Behavioral Variant Frontotemporal Dementia in the Context of Progressive Apraxia of Speech: A Clinico-Neuroimaging Case–Control Study
Abstract
1. Introduction
2. Methods
2.1. Protocol and Patient Consent
2.2. Participants and Recruitment Criteria
2.3. Clinical Data
2.4. Speech and Language Data
2.5. Assessing bvFTD Criteria
2.6. Neuroimaging Data
2.7. Statistical Analysis
3. Results
3.1. Demographic and Clinical Findings
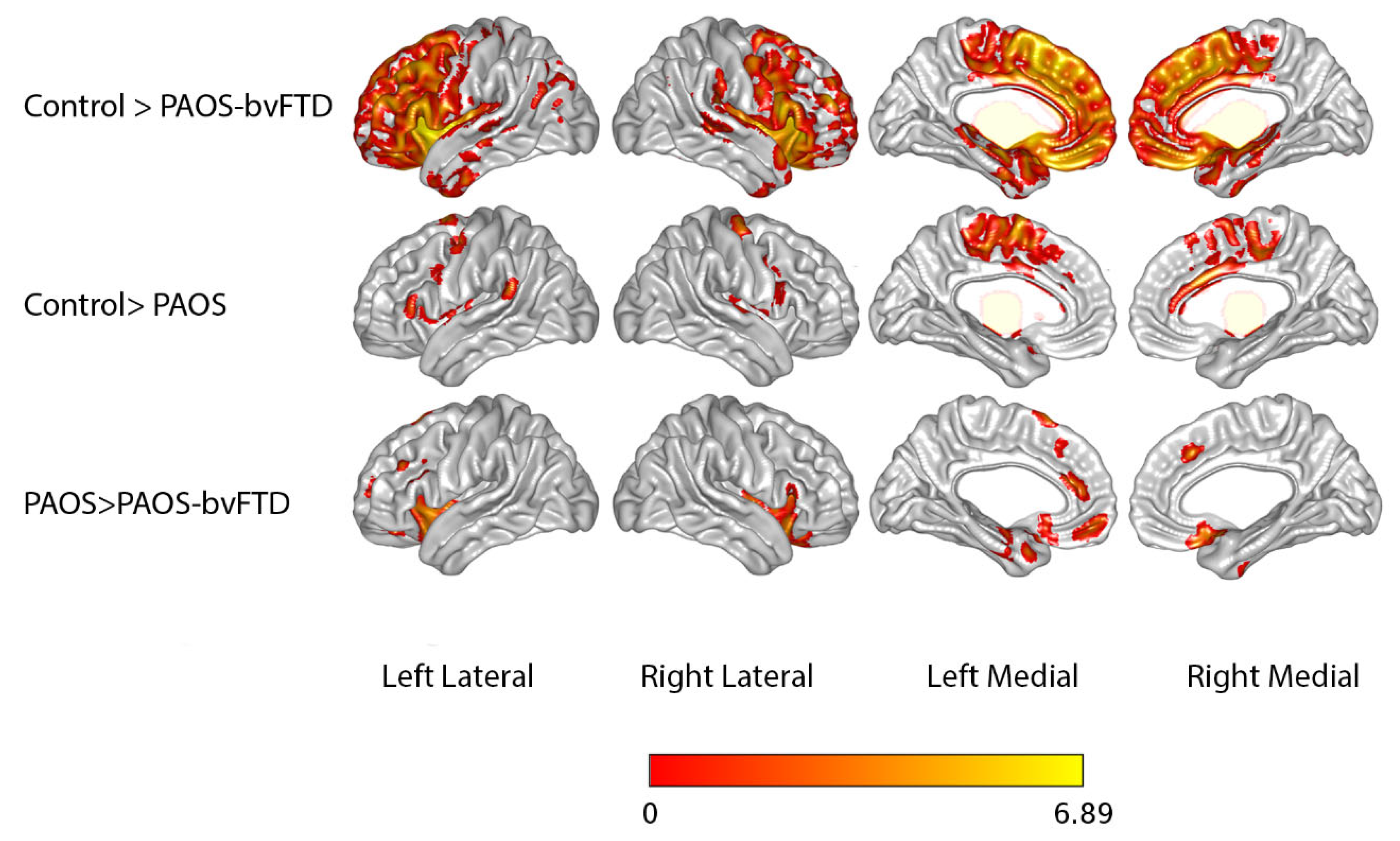
3.2. Neuroimaging Findings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duffy, J.R. Motor Speech Disorders: Substrates, Differential Diagnosis, and Management; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Darley, F.L.; Aronson, A.E.; Brown, J.R. Motor Speech Disorders; Elsevier Mosby: St. Louis, MO, USA, 1975. [Google Scholar]
- Josephs, K.A.; Duffy, J.R.; Strand, E.A.; Machulda, M.M.; Senjem, M.L.; Master, A.V.; Lowe, V.J.; Jack, C.R., Jr.; Whitwell, J.L. Characterizing a neurodegenerative syndrome: Primary progressive apraxia of speech. Brain 2012, 135, 1522–1536. [Google Scholar] [CrossRef]
- Josephs, K.A.; Duffy, J.R.; Strand, E.A.; Machulda, M.M.; Senjem, M.L.; Gunter, J.L.; Schwarz, C.G.; Reid, R.I.; Spychalla, A.J.; Lowe, V.J.; et al. The evolution of primary progressive apraxia of speech. Brain 2014, 137, 2783–2795. [Google Scholar] [CrossRef]
- Dang, J.; Graff-Radford, J.; Duffy, J.R.; Utianski, R.L.; Clark, H.M.; Stierwalt, J.A.; Whitwell, J.L.; Josephs, K.A.; Botha, H. Progressive apraxia of speech: Delays to diagnosis and rates of alternative diagnoses. J. Neurol. 2021, 268, 4752–4758. [Google Scholar] [CrossRef]
- Neary, D.; Snowden, J.S.; Gustafson, L.; Passant, U.; Stuss, D.; Black, S.; Freedman, M.; Kertesz, A.; Robert, P.H.; Albert, M.; et al. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology 1998, 51, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.K.; Diehl, J.; Mendez, M.F.; Neuhaus, J.; Shapira, J.S.; Forman, M.; Chute, D.J.; Roberson, E.D.; Pace-Savitsky, C.; Neumann, M.; et al. Frontotemporal lobar degeneration: Demographic characteristics of 353 patients. Arch. Neurol. 2005, 62, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Rascovsky, K.; Hodges, J.R.; Knopman, D.; Mendez, M.F.; Kramer, J.H.; Neuhaus, J.; van Swieten, J.C.; Seelaar, H.; Dopper, E.G.; Onyike, C.U.; et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011, 134, 2456–2477. [Google Scholar] [CrossRef] [PubMed]
- Whitwell, J.L.; Przybelski, S.A.; Weigand, S.D.; Ivnik, R.J.; Vemuri, P.; Gunter, J.L.; Senjem, M.L.; Shiung, M.M.; Boeve, B.F.; Knopman, D.S.; et al. Distinct anatomical subtypes of the behavioural variant of frontotemporal dementia: A cluster analysis study. Brain 2009, 132, 2932–2946. [Google Scholar] [CrossRef]
