Early Prediction of Cerebral Vasospasm After Aneurysmal Subarachnoid Hemorrhage Using a Machine Learning Model and Interactive Web Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Definition of Cerebral Vasospasm
2.2. Features and Data Preprocessing
2.3. Statistical Analysis
2.4. Predictive Modeling
2.5. Data and Code Availability
2.6. Web-Based Risk Estimation Interface
3. Results
3.1. Prediction of Cerebral Vasospasm
3.2. Feature Importance in Logistic Regression
4. Discussion
Limitations and Future Directions
5. Conclusions
- Multicenter prospective validation: Confirm the model’s generalizability in larger, independent patient cohorts across multiple institutions.
- Integration of multimodal data: Incorporate advanced imaging (e.g., perfusion imaging) and biomarker information to further improve predictive performance.
- Continuous learning systems: Develop dynamic models capable of updating performance as new data become available over time.
- Clinical workflow integration: Evaluate the usability and impact of the web-based application in real clinical settings.
- Outcome improvement: Assess whether early vasospasm risk identification leads to optimized monitoring strategies and improved patient outcomes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SAH | spontaneous subarachnoid hemorrhage |
| aSAH | aneurysmal subarachnoid hemorrhage |
| EVD | external ventricular drainage |
| DALYs | disability-adjusted life years |
| DCI | delayed cerebral ischemia |
| WFNS | World Federation of Neurological Societies |
| ML | machine learning |
| CNS | central nervous system |
| CTA | computed tomography angiography |
| MRA | magnetic resonance angiography |
| DSA | digital subtraction angiography |
| TCD | transcranial Doppler |
| MCA | middle cerebral artery |
| SVMs | support vector machines |
| KNN | k-nearest neighbors |
| ACom | anterior communicating artery |
| SMOTE | Synthetic Minority Oversampling Technique |
| CV | cross-validation |
| AUC-ROC), | area under the receiver operating characteristic curve |
| AUC-PR | area under the precision–recall curve |
| AP | average precision |
| bACC | balanced accuracy |
| PPV | positive predictive value |
| NPV | negative predictive value |
| TPR | true positive rate |
| TNR | true negative rate |
| PR | precision–recall |
| MIT | Massachusetts Institute of Technology |
| ACA | Anterior Cerebral Artery |
| AICA | Anterior Inferior Cerebellar Artery |
| BA | Basilar Artery |
| ICA | Internal Carotid Artery |
| PCA | Posterior Cerebral Artery |
| PCom | Posterior Communicating Artery |
| PICA | Posterior Inferior Cerebellar Artery |
| SCA | Superior Cerebellar Artery |
| VA | Vertebral Artery |
References
- Hoh, B.L.; Ko, N.U.; Amin-Hanjani, S.; Chou, S.H.-Y.; Cruz-Flores, S.; Dangayach, N.S.; Derdeyn, C.P.; Du, R.; Hänggi, D.; Hetts, S.W.; et al. 2023 Guideline for the Management of Patients with Aneurysmal Subarachnoid Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke 2023, 54, e314–e370. [Google Scholar] [CrossRef] [PubMed]
