1. Introduction
Spasticity is one of the hallmark features of spinal circuit dysregulation after spinal cord injury (SCI) [
1]. Maladaptive changes in spinal cord circuitry result in excessive excitation to motoneurons, partly due to diminished influence from inhibitory circuits [
2]. When activated, sensory afferents have a robust inhibitory influence on spinal circuits. After SCI, paresis and paralysis result in decreased movement and an associated reduction in movement-related afferent input.
Noninvasive electrical stimulation over the vertebrae, termed transcutaneous spinal stimulation (TSS), activates dorsal (posterior) spinal nerve roots [
3]. TSS appears to have robust effects on spasticity and has the advantage that stimulation parameters can be customized or “tuned” to the individual [
4,
5,
6]. However, optimal pulse parameters for spasticity reduction are not known. Despite the changes to spinal networks after SCI, spinal circuits continue to respond to temporal variations in sensory input. The majority of interventional TSS studies for spasticity reduction use a uniform 50 Hz frequency delivered continuously [
7]. A recent in vivo computational modeling and preclinical study concluded that both 50 Hz continuous and patterned spinal stimulation promoted inhibition in hyperexcitable dorsal horn neurons, which likely contribute to pain and spasticity after SCI [
8]. Moreover, neuromodulation studies at all levels of the neuraxis suggest benefits associated with the use of burst stimulation. In a comparison study, patterned afferent stimulation augmented inhibition within spinal reflex circuits, whereas uniform stimulation demonstrated no effect [
9].
Beyond the temporal pattern of stimulation, another customizable parameter of TSS is the electrode location used to target lower extremity muscle activation. To date, studies of TSS have specified a single spinal level for cathodal stimulation, the T11/12 spinous interspace, with anodes placed anteriorly to direct current towards the spinal nerve roots [
10]. The T11/12 electrode location preferentially activates the dorsal roots of the lumbar enlargement of the spinal cord, specifically, the afferents associated with monosynaptic activation of the motoneuron pools of the quadriceps muscles [
11,
12]. While spasticity of the quadriceps muscles is often problematic for persons with SCI, spasticity in the form of clonus of the soleus muscle can also be problematic and has been the target of numerous studies [
13]. The addition of a second cathodal electrode over the L1/2 spinous interspace has the potential to modulate the more caudal area of the lumbar spinal cord, where the motoneuron pools for the soleus muscle are located [
14].
The purpose of this study was to assess how TSS pulse parameters and electrode placement affect spasticity in participants with SCI who exhibit objectively measurable lower extremity spasticity. We assessed the immediate, within-session effects of 50 Hz continuous TSS delivered through electrodes placed at single and dual sites and 50 Hz burst TSS at a single site. The primary outcome of interest was change in quadriceps muscle spasticity as measured using the pendulum test first swing excursion (FSE). Change in soleus muscle spasticity as measured by the ankle clonus drop test, first drop excursion (FDE) was used as a secondary outcome to provide insight into the effects of an additional caudal lumbar electrode. Finally, we examined the relationship between baseline spasticity and TSS responsiveness to determine whether biomechanical measures of quadriceps and soleus hyperreflexia can serve as biomarkers to guide clinical decision-making.
2. Materials and Methods
This study was conducted with ethical approval from the Shepherd Center Research Review Committee. All participants gave their written informed consent prior to study enrollment, in accordance with the Declaration of Helsinki. This study was registered with clinicaltrials.gov (23 January 2020; NCT04243044). Complete details of the study methods are described in a prior publication, including acquisition of neurophysiologic reflex responses and interventional stimulation protocol [
15].
2.1. Participants
Participants were enrolled if they met the following inclusion criteria: at least 16 years of age with SCI ≥ 3 months duration, regardless of injury severity, and presence of at least mild spasticity in either lower extremity. Spasticity was indicated by a pendulum test FSE ≤ 77°, or ≥5 beats of clonus on the ankle clonus drop test. Individuals with any of the following were excluded from participation: neurological level of injury below T12, progressive or potentially progressive spinal lesions, history of cardiovascular irregularities, active cancer or history of cancer, current pregnancy, implanted stimulators of any type, difficulty following instructions, or orthopedic pathology that would limit the ability to interpret study outcome measures (i.e., knee or hip flexion contractures > 10°).
At enrollment, the pendulum test and ankle clonus drop test were performed to identify the presence of spasticity. The most spastic lower extremity was determined as the lower extremity with the smaller pendulum test FSE, or if the participant did not exhibit quadriceps spasticity, as the lower extremity with the smaller ankle clonus drop test FDE. The same lower extremity was tested for all sessions. All participants were required to meet the spasticity inclusion criteria at the beginning of each session.
2.2. Study Design
This was a randomized, crossover study of a single session of each of three TSS conditions: single-site continuous stimulation (SS-CONT), single-site burst stimulation (SS-BURST), and dual-site continuous stimulation (DS-CONT). Biomechanical measures of quadriceps and soleus spasticity were captured before and immediately after the application of TSS to determine the effects of each TSS condition. There was a minimum of 48 h between sessions to reduce the potential for carryover effects.
2.3. Intervention
For the SS-CONT and SS-BURST conditions, stimulation was delivered through a single 5 cm round self-adhesive electrode (cathode) placed over the T11/12 spinous interspace. For the DS-CONT condition, stimulation was delivered through two 5 cm round self-adhesive electrodes (cathodes)—one over the T11/12 spinous interspace and one over either the L1/2 spinous interspace or the L2/3 spinous interspace. Two interconnected rectangular electrodes (5 × 9 cm each) were placed paraumbilically as anodes. SS-CONT stimulation: continuous and uniform 50 Hz charge-balanced, biphasic stimulation; SS-BURST stimulation: 4 bursts per second of 50 Hz pulses (130 ms train, 120 ms inter-train interval, 7 pulses/train) charge-balanced, biphasic stimulation; DS-CONT stimulation: continuous and uniform 50 Hz charge-balanced, biphasic stimulation at two locations as described above. An illustrative representation of the electrode configuration for all three conditions has been previously published [
15].
All stimulation conditions (Vectra Genisys, Chattanooga/DJO, Carlsbad, CA, USA) were applied with a pulse width of 1 ms per phase for 30 min. Stimulation was delivered while participants remained in a supine position, with skin being checked intermittently for signs of adverse reactions.
2.4. Posterior Root-Muscle Reflex and Stimulation Intensity
Posterior root-muscle (PRM) reflexes were obtained to guide the stimulation intensity for each TSS condition. Electromyographic activity (EMG) was recorded from the soleus muscle using surface electrodes (MA300, Motion Lab Systems, Baton Rouge, LA, USA) and digitized (Power 1401, Cambridge Electronic Design, Cambridge, UK) at a sampling rate of 2 kHz using data capture software (Signal, Cambridge Electronic Design, Cambridge, UK).