- Garcia-Guaqueta, D.P.; Botha, H.; Utianski, R.L.; Duffy, J.R.; Clark, H.M.; Goodrich, A.W.; Pham, N.T.T.; Machulda, M.M.; Baker, M.; Rademakers, R.; et al. Progression to corticobasal syndrome: A longitudinal study of patients with nonfluent primary progressive aphasia and primary progressive apraxia of speech. J. Neurol. 2024, 271, 4168–4179. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Litvan, I.; Lang, A.E.; Bak, T.H.; Bhatia, K.P.; Borroni, B.; Boxer, A.L.; Dickson, D.W.; Grossman, M.; Hallett, M.; et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013, 80, 496–503. [Google Scholar] [CrossRef]
- Hoglinger, G.U.; Respondek, G.; Stamelou, M.; Kurz, C.; Josephs, K.A.; Lang, A.E.; Mollenhauer, B.; Muller, U.; Nilsson, C.; Whitwell, J.L.; et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov. Disord. 2017, 32, 853–864. [Google Scholar] [CrossRef]
- Josephs, K.A.; Duffy, J.R.; Clark, H.M.; Utianski, R.L.; Strand, E.A.; Machulda, M.M.; Botha, H.; Martin, P.R.; Pham, N.T.T.; Stierwalt, J.; et al. A molecular pathology, neurobiology, biochemical, genetic and neuroimaging study of progressive apraxia of speech. Nat. Commun. 2021, 12, 3452. [Google Scholar] [CrossRef]
- Meade, G.; Machulda, M.M.; Clark, H.M.; Duffy, J.R.; Botha, H.; Whitwell, J.L.; Josephs, K.A.; Utianski, R.L. Identifying and Addressing Functional Communication Challenges in Patients with Behavioral Variant Frontotemporal Dementia. Am. J. Speech Lang. Pathol. 2024, 33, 1573–1589. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bedirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef] [PubMed]
- Kertesz, A. Western Aphasia Battery—Revised; Pearson Education Limited: London, UK, 2007. [Google Scholar]
- Lansing, A.E.; Ivnik, R.J.; Cullum, C.M.; Randolph, C. An empirically derived short form of the Boston naming test. Arch. Clin. Neuropsychol. 1999, 14, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Whitwell, J.L.; Master, A.V.; Avula, R.; Kantarci, K.; Eggers, S.D.; Edmonson, H.A.; Jack, C.R., Jr.; Josephs, K.A. Clinical correlates of white matter tract degeneration in progressive supranuclear palsy. Arch. Neurol. 2011, 68, 753–760. [Google Scholar] [CrossRef]
- Boyd, C.D.; Tierney, M.; Wassermann, E.M.; Spina, S.; Oblak, A.L.; Ghetti, B.; Grafman, J.; Huey, E. Visuoperception test predicts pathologic diagnosis of Alzheimer disease in corticobasal syndrome. Neurology 2014, 83, 510–519. [Google Scholar] [CrossRef]
- Kertesz, A.; Davidson, W.; Fox, H. Frontal behavioral inventory: Diagnostic criteria for frontal lobe dementia. Can. J. Neurol. Sci. 1997, 24, 29–36. [Google Scholar] [CrossRef]