- Steiner, T.; Juvela, S.; Unterberg, A.; Jung, C.; Forsting, M.; Rinkel, G. European Stroke Organization Guidelines for the Management of Intracranial Aneurysms and Subarachnoid Haemorrhage. Cerebrovasc. Dis. 2013, 35, 93–112. [Google Scholar] [CrossRef] [PubMed]
- Etminan, N.; Chang, H.-S.; Hackenberg, K.; de Rooij, N.K.; Vergouwen, M.D.I.; Rinkel, G.J.E.; Algra, A. Worldwide Incidence of Aneurysmal Subarachnoid Hemorrhage According to Region, Time Period, Blood Pressure, and Smoking Prevalence in the Population: A Systematic Review and Meta-Analysis. JAMA Neurol. 2019, 76, 588–597. [Google Scholar] [CrossRef] [PubMed]
- GBD 2021 Global Subarachnoid Hemorrhage Risk Factors Collaborators. Global, Regional, and National Burden of Nontraumatic Subarachnoid Hemorrhage: The Global Burden of Disease Study 2021. JAMA Neurol. 2025, 82, 765–787. [Google Scholar] [CrossRef]
- Thilak, S.; Brown, P.; Whitehouse, T.; Gautam, N.; Lawrence, E.; Ahmed, Z.; Veenith, T. Diagnosis and Management of Subarachnoid Haemorrhage. Nat. Commun. 2024, 15, 1850. [Google Scholar] [CrossRef]
- Robba, C.; Busl, K.M.; Claassen, J.; Diringer, M.N.; Helbok, R.; Park, S.; Rabinstein, A.; Treggiari, M.; Vergouwen, M.D.I.; Citerio, G. Contemporary Management of Aneurysmal Subarachnoid Haemorrhage. An Update for the Intensivist. Intensive Care Med. 2024, 50, 646–664. [Google Scholar] [CrossRef]
- Chalet, F.-X.; Briasoulis, O.; Manalastas, E.J.; Talbot, D.A.; Thompson, J.C.; Macdonald, R.L. Clinical Burden of Angiographic Vasospasm and Its Complications After Aneurysmal Subarachnoid Hemorrhage: A Systematic Review. Neurol. Ther. 2023, 12, 371–390. [Google Scholar] [CrossRef]
- Nwafor, D.C.; Brichacek, A.L.; Rallo, M.S.; Bidwai, N.; Marsh, R.A. Subarachnoid Hemorrhage: New Insights on Pathogenesis. Front. Stroke 2023, 2, 1110506. [Google Scholar] [CrossRef]
- Schenck, H.; Netti, E.; Teernstra, O.; De Ridder, I.; Dings, J.; Niemelä, M.; Temel, Y.; Hoogland, G.; Haeren, R. The Role of the Glycocalyx in the Pathophysiology of Subarachnoid Hemorrhage-Induced Delayed Cerebral Ischemia. Front. Cell Dev. Biol. 2021, 9, 731641. [Google Scholar] [CrossRef]
- Taylor, R.R.; Keane, R.W.; Guardiola, B.; Martí, R.; Alegre, D.; Dietrich, W.D.; Perez-Barcena, J.; de Rivero Vaccari, J.P. Acute Neurovascular Inflammatory Profile in Patients with Aneurysmal Subarachnoid Hemorrhage. Biomolecules 2025, 15, 613. [Google Scholar] [CrossRef]
- Mielke, D.; Döring, K.; Behme, D.; Psychogios, M.N.; Rohde, V.; Malinova, V. The Impact of Endovascular Rescue Therapy on the Clinical and Radiological Outcome After Aneurysmal Subarachnoid Hemorrhage: A Safe and Effective Treatment Option for Hemodynamically Relevant Vasospasm? Front. Neurol. 2022, 13, 838456. [Google Scholar] [CrossRef]
- Mao, M.; Zhou, R.; Chen, Y.; Wei, J.; Lin, M.; Li, W. Construction of a Nomogram Model for Predicting Delayed Cerebral Ischemia in Aneurysmal Subarachnoid Hemorrhage Patients. Sci. Rep. 2025, 15, 17739. [Google Scholar] [CrossRef]