Monophasic, rectangular pulses of 1 ms pulse width were delivered at the T11/12 interspinous space using a constant-current stimulator (Digitimer DS7AH, Hertforshire, UK). Pairs of stimuli with a 50 ms interstimulus interval (Grass S88X, Natus Neurology, Middleton, WI, USA) enabled verification of the reflex nature of the response through the presence of post-activation depression of the second stimulus response [
11]. Reflex threshold (RT) was defined as the stimulation intensity that evoked responses in the soleus ≥ 100 μV peak-to-peak amplitude in at least 3 of 5 responses. Responses to five stimuli were collected at RT. TSS intensity was set to 0.8 × T or the highest intensity tolerable by the participant if they could not receive stimulation at 0.8 × RT.
2.5. Biomechanical Measurement of Spasticity
Within-session effects of TSS on spasticity were evaluated using biomechanical measurements of reflex-generated responses at the knee and ankle. Testing was completed before and immediately after TSS application.
The pendulum test assessed stretch-induced quadriceps spasticity [
5,
6,
16,
17,
18]. Participants were positioned semi-reclined with the lower leg pendant over the edge of the mat and the shoe removed. To record knee angle during testing, an electrogoniometer (SG150, Biometrics Ltd., Newport, UK) was affixed to the test leg with the arms aligned with the midline of the thigh and lower leg, and the axis aligned with the knee joint center. The non-test leg was supported with the knee extended. Grasping the heel of the test leg, the examiner extended the knee and held the leg in this position for 60 s to allow movement-related excitability to dissipate. The examiner then released the heel, allowing the test leg to swing with gravity. FSE, the angle at which the knee first reversed from flexion to extension, was acquired and analyzed using Spike software (Cambridge Electronic Design Limited, Cambridge, UK). A larger FSE angle indicates less spasticity.
The ankle clonus drop test assessed stretch-induced soleus spasticity [
19]. Participants were seated at the edge of a mat with knees at 90° flexion and back supported in a fully upright position to allow participants to relax their trunk and upper extremities during testing. The mat height was adjusted so the posterior surface of the upper leg was not touching the mat to allow sufficient distance between the mat and the leg when dropped. An electrogoniometer (SG150 or SG110, Biometrics Ltd., Newport, UK) was used to record the ankle angle of the test leg. The arms of the goniometer were aligned with the midline of the lower leg and the fifth metatarsal, and the axis was placed in the center of the ankle joint. Grasping the leg below the knee joint, the leg was lifted 10 cm above the resting position. The forefoot was positioned to strike the edge of a 6” platform when released, causing a stretch of the soleus. FDE, the angle at which the ankle first reversed from dorsiflexion to plantarflexion, was acquired and analyzed using Spike software (Cambridge Electronic Design Limited, Cambridge, UK). A larger FDE angle indicates less spasticity.
Previous literature has identified differences in biomechanical spasticity measurement due to repeated measurement [
16,
20,
21]. Therefore, to obtain a comprehensive representation of stretch-induced quadriceps and soleus spasticity, the FSE and FDE of both the first trial (FSE
T1 and FDE
T1) and the average FSE and FDE of 3 trials (FSE
avg and FDE
avg) of each test session were used for analysis.
2.6. Data Analysis
Analyses were executed using SPSS version 28 (IBM, London, UK). Data are presented as the mean ± SD. Between- and within-condition analyses were completed. When no significant between-condition differences were observed, data were collapsed across all conditions. To determine the effect of spasticity severity on responsiveness to TSS, participants were then subgrouped based on the median baseline FSE or FDE values. The subgroups were high-spasticity: FSE
avg < 59.0° and FSE
T1 < 56.5°, FDE
avg < 38.4° and FDE
T1 < 38.6°; and low-spasticity: FSE
avg ≥ 59.0° and FSE
T1 ≥ 56.5°, FDE
avg ≥ 38.4° and FDE
T1 ≥ 38.6°. Spasticity is known to vary daily [
18]; therefore, it was necessary to subgroup participants for each outcome measure within a condition, as some participants who demonstrated high spasticity at baseline for one condition may have demonstrated low spasticity for other conditions, and vice versa. In each spasticity subgroup, between- and within-condition analyses were first completed. When no significant between-condition differences were observed within a subgroup, data were collapsed across all conditions.
All analyses were performed for FSEavg, FDEavg, FSET1, and FDET1. One-way ANOVAs were performed to determine differences between baseline values of the first, second, and third sessions and identify the presence of order effects. Paired t-tests were used to test for within-condition differences between pre- and post-intervention values for each condition. One-way ANOVAs were performed to determine differences between baseline values of the three stimulation conditions and to determine between-condition differences in change from baseline. Independent t-tests were used to identify differences in change from baseline between high- and low-spasticity subgroups. One-way ANOVAs were used to determine differences in change from baseline to post-intervention between conditions for the high- and low-spasticity subgroups. Post hoc Tukey tests were performed to identify any significant differences between the three conditions.
Effect sizes for pre-post change in each outcome measure were calculated using Cohen’s
d based on the pooled variance of the compared values. Effect sizes were categorized as small (0.2), moderate (0.5), or large (0.8) [
22]. Effect sizes offer insight into the magnitude of the outcome regardless of statistical significance and allow comparison between intervention conditions regarding change [
23]. Comparison between conditions allows meaningful decisions for research translation into clinical practice.
Pearson correlations (
r) were calculated to determine the relationship between change scores and baseline measures for each TSS condition and when data were collapsed across conditions. Pearson correlations were also calculated to determine the relationship between measures of knee and ankle spasticity. Designations of correlation coefficients were no relationship (<0.25), fair (0.26–0.5), moderate (0.51–0.75), and good (>0.76) [
24]. For all analyses, significance was set at α = 0.05. Increases in FSE and FDE are represented as positive values, indicating increased joint angles (i.e., decrease in spasticity), whereas decreases are represented by negative values, indicating decreased joint angles (i.e., increase in spasticity). All data are presented first as FSE
avg or FDE
avg, followed by FSE
T1 or FDE
T1.
3. Results
Out of 21 participants enrolled, usable data were obtained from 17 participants. Individual movement limitations restricted the collection of all outcome measures for the 17 participants. Of the 17 participants with usable data, 13 participants completed all three conditions, 2 participants completed 2 conditions, and 2 participants completed only one condition. Participant randomization and withdrawal can be found in the previously published CONSORT diagram [
15]. Participant demographics have been previously published [
15].