- Hokelekli, F.O.; Duffy, J.R.; Clark, H.M.; Utianski, R.L.; Botha, H.; Stierwalt, J.A.; Strand, E.A.; Machulda, M.M.; Whitwell, J.L.; Josephs, K.A. Cross-Sectional and Longitudinal Assessment of Behavior in Primary Progressive Apraxia of Speech and Agrammatic Aphasia. Dement. Geriatr. Cogn. Disord. 2022, 51, 193–202. [Google Scholar] [CrossRef]
- Cummings, J.L.; Mega, M.; Gray, K.; Rosenberg-Thompson, S.; Carusi, D.A.; Gornbein, J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 1994, 44, 2308–2314. [Google Scholar] [CrossRef]
- Kaufer, D.I.; Cummings, J.L.; Ketchel, P.; Smith, V.; MacMillan, A.; Shelley, T.; Lopez, O.L.; DeKosky, S.T. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J. Neuropsychiatry Clin. Neurosci. 2000, 12, 233–239. [Google Scholar] [CrossRef]
- Dubois, B.; Slachevsky, A.; Litvan, I.; Pillon, B. The FAB: A Frontal Assessment Battery at bedside. Neurology 2000, 55, 1621–1626. [Google Scholar] [CrossRef] [PubMed]
- Reitan, R.M. Validity of the Trail Making Test as an Indicator of Organic Brain Damage. Percept. Mot. Ski. 1958, 8, 271–276. [Google Scholar] [CrossRef]
- de Zubicaray, G.; Ashton, R. Nelson’s (1976) Modified Card Sorting Test: A review. Clin. Neuropsychol. 1996, 10, 245–254. [Google Scholar] [CrossRef]
- Golbe, L.I.; Ohman-Strickland, P.A. A clinical rating scale for progressive supranuclear palsy. Brain 2007, 130, 1552–1565. [Google Scholar] [CrossRef] [PubMed]
- Whitwell, J.L.; Stevens, C.A.; Duffy, J.R.; Clark, H.M.; Machulda, M.M.; Strand, E.A.; Martin, P.R.; Utianski, R.L.; Botha, H.; Spychalla, A.J.; et al. An Evaluation of the Progressive Supranuclear Palsy Speech/Language Variant. Mov. Disord. Clin. Pract. 2019, 6, 452–461. [Google Scholar] [CrossRef]
- Fillenbaum, G.G.; Peterson, B.; Morris, J.C. Estimating the validity of the clinical Dementia Rating Scale: The CERAD experience. Consortium to Establish a Registry for Alzheimer’s Disease. Aging 1996, 8, 379–385. [Google Scholar] [CrossRef]
- Hughes, C.P.; Berg, L.; Danziger, W.L.; Coben, L.A.; Martin, R.L. A new clinical scale for the staging of dementia. Br. J. Psychiatry 1982, 140, 566–572. [Google Scholar] [CrossRef]
- Josephs, K.A.; Tsuboi, Y.; Cookson, N.; Watt, H.; Dickson, D.W. Apolipoprotein E epsilon 4 is a determinant for Alzheimer-type pathologic features in tauopathies, synucleinopathies, and frontotemporal degeneration. Arch. Neurol. 2004, 61, 1579–1584. [Google Scholar] [CrossRef]
- Loonstra, A.S.; Tarlow, A.R.; Sellers, A.H. COWAT metanorms across age, education, and gender. Appl. Neuropsychol. 2001, 8, 161–166. [Google Scholar] [CrossRef]
- Botha, H.; Duffy, J.R.; Strand, E.A.; Machulda, M.M.; Whitwell, J.L.; Josephs, K.A. Nonverbal oral apraxia in primary progressive aphasia and apraxia of speech. Neurology 2014, 82, 1729–1735. [Google Scholar] [CrossRef]
