Functional and Structural Connectivity Correlates of Axial Symptom Outcomes After Pallidal Deep Brain Stimulation in Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Surgical Procedure and Electrode Location
2.3. Assessments
2.4. Imaging Technique and MRI Data Analysis
2.5. Statistical Analysis
3. Results
3.1. Study Population
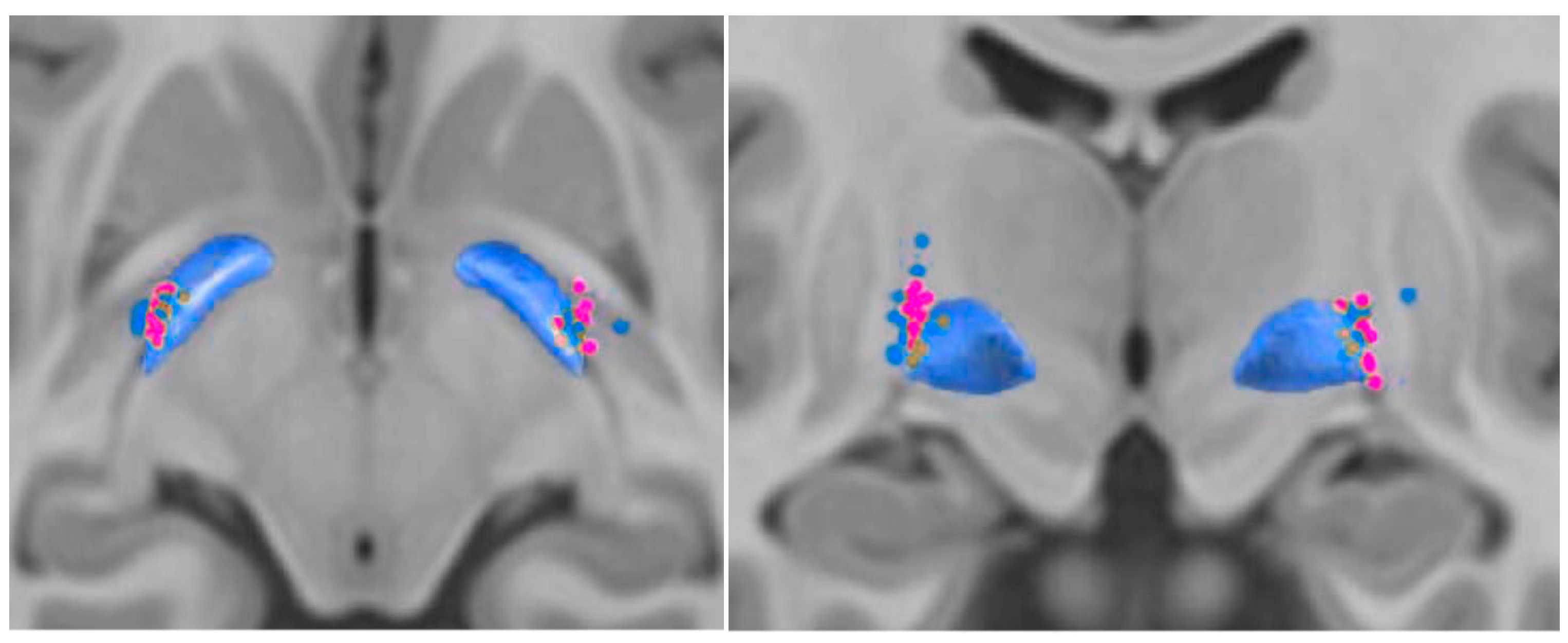
3.2. Contact Position
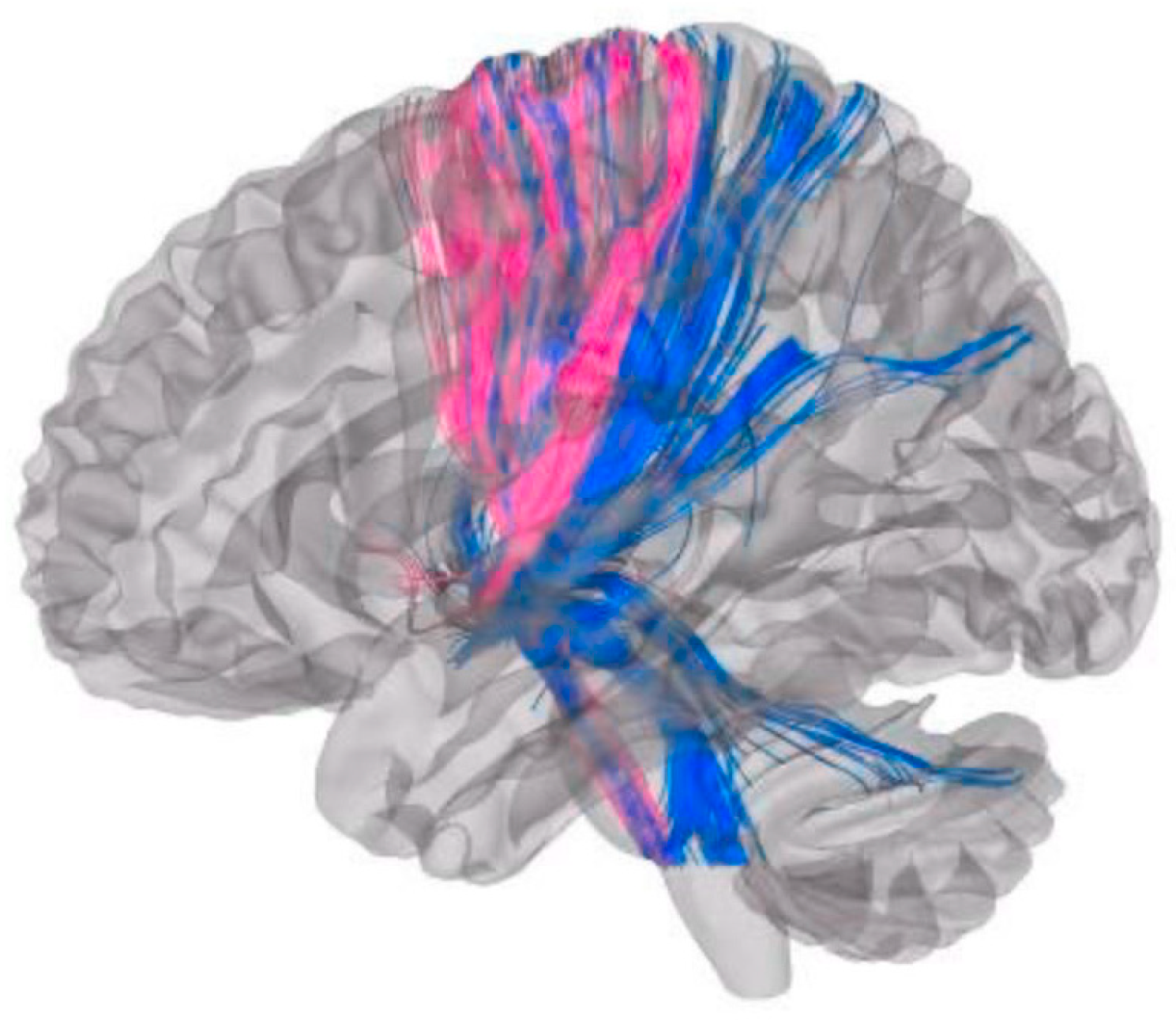
3.3. Structural Connectivity and Fiber Tract Count
3.3.1. Axial Gait Scores
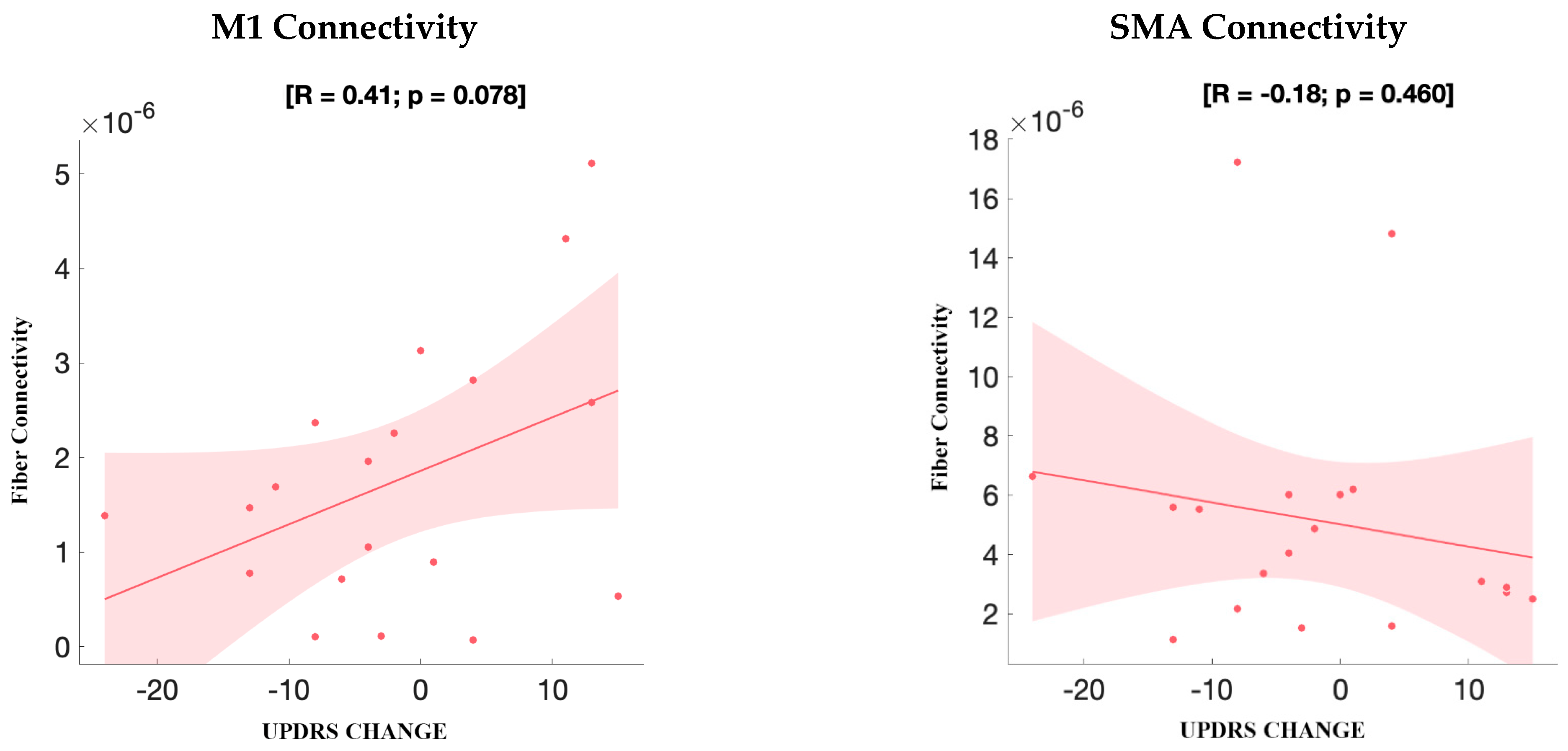
3.3.2. UPDRS Improvement
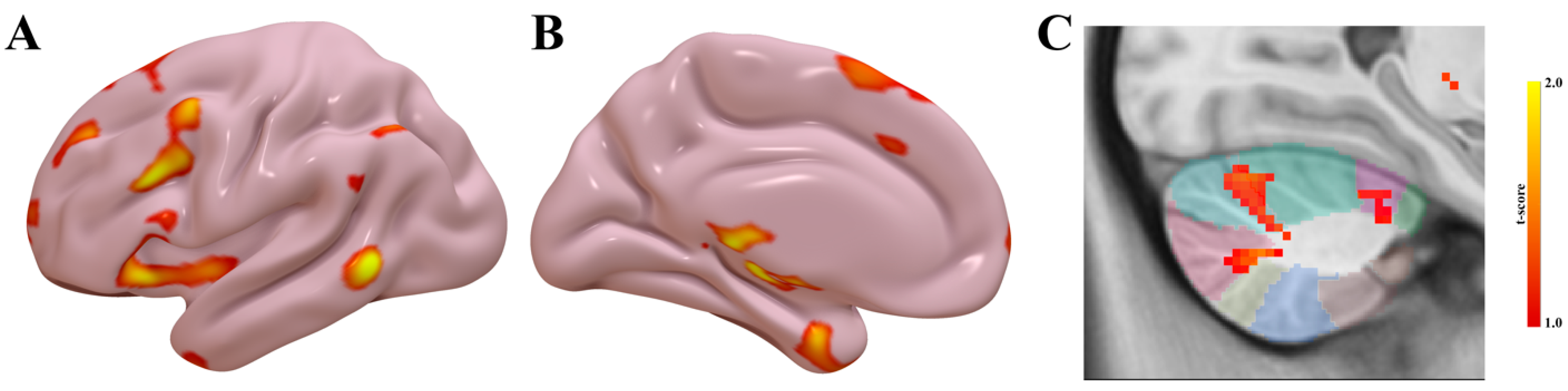
3.4. Functional Connectivity
4. Discussion
4.1. Integration of Stimulation Parameters and Connectivity Insights
4.2. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC–PC | Anterior commissure–posterior commissure |
| AAL | Automated Anatomical Labeling (atlas) |
| ANTs | Advanced Normalization Tools |
| BOLD | Blood oxygen level–dependent |
| COG | Center of gravity |
| DBS | Deep brain stimulation |
| DSI | Diffusion Spectrum Imaging |
| fMRI | Functional magnetic resonance imaging |
| FSL | FMRIB Software Library |
| GPi | Globus pallidus internus |
| GSP | Brain Genomics Superstruct Project |
| HCP | Human Connectome Project |
| INFORM | Movement disorders database at the University of Florida |
| LEDD | Levodopa equivalent daily dose |
| LFP | Local field potential |
| M1 | Primary motor cortex |
| MEG | Magnetoencephalography |
| MNI | Montreal Neurological Institute (template space) |
| MP-RAGE | Magnetization-prepared rapid gradient-echo |
| MRI | Magnetic resonance imaging |
| PaCER | Automated method for electrode trajectory and contact reconstruction |
| PD | Parkinson’s disease |
| QA | Quantitative anisotropy |
| ROI | Region of interest |
| SD | Standard deviation |
| SMA | Supplementary motor area |
| SPM | Statistical Parametric Mapping |
| STN | Subthalamic nucleus |
| SUIT | Spatially Unbiased Infratentorial Template (cerebellar atlas) |
| UPDRS | Unified Parkinson’s Disease Rating Scale |
| VTA | Volume(s) of tissue activated |
References
- Ramirez-Zamora, A.; Ostrem, J.L. Globus Pallidus Interna or Subthalamic Nucleus Deep Brain Stimulation for Parkinson Disease: A Review. JAMA Neurol. 2018, 75, 367–372. [Google Scholar] [CrossRef]
- Fox, M.D.; Buckner, R.L.; Liu, H.; Chakravarty, M.M.; Lozano, A.M.; Pascual-Leone, A. Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proc. Natl. Acad. Sci. USA 2014, 111, E4367–E4375. [Google Scholar] [CrossRef]
- Henderson, J.M. “Connectomic surgery”: Diffusion tensor imaging (DTI) tractography as a targeting modality for surgical modulation of neural networks. Front. Integr. Neurosci. 2012, 6, 15. [Google Scholar] [CrossRef]