Of the 82 instances in which the pendulum test was performed, accounting for all participants, conditions, and timepoints, we observed the smallest FSE (i.e., highest quadriceps spasticity) in 46 instances for trial one, 19 for trial two, and 17 for trial three. Of the 90 instances in which the ankle clonus drop test was performed, we observed the smallest FDE (i.e., highest soleus spasticity) in 22 instances for trial one, 33 for trial two, and 35 for trial three.
No order effects for session were identified for FSEavg, FSET1, FDEavg, or FDET1, respectively (F(2,38) = 0.52 p = 0.60, F(2,38) = 0.68 p = 0.51, F(2,42) = 1.74 p = 0.19, F(2,42) = 1.76 p = 0.18).
3.1. Stretch-Induced Spasticity of the Quadriceps
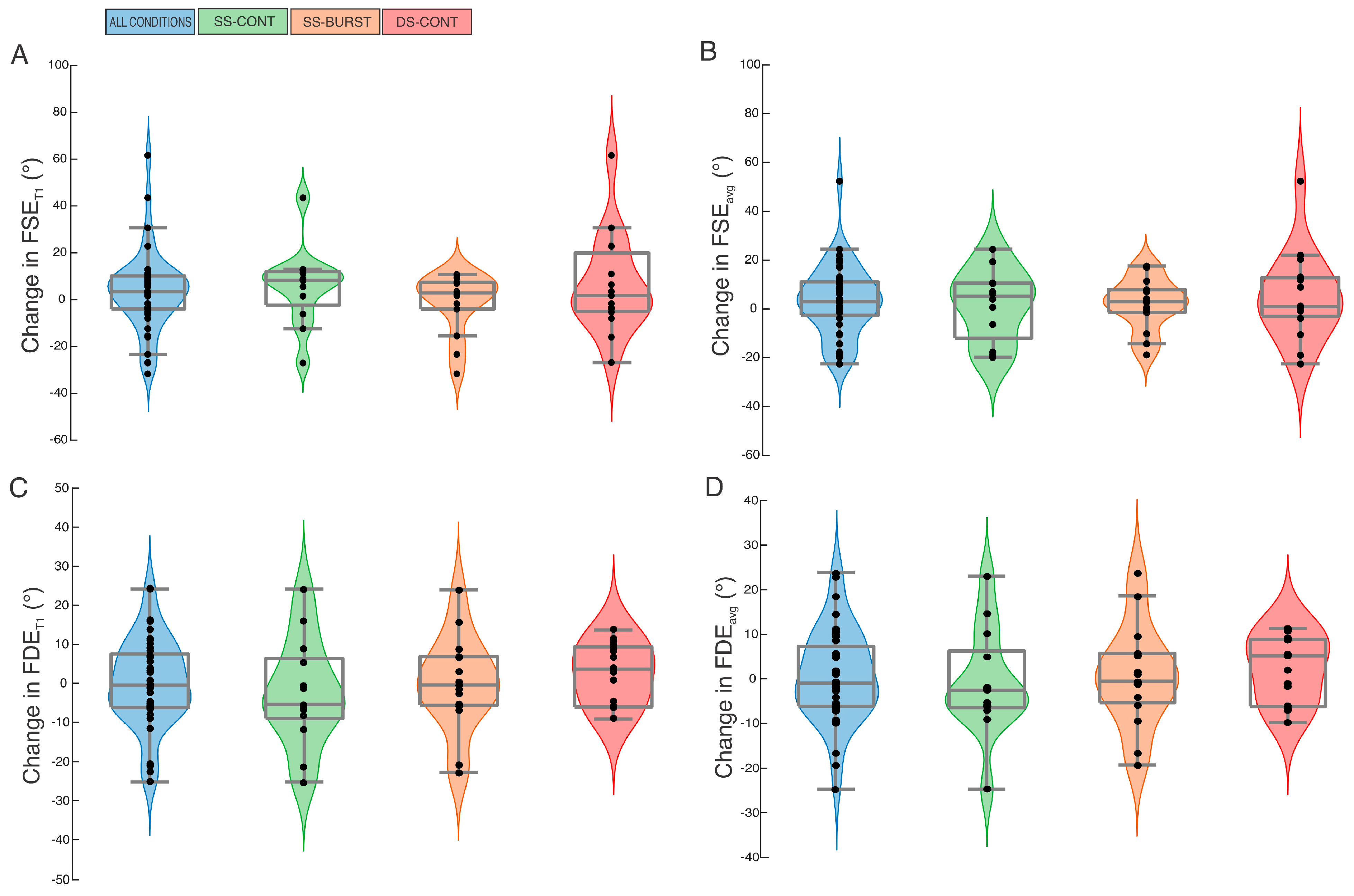
3.1.1. Whole Group Analysis
At baseline, no differences were found for FSE
avg or FSE
T1 among SS-CONT, DS-CONT, and SS-BURST (F(2,38) = 0.16,
p = 0.86, F(2,38) = 0.02,
p = 0.98) (
Table 1). Between-condition analysis identified no difference in pre-post change in FSE
avg or FSE
T1 between the three intervention conditions (F(2,38) = 0.21,
p = 0.81; F(2,38) = 0.86,
p = 0.43). Within-condition pre-post analysis showed all conditions demonstrated small or no effect and non-significant increases in FSE
avg and FSE
T1 after intervention, except SS-BURST for the FSE
T1 comparison (
Table 2,
Figure 1A,B). SS-BURST demonstrated a non-significant decrease in FSE
T1 with no effect. The largest effect size for change in FSE
avg was demonstrated after SS-CONT stimulation; the largest effect size for change in FSE
T1 was observed after DS-CONT stimulation (
Table 2,
Figure 1A,B). When data were collapsed across intervention conditions, there was no significant change in FSE
avg or FSE
T1 (
Table 2,
Figure 1A,B).
3.1.2. High-Spasticity Subgroup Analysis
The mean baseline FSE
avg and FSE
T1 for the high-spasticity subgroup were 45.8 ± 7.3° and 43.8 ± 7.0°, respectively. Between-condition analysis showed no difference in the change in mean FSE
avg and FSE
T1 between intervention conditions (FSE
avg: F(2,18) = 1.0,
p = 0.38; FSE
T1: F(2,18) = 1.03,
p = 0.38). Within-condition analysis showed large, significant increases in mean FSE
T1 after DS-CONT and SS-BURST stimulation (
Table 3). Although non-significant, there was a moderate effect after SS-CONT stimulation for FSE
avg and FSE
T1 (
Table 3). When data were collapsed across intervention conditions, moderate and significant increases were observed in both mean FSE
avg and FSE
T1 (
Table 3).