- Duffy, J.R.; Strand, E.A.; Josephs, K.A. Motor Speech Disorders Associated with Primary Progressive Aphasia. Aphasiology 2014, 28, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Seeley, W.W.; Crawford, R.; Rascovsky, K.; Kramer, J.H.; Weiner, M.; Miller, B.L.; Gorno-Tempini, M.L. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch. Neurol. 2008, 65, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Wang, J.; He, Y. BrainNet Viewer: A network visualization tool for human brain connectomics. PLoS ONE 2013, 8, e68910. [Google Scholar] [CrossRef] [PubMed]
- Josephs, K.A.; Duffy, J.R.; Strand, E.A.; Machulda, M.M.; Senjem, M.L.; Lowe, V.J.; Jack, C.R., Jr.; Whitwell, J.L. Syndromes dominated by apraxia of speech show distinct characteristics from agrammatic PPA. Neurology 2013, 81, 337–345. [Google Scholar] [CrossRef]
- Snowden, J.S.; Bathgate, D.; Varma, A.; Blackshaw, A.; Gibbons, Z.C.; Neary, D. Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J. Neurol. Neurosurg. Psychiatry 2001, 70, 323–332. [Google Scholar] [CrossRef]
- Liu, X.; de Boer, S.C.M.; Cortez, K.; Poos, J.M.; Illán-Gala, I.; Heuer, H.; Forsberg, L.K.; Casaletto, K.; Memel, M.; Appleby, B.S.; et al. Sex differences in clinical phenotypes of behavioral variant frontotemporal dementia. Alzheimers Dement. 2025, 21, e14608. [Google Scholar] [CrossRef]
- Bozeat, S.; Gregory, C.A.; Ralph, M.A.; Hodges, J.R. Which neuropsychiatric and behavioural features distinguish frontal and temporal variants of frontotemporal dementia from Alzheimer’s disease? J. Neurol. Neurosurg. Psychiatry 2000, 69, 178–186. [Google Scholar] [CrossRef]
- Josephs, K.A. Frontotemporal dementia and related disorders: Deciphering the enigma. Ann. Neurol. 2008, 64, 4–14. [Google Scholar] [CrossRef]
- Whitwell, J.L.; Duffy, J.R.; Strand, E.A.; Xia, R.; Mandrekar, J.; Machulda, M.M.; Senjem, M.L.; Lowe, V.J.; Jack, C.R., Jr.; Josephs, K.A. Distinct regional anatomic and functional correlates of neurodegenerative apraxia of speech and aphasia: An MRI and FDG-PET study. Brain Lang. 2013, 125, 245–252. [Google Scholar] [CrossRef]
- Jones, K.E.; Graff-Radford, J.; Utianski, R.L.; Duffy, J.R.; Clark, H.M.; Machulda, M.M.; Dickson, D.W.; Whitwell, J.L.; Josephs, K.A.; Botha, H. Pick’s disease presenting as progressive apraxia of speech: Atypical clinical and neuroimaging features in three autopsy-confirmed cases. Clin. Neurol. Neurosurg. 2025, 256, 109018. [Google Scholar] [CrossRef]
| Criteria | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | Median or % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time from PAOS onset to when bvFTD criteria were met (years) | 3 * | 1 * | 2 | 5 | 1 | 4 | 2 * | 3 | 4 | 9 | 2 | 3 |
| Visit no. when bvFTD criteria were met | 1 | 1 | 1 | 1 | 1 | 2 | 3 | 2 | 2 | 3 | 1 | 1 |