- Lozano, A.M.; Lipsman, N. Probing and regulating dysfunctional circuits using deep brain stimulation. Neuron 2013, 77, 406–424. [Google Scholar] [CrossRef]
- Litvak, V.; Jha, A.; Eusebio, A.; Oostenveld, R.; Foltynie, T.; Limousin, P.; Zrinzo, L.; Hariz, M.I.; Friston, K.; Brown, P. Resting oscillatory cortico-subthalamic connectivity in patients with Parkinson’s disease. Brain 2011, 134, 359–374. [Google Scholar] [CrossRef] [PubMed]
- Oswal, A.; Jha, A.; Neal, S.; Reid, A.; Bradbury, D.; Aston, P.; Limousin, P.; Foltynie, T.; Zrinzo, L.; Brown, P.; et al. Analysis of simultaneous MEG and intracranial LFP recordings during Deep Brain Stimulation: A protocol and experimental validation. J. Neurosci. Methods 2016, 261, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Accolla, E.A.; Herrojo Ruiz, M.; Horn, A.; Schneider, G.H.; Schmitz-Hubsch, T.; Draganski, B.; Kuhn, A.A. Brain networks modulated by subthalamic nucleus deep brain stimulation. Brain 2016, 139, 2503–2515. [Google Scholar] [CrossRef]
- Pouratian, N.; Zheng, Z.; Bari, A.A.; Behnke, E.; Elias, W.J.; Desalles, A.A. Multi-institutional evaluation of deep brain stimulation targeting using probabilistic connectivity-based thalamic segmentation. J. Neurosurg. 2011, 115, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, K.; Tang, C.; Ma, Y.; Dhawan, V.; Mattis, P.; Edwards, C.; Kaplitt, M.G.; Feigin, A.; Eidelberg, D. Network modulation in the treatment of Parkinson’s disease. Brain 2006, 129, 2667–2678. [Google Scholar] [CrossRef]
- Gradinaru, V.; Mogri, M.; Thompson, K.R.; Henderson, J.M.; Deisseroth, K. Optical deconstruction of parkinsonian neural circuitry. Science 2009, 324, 354–359. [Google Scholar] [CrossRef]
- Horn, A.; Reich, M.; Vorwerk, J.; Li, N.; Wenzel, G.; Fang, Q.; Schmitz-Hubsch, T.; Nickl, R.; Kupsch, A.; Volkmann, J.; et al. Connectivity Predicts deep brain stimulation outcome in Parkinson disease. Ann. Neurol. 2017, 82, 67–78. [Google Scholar] [CrossRef]
- Lai, Y.; He, N.; Wei, H.; Deng, L.; Zhou, H.; Li, J.; Kaiser, M.; Zhang, C.; Li, D.; Sun, B. Value of functional connectivity in outcome prediction for pallidal stimulation in Parkinson disease. J. Neurosurg. 2023, 138, 27–37. [Google Scholar] [CrossRef]
- Rajamani, N.; Friedrich, H.; Butenko, K.; Dembek, T.; Lange, F.; Navratil, P.; Zvarova, P.; Hollunder, B.; de Bie, R.M.A.; Odekerken, V.J.J.; et al. Deep brain stimulation of symptom-specific networks in Parkinson’s disease. Nat. Commun. 2024, 15, 4662. [Google Scholar] [CrossRef]