3.1.3. Low-Spasticity Subgroup Analysis
The mean baseline FSE
avg and FSE
T1 for the low-spasticity subgroup were 81.2 ± 17.6° and 79.9 ± 20.3°, respectively. Between-conditions analysis showed no difference in the change in mean FSE
avg and FSE
T1 between intervention conditions (FSE
avg: F(2,17) = 0.43,
p = 0.66; FSE
T1: F(2,17) = 0.33,
p = 0.72). Within-condition analysis identified DS-CONT stimulation demonstrated the largest mean decrease in FSE
avg and FSE
T1 with small and moderate effects, respectively (
Table 3). When data were collapsed across intervention conditions, mean FSE
avg and FSE
T1 demonstrated no significant decrease (
Table 3).
3.2. Stretch-Induced Spasticity of the Soleus
3.2.1. Whole Group Analysis
At baseline, no differences were found for FDE
avg or FDE
T1 among the three intervention conditions (F(2,42) = 1.98,
p = 0.15; F(2,42) = 2.01,
p = 0.15) (
Table 1). Between-condition analysis identified no difference in pre-post change in FDE
avg and FDE
T1 between the three intervention conditions (F(2,42) = 0.37,
p = 0.69; F(2,42) = 0.70,
p = 0.50). Within-condition pre-post analysis showed no significant change with small or no effect in FDE
avg or FDE
T1 for any condition (
Table 2,
Figure 1C,D). SS-CONT demonstrated the largest effect size, albeit small, for both change in FDE
avg and FDE
T1 (
Table 2,
Figure 1C,D). When data were collapsed across intervention conditions, there was no significant change in FDE
avg or FDE
T1 (
Table 2,
Figure 1C,D).
3.2.2. High-Spasticity Subgroup Analysis
The mean baseline FDE
avg and FDE
T1 for the high-spasticity subgroup were 33.1 ± 4.5° and 33.6 ± 4.6°, respectively. There were no significant differences in change between conditions for FDE
avg or FDE
T1 (F(2,20) = 0.49,
p = 0.62; F(2,20) = 0.60,
p = 0.56). Within-condition analysis showed the largest mean increases in FDE
avg and FDE
T1 with moderate effect were observed after DS-CONT stimulation (
Table 3). Mean FDE
avg and FDE
T1 also demonstrated a small non-significant increase after SS-CONT stimulation (
Table 3). No significance or effect was demonstrated after SS-BURST stimulation (
Table 3). When data were collapsed across intervention conditions, a small, non-significant increase in both mean FDE
avg and FDE
T1 was observed (
Table 3).
3.2.3. Low-Spasticity Subgroup Analysis
The mean baseline FDE
avg and FDE
T1 for the low-spasticity subgroup were 45.7 ± 7.0° and 46.7 ± 8.1°, respectively. Between-condition analysis showed significant differences in change for FDE
T1 only (FDE
avg: F(2,19) = 2.53,
p = 0.10; FDE
T1 F(2,19) = 4.00,
p = 0.04). Post hoc comparisons revealed significant differences between SS-CONT and DS-CONT stimulation only (FDE
T1:
p = 0.03). Within-condition analysis shows SS-CONT stimulation demonstrated a small, non-significant increase in FDE
avg and FDE
T1 (
Table 3). DS-CONT stimulation demonstrated a large, significant decrease in both FDE
avg and FDE
T1 (
Table 3). When data were collapsed across intervention conditions, no change was observed in mean FDE
avg and FDE
T1 (
Table 3).
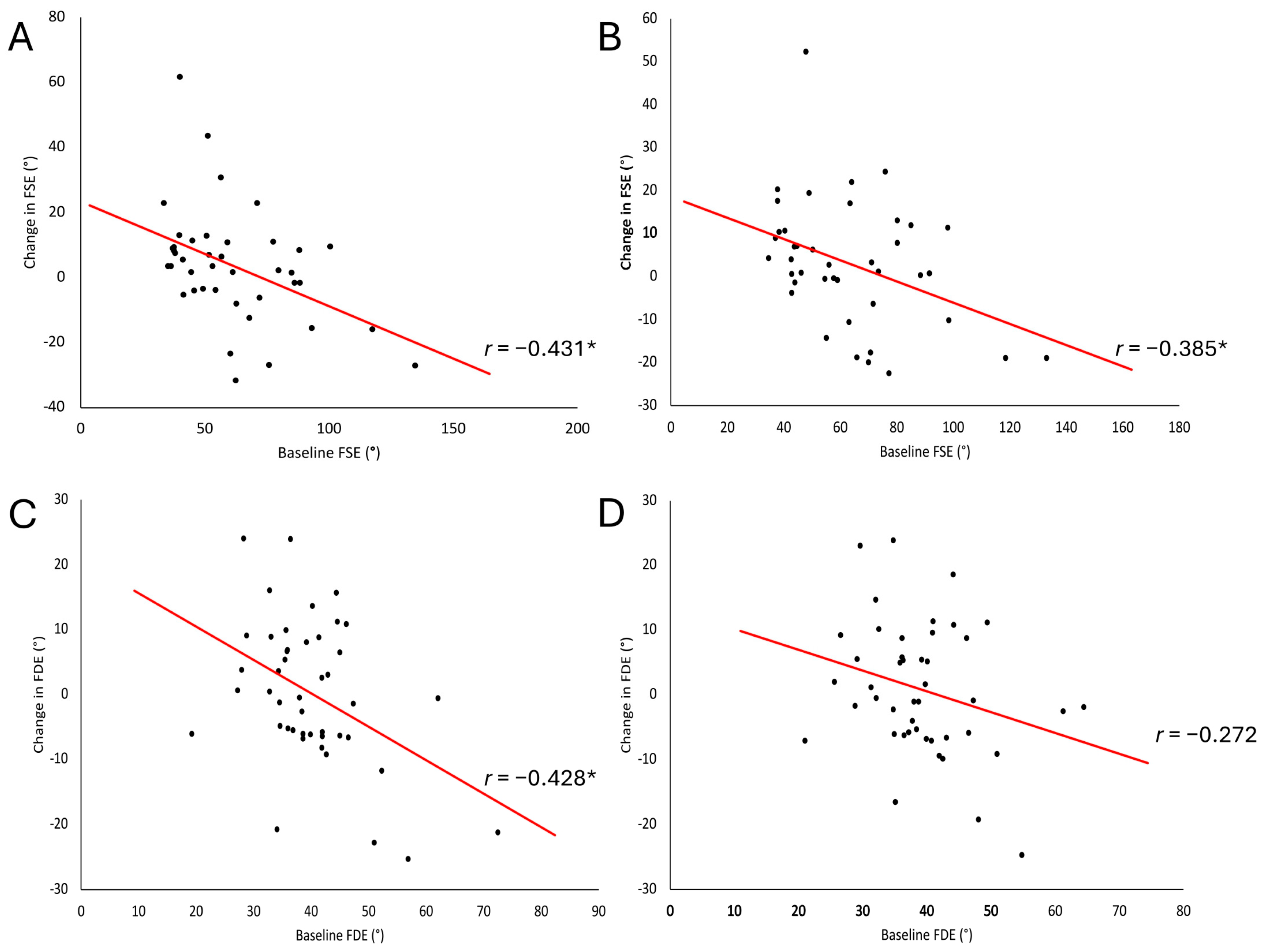
3.3. Correlations of Baseline Spasticity and Change in Biomechanical Measures
Participants with higher baseline spasticity (smaller FSE and FDE) experienced greater change, as indicated by negative correlations when data were collapsed across intervention conditions. Correlations between baseline FSE
avg and FSE
T1 and change in the respective measures were fair and significant when data were collapsed across intervention conditions (
Figure 2A,B). Within-condition analysis demonstrated a fair and significant relationship in the SS-CONT condition between baseline FSE
T1 and change in FSE
T1 and a moderate and significant relationship for the DS-CONT condition (
Table 4). In addition, baseline FSE
avg and change in FSE
avg demonstrated a significant relationship in the DS-CONT condition only, which was moderate (
Table 4). Baseline FDE
avg and change in FDE
avg demonstrated no relationship (
Figure 2D) when data were collapsed across intervention conditions. Within-condition analysis demonstrated a moderate and significant relationship between baseline FDE
avg and change in FDE
avg (
Table 4). Correlation between baseline FDE
T1 and change in FDE
T1 was fair and significant (
Figure 2C) when data were collapsed across intervention conditions and moderate and significant in the DS-CONT condition. No relationships were found between measures of spasticity at the knee and the ankle at baseline. No relationships were found between measures of change in spasticity post-intervention at the knee and the ankle.