| Possible bvFTD (any 3 from 1 to 6 should be met) | ||||||||||||
| 1. Disinhibition | + | + | + | + | + | + | + | + | + | + | + | 100% |
| 2. Apathy | + | + | + | + | + | − | + | + | + | + | + | 90.9% |
| 3. Loss of empathy/sympathy | + | + | + | + | − | + | + | + | + | + | + | 90.9% |
| 4. Stereotyped behavior | − | + | + | + | − | − | + | − | + | + | + | 63.6% |
| 5. Hyperorality | + | + | − | + | − | + | + | + | + | + | − | 72.7% |
| 6. Neuropsychological features (6a–6c should all be met) | + | − | + | + | + | + | + | − | − | + | + | 72.7% |
| 6a. Impaired executive function | + | + | + | + | + | + | + | − | + | + | + | 90.9% |
| 6b. Relative sparing of episodic memory | + | − | + | + | + | + | + | + | + | + | + | 90.9% |
| 6c. Relative sparing of visuospatial function | + | + | + | + | + | + | + | + | − | + | + | 90.9% |
| Probable bvFTD (all 3 should be met) | ||||||||||||
| 7. Meets possible bvFTD criteria | + | + | + | + | + | + | + | + | + | + | + | 100% |
| 8. Exhibits significant social decline | + | + | + | + | + | + | + | + | + | + | + | 100% |
| 9. Imaging findings (frontal/temporal atrophy or hypometabolism) | + | + | + | + | + | + | + | + | + | + | + | 100% |
| Exclusionary Criteria (all 3 should be (−) for probable) | ||||||||||||
| 10. Other prominent non-degenerative or medical disorders | − | − | − | − | − | − | − | − | − | − | − | 100% |
| 11. Pronounced behavioral disturbances from other psychiatric disorders | − | − | − | − | − | − | − | − | − | − | − | 100% |
| 12. Biomarkers for Alzheimer’s or other degenerative diseases | − | − | − | − | − | − | − | − | − | − | − | 100% |
| Variable | PAOS-bvFTD (N = 11) | PAOS (N = 11) | p-Value | |
|---|---|---|---|---|
| Demographic features | ||||
| Gender: Female | 6 (55%) | 6 (55%) | 0.99 | |
| Race: White | 11 (100%) | 10 (90.9%) | 0.31 | |
| Ethnicity: Hispanic or Latino | 0 (0%) | 0 (0%) | 0.99 | |
| Handedness: Right | 11 (100%) | 9 (81.8) | 0.14 | |
| Education (years) | 16 (13, 17) | 16 (13, 17) | 0.89 | |
| Apo E4 (+) | 2 (22%) | 3 (33%) | 0.60 | |
| Age at Visit, years | 62 (53, 73) | 64 (57, 71) | 0.87 | |
| Age at Onset, years | 57 (49, 69) | 59 (50, 66) | 0.97 | |
| Time from Onset to Visit (years) | 3.8 (2.7, 4.2) | 5.2 (4.7, 6.5) | 0.06 | |
| Neurological and neuropsychological testing | ||||
| MoCA (/30) | 19.0 (15.5, 25.5) | 24.0 (20.0, 25.0) | 0.13 | |
| NPI-Q (/36) | 4.0 (3.5, 10.0) | 1.5 (1.0, 2.8) | <0.01 | |
| 20-BAS (/20) | 4.5 (3.3, 5.0) | 1.0 (0.0, 1.0) | <0.01 | |
| Frontal Behavioral Inventory (/72) | 33.0 (29.0, 35.5) | 10.0 (8.0, 17.0) | <0.01 | |
| Frontal Assessment Battery (/18) | 13.0 (6.0, 16.5) | 16.0 (13.0, 17.0) | 0.26 | |
| MDS-UPDRS III (/120) | 10.0 (5.0, 11.5) | 11.0 (8.5, 18.0) | 0.13 | |