- Eisenstein, S.A.; Koller, J.M.; Black, K.D.; Campbell, M.C.; Lugar, H.M.; Ushe, M.; Tabbal, S.D.; Karimi, M.; Hershey, T.; Perlmutter, J.S.; et al. Functional anatomy of subthalamic nucleus stimulation in Parkinson disease. Ann. Neurol. 2014, 76, 279–295. [Google Scholar] [CrossRef]
- Greenhouse, I.; Gould, S.; Houser, M.; Hicks, G.; Gross, J.; Aron, A.R. Stimulation at dorsal and ventral electrode contacts targeted at the subthalamic nucleus has different effects on motor and emotion functions in Parkinson’s disease. Neuropsychologia 2011, 49, 528–534. [Google Scholar] [CrossRef]
- Akram, H.; Sotiropoulos, S.N.; Jbabdi, S.; Georgiev, D.; Mahlknecht, P.; Hyam, J.; Foltynie, T.; Limousin, P.; De Vita, E.; Jahanshahi, M.; et al. Subthalamic deep brain stimulation sweet spots and hyperdirect cortical connectivity in Parkinson’s disease. Neuroimage 2017, 158, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M.; Mentis, M.J.; Ma, Y.; Dhawan, V.; Antonini, A.; Lang, A.E.; Lozano, A.M.; Hammerstad, J.; Lyons, K.; Koller, W.C.; et al. Networks mediating the clinical effects of pallidal brain stimulation for Parkinson’s disease: A PET study of resting-state glucose metabolism. Brain 2001, 124, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Malekmohammadi, M.; AuYong, N.; Ricks-Oddie, J.; Bordelon, Y.; Pouratian, N. Pallidal deep brain stimulation modulates excessive cortical high beta phase amplitude coupling in Parkinson disease. Brain Stimul. 2018, 11, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; de Hemptinne, C.; Miocinovic, S.; Ostrem, J.L.; Galifianakis, N.B.; San Luciano, M.; Starr, P.A. Pallidal Deep-Brain Stimulation Disrupts Pallidal Beta Oscillations and Coherence with Primary Motor Cortex in Parkinson’s Disease. J. Neurosci. 2018, 38, 4556–4568. [Google Scholar] [CrossRef]
- Li, Z.; Lai, Y.; Li, J.; He, N.; Li, D.; Yan, F.; Zhang, Y.; Zhang, C.; Sun, B.; Wei, H. BOLD frequency-dependent alterations in resting-state functional connectivity by pallidal deep brain stimulation in patients with Parkinson’s disease. J. Neurosurg. 2023, 139, 1354–1365. [Google Scholar] [CrossRef]
- Johnson, K.A.; Cagle, J.N.; Lopes, J.L.; Wong, J.K.; Okun, M.S.; Gunduz, A.; Wagle Shukla, A.; Hilliard, J.D.; Foote, K.D.; de Hemptinne, C. Globus pallidus internus deep brain stimulation evokes resonant neural activity in Parkinson’s disease. Brain Commun. 2023, 5, fcad025. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, D.; Fan, H.; Xie, H.; Zhang, Q.; Qin, G.; Jiang, Y.; Meng, F.; Yin, Z.; Yang, A.; et al. The effect of pallidal stimulation on sleep outcomes and related brain connectometries in Parkinson’s disease. NPJ Park. Dis. 2024, 10, 212. [Google Scholar] [CrossRef]