4. Discussion
This study compared the single-session effects of three TSS conditions (SS-CONT, DS-CONT, and SS-BURST) to assess their influence on spasticity in participants with SCI who experience hyperreflexia of the muscles about the knee and/or ankle. Biomechanical measures of stretch-induced reflex excitability were used to assess spasticity. Quadriceps spasticity did not change significantly from baseline to post-TSS intervention in any of the conditions when data from the whole group were analyzed together. The largest effect sizes for change in quadriceps spasticity post-intervention were for the SS-CONT condition for FSEavg and for the DS-CONT condition for FSET1. In whole group analysis, the largest decrease in soleus spasticity from baseline to post-TSS intervention was observed in the SS-CONT condition. Moreover, the change in soleus spasticity after SS-CONT was associated with small effect sizes for both measures, FDEavg and FDET1.
4.1. Effects of Baseline Spasticity on Responsiveness to TSS
Prior studies have shown that responsiveness to afferent stimulation is influenced by the baseline level of spasticity [
6,
18]. The results of the current study are consistent with those findings. When combining all participants and conditions, there was a significant fair relationship between baseline quadriceps spasticity (FSE
avg and FSE
T1) and the magnitude of change post-intervention. For soleus spasticity, there was also a significant fair relationship between baseline FDE
T1 and change post-intervention; however, there was no relationship between baseline FDE
avg and magnitude of change post-intervention when data were collapsed across conditions. In addition, with all intervention conditions combined, the high-spasticity subgroup (FSE
avg < 59.0° and FSE
T1 < 56.5°) demonstrated a significant decrease in quadriceps spasticity, whereas there was no change in the low-spasticity subgroup (FSE
avg ≥ 59.0° and FSE
T1 ≥ 56.5°). In the high-spasticity subgroup, spasticity decreased significantly for DS-CONT and SS-BURST. There was no significant change in soleus spasticity in the high-spasticity subgroup (FDE
avg < 38.4° and FDE
T1 < 38.6°) or the low-spasticity subgroup (FDE
avg ≥ 38.4° and FDE
T1 ≥ 38.6°) from baseline to post-intervention. DS-CONT stimulation significantly increased soleus spasticity in the low-spasticity subgroup.
4.2. Differences in Current Delivery Between Conditions
Differences in total current delivered during spinal stimulation may account for differences observed between intervention conditions. Stimulation intensity for each condition was maintained below motor threshold to activate only large-diameter afferent fibers. Electrical fields targeting spinal circuits have been shown to modulate firing patterns of spinal neurons in an intensity dose-dependent manner [
25,
26]. Although intensity was consistently 0.8 × RT for each condition, DS-CONT stimulation was delivered at 0.8 × RT at each of the two electrodes, effectively doubling the total charge delivered. Moreover, SS-BURST stimulation included “off” time, thereby reducing the total charge delivery in comparison to continuous stimulation.
The SS-BURST condition demonstrated less effect on quadriceps spasticity than SS-CONT for within-condition, whole-group analyses. This is not consistent with previous literature demonstrating a greater inhibitory effect on mechanisms of spasticity after patterned stimulation compared to uniform/continuous stimulation [
9]. However, SS-BURST stimulation demonstrated a large, significant positive effect for FSE
T1 for the high spasticity subgroup in contrast to a small, non-significant negative effect in the low spasticity subgroup. Stimulation pattern, therefore, had an influence on neural circuits that was not evident until participants were stratified into high and low spasticity subgroups. Of note, the total charge in the SS-BURST condition was less than that of the SS-CONT and DS-CONT conditions. Taken together, this may indicate that the introduction of a pattern into the stimulation may preferentially activate inhibitory spinal circuits responsible for quadriceps spasticity reduction, but only in individuals with high spasticity, and may require less overall charge delivery to demonstrate effect.
4.3. Dual-Site Electrodes Preferentially Target the Soleus
Preferential activation of spinal circuits can be achieved, dependent upon the level of the spinal cord at which stimulation is applied [
14]. The probability of activating circuits associated with the quadriceps is greatest at L2–L4 spinal levels and S1/2 for the soleus [
14]. Whole group analyses demonstrated a decrease in ankle spasticity after SS-CONT stimulation. This suggests the SS-CONT condition was capable of modulating more caudal dorsal nerve roots, most likely through current dispersion at multiple spinal levels [
27]. The beneficial effects of SS-CONT stimulation on ankle spasticity, as indicated by a small effect size (FSE
avg d = 0.23, FSE
T1 d = 0.38), contrast with results from previous work, potentially due to methodological differences [
28]. Estes et al. combined locomotor training and continuous spinal stimulation and found no effect, as indicated by an effect size of
d = 0.06 on soleus spasticity of the more impaired limb as measured by the number of oscillations in the ankle clonus drop test.