| WAB-Praxis (/60) | 57.0 (42.5, 58.5) | 58.0 (54.5, 59.0) | 0.41 | |
| PSPRS (/100) | 12.0 (7.3, 14.5) | 14.0 (7.8, 19.0) | 0.52 | |
| PSIS (/5) | 0.0 (0.0, 1.0) | 1.0 (0.0, 1.0) | 0.55 | |
| TMT-B | 101.0 (65.0, 205.0) | 251.0 (94.0, 300.0) | 0.19 | |
| mWCST-CAT | 22.0 (19.0, 55.0) | 37.5 (32.3, 49.8) | 0.32 | |
| VOSP Letters (/20) | 20 (20.0, 20.0) | 20 (20.0, 20.0) | 0.99 | |
| Speech and language testing | ||||
| Boston Naming Test (/15) | 14.0 (12.0, 14.0) | 13.0 (12.0, 14.8) | 0.97 | |
| Letter Fluency (f) (/27) | 5.0 (1.5, 5.5) | 6.0 (4.5, 10.0) | 0.15 | |
| WAB-AQ (/100) | 91.5 (81.3, 96.2) | 94.2 (90.8, 96.2) | 0.49 | |
| WAB Animal Fluency (/20) | 10.0 (7.0, 19.0) | 15.5 (10.5, 17.0) | 0.46 | |
| ASRS-3 Total (/52) | 23.0 (19.5, 24.5) | 25.0 (18.5, 30.0) | 0.51 | |
| ASRS-3 Phonetic Subscore (/16) | 8.0 (6.5, 9.3) | 12.0 (6.0, 14.0) | 0.25 | |
| ASRS-3 Prosodic Subscore (/16) | 7.0 (6.5, 8.5) | 6.0 (4.5, 10.0) | 0.65 | |
| NVOA (/32) | 23.0 (11.0, 30.0) | 27.5 (24.0, 30.0) | 0.37 | |
| AOS type: | Mixed | 1 (9.1%) | 1 (9.1%) | 0.82 |
| Phonetic | 8 (72.7%) | 9 (81.8%) | ||
| Prosodic | 2 (18.2%) | 1 (9.1%) | ||
| AOS Severity (/4) | 2.0 (1.8, 3.0) | 2.3 (1.3, 3.4) | 0.86 | |
| Aphasia Present, N (%) | 7 (64%) | 7 (64%) | 0.99 | |
| Aphasia Severity (/4) | 1.0 (1.0, 2.0) | 1.0 (0.25, 1.0) | 0.21 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, N.; Bhaskaran, J.; Duffy, J.R.; Clark, H.M.; Machulda, M.M.; Dickson, D.W.; Whitwell, J.L.; Josephs, K.A. Behavioral Variant Frontotemporal Dementia in the Context of Progressive Apraxia of Speech: A Clinico-Neuroimaging Case–Control Study. Brain Sci. 2025, 15, 1169. https://doi.org/10.3390/brainsci15111169
Hossain N, Bhaskaran J, Duffy JR, Clark HM, Machulda MM, Dickson DW, Whitwell JL, Josephs KA. Behavioral Variant Frontotemporal Dementia in the Context of Progressive Apraxia of Speech: A Clinico-Neuroimaging Case–Control Study. Brain Sciences. 2025; 15(11):1169. https://doi.org/10.3390/brainsci15111169
Chicago/Turabian StyleHossain, Nadia, Jerusha Bhaskaran, Joseph R. Duffy, Heather M. Clark, Mary M. Machulda, Dennis W. Dickson, Jennifer L. Whitwell, and Keith A. Josephs. 2025. "Behavioral Variant Frontotemporal Dementia in the Context of Progressive Apraxia of Speech: A Clinico-Neuroimaging Case–Control Study" Brain Sciences 15, no. 11: 1169. https://doi.org/10.3390/brainsci15111169
APA StyleHossain, N., Bhaskaran, J., Duffy, J. R., Clark, H. M., Machulda, M. M., Dickson, D. W., Whitwell, J. L., & Josephs, K. A. (2025). Behavioral Variant Frontotemporal Dementia in the Context of Progressive Apraxia of Speech: A Clinico-Neuroimaging Case–Control Study. Brain Sciences, 15(11), 1169. https://doi.org/10.3390/brainsci15111169