- Lawton, M.T.; Vates, G.E. Subarachnoid Hemorrhage. N. Engl. J. Med. 2017, 377, 257–266. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Mai, T.D.; Vu, L.D.; Dao, C.X.; Ngo, H.M.; Hoang, H.B.; Tran, T.A.; Pham, T.Q.; Pham, D.T.; Nguyen, M.H.; et al. Validation of the Accuracy of the Modified World Federation of Neurosurgical Societies Subarachnoid Hemorrhage Grading Scale for Predicting the Outcomes of Patients with Aneurysmal Subarachnoid Hemorrhage. PLoS ONE 2023, 18, e0289267. [Google Scholar] [CrossRef] [PubMed]
- Rajpurkar, P.; Chen, E.; Banerjee, O.; Topol, E.J. AI in Health and Medicine. Nat. Med. 2022, 28, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Samajdar, S.; Chatterjee, R.; Mukherjee, S.; Dey, A.; Saboo, B.; Pal, J.; Joshi, S.; Chakrabarti, N. Artificial Intelligence in Healthcare: Current Trends and Future Directions. Curr. Med. Issues 2025, 23, 53–60. [Google Scholar] [CrossRef]
- Amann, J.; Blasimme, A.; Vayena, E.; Frey, D.; Madai, V.I.; The Precise4Q Consortium. Explainability for Artificial Intelligence in Healthcare: A Multidisciplinary Perspective. BMC Med. Inform. Decis. Mak. 2020, 20, 310. [Google Scholar] [CrossRef]
- Kitsios, F.; Kamariotou, M.; Syngelakis, A.I.; Talias, M.A. Recent Advances of Artificial Intelligence in Healthcare: A Systematic Literature Review. Appl. Sci. 2023, 13, 7479. [Google Scholar] [CrossRef]
- Beam, A.L.; Drazen, J.M.; Kohane, I.S.; Leong, T.-Y.; Manrai, A.K.; Rubin, E.J. Artificial Intelligence in Medicine. N. Engl. J. Med. 2023, 388, 1220–1221. [Google Scholar] [CrossRef]
- Stahlschmidt, S.R.; Ulfenborg, B.; Synnergren, J. Multimodal Deep Learning for Biomedical Data Fusion: A Review. Brief. Bioinform. 2022, 23, bbab569. [Google Scholar] [CrossRef]
- Zarrin, D.A.; Suri, A.; McCarthy, K.; Gaonkar, B.; Wilson, B.R.; Colby, G.P.; Freundlich, R.E.; Gabel, E. Machine Learning Predicts Cerebral Vasospasm in Patients with Subarachnoid Haemorrhage. eBioMedicine 2024, 105, 105206. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, P.; Luo, P.; Jiang, X. Machine Learning for the Early Prediction of Delayed Cerebral Ischemia in Patients with Subarachnoid Hemorrhage: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2025, 27, e54121. [Google Scholar] [CrossRef] [PubMed]
- Schwarting, J.; Trost, D.; Albrecht, C.; Jörger, A.-K.; Zimmer, C.; Wostrack, M.; Meyer, B.; Bodden, J.; Boeckh-Behrens, T. Risk Identification for the Development of Large-Artery Vasospasm after Aneurysmatic Subarachnoid Hemorrhage—A Multivariate, Risk-, and Location-Adjusted Prediction Model. J. Neurointerv. Surg. 2024, 16, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Chen, J.; Wang, W.; Zhang, L.; Teng, Y.; Yang, C.; Wang, H.; Tao, Y.; Chen, Z.; Li, R.; et al. Predicting Who Has Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Hemorrhage Using Machine Learning Approach: A Multicenter, Retrospective Cohort Study. BMC Neurol. 2024, 24, 177. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, H.A.; Safwan, Z.; Nabi, R. Advancing Grading and Outcome Prediction in Aneurysmal Subarachnoid Hemorrhage: Harnessing Artificial Intelligence and Machine Learning for Precision Healthcare. Neurosurg. Rev. 2024, 47, 326. [Google Scholar] [CrossRef]