In the high spasticity subgroup, DS-CONT stimulation resulted in decreased ankle spasticity with a moderate effect size. The small increase in soleus spasticity observed in the whole group analysis of the DS-CONT condition may be attributable to the high variability in baseline spasticity when all participants were combined. Subsequently, subgroup analysis revealed participants with high spasticity at the soleus benefited from the addition of the second electrode, as demonstrated by a larger effect on FDEavg and FDET1 after DS-CONT stimulation compared to SS-CONT stimulation. In contrast, there was a significant increase in spasticity in participants with low spasticity after DS-CONT stimulation. Afferent fibers associated with the soleus were likely recruited with the addition of a lumbar electrode for dual-site stimulation, promoting neuromodulation of soleus spinal circuits.
4.4. Activation of Multisegmental Inhibitory Circuits
Neuronal connections between the rostral and caudal motoneuron pools are responsible for excitation and inhibition of circuits that regulate interlimb and intralimb timing [
29,
30,
31,
32,
33]. Specifically, the spinal networks related to the quadriceps exert heteronymous influence on the soleus in humans [
33,
34]. An inhibitory effect of rostral spinal circuits on caudal spinal circuits, dependent upon temporal characteristics of each stimulus, has also been demonstrated during dual-site stimulation [
34]. Moreover, single-site stimulation at either thoracic or lumbar electrode locations resulted in larger evoked responses and corresponding muscle forces at proximal and distal muscles compared to multi-site stimulation [
35]. In our study, each electrode (thoracic and lumbar) provided 50 Hz stimulation independently. The relative timing (rostral followed by caudal or caudal followed by rostral) of stimulating pulses from each of the two electrodes in the DS-CONT was therefore not consistent, potentially producing varying timing of activation between rostral (quadriceps) and caudal (soleus) spinal circuits. Single-site stimulation (SS-CONT) resulted in the largest decrease in ankle spasticity with all participants combined. Conversely, dual-site stimulation (DS-CONT) resulted in no significant change with all participants combined or in the high-spasticity subgroup and a significant increase in ankle spasticity in the low-spasticity subgroup. It is possible that spatiotemporal-dependent characteristics of dual-site stimulation, i.e., location and timing of stimulation at each electrode, may account for the lack of significant effect observed in the DS-CONT condition.
4.5. Delayed Effect of TSS on Quadriceps and Soleus Spasticity
The magnitude of TSS effects depends on the timing of post-intervention measurement [
6,
18]. A previous study of the persistent effects of a single session of TSS demonstrates a significant decrease in quadriceps spasticity for participants categorized as having high spasticity immediately following and 45 min post-intervention [
6]. The stimulation in these studies was delivered continuously at a single site at a frequency of 50 Hz with a pulse width of 400 μs for 15 min. However, the antispasmodic effect was non-significant at the 15-min post-intervention measurement time point. The biomechanical measurements of spasticity, the pendulum test and the ankle clonus drop test, were assessed in the current study at a single time point, approximately 15–30 min post-intervention. Due to the post-assessment timing in the current study, the previously observed time-dependent effect may account for the lack of significance in the change in spasticity of the quadriceps found in post-intervention measurement for the single-site (SS-CONT and SS-BURST) conditions. It is likely that the timing of the post-TSS assessment would have a similar influence on outcomes at the quadriceps and at the soleus.
4.6. TSS Overcomes Immobility-Induced Spasticity
Therapeutic interventions that involve activation of sensory afferents through movement, as well as devices that stimulate these same afferents, have been shown to reduce spasticity [
5]. By comparison, imposed immobility of participants remaining in a supine position for the 30-min duration of sham stimulation resulted in increased spasticity [
5]. Therefore, the anti-spasmodic effect of spinal stimulation must overcome the spasticity-inducing effects of immobility. While no sham condition was included in the current study, the low-spasticity subgroup may have experienced the negative effects of immobility during intervention. Movement and activity are known to have antispasmodic effects [
5,
36]. Multiple studies of spinal stimulation for motor function have combined the intervention with task practice [
28,
37,
38]. For this reason, it may be advisable to combine spinal stimulation with movement-based interventions to augment its antispasmodic effects.
4.7. Limitations
This study serves as a foundation to identify appropriate dosing parameters for the use of spinal stimulation to reduce spasticity. However, several limitations must be addressed to assist in the clinical translation of spinal stimulation used in this study. First, individuals with spasticity at the knee or the ankle were included in this study. Participants were not required to demonstrate spasticity at both joints. At enrollment and prior to each session, individuals who exhibited spasticity at the knee may not have exhibited spasticity at the ankle, and vice versa. Despite this, measures were taken at both joints during each session, which may explain the lack of relationship found between spasticity of the knee and ankle in this study. Second, the pendulum test and ankle clonus drop test measure a single component of spasticity, hyperreflexia, around a single joint in isolation. In a survey of people with tetraplegia and paraplegia after SCI, stiffness was reported to be more problematic than spontaneous spasms and hyperreflexia for both levels of paresis [
39]. Clinical measurement of spasticity presentation is limited to assessment of stretch-induced ordinal measures; further research is needed to quantify qualities of spasticity that have the greatest impact on lived experience. The current study did not assess the persistence of any anti-spasmodic effect beyond the single immediate post-intervention time point. Identifying therapeutic interventions to reduce dependence upon pharmaceuticals, which are often associated with negative side effects, requires an understanding of the duration of effects. Moreover, this was a single-session study that used a stimulation intensity below motor threshold. Further research is needed to elaborate upon the potential benefits of multi-session use of spinal stimulation applied at various intensities to establish its efficacy with consistent use in a clinical or home setting.
5. Conclusions
In the current study, we identified that when data were collapsed across conditions, TSS did not result in a significant reduction in quadriceps or soleus spasticity. Continuous stimulation at both single- and dual-sites, SS-CONT and DS-CONT, respectively, were associated with the largest effect on quadriceps spasticity when all participants were combined. Based on our findings, the metric used to assess quadriceps and soleus spasticity influences the study outcome. We have demonstrated that the TSS condition with the largest effect on quadriceps spasticity differs when using the first trial or the average of three trials of the pendulum test. We recommend the use of the average of three trials when assessing quadriceps spasticity via the pendulum test, as the first trial did not always result in the smallest FSE. Although the TSS condition with the largest effect on soleus spasticity did not differ when using the first trial or the average of three trials of the ankle clonus test, we recommend the use of the average of three trials when assessing soleus spasticity via the ankle clonus drop test. By using the average of three trials, the known variability of spasticity can be accounted for.
TSS was demonstrated to reduce spasticity in a severity-dependent manner. Identification of spasticity severity at the knee and ankle is therefore critical prior to TSS application. We recommend the use of SS-CONT stimulation when unable to obtain an objective measurement of spasticity severity. Although DS-CONT and SS-CONT stimulation demonstrated the largest effect on quadriceps spasticity when low and high spasticity subgroups were considered together, DS-CONT stimulation, however, resulted in an increase in spasticity in the low spasticity subgroup. If spasticity severity is known to be high, we recommend the use of DS-CONT stimulation for reduction in quadriceps spasticity. We also recommend the use of DS-CONT stimulation when targeting spasticity of the soleus, but only when an individual presents with high spasticity severity.