- Higuchi, M.A.; Martinez-Ramirez, D.; Morita, H.; Topiol, D.; Bowers, D.; Ward, H.; Warren, L.; DeFranco, M.; Hicks, J.A.; Hegland, K.W.; et al. Interdisciplinary Parkinson’s Disease Deep Brain Stimulation Screening and the Relationship to Unintended Hospitalizations and Quality of Life. PLoS ONE 2016, 11, e0153785. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Sudhyadhom, A.; Okun, M.S.; Foote, K.D.; Rahman, M.; Bova, F.J. A Three-dimensional Deformable Brain Atlas for DBS Targeting. I. Methodology for Atlas Creation and Artifact Reduction. Open Neuroimaging J. 2012, 6, 92–98. [Google Scholar] [CrossRef]
- Bonenfant, J.; Drapier, S.; Houvenaghel, J.F.; Naudet, F.; Haegelen, C.; Sauleau, P.; Verin, M. Pallidal stimulation in Parkinson’s patients with contraindications to subthalamic target: A 3 years follow-up. Park. Relat. Disord. 2017, 34, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Thevathasan, W.; Pogosyan, A.; Hyam, J.A.; Jenkinson, N.; Bogdanovic, M.; Coyne, T.J.; Silburn, P.A.; Aziz, T.Z.; Brown, P. A block to pre-prepared movement in gait freezing, relieved by pedunculopontine nucleus stimulation. Brain 2011, 134, 2085–2095. [Google Scholar] [CrossRef]
- Tomlinson, C.L.; Stowe, R.; Patel, S.; Rick, C.; Gray, R.; Clarke, C.E. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord. 2010, 25, 2649–2653. [Google Scholar] [CrossRef]
- Jost, S.T.; Kaldenbach, M.A.; Antonini, A.; Martinez-Martin, P.; Timmermann, L.; Odin, P.; Katzenschlager, R.; Borgohain, R.; Fasano, A.; Stocchi, F.; et al. Levodopa Dose Equivalency in Parkinson’s Disease: Updated Systematic Review and Proposals. Mov. Disord. 2023, 38, 1236–1252. [Google Scholar] [CrossRef]
- Avants, B.B.; Epstein, C.L.; Grossman, M.; Gee, J.C. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 2008, 12, 26–41. [Google Scholar] [CrossRef]
- Fonov, V.; Evans, A.C.; Botteron, K.; Almli, C.R.; McKinstry, R.C.; Collins, D.L.; Brain Development Cooperative, G. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage 2011, 54, 313–327. [Google Scholar] [CrossRef]
- Schonecker, T.; Kupsch, A.; Kuhn, A.A.; Schneider, G.H.; Hoffmann, K.T. Automated optimization of subcortical cerebral MR imaging-atlas coregistration for improved postoperative electrode localization in deep brain stimulation. AJNR Am. J. Neuroradiol. 2009, 30, 1914–1921. [Google Scholar] [CrossRef]