- Sen, R.D.; McGrath, M.C.; Shenoy, V.S.; Meyer, R.M.; Park, C.; Fong, C.T.; Lele, A.V.; Kim, L.J.; Levitt, M.R.; Wang, L.L.; et al. A Dynamic Machine Learning Model to Predict Angiographic Vasospasm After Aneurysmal Subarachnoid Hemorrhage. Neurosurgery 2025, 1–8. [Google Scholar] [CrossRef]
- Shu, L.; Yan, H.; Wu, Y.; Yan, T.; Yang, L.; Zhang, S.; Chen, Z.; Liao, Q.; Yang, L.; Xiao, B.; et al. Explainable Machine Learning in Outcome Prediction of High-Grade Aneurysmal Subarachnoid Hemorrhage. Aging 2024, 16, 4654–4669. [Google Scholar] [CrossRef]
- Salman, S.; Gu, Q.; Sharma, R.; Wei, Y.; Dherin, B.; Reddy, S.; Tawk, R.; Freeman, W.D. Artificial Intelligence and Machine Learning in Aneurysmal Subarachnoid Hemorrhage: Future Promises, Perils, and Practicalities. J. Neurol. Sci. 2023, 454, 120832. [Google Scholar] [CrossRef]
- Findlay, J.M. Current Management of Aneurysmal Subarachnoid Hemorrhage. Neurol. Int. 2025, 17, 36. [Google Scholar] [CrossRef]
- Li, K.; Barras, C.D.; Chandra, R.V.; Kok, H.K.; Maingard, J.T.; Carter, N.S.; Russell, J.H.; Lai, L.; Brooks, M.; Asadi, H. A Review of the Management of Cerebral Vasospasm After Aneurysmal Subarachnoid Hemorrhage. World Neurosurg. 2019, 126, 513–527. [Google Scholar] [CrossRef]
- Merkel, H.; Lindner, D.; Gaber, K.; Ziganshyna, S.; Jentzsch, J.; Mucha, S.; Gerhards, T.; Sari, S.; Stock, A.; Vothel, F.; et al. Standardized Classification of Cerebral Vasospasm after Subarachnoid Hemorrhage by Digital Subtraction Angiography. J. Clin. Med. 2022, 11, 2011. [Google Scholar] [CrossRef]
- Greenberg, M.S. Greenberg’s Handbook of Neurosurgery; Thieme: Stuttgart, Germany, 2023; ISBN 978-1-68420-504-2. [Google Scholar]
- Raymond, J.; Létourneau-Guillon, L.; Darsaut, T.E. Angiographic Vasospasm and Delayed Cerebral Ischemia after Subarachnoid Hemorrhage: Moving from Theoretical to Practical Research Pertinent to Neurosurgical Care. Neurochirurgie 2022, 68, 363–366. [Google Scholar] [CrossRef]
- Kumar, G.; Shahripour, R.B.; Harrigan, M.R. Vasospasm on Transcranial Doppler Is Predictive of Delayed Cerebral Ischemia in Aneurysmal Subarachnoid Hemorrhage: A Systematic Review and Meta-Analysis. J. Neurosurg. 2016, 124, 1257–1264. [Google Scholar] [CrossRef]
- Schenck, H.; van Craenenbroeck, C.; van Kuijk, S.; Gommer, E.; Veldeman, M.; Temel, Y.; Aries, M.; Mess, W.; Haeren, R. Systematic Review and Meta-Analysis of Transcranial Doppler Biomarkers for the Prediction of Delayed Cerebral Ischemia Following Subarachnoid Hemorrhage. J. Cereb. Blood Flow Metab. 2025, 45, 1031–1047. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Liu, Z.; Li, G.; Zhou, L.; Huang, K.; Wu, Z.; Zhan, R.; Shen, J. Inflammation and Oxidative Stress: Potential Targets for Improving Prognosis After Subarachnoid Hemorrhage. Front. Cell. Neurosci. 2021, 15, 739506. [Google Scholar] [CrossRef] [PubMed]
- Stragier, H.; Vandersmissen, H.; Ordies, S.; Thiessen, S.; Mesotten, D.; Peuskens, D.; Ten Cate, H. Pathophysiological Mechanisms Underlying Early Brain Injury and Delayed Cerebral Ischemia in the Aftermath of Aneurysmal Subarachnoid Hemorrhage: A Comprehensive Analysis. Front. Neurol. 2025, 16, 1587091. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Lu, K. Bidirectional Regulation of Nitric Oxide and Endothelin-1 in Cerebral Vasospasm: Mechanisms and Therapeutic Perspectives. Future Pharmacol. 2025, 5, 59. [Google Scholar] [CrossRef]