Author Contributions
Conceptualization, E.B.S., J.A.I. and E.C.F.-F.; methodology, E.B.S., J.A.I. and E.C.F.-F.; formal analysis, E.B.S. and J.A.I.; writing—original draft preparation, E.B.S.; writing—review and editing, J.A.I. and E.C.F.-F.; funding acquisition, E.C.F.-F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NIH National Institute of Child Health and Human Development (NICHD) R01 HD101812-01 (E.C.F.-F.).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Shepherd Center Research Review Committee (protocol code 851 and 8 July 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors thank all research participants who volunteered their time to participate in this study. We also thank Kelly Thatcher, PT, DPT, NCS, and Oliver Daliet IV, MS, for their recruitment efforts and assistance with conducting research sessions.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| TSS | Transcutaneous spinal stimulation |
| SCI | Spinal cord injury |
| PRM | Posterior root-muscle |
| SS-CONT | Single-site continuous |
| SS-BURST | Single-site burst |
| DS-CONT | Dual-site continuous |
| RT | Reflex threshold |
| FDE | First drop excursion |
| FSE | First swing excursion |
| RA | Response amplitude |
| EMG | Electromyography |
References
- Nathan, P.W. Factors affecting spasticity. Int. Rehabil. Med. 1980, 2, 27–30. [Google Scholar] [CrossRef]
- Elbasiouny, S.M.; Moroz, D.; Bakr, M.M.; Mushahwar, V.K. Management of spasticity after spinal cord injury: Current techniques and future directions. Neurorehabilit. Neural Repair 2010, 24, 23–33. [Google Scholar] [CrossRef]
- Hofstoetter, U.S.; Freundl, B.; Binder, H.; Minassian, K. Common neural structures activated by epidural and transcutaneous lumbar spinal cord stimulation: Elicitation of posterior root-muscle reflexes. PLoS ONE 2018, 13, e0192013. [Google Scholar] [CrossRef]
- Hofstoetter, U.S.; Freundl, B.; Danner, S.M.; Krenn, M.J.; Mayr, W.; Binder, H.; Minassian, K. Transcutaneous Spinal Cord Stimulation Induces Temporary Attenuation of Spasticity in Individuals with Spinal Cord Injury. J. Neurotrauma 2020, 37, 481–493. [Google Scholar] [CrossRef]
- Estes, S.P.; Iddings, J.A.; Field-Fote, E.C. Priming Neural Circuits to Modulate Spinal Reflex Excitability. Front. Neurol. 2017, 8, 17. [Google Scholar] [CrossRef]
- Sandler, E.B.; Condon, K.; Field-Fote, E.C. Efficacy of Transcutaneous Spinal Stimulation versus Whole Body Vibration for Spasticity Reduction in Persons with Spinal Cord Injury. J. Clin. Med. 2021, 10, 3267. [Google Scholar] [CrossRef]
- Massey, S.; Vanhoestenberghe, A.; Duffell, L. Neurophysiological and clinical outcome measures of the impact of electrical stimulation on spasticity in spinal cord injury: Systematic review and meta-analysis. Front. Rehabil. Sci. 2022, 3, 1058663. [Google Scholar] [CrossRef]
- Gilbert, J.E.; Zhang, T.; Esteller, R.; Grill, W.M. Evaluating optimized temporal patterns of spinal cord stimulation (SCS). Brain Stimul. 2022, 15, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.A.; Field-Fote, E.C.; Floeter, M.K. Patterned sensory stimulation induces plasticity in reciprocal ia inhibition in humans. J. Neurosci. Off. J. Soc. Neurosci. 2003, 23, 2014–2018. [Google Scholar] [CrossRef] [PubMed]
- Thatcher, K.L.; Nielsen, K.E.; Sandler, E.B.; Daliet, O.J.; Iddings, J.A.; Field-Fote, E.C. Optimizing Transcutaneous Spinal Stimulation: Excitability of Evoked Spinal Reflexes is Dependent on Electrode Montage. J. Neuroeng. Rehabil. 2025, 22, 2. [Google Scholar] [CrossRef] [PubMed]
- Minassian, K.; Persy, I.; Rattay, F.; Dimitrijevic, M.R.; Hofer, C.; Kern, H. Posterior root-muscle reflexes elicited by transcutaneous stimulation of the human lumbosacral cord. Muscle Nerve 2007, 35, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Sayenko, D.G.; Atkinson, D.A.; Dy, C.J.; Gurley, K.M.; Smith, V.L.; Angeli, C.; Harkema, S.J.; Edgerton, V.R.; Gerasimenko, Y.P. Spinal segment-specific transcutaneous stimulation differentially shapes activation pattern among motor pools in humans. J. Appl. Physiol. 2015, 118, 1364–1374. [Google Scholar] [CrossRef]
- Hope, J.M.; Koter, R.Z.; Estes, S.P.; Field-Fote, E.C. Disrupted Ankle Control and Spasticity in Persons with Spinal Cord Injury: The Association Between Neurophysiologic Measures and Function. A Scoping Review. Front. Neurol. 2020, 11, 166. [Google Scholar] [CrossRef]
- Hofstoetter, U.S.; Perret, I.; Bayart, A.; Lackner, P.; Binder, H.; Freundl, B.; Minassian, K. Spinal motor mapping by epidural stimulation of lumbosacral posterior roots in humans. iScience 2021, 24, 101930. [Google Scholar] [CrossRef]
- Sandler, E.B.; Iddings, J.A.; Minassian, K.; Field-Fote, E.C. Transcutaneous Spinal Stimulation Modulates Spinal Reflex Circuit Excitability in Persons with Spinal Cord Injury. Biomedicines 2025, 13, 2195. [Google Scholar] [CrossRef]
- Zarkou, A.; Field-Fote, E.C. The influence of physiologic and atmospheric variables on spasticity after spinal cord injury. NeuroRehabilitation 2021, 48, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Fowler, E.G.; Nwigwe, A.I.; Ho, T.W. Sensitivity of the pendulum test for assessing spasticity in persons with cerebral palsy. Dev. Med. Child Neurol. 2000, 42, 182–189. [Google Scholar] [CrossRef]