- Husch, A.; Petersen, M.V.; Gemmar, P.; Goncalves, J.; Hertel, F. PaCER—A fully automated method for electrode trajectory and contact reconstruction in deep brain stimulation. Neuroimage Clin. 2018, 17, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Horn, A.; Li, N.; Dembek, T.A.; Kappel, A.; Boulay, C.; Ewert, S.; Tietze, A.; Husch, A.; Perera, T.; Neumann, W.J.; et al. Lead-DBS v2: Towards a comprehensive pipeline for deep brain stimulation imaging. Neuroimage 2019, 184, 293–316. [Google Scholar] [CrossRef]
- Rolls, E.T.; Joliot, M.; Tzourio-Mazoyer, N. Implementation of a new parcellation of the orbitofrontal cortex in the automated anatomical labeling atlas. Neuroimage 2015, 122, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Tench, C.; Wongwandee, M.; Schwarz, S.T.; Bajaj, N.; Auer, D.P. Coordinate based meta-analysis of motor functional imaging in Parkinson’s: Disease-specific patterns and modulation by dopamine replacement and deep brain stimulation. Brain Imaging Behav. 2020, 14, 1263–1280. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Chen, F.; Liu, X.; Jiang, C.; Hu, X.; Ma, L.; Xu, Z. STN versus GPi deep brain stimulation for dyskinesia improvement in advanced Parkinson’s disease: A meta-analysis of randomized controlled trials. Clin. Neurol. Neurosurg. 2021, 201, 106450. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.Y.; Wang, K.L.; Hu, W.; Eisinger, R.S.; Han, A.; Han, C.L.; Wang, Q.; Michitomo, S.; Zhang, J.G.; Wang, F.; et al. Pallidal versus subthalamic nucleus deep brain stimulation for levodopa-induced dyskinesia. Ann. Clin. Transl. Neurol. 2020, 7, 59–68. [Google Scholar] [CrossRef]
- Trost, M.; Su, S.; Su, P.; Yen, R.F.; Tseng, H.M.; Barnes, A.; Ma, Y.; Eidelberg, D. Network modulation by the subthalamic nucleus in the treatment of Parkinson’s disease. Neuroimage 2006, 31, 301–307. [Google Scholar] [CrossRef]
- Kahan, J.; Mancini, L.; Urner, M.; Friston, K.; Hariz, M.; Holl, E.; White, M.; Ruge, D.; Jahanshahi, M.; Boertien, T.; et al. Therapeutic subthalamic nucleus deep brain stimulation reverses cortico-thalamic coupling during voluntary movements in Parkinson’s disease. PLoS ONE 2012, 7, e50270. [Google Scholar] [CrossRef]
- Zhao, S.; Li, G.; Tong, C.; Chen, W.; Wang, P.; Dai, J.; Fu, X.; Xu, Z.; Liu, X.; Lu, L.; et al. Full activation pattern mapping by simultaneous deep brain stimulation and fMRI with graphene fiber electrodes. Nat. Commun. 2020, 11, 1788. [Google Scholar] [CrossRef]
- Lai, H.Y.; Younce, J.R.; Albaugh, D.L.; Kao, Y.C.; Shih, Y.Y. Functional MRI reveals frequency-dependent responses during deep brain stimulation at the subthalamic nucleus or internal globus pallidus. Neuroimage 2014, 84, 11–18. [Google Scholar] [CrossRef]