- Zhang, Y.; Khan, S.; Liu, Y.; Wu, G.; Yong, V.W.; Xue, M. Oxidative Stress Following Intracerebral Hemorrhage: From Molecular Mechanisms to Therapeutic Targets. Front. Immunol. 2022, 13, 847246. [Google Scholar] [CrossRef]
- Jarocka-Karpowicz, I.; Syta-Krzyżanowska, A.; Kochanowicz, J.; Mariak, Z.D. Clinical Prognosis for SAH Consistent with Redox Imbalance and Lipid Peroxidation. Molecules 2020, 25, 1921. [Google Scholar] [CrossRef]
- Darkwah Oppong, M.; Iannaccone, A.; Gembruch, O.; Pierscianek, D.; Chihi, M.; Dammann, P.; Köninger, A.; Müller, O.; Forsting, M.; Sure, U.; et al. Vasospasm-Related Complications after Subarachnoid Hemorrhage: The Role of Patients’ Age and Sex. Acta Neurochir. 2018, 160, 1393–1400. [Google Scholar] [CrossRef]
- Fragata, I.; Canto-Moreira, N.; Canhão, P. Ischemic Lesions in Acute and Subacute Perimesencephalic Subarachnoid Hemorrhage. AJR Am. J. Roentgenol. 2019, 212, 418–424. [Google Scholar] [CrossRef]
- Lee, S.U.; Hong, E.P.; Kim, B.J.; Kim, S.E.; Jeon, J.P. Delayed Cerebral Ischemia and Vasospasm After Spontaneous Angiogram-Negative Subarachnoid Hemorrhage: An Updated Meta-Analysis. World Neurosurg. 2018, 115, e558–e569. [Google Scholar] [CrossRef] [PubMed]
- Harrod, C.G.; Bendok, B.R.; Batjer, H.H. Prediction of Cerebral Vasospasm in Patients Presenting with Aneurysmal Subarachnoid Hemorrhage: A Review. Neurosurgery 2005, 56, 633–654. [Google Scholar] [CrossRef]
- Ramos, L.A.; van der Steen, W.E.; Sales Barros, R.; Majoie, C.B.L.M.; van den Berg, R.; Verbaan, D.; Vandertop, W.P.; Zijlstra, I.J.A.J.; Zwinderman, A.H.; Strijkers, G.J.; et al. Machine Learning Improves Prediction of Delayed Cerebral Ischemia in Patients with Subarachnoid Hemorrhage. J. Neurointerv. Surg. 2019, 11, 497–502. [Google Scholar] [CrossRef]
- Kim, K.H.; Koo, H.-W.; Lee, B.-J.; Sohn, M.-J. Analysis of Risk Factors Correlated with Angiographic Vasospasm in Patients with Aneurysmal Subarachnoid Hemorrhage Using Explainable Predictive Modeling. J. Clin. Neurosci. 2021, 91, 334–342. [Google Scholar] [CrossRef]
- Gollwitzer, M.; Steindl, M.; Stroh, N.; Hauser, A.; Sardi, G.; Rossmann, T.; Aspalter, S.; Rauch, P.; Sonnberger, M.; Gruber, A.; et al. Machine Learning-Based Prediction of Chronic Shunt-Dependent Hydrocephalus After Spontaneous Subarachnoid Hemorrhage. World Neurosurg. 2024, 192, e124–e133. [Google Scholar] [CrossRef]
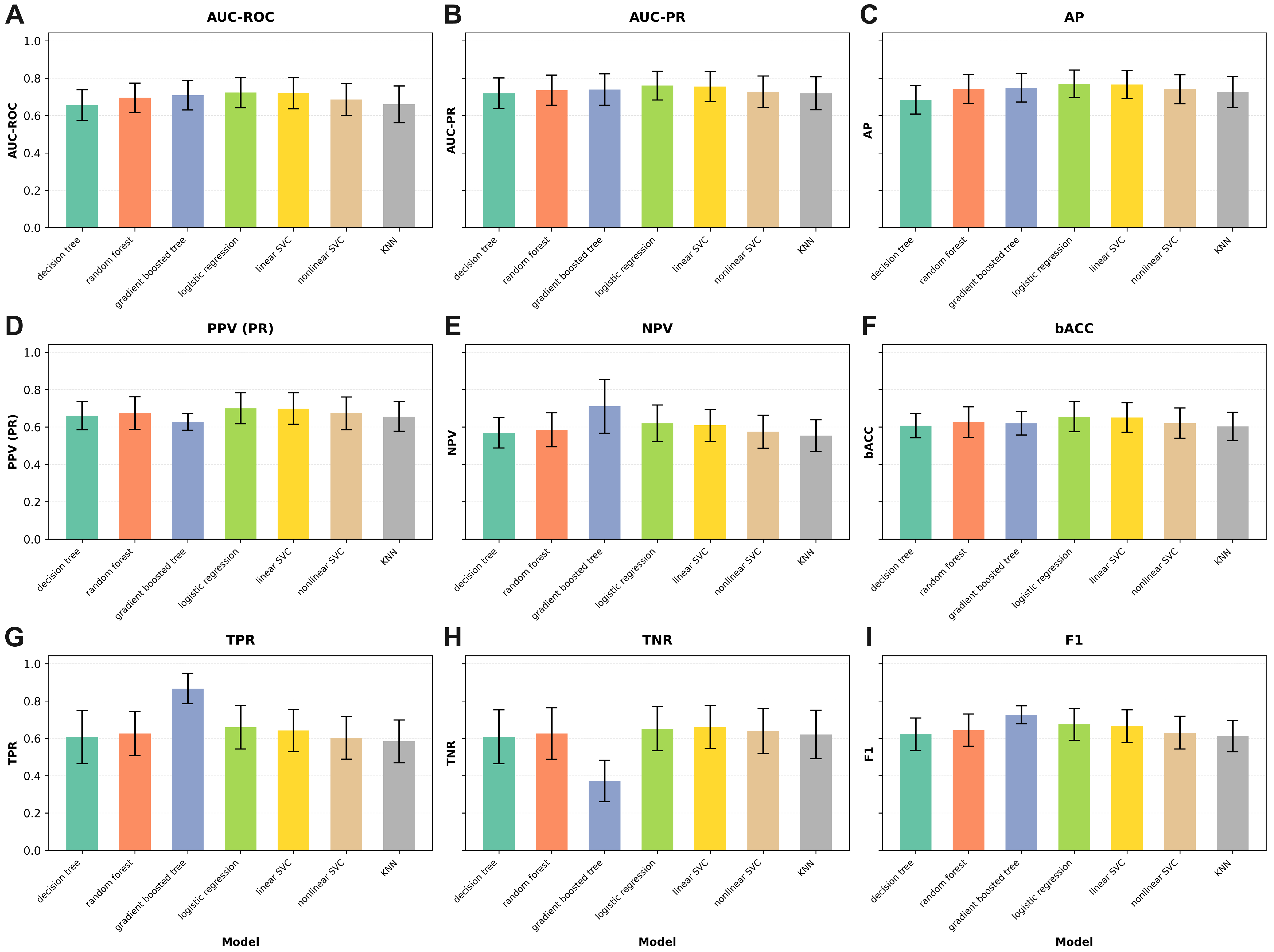
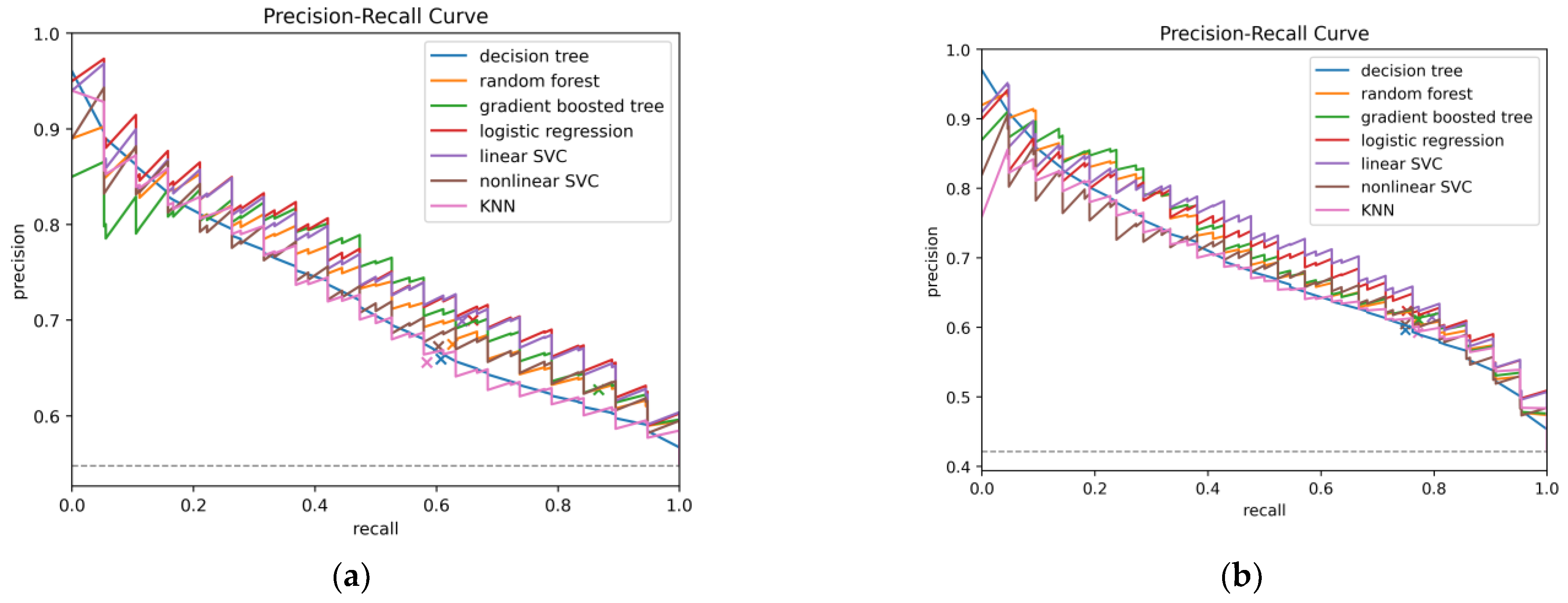

| Model | AUC-ROC | AUC-PR | AP | PPV (PR) | NPV | bACC | TPR | TNR | F1 |
|---|---|---|---|---|---|---|---|---|---|
| decision tree | 0.656 ± 0.082 | 0.719 ± 0.082 | 0.685 ± 0.077 | 0.660 ± 0.075 | 0.570 ± 0.082 | 0.607 ± 0.065 | 0.607 ± 0.142 | 0.608 ± 0.144 | 0.622 ± 0.087 |
| random forest | 0.695 ± 0.079 | 0.736 ± 0.081 | 0.742 ± 0.077 | 0.675 ± 0.087 | 0.585 ± 0.091 | 0.626 ± 0.082 | 0.626 ± 0.118 | 0.626 ± 0.138 | 0.644 ± 0.086 |
