV has a greater pain-relieving effect, followed by IPL and IA | W3—PNS and CNS: ↓ Pro-inflammatory cytokines, macrophages/microglia markers, GFAP and ATF3. Note: IV has a greater pain-relieving effect, followed by IPL and IA | [110] |
| intrarticular, 15 µL | |||||||||
| Intraplantar, 15 µL | |||||||||
| db/db mice | mouse BM-MSC | EVs | Processing: concentrated Storage: −80 °C or freshly used | W20 + W21 + W22 + W23 | 1 × 109 particles (total dose 4 × 109 particles) | Intravenous, N/A | ↓ Mechanical allodynia (von Frey filaments) ↓ Thermal hyperalgesia (plantar test) | W24—PNS: ↓ inflammatory macrophages markers ↑ neurological function and recovery | [111] |
| CCI (rat) | rat BM-MSC | whole | Processing: N/A Storage: −80 °C | W0 (preventive treatment—one day before CCI and after 7 and 11 days) | N/A | Intraperitoneal, 1 mL | ↓ Mechanical allodynia (von Frey filaments) ↓ Thermal hyperalgesia (hot plate test) | W2—CNS: ↓ Purinergic receptors (P2X4 and P2X7) | [112] |
| SNL (rat) | Thera101 (purchased from Theratome Bio, Inc.) | N/A | N/A | W0 (preventive treatment) | 1–2 mg/kg | Directly to the injured nerve, 350–450 µL | ↓ Mechanical allodynia (von Frey test) only acute evaluations (30, 60, 120 min) | N/A | [113] |
| PNL (mouse) | human SHED | whole | N/A | W0: every day for a week | 1 × 105 (total dose 7 × 105) | Intravenous, N/A | ↓ Mechanical allodynia (von Frey test) | W1—PNS and CNS: ↓ Pro-inflammatory cytokines and microglia markers ↑ Anti-inflammatory markers | [114] |
| W1: every day for a week | 1 × 105 (total dose 7 × 105) | Intravenous, N/A | ↓ Mechanical allodynia (von Frey test) | N/A | |||||
| W2: every day for a week | 1 × 105 (total dose 7 × 105) | Intravenous, N/A | ↓ Mechanical allodynia (von Frey test) | N/A | |||||
| similar to CCI damage induced by biomaterial implant containing TNF (rat) | rat ASC | whole | Processing: concentrated Storage: N/A | W0: biomaterial implant containing secretome in combination with TNF (preventive treatment) | N/A | Biomaterial implant at sciatic nerve level | N/A | W3—PNS: prevention of nerve demyelination and morphological alteration | [115] |
| IC(rat) | human UC-MSC | EVs | N/A | W1: 3 doses on alternate days | 20 μg (total dose 60 μg) | Intrathecal, 20 µL | ↓ Mechanical allodynia (von Frey filaments) | N/A—CNS: ↓ Pro-inflammatory cytokines and glia markers | [116] |
| TMJ-OA—MIA (rat) | human DPSC | whole | Processing: concentrated Storage: −80 °C | W4 + W5 + W6 (i.e., once a week for 3 weeks) | N/A | N/A, 100 µL | ↓ Mechanical allodynia (von Frey filaments) | W8 and W12—TMJ: ↓ inflammation ↑ extracellular matrix and subchondral bone repair and regeneration | [117] |
| CCI (rat) | human UC-MSC | EVs | Processing: concentrated Storage: −80 °C | W0: on day 2, 4 and 6 after CCI | 5 µg (total dose 15 µg) | Intrathecal, 25 µL | ↓ Mechanical allodynia (von Frey filaments) | W1: ↓ Pro-inflammatory cytokines and microglia markers | [118,119] |
| CCI (rat) | rat BM-MSC | whole | Processing: N/A Storage: none, freshly used | W0 (preventive treatment—for 3 consecutive days from the day pre-CCI) | N/A | Intraperitoneal, 1 mL | ↓ Mechanical allodynia (von Frey filaments) ↓ Thermal hyperalgesia (hot plate test) | W2: CNS ↓ Pro-inflammatory cytokines | [120] |
| Model | Secretome Source |
Type and Administration
Method | Effect | Ref. |
|---|---|---|---|---|
| DRG neurons from naive rats exposed to high glucose concentrations (diabetic polyneuropathy) | human ASC | whole, in vitro | ↓ apoptosis | [130] |
| Ex vivo: DRG neurons isolated from db/db mice (diabetic polyneuropathy) | human ASC | whole, in vivo | ↑ neurite arborization | [108] |
| DRG neurons isolated from db/db mice (diabetic polyneuropathy) | human ASC | whole, in vitro | ↑ neurite arborization | [131] |
| Cerebral organoids (CeO) | human BM-MSC | whole, in vitro | ↑ MOR expression ↑ neurogenesis and astrogenesis | [132] |
| Human AF cells from IS or DD patients, stimulated with mechanical stress, IL-1βor combined (chronic back pain) | N/A | whole, in vitro | ↓ inflammation ↑ tissue regeneration | [133] |
