Baseline Neuropsychological Characteristics of Adolescents and Young Adults with Down Syndrome Who Participated in Two Clinical Trials of the Drug Memantine
Abstract
1. Introduction
2. Materials and Methods
3. Results
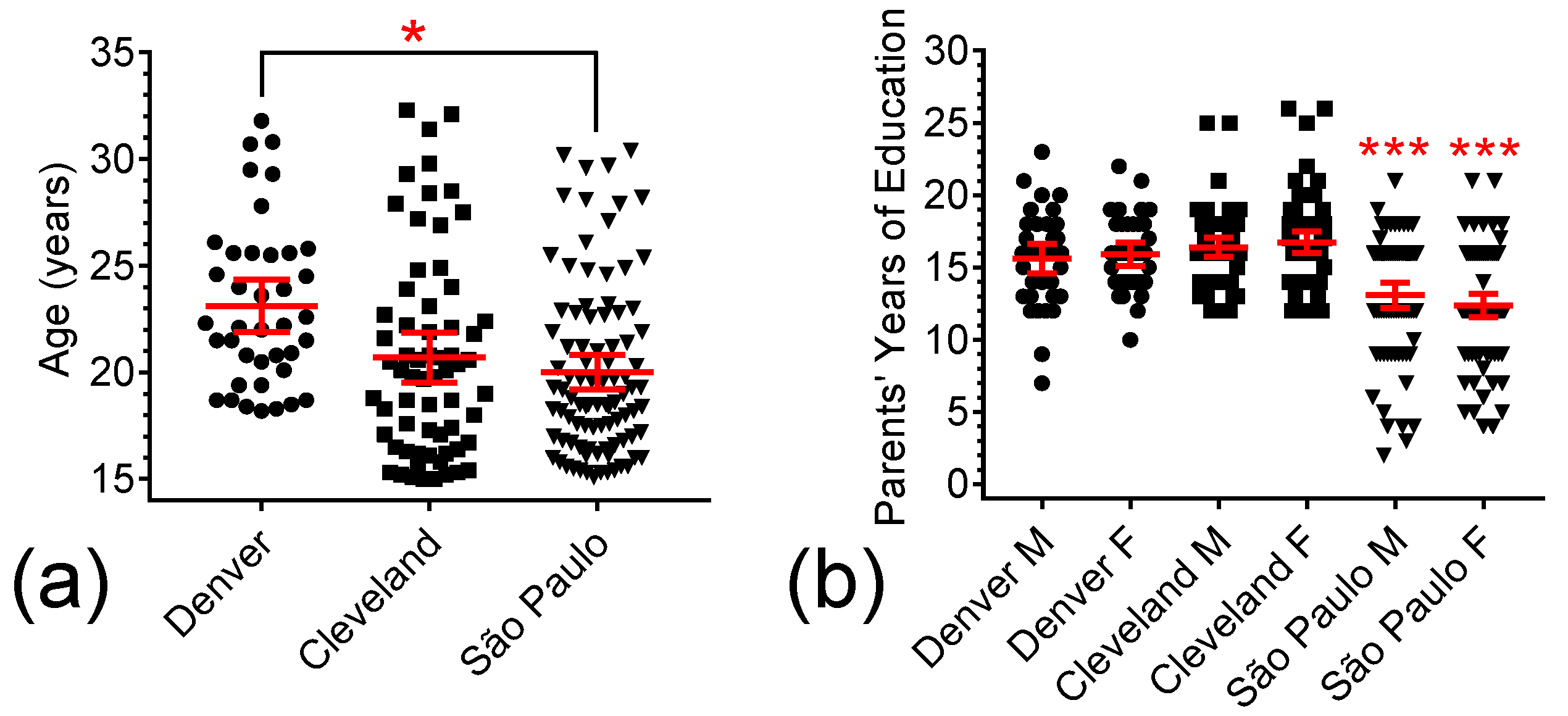
3.1. Demographics
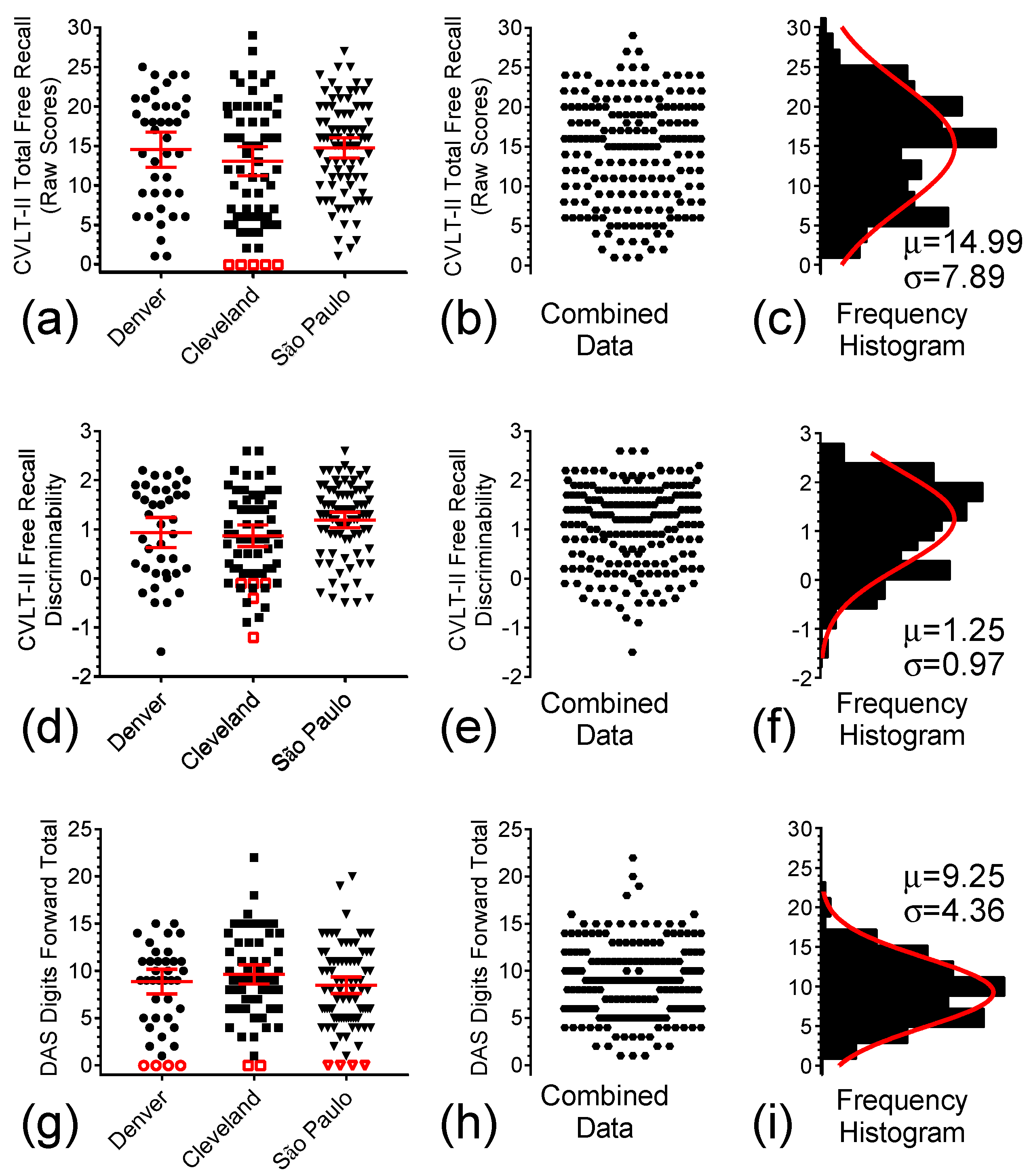
3.2. Episodic Verbal Long-Term Memory and Working/Short-Term Memory as Assessed by the CVLT-II-sf and the DAS-II Recall of Digits Forward
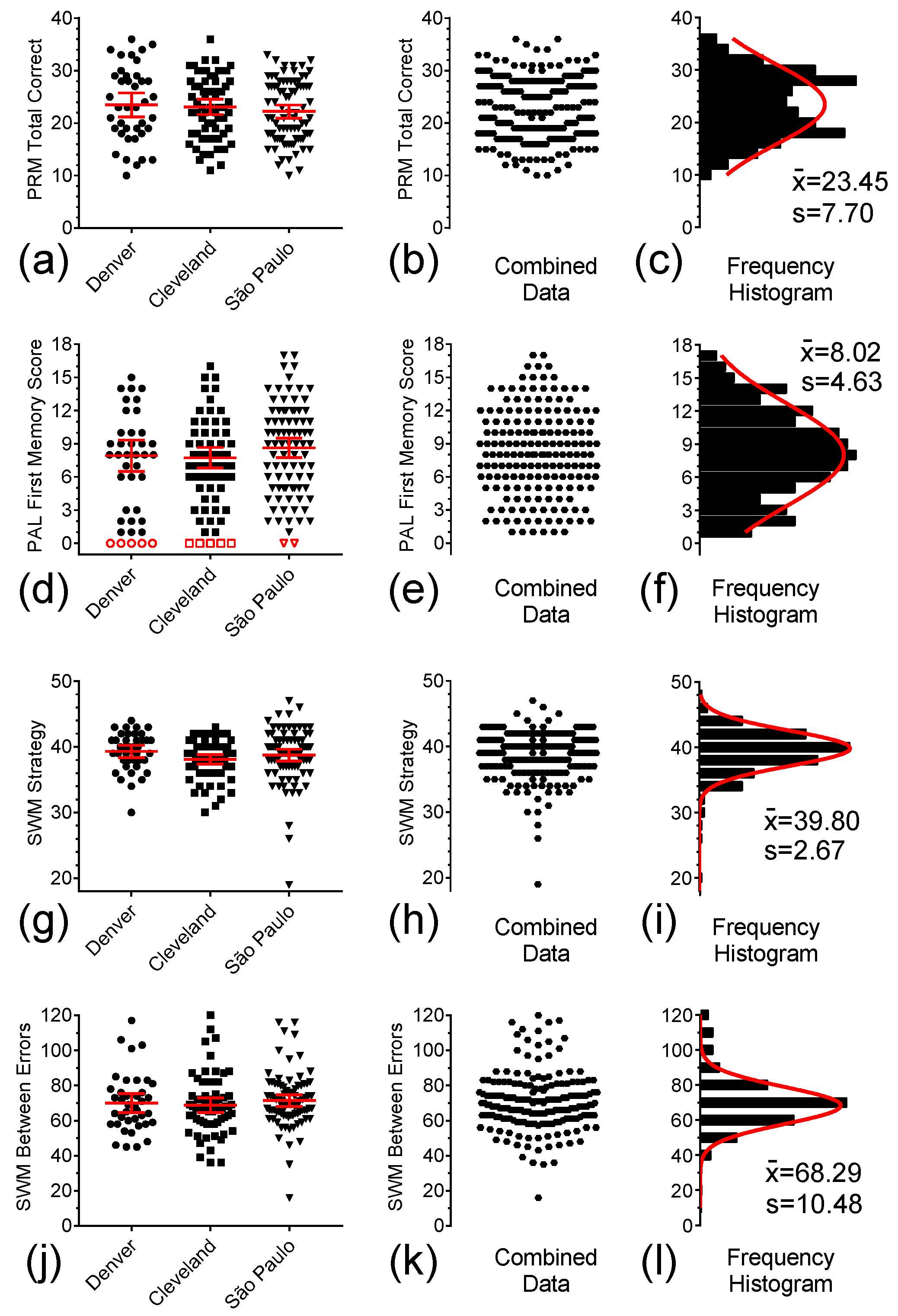
3.3. CANTAB PRM, PAL, and SWM
3.4. SSP and Go-No-Go
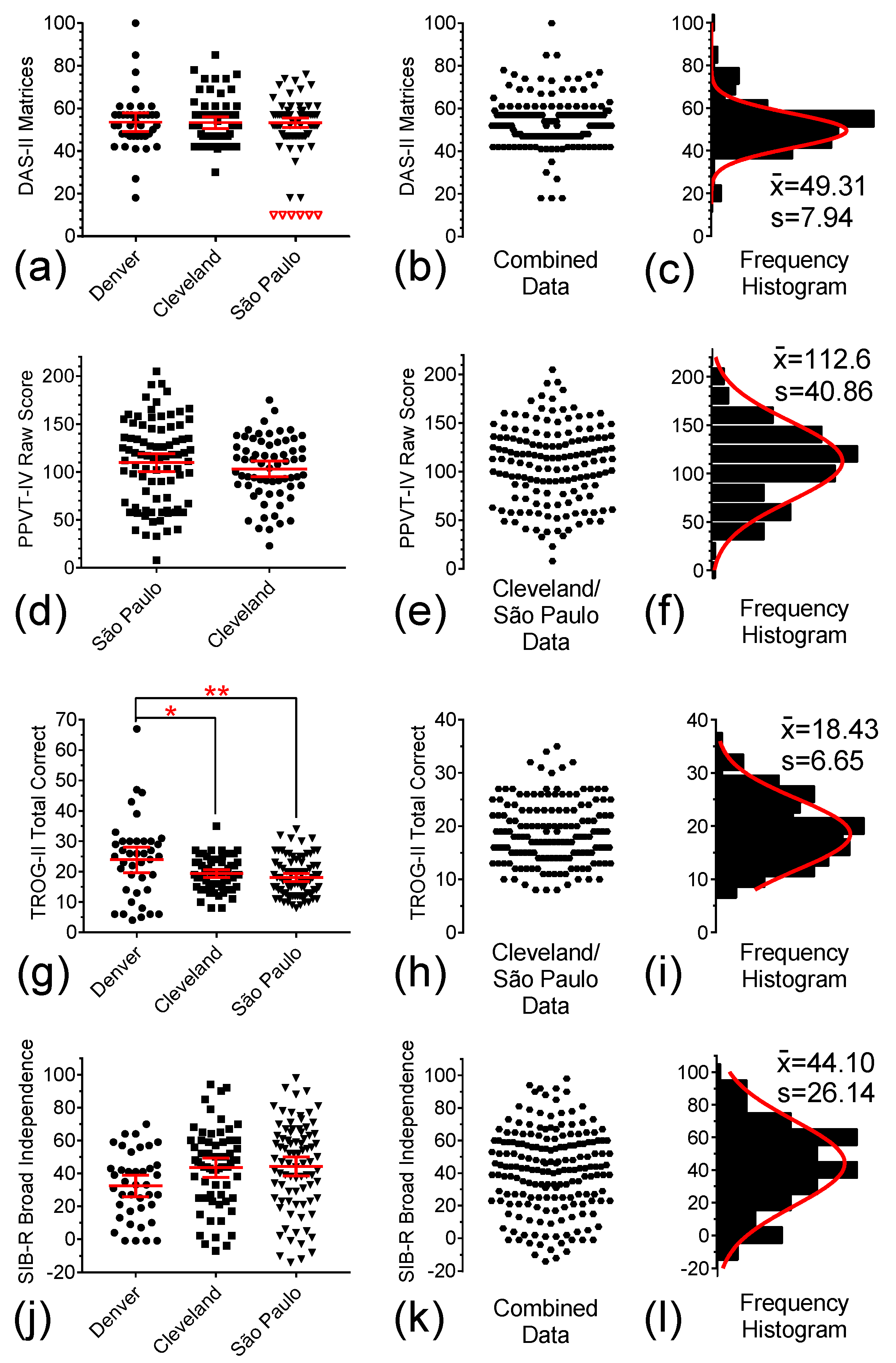
3.5. DAS-II Matrices, PPVT, TROG-II, and SIB-R
3.6. Test Battery’s Psychometric Landscape
3.6.1. Additional Descriptive Statistics
3.6.2. Multiple Regression Analysis
3.6.3. Test–Retest Reliability
3.6.4. Potential Functional Clustering of the Different Measures
3.6.5. Primacy and Recency Effects for the CVLT-II-sf
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DS | Down syndrome |
| AD | Alzheimer’s disease |
| DSAD | Alzheimer’s disease associated with Down syndrome |
| NMDA | N-Methyl-D-aspartate |
| ID | Intellectual Disability |
| CVLT-II | California Verbal Learning Test 2nd Edition |
| CVLT-II-sf | California Verbal Learning Test 2nd Edition short form |