| gradient boosted tree | 0.709 ± 0.079 | 0.739 ± 0.084 | 0.749 ± 0.077 | 0.628 ± 0.045 | 0.711 ± 0.144 | 0.620 ± 0.063 | 0.867 ± 0.081 | 0.372 ± 0.111 | 0.726 ± 0.048 |
| logistic regression | 0.723 ± 0.082 | 0.760 ± 0.077 | 0.770 ± 0.073 | 0.700 ± 0.083 | 0.620 ± 0.098 | 0.656 ± 0.081 | 0.660 ± 0.117 | 0.652 ± 0.118 | 0.675 ± 0.085 |
| linear SVC | 0.720 ± 0.084 | 0.755 ± 0.080 | 0.766 ± 0.075 | 0.699 ± 0.084 | 0.609 ± 0.086 | 0.651 ± 0.079 | 0.642 ± 0.113 | 0.661 ± 0.115 | 0.665 ± 0.087 |
| nonlinear SVC | 0.686 ± 0.085 | 0.728 ± 0.084 | 0.740 ± 0.078 | 0.673 ± 0.088 | 0.575 ± 0.088 | 0.621 ± 0.081 | 0.603 ± 0.114 | 0.639 ± 0.120 | 0.631 ± 0.088 |
| KNN | 0.660 ± 0.098 | 0.719 ± 0.088 | 0.725 ± 0.083 | 0.656 ± 0.079 | 0.554 ± 0.085 | 0.603 ± 0.076 | 0.584 ± 0.115 | 0.621 ± 0.130 | 0.612 ± 0.084 |
| Model | AUC-ROC | AUC-PR | AP | PPV (PR) | NPV | bACC | TPR | TNR | F1 |
|---|---|---|---|---|---|---|---|---|---|
| decision tree | 0.734 ± 0.071 | 0.688 ± 0.083 | 0.630 ± 0.084 | 0.596 ± 0.058 | 0.786 ± 0.082 | 0.689 ± 0.061 | 0.750 ± 0.132 | 0.629 ± 0.087 | 0.659 ± 0.075 |
| random forest | 0.764 ± 0.065 | 0.709 ± 0.083 | 0.702 ± 0.087 | 0.611 ± 0.057 | 0.799 ± 0.072 | 0.704 ± 0.057 | 0.769 ± 0.108 | 0.640 ± 0.083 | 0.677 ± 0.065 |
| gradient boosted tree | 0.769 ± 0.062 | 0.712 ± 0.077 | 0.710 ± 0.079 | 0.611 ± 0.056 | 0.801 ± 0.075 | 0.706 ± 0.058 | 0.773 ± 0.106 | 0.639 ± 0.077 | 0.680 ± 0.064 |
| logistic regression | 0.786 ± 0.064 | 0.717 ± 0.088 | 0.728 ± 0.082 | 0.624 ± 0.060 | 0.791 ± 0.066 | 0.709 ± 0.056 | 0.752 ± 0.093 | 0.666 ± 0.078 | 0.679 ± 0.061 |
| linear SVC | 0.791 ± 0.062 | 0.727 ± 0.088 | 0.737 ± 0.082 | 0.615 ± 0.055 | 0.814 ± 0.068 | 0.714 ± 0.054 | 0.796 ± 0.086 | 0.632 ± 0.079 | 0.692 ± 0.055 |
| nonlinear SVC | 0.762 ± 0.059 | 0.684 ± 0.076 | 0.698 ± 0.071 | 0.605 ± 0.060 | 0.784 ± 0.073 | 0.693 ± 0.056 | 0.749 ± 0.108 | 0.637 ± 0.092 | 0.665 ± 0.062 |
| KNN | 0.759 ± 0.063 | 0.680 ± 0.087 | 0.685 ± 0.079 | 0.592 ± 0.057 | 0.793 ± 0.080 | 0.691 ± 0.061 | 0.772 ± 0.108 | 0.610 ± 0.080 | 0.667 ± 0.066 |
| Characteristic | Overall N = 345 1 | Vasospasm: Yes N = 189 1 | Vasospasm: No N = 156 1 | p-Value 2 |
|---|---|---|---|---|
| Age at aSAH | 56 ± 13 | 52 ± 12 | 60 ± 13 | <0.001 |
| Sex | >0.9 | |||
| Female | 236/345 (68%) | 130/189 (69%) | 106/156 (68%) | |
| Male | 109/345 (32%) | 59/189 (31%) | 50/156 (32%) | |
| Hunt & Hess grade | 0.001 | |||
| 1 | 77/345 (22%) | 30/189 (16%) | 47/156 (30%) | |
| 2 | 113/345 (33%) | 58/189 (31%) | 55/156 (35%) | |
| 3 | 69/345 (20%) | 41/189 (22%) | 28/156 (18%) | |
| 4 | 69/345 (20%) | 50/189 (26%) | 19/156 (12%) | |
| 5 | 17/345 (4.9%) | 10/189 (5.3%) | 7/156 (4.5%) | |
| Fisher grade | 0.001 | |||
| 1 | 9/345 (2.6%) | 5/189 (2.6%) | 4/156 (2.6%) | |
| 2 | 25/345 (7.2%) | 5/189 (2.6%) | 20/156 (13%) | |
| 3 | 112/345 (32%) | 58/189 (31%) | 54/156 (35%) | |
| 4 | 199/345 (58%) | 121/189 (64%) | 78/156 (50%) | |
| Aneurysm location | 0.2 | |||
| ACA | 35/345 (10%) | 16/189 (8.5%) | 19/156 (12%) | |
| ACom | 120/345 (35%) | 56/189 (30%) | 64/156 (41%) | |
| AICA | 1/345 (0.3%) | 1/189 (0.5%) | 0/156 (0%) | |
| BA | 23/345 (6.7%) | 15/189 (7.9%) | 8/156 (5.1%) | |