- Middlebrooks, E.H.; Domingo, R.A.; Vivas-Buitrago, T.; Okromelidze, L.; Tsuboi, T.; Wong, J.K.; Eisinger, R.S.; Almeida, L.; Burns, M.R.; Horn, A.; et al. Neuroimaging Advances in Deep Brain Stimulation: Review of Indications, Anatomy, and Brain Connectomics. AJNR Am. J. Neuroradiol. 2020, 41, 1558–1568. [Google Scholar] [CrossRef]
- Saadon-Grosman, N.; Du, J.; Kosakowski, H.L.; Angeli, P.A.; DiNicola, L.M.; Eldaief, M.C.; Buckner, R.L. Within-individual organization of the human cognitive cerebellum: Evidence for closely juxtaposed, functionally specialized regions. Sci. Adv. 2024, 10, eadq4037. [Google Scholar] [CrossRef]
- Begue, I.; Elandaloussi, Y.; Delavari, F.; Cao, H.; Moussa-Tooks, A.; Roser, M.; Coupe, P.; Leboyer, M.; Kaiser, S.; Houenou, J.; et al. The Cerebellum and Cognitive Function: Anatomical Evidence from a Transdiagnostic Sample. Cerebellum 2024, 23, 1399–1410. [Google Scholar] [CrossRef]
- Stoodley, C.J.; Valera, E.M.; Schmahmann, J.D. Functional topography of the cerebellum for motor and cognitive tasks: An fMRI study. Neuroimage 2012, 59, 1560–1570. [Google Scholar] [CrossRef] [PubMed]
- Solstrand Dahlberg, L.; Lungu, O.; Doyon, J. Cerebellar Contribution to Motor and Non-motor Functions in Parkinson’s Disease: A Meta-Analysis of fMRI Findings. Front. Neurol. 2020, 11, 127. [Google Scholar] [CrossRef] [PubMed]
- Gardoni, A.; Agosta, F.; Sarasso, E.; Basaia, S.; Canu, E.; Leocadi, M.; Castelnovo, V.; Tettamanti, A.; Volonte, M.A.; Filippi, M. Cerebellar alterations in Parkinson’s disease with postural instability and gait disorders. J. Neurol. 2023, 270, 1735–1744. [Google Scholar] [CrossRef]
- Fukuda, M.; Mentis, M.; Ghilardi, M.F.; Dhawan, V.; Antonini, A.; Hammerstad, J.; Lozano, A.M.; Lang, A.; Lyons, K.; Koller, W.; et al. Functional correlates of pallidal stimulation for Parkinson’s disease. Ann. Neurol. 2001, 49, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Kipping, J.A.; Grodd, W.; Kumar, V.; Taubert, M.; Villringer, A.; Margulies, D.S. Overlapping and parallel cerebello-cerebral networks contributing to sensorimotor control: An intrinsic functional connectivity study. Neuroimage 2013, 83, 837–848. [Google Scholar] [CrossRef]
- Zhang, C.; Lai, Y.; Li, J.; He, N.; Liu, Y.; Li, Y.; Li, H.; Wei, H.; Yan, F.; Horn, A.; et al. Subthalamic and Pallidal Stimulations in Patients with Parkinson’s Disease: Common and Dissociable Connections. Ann. Neurol. 2021, 90, 670–682. [Google Scholar] [CrossRef]