| AF and NP cells from healthy donors stimulated with TNF-α (chronic back pain) | human ASC | whole, EVs or EV-free secretome, in vitro | ↓ degeneration | [134] |
| Immortalized microglial cell line stimulated with LPS (generic model of neuroinflammation/pain) | human UC-MSC | whole, in vitro | ↓ pro-inflammatory cytokines and microgliosis | [118] |
| Type of Study | Disease | Secretome Source | Secretome Origin | Treatment Strategy | Treatment Effect | Ref. |
|---|---|---|---|---|---|---|
| N/A | MSK pain | ASC | Self-produced | Local administration. First treatment: 27 body areas; second treatment: 7 sites. | Pain assessed with NRS: pain reduction after 15 min, which was maintained up to 4 weeks later. | [140] |
| Open-label clinical study | OA knee | UC-MSC | Purchased, GMP certified. | Intra-articular administration (once weekly for 5 weeks). | Reduction of VAS and WOMAC scores up to 12 weeks post-treatment. Reduction of serum MMP-3 levels and an increase in TGF-β levels. | [141] |
| Case report | CLBP | UC-MSC | N/A | Unique multi-site treatment (in vertebrae from T12 to S5, epidural region of the sacral spine and piriformis muscles). | Before administration, the patient underwent PLDD. Pain reduction lasted for up to six months. The impact of the secretome alone cannot be determined in a control group (no treatment). | [142] |
| Randomized controlled clinical trial | TBPI | UC-MSC | Self-produced | Single administration at the neuromuscular junction of the median nerve-superficial flexor digitorum muscle. | Before administration, patients underwent nerve transfer surgery. A reduction in postoperative pain was documented. The impact of the secretome cannot be determined due to the lack of a control group (no treatment). | [143] |
| NCT Number | Condition | Secretome Source | Secretome Origin | Treatment Strategy | Pain Measurement | Study Status |
|---|---|---|---|---|---|---|
| NCT05909488 | RP | UC-MSC | N/A | Peribulbar injection of 1.5 or 5 × 106 UC-MSCs resuspended in CM | 6 months after injection: evaluation of the level of pain felt by patients | not yet recruiting |
| NCT06688318 | OA knee | UC-MSC | N/A | Intra-articular injection of CM from hypoxic UC-MSCs | 2, 4 and 6 months after injection: KOOS and WOMAC scores | active, not recruiting |
| NCT05579665 | OA knee | UC-MSC | N/A | Intra-articular injection under ultrasound guidance of CM from UC-MSCs (once weekly for 5 weeks) | pre-treatment, and 3 and 6 months after injection: VAS and WOMAC scores | completed [141] |
| NCT04314661 | OA knee | UC-MSC | N/A | Intra-articular injection of 10 × 106 UC-MSCs and 2 cc secretome twice with a 2-week interval, or 2 cc secretome, 10 × 106 UC-MSCs and 2 cc secretome with a 2-week interval | 1, 3 and 6 months after injection: VAS and WOMAC scores | unknown status |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amodeo, G.; Galimberti, G.; Niada, S.; Giannasi, C.; Della Morte, E.; Franchi, S.; Riboldi, B.; Ceruti, S.; Brini, A.T.; Sacerdote, P. Cell-Free Therapies for Chronic Pain: The Rise of the Mesenchymal Stem Cell Secretome. Brain Sci. 2025, 15, 1263. https://doi.org/10.3390/brainsci15121263
Amodeo G, Galimberti G, Niada S, Giannasi C, Della Morte E, Franchi S, Riboldi B, Ceruti S, Brini AT, Sacerdote P. Cell-Free Therapies for Chronic Pain: The Rise of the Mesenchymal Stem Cell Secretome. Brain Sciences. 2025; 15(12):1263. https://doi.org/10.3390/brainsci15121263
Chicago/Turabian StyleAmodeo, Giada, Giulia Galimberti, Stefania Niada, Chiara Giannasi, Elena Della Morte, Silvia Franchi, Benedetta Riboldi, Stefania Ceruti, Anna Teresa Brini, and Paola Sacerdote. 2025. "Cell-Free Therapies for Chronic Pain: The Rise of the Mesenchymal Stem Cell Secretome" Brain Sciences 15, no. 12: 1263. https://doi.org/10.3390/brainsci15121263
APA StyleAmodeo, G., Galimberti, G., Niada, S., Giannasi, C., Della Morte, E., Franchi, S., Riboldi, B., Ceruti, S., Brini, A. T., & Sacerdote, P. (2025). Cell-Free Therapies for Chronic Pain: The Rise of the Mesenchymal Stem Cell Secretome. Brain Sciences, 15(12), 1263. https://doi.org/10.3390/brainsci15121263