| ICA | 37/345 (11%) | 20/189 (11%) | 17/156 (11%) | |
| MCA | 69/345 (20%) | 42/189 (22%) | 27/156 (17%) | |
| PCA | 5/345 (1.4%) | 2/189 (1.1%) | 3/156 (1.9%) | |
| PCom | 39/345 (11%) | 24/189 (13%) | 15/156 (9.6%) | |
| PICA | 10/345 (2.9%) | 7/189 (3.7%) | 3/156 (1.9%) | |
| SCA | 1/345 (0.3%) | 1/189 (0.5%) | 0/156 (0%) | |
| VA | 5/345 (1.4%) | 5/189 (2.6%) | 0/156 (0%) | |
| Aneurysm circulation | 0.060 | |||
| Anterior | 300/345 (87%) | 158/189 (84%) | 142/156 (91%) | |
| Posterior | 45/345 (13%) | 31/189 (16%) | 14/156 (9.0%) | |
| Aneurysm maximum diameter (mm) | 6.1 ± 3.5 | 6.0 ± 3.4 | 6.1 ± 3.6 | >0.9 |
| Aneurysm dome height (mm) | 4.90 ± 2.99 | 4.93 ± 3.11 | 4.87 ± 2.84 | 0.8 |
| Treatment for aSAH | 0.6 | |||
| Clipping | 47/345 (14%) | 27/189 (14%) | 20/156 (13%) | |
| Coiling | 288/345 (83%) | 155/189 (82%) | 133/156 (85%) | |
| Untreated | 10/345 (2.9%) | 7/189 (3.7%) | 3/156 (1.9%) | |
| EVD implanted | 151/345 (44%) | 98/189 (52%) | 53/156 (34%) | 0.001 |
| CNS infection during treatment | 32/345 (9.3%) | 20/189 (11%) | 12/156 (7.7%) | 0.5 |
| Characteristic | Overall N = 503 1 | Vasospasm: Yes N = 212 1 | Vasospasm: No N = 291 1 | p-Value 2 |
|---|---|---|---|---|
| Age | 55 ± 13 | 52 ± 12 | 58 ± 13 | <0.001 |
| Sex | 0.2 | |||
| Female | 311/503 (62%) | 139/212 (66%) | 172/291 (59%) | |
| Male | 192/503 (38%) | 73/212 (34%) | 119/291 (41%) | |
| aSAH | 345/503 (69%) | 189/212 (89%) | 156/291 (54%) | <0.001 |
| Hunt & Hess grade | <0.001 | |||
| 1 | 160/503 (32%) | 40/212 (19%) | 120/291 (41%) | |
| 2 | 161/503 (32%) | 63/212 (30%) | 98/291 (34%) | |
| 3 | 84/503 (17%) | 47/212 (22%) | 37/291 (13%) | |
| 4 | 80/503 (16%) | 52/212 (25%) | 28/291 (9.6%) | |
| 5 | 18/503 (3.6%) | 10/212 (4.7%) | 8/291 (2.7%) | |
| Fisher grade | <0.001 | |||
| 1 | 20/503 (4.0%) | 5/212 (2.4%) | 15/291 (5.2%) | |
| 2 | 50/503 (9.9%) | 7/212 (3.3%) | 43/291 (15%) | |
| 3 | 195/503 (39%) | 71/212 (33%) | 124/291 (43%) | |
| 4 | 238/503 (47%) | 129/212 (61%) | 109/291 (37%) | |
| Aneurysm location | 0.2 | |||
| ACA | 35/345 (10%) | 16/189 (8.5%) | 19/156 (12%) | |
| ACom | 120/345 (35%) | 56/189 (30%) | 64/156 (41%) | |
| AICA | 1/345 (0.3%) | 1/189 (0.5%) | 0/156 (0%) | |
| BA | 23/345 (6.7%) | 15/189 (7.9%) | 8/156 (5.1%) | |
| ICA | 37/345 (11%) | 20/189 (11%) | 17/156 (11%) | |
| MCA | 69/345 (20%) | 42/189 (22%) | 27/156 (17%) | |
| PCA | 5/345 (1.4%) | 2/189 (1.1%) | 3/156 (1.9%) | |
| PCom | 39/345 (11%) | 24/189 (13%) | 15/156 (9.6%) | |
| PICA | 10/345 (2.9%) | 7/189 (3.7%) | 3/156 (1.9%) | |
| SCA | 1/345 (0.3%) | 1/189 (0.5%) | 0/156 (0%) | |
| VA | 5/345 (1.4%) | 5/189 (2.6%) | 0/156 (0%) | |
| Aneurysm circulation | 0.060 | |||
| Anterior | 300/345 (87%) | 158/189 (84%) | 142/156 (91%) | |
| Posterior | 45/345 (13%) | 31/189 (16%) | 14/156 (9.0%) | |
| Aneurysm maximum diameter (mm) | 6.1 ± 3.5 | 6.0 ± 3.4 | 6.1 ± 3.6 | >0.9 |
| Aneurysm dome height (mm) | 4.90 ± 2.99 | 4.93 ± 3.11 | 4.87 ± 2.84 | 0.8 |
| Treatment for aSAH | 0.6 | |||
| Clipping | 47/345 (14%) | 27/189 (14%) | 20/156 (13%) | |
| Coiling | 288/345 (83%) | 155/189 (82%) | 133/156 (85%) | |