- Estes, S.; Iddings, J.A.; Ray, S.; Kirk-Sanchez, N.J.; Field-Fote, E.C. Comparison of Single-Session Dose Response Effects of Whole Body Vibration on Spasticity and Walking Speed in Persons with Spinal Cord Injury. Neurother. J. Am. Soc. Exp. Neurother. 2018, 15, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Manella, K.J.; Roach, K.E.; Field-Fote, E.C. Temporal Indices of Ankle Clonus and Relationship to Electrophysiologic and Clinical Measures in Persons with Spinal Cord Injury. J. Neurol. Phys. Ther. JNPT 2017, 41, 229–238. [Google Scholar] [CrossRef]
- Willaert, J.; Desloovere, K.; Van Campenhout, A.; Ting, L.H.; De Groote, F. Movement History Influences Pendulum Test Kinematics in Children with Spastic Cerebral Palsy. Front. Bioeng. Biotechnol. 2020, 8, 920. [Google Scholar] [CrossRef]
- De Groote, F.; Blum, K.P.; Horslen, B.C.; Ting, L.H. Interaction between muscle tone, short-range stiffness and increased sensory feedback gains explains key kinematic features of the pendulum test in spastic cerebral palsy: A simulation study. PLoS ONE 2018, 13, e0205763. [Google Scholar] [CrossRef]
- Sullivan, G.M.; Feinn, R. Using Effect Size-or Why the P Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef]
- Kraemer, H.C.; Morgan, G.A.; Leech, N.L.; Gliner, J.A.; Vaske, J.J.; Harmon, R.J. Measures of clinical significance. J. Am. Acad. Child Adolesc. Psychiatry 2003, 42, 1524–1529. [Google Scholar] [CrossRef]
- Portney, L.G. Foundations of Clinical Research: Applications to Evidence-Based Practice; F.A. Davis: Philadelphia, PA, USA, 2020. [Google Scholar]
- Danner, S.M.; Zhang, H.; Shevtsova, N.A.; Borowska-Fielding, J.; Deska-Gauthier, D.; Rybak, I.A.; Zhang, Y. Spinal V3 Interneurons and Left-Right Coordination in Mammalian Locomotion. Front. Cell. Neurosci. 2019, 13, 516. [Google Scholar] [CrossRef]
- Powell, E.S.; Korupolu, R.; Westgate, P.M.; Carrico, C.; Reddy, L.; Sawaki, L. Dose-response relationship of transcutaneous spinal direct current stimulation in healthy humans: A proof of concept study. NeuroRehabilitation 2018, 43, 369–376. [Google Scholar] [CrossRef]
- Danner, S.M.; Hofstoetter, U.S.; Ladenbauer, J.; Rattay, F.; Minassian, K. Can the human lumbar posterior columns be stimulated by transcutaneous spinal cord stimulation? A modeling study. Artif. Organs 2011, 35, 257–262. [Google Scholar] [CrossRef]
- Estes, S.; Zarkou, A.; Hope, J.M.; Suri, C.; Field-Fote, E.C. Combined Transcutaneous Spinal Stimulation and Locomotor Training to Improve Walking Function and Reduce Spasticity in Subacute Spinal Cord Injury: A Randomized Study of Clinical Feasibility and Efficacy. J. Clin. Med. 2021, 10, 1167. [Google Scholar] [CrossRef] [PubMed]
- Lyle, M.A.; Cuadra, C.; Wolf, S.L. Quadriceps muscle stimulation evokes heteronymous inhibition onto soleus with limited Ia activation compared to femoral nerve stimulation. Exp. Brain Res. 2022, 240, 2375–2388. [Google Scholar] [CrossRef]
- Cuadra, C.; De Boef, A.; Luong, S.; Wolf, S.L.; Nichols, T.R.; Lyle, M.A. Reduced inhibition from quadriceps onto soleus after acute quadriceps fatigue suggests Golgi tendon organ contribution to heteronymous inhibition. Eur. J. Neurosci. 2024, 60, 4317–4331. [Google Scholar] [CrossRef] [PubMed]
- Bergmans, J.; Delwaide, P.J.; Gadea-Ciria, M. Short-latency effects of low-threshold muscular afferent fibers on different motoneuronal pools of the lower limb in man. Exp. Neurol. 1978, 60, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Hultborn, H.; Meunier, S.; Morin, C.; Pierrot-Deseilligny, E. Assessing changes in presynaptic inhibition of I a fibres: A study in man and the cat. J. Physiol. 1987, 389, 729–756. [Google Scholar] [CrossRef]
- Crone, C.; Hultborn, H.; Mazières, L.; Morin, C.; Nielsen, J.; Pierrot-Deseilligny, E. Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex size: A study in man and the cat. Exp. Brain Res. 1990, 81, 35–45. [Google Scholar] [CrossRef]
- Sayenko, D.G.; Atkinson, D.A.; Floyd, T.C.; Gorodnichev, R.M.; Moshonkina, T.R.; Harkema, S.J.; Edgerton, V.R.; Gerasimenko, Y.P. Effects of paired transcutaneous electrical stimulation delivered at single and dual sites over lumbosacral spinal cord. Neurosci. Lett. 2015, 609, 229–234. [Google Scholar] [CrossRef]
- Tran, K.; Steele, A.; Crossnoe, R.; Martin, C.; Sayenko, D.G. Multi-site lumbar transcutaneous spinal cord stimulation: When less is more. Neurosci. Lett. 2024, 820, 137579. [Google Scholar] [CrossRef]
- Field-Fote, E.C.; Furbish, C.L.; Tripp, N.E.; Zanca, J.; Dyson-Hudson, T.; Kirshblum, S.; Heinemann, A.; Chen, D.; Felix, E.; Worobey, L.; et al. Characterizing the Experience of Spasticity after Spinal Cord Injury: A National Survey Project of the Spinal Cord Injury Model Systems Centers. In Archives of Physical Medicine and Rehabilitation; Elsevier Inc.: Philadelphia, PA, USA, 2021. [Google Scholar]
- Moritz, C.; Field-Fote, E.C.; Tefertiller, C.; van Nes, I.; Trumbower, R.; Kalsi-Ryan, S.; Purcell, M.; Janssen, T.W.J.; Krassioukov, A.; Morse, L.R.; et al. Non-invasive spinal cord electrical stimulation for arm and hand function in chronic tetraplegia: A safety and efficacy trial. Nat. Med. 2024, 30, 1276–1283. [Google Scholar] [CrossRef]
- Hofstoetter, U.S.; Hofer, C.; Kern, H.; Danner, S.M.; Mayr, W.; Dimitrijevic, M.R.; Minassian, K. Effects of transcutaneous spinal cord stimulation on voluntary locomotor activity in an incomplete spinal cord injured individual. Biomed. Tech. Biomed. Eng. 2013, 58, 5–7. [Google Scholar] [CrossRef]
- McKay, W.B.; Sweatman, W.M.; Field-Fote, E.C. The experience of spasticity after spinal cord injury: Perceived characteristics and impact on daily life. In Spinal Cord; Springer: New York, NY, USA, 2018; Volume 56, pp. 478–486. [Google Scholar]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).