- DiRisio, A.C.; Avecillas-Chasin, J.M.; Platt, S.; Jimenez-Shahed, J.; Figee, M.; Mayberg, H.S.; Choi, K.S.; Kopell, B.H. White matter connectivity of subthalamic nucleus and globus pallidus interna targets for deep brain stimulation. J. Neurosurg. 2023, 139, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.K.; Middlebrooks, E.H.; Grewal, S.S.; Almeida, L.; Hess, C.W.; Okun, M.S. A Comprehensive Review of Brain Connectomics and Imaging to Improve Deep Brain Stimulation Outcomes. Mov. Disord. 2020, 35, 741–751. [Google Scholar] [CrossRef]
- Muller, J.; Alizadeh, M.; Mohamed, F.B.; Riley, J.; Pearce, J.J.; Trieu, B.; Liang, T.W.; Romo, V.; Sharan, A.; Wu, C. Clinically applicable delineation of the pallidal sensorimotor region in patients with advanced Parkinson’s disease: Study of probabilistic and deterministic tractography. J. Neurosurg. 2019, 131, 1520–1531. [Google Scholar] [CrossRef]
- Dembek, T.A.; Roediger, J.; Horn, A.; Reker, P.; Oehrn, C.; Dafsari, H.S.; Li, N.; Kühn, A.A.; Fink, G.R.; Visser-Vandewalle, V.; et al. Probabilistic sweet spots predict motor outcome for deep brain stimulation in Parkinson disease. Ann. Neurol. 2019, 86, 527–538. [Google Scholar] [CrossRef]
- Wang, Q.; Akram, H.; Muthuraman, M.; Gonzalez-Escamilla, G.; Sheth, S.A.; Oxenford, S.; Yeh, F.-C.; Groppa, S.; Vanegas-Arroyave, N.; Zrinzo, L.; et al. Normative vs. patient-specific brain connectivity in deep brain stimulation. NeuroImage 2021, 224, 117307. [Google Scholar] [CrossRef]
- Cacciola, A.; Milardi, D.; Bertino, S.; Basile, G.A.; Calamuneri, A.; Chillemi, G.; Rizzo, G.; Anastasi, G.; Quartarone, A. Structural connectivity-based topography of the human globus pallidus: Implications for therapeutic targeting in movement disorders. Mov. Disord. 2019, 34, 987–996. [Google Scholar] [CrossRef]
- Milardi, D.; Gaeta, M.; Marino, S.; Arrigo, A.; Vaccarino, G.; Mormina, E.; Rizzo, G.; Milazzo, C.; Finocchio, G.; Baglieri, A.; et al. Basal ganglia network by constrained spherical deconvolution: A possible cortico-pallidal pathway? Mov. Disord. 2015, 30, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Nambu, A.; Tokuno, H.; Takada, M. Functional significance of the cortico-subthalamo-pallidal ‘hyperdirect’ pathway. Neurosci. Res. 2002, 43, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Kindred, J.H.; Tuulari, J.J.; Bucci, M.; Kalliokoski, K.K.; Rudroff, T. Walking speed and brain glucose uptake are uncoupled in patients with multiple sclerosis. Front. Hum. Neurosci. 2015, 9, 84. [Google Scholar] [CrossRef]
| Improving (n = 10) Mean ± SD (Range) | Worsening (n = 9) Mean ± SD (Range) | p-Value | |
|---|---|---|---|
| Gender, M/F | 7/3 | 6/3 | 1.000 |
| Age of onset (years) | 45.0 ± 6.6 (37–55) | 47.9 ± 7.5 (37–59) | 0.384 |
| Age at surgery (years) | 58.7 ± 8.5 (47–68) | 62.4 ± 6.4 (55–74) | 0.297 |