| Untreated | 10/345 (2.9%) | 7/189 (3.7%) | 3/156 (1.9%) | |
| EVD implanted | 184/503 (37%) | 107/212 (50%) | 77/291 (26%) | <0.001 |
| CNS infection during treatment | 40/503 (8.0%) | 21/212 (9.9%) | 19/291 (6.5%) | 0.2 |
| Feature | Odds Ratio | Standardized Coefficient |
|---|---|---|
| Age at aSAH | 0.951 ± 0.021 | −0.659 ± 0.282 |
| Hunt & Hess grade | 1.268 ± 0.116 | 0.275 ± 0.112 |
| Fisher grade | 1.379 ± 0.180 | 0.231 ± 0.101 |
| CNS infection | 1.152 ± 0.137 | 0.039 ± 0.033 |
| EVD implanted | 1.480 ± 0.217 | 0.189 ± 0.077 |
| Aneurysm | 1.000 ± 0.000 | 0.000 ± 0.000 |
| Aneurysm maximum diameter | 0.959 ± 0.025 | −0.146 ± 0.090 |
| Aneurysm dome height | 1.002 ± 0.016 | 0.005 ± 0.045 |
| Female sex | 1.116 ± 0.111 | 0.049 ± 0.045 |
| ACom location | 0.640 ± 0.142 | −0.223 ± 0.098 |
| Clipping | 0.925 ± 0.140 | −0.032 ± 0.069 |
| Coiling | 0.832 ± 0.128 | −0.074 ± 0.072 |
| Feature | Odds Ratio | Standardized Coefficient |
|---|---|---|
| Age at SAH | 0.960 ± 0.019 | −0.546 ± 0.259 |
| Hunt & Hess grade | 1.244 ± 0.108 | 0.251 ± 0.105 |
| Fisher grade | 1.359 ± 0.166 | 0.240 ± 0.104 |
| CNS infection | 1.010 ± 0.108 | 0.001 ± 0.029 |
| EVD implanted | 1.577 ± 0.269 | 0.212 ± 0.089 |
| Aneurysm | 4.716 ± 3.801 | 0.588 ± 0.349 |
| Aneurysm maximum diameter | 0.975 ± 0.031 | −0.102 ± 0.128 |
| Aneurysm dome height | 1.015 ± 0.015 | 0.050 ± 0.049 |
| Female sex | 1.007 ± 0.063 | 0.002 ± 0.030 |
| ACom location | 0.691 ± 0.176 | −0.171 ± 0.103 |
| Clipping | 1.418 ± 0.370 | 0.091 ± 0.080 |
| Coiling | 1.355 ± 0.320 | 0.134 ± 0.133 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gollwitzer, M.; Mazanec, V.; Steindl, M.; Atli, B.; Stroh-Holly, N.; Hauser, A.; Sardi, G.; Rossmann, T.; Aspalter, S.; Rauch, P.; et al. Early Prediction of Cerebral Vasospasm After Aneurysmal Subarachnoid Hemorrhage Using a Machine Learning Model and Interactive Web Application. Brain Sci. 2025, 15, 1187. https://doi.org/10.3390/brainsci15111187
Gollwitzer M, Mazanec V, Steindl M, Atli B, Stroh-Holly N, Hauser A, Sardi G, Rossmann T, Aspalter S, Rauch P, et al. Early Prediction of Cerebral Vasospasm After Aneurysmal Subarachnoid Hemorrhage Using a Machine Learning Model and Interactive Web Application. Brain Sciences. 2025; 15(11):1187. https://doi.org/10.3390/brainsci15111187
Chicago/Turabian StyleGollwitzer, Maria, Vanessa Mazanec, Markus Steindl, Baran Atli, Nico Stroh-Holly, Anna Hauser, Gracija Sardi, Tobias Rossmann, Stefan Aspalter, Philip Rauch, and et al. 2025. "Early Prediction of Cerebral Vasospasm After Aneurysmal Subarachnoid Hemorrhage Using a Machine Learning Model and Interactive Web Application" Brain Sciences 15, no. 11: 1187. https://doi.org/10.3390/brainsci15111187
APA StyleGollwitzer, M., Mazanec, V., Steindl, M., Atli, B., Stroh-Holly, N., Hauser, A., Sardi, G., Rossmann, T., Aspalter, S., Rauch, P., Horner, E., Sonnberger, M., Gruber, A., & Gmeiner, M. (2025). Early Prediction of Cerebral Vasospasm After Aneurysmal Subarachnoid Hemorrhage Using a Machine Learning Model and Interactive Web Application. Brain Sciences, 15(11), 1187. https://doi.org/10.3390/brainsci15111187