| Follow-up from baseline to the 36-month timepoint (months) | 53.2 ± 12.9 (43.1– 85.6) | 55.0 ± 10.4 (45.2–73.5) | 0.740 |
| Duration of PD at baseline (years) | 21.0 ± 5.3 (13–21) | 24.4 ± 7.9 (12–34) | 0.274 |
| UPDRS-III baseline ** | |||
| Off-medication | 43.6 ±11.2 (28–68) | 41.9 ± 6.6 (34–54) | 0.695 |
| On-medication | 26.2 ±13.2 (15–58) | 23.4 ± 10.7 (8–42) | 0.627 |
| Axial gait score | |||
| Off-medication | 6.8 ± 2.2 (4–10) | 5.1 ± 3.1 (0–10) | 0.185 |
| On-medication | 3.8 ± 3.0 (0–9) | 3.2 ± 2.7 (0–7) | 0.666 |
| Hoehn & Yahr | |||
| Off-medication | 3.0 ± 0.7 (2–4) | 2.8 ± 0.6 (2–4) | 0.702 |
| On-medication | 2.6 ± 0.6 (2–4) | 2.5 ± 0.6 (2–4) | 0.737 |
| LEDD (mg) | 1299.6 ± 655.9 (300–2375) | 1169.8 ± 698.4 (532–2429) | 0.681 |
| ROI | Mean (Better) | Mean (Worse) | Δ (Better–Worse) | t (df = 17) | p | Pooled SD | 95% CI for Δ | Hedges’ g |
|---|---|---|---|---|---|---|---|---|
| Cerebellum Crus 2 (Ipsilateral) | 31.6 | 47.6 | −16.0 | 2.53 | 0.02 | 13.8 | −29.3 to −2.7 | −1.11 |
| Putamen (Ipsilateral) | 68.6 | 46.7 | +21.9 | 3.92 | 0.001 | 12.2 | +10.1 to +33.7 | +1.72 |
| Pallidum (Ipsilateral) | 116.2 | 96.9 | +19.3 | 3.00 | 0.005 | 14.0 | +5.7 to +32.9 | +1.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez Rodriguez Garcia, G.; Middlebrooks, E.; Mei, S.; Tsuboi, T.; Wong, J.; Burns, M.; de Hemptinne, C.; Ramirez-Zamora, A. Functional and Structural Connectivity Correlates of Axial Symptom Outcomes After Pallidal Deep Brain Stimulation in Parkinson’s Disease. Brain Sci. 2025, 15, 1245. https://doi.org/10.3390/brainsci15111245
Perez Rodriguez Garcia G, Middlebrooks E, Mei S, Tsuboi T, Wong J, Burns M, de Hemptinne C, Ramirez-Zamora A. Functional and Structural Connectivity Correlates of Axial Symptom Outcomes After Pallidal Deep Brain Stimulation in Parkinson’s Disease. Brain Sciences. 2025; 15(11):1245. https://doi.org/10.3390/brainsci15111245
Chicago/Turabian StylePerez Rodriguez Garcia, Gilberto, Erik Middlebrooks, Shanshan Mei, Takashi Tsuboi, Joshua Wong, Matthew Burns, Coralie de Hemptinne, and Adolfo Ramirez-Zamora. 2025. "Functional and Structural Connectivity Correlates of Axial Symptom Outcomes After Pallidal Deep Brain Stimulation in Parkinson’s Disease" Brain Sciences 15, no. 11: 1245. https://doi.org/10.3390/brainsci15111245
APA StylePerez Rodriguez Garcia, G., Middlebrooks, E., Mei, S., Tsuboi, T., Wong, J., Burns, M., de Hemptinne, C., & Ramirez-Zamora, A. (2025). Functional and Structural Connectivity Correlates of Axial Symptom Outcomes After Pallidal Deep Brain Stimulation in Parkinson’s Disease. Brain Sciences, 15(11), 1245. https://doi.org/10.3390/brainsci15111245