| CVLT-III | California Verbal Learning Test 3rd Edition |
| DAS-II | Differential Ability Scales-Second Edition |
| CANTAB | Cambridge Neuropsychological Test Automated Battery |
| PRM | Pattern Recognition Memory |
| PAL | Paired Associates Learning |
| RBMT | Rivermead Behavioral Memory Test |
| SWM | Spatial Working Memory |
| SSP | Spatial Span |
| PPVT-III | Peabody Picture Vocabulary Test-3rd edition |
| PPVT-IV | Peabody Picture Vocabulary Test-4th edition |
| TROG-II | Test of Reception of Grammar-2nd edition |
| SIB-R | Scales of Independent Behavior–Revised |
| ANOVA | Analysis of Variance |
| PCA | Principal Component Analysis |
| ICC | Intra-class Correlation |
| CI | Confidence Interval |
| AVLT | Auditory Verbal Learning Test |
Appendix A
- Inclusion and Exclusion Criteria (these applied to both the Pilot and Follow-up Memantine Trials): participants who fulfilled all the inclusion criteria and none of the exclusion criteria listed below were accepted for enrollment. The benefits and risks of participation in the studies were explained to all subjects and respective caregivers prior to screening for the studies. At that time, written informed consent was obtained from all parties prior to initiating formal screening for the studies.Inclusion Criteria: Cytogenetically documented trisomy 21 or complete unbalanced translocation of Chromosome 21. Mosaic DS and partial translocations were excluded from the study. No pregnancy by serum testing at screening. Females of child-bearing potential, sexually active had to be practicing a reliable method of birth control. Pregnancy tests were performed at all follow-up medical visits. Laboratory findings within normal limits or judged clinically insignificant at baseline. Vital signs within normal limits for age (stable, medically treated hypotension was allowed). Electrocardiogram (ECG) had to demonstrate predominately normal sinus rhythm. (Minor abnormalities documented as clinically insignificant were allowed.) Participants who have received any experimental drug for DS had to undergo a washout. All participants had to be in general good health; be able to swallow oral medication; have a reliable caregiver or family member who agrees to accompany the participant to all visits, provides information about the participant as required by the protocol, and ensures compliance; and be sufficiently proficient in English to reliably complete the study assessments.Exclusion Criteria: Participant weighing less than 40 kg. Current psychiatric or neurologic diagnosis other than DS (e.g., schizophrenia, bipolar disorder, clinical dementia). Current treatment with psychotropic drugs. Drug or alcohol abuse or dependence. Significant suicide risk or who would require treatment with electro-convulsive therapy or with psychotropic drugs during the study or who have received treatment with a depot neuroleptic drug within 6 months of entering the study. Current or expected hospitalization or residence in a skilled nursing facility (could reside in group homes or other residential settings with no skilled nursing). Active or clinically significant conditions affecting absorption, distribution, or metabolism of study drug (e.g., inflammatory bowel disease, celiac disease, or renal insufficiency). Significant allergies to or other significant intolerance of or with contraindications to memantine therapy, or their ingredients, as stated in the prescribing information. Expected to require general anesthetics during the course of the study. Presence or recent history of seizure disorder (<3 years). Clinically significant and/or clinically unstable systemic disease. [Those with controlled hypothyroidism had to be on a stable dose of medication for at least 3 months prior to screening and have normal serum thyroid-stimulating hormone (TSH) levels at screening; and those with controlled diabetes mellitus had to have a hemoglobin A1C (HbA1c) reading of <8.0% and a random serum glucose value of <170 mg/dL.] Severe infections or a major surgical operation within 3 months prior to screening. History of persistent cognitive deficits immediately following head trauma. Donation of blood or blood products in less than 30 days prior to screening or while participating in the study. Inability to comply with the protocol or perform the outcomes measures due to hearing or visual impairment or other clinically relevant issues.
Appendix B
| Word List (English) | Word List (Portuguese) |
|---|---|
| hat | chapéu |
| cherries | uvas |
| wrench | martelo |
| sweater | blusa |
| lemon | limão |
| pliers | alicate |
| belt | cinto |
| peaches | peras |
| drill | serrote |
| Long-term Recognition Intrusion Lists (Participants are asked whether they have heard the following words) | |
| List 1 (English) | List 1 (Portuguese) |
| newspaper | jornal |
| wrench | martelo |
| apple | maçã |
| screwdriver | parafuso |
| peaches | peras |
| shirt | camisa |
| typewriter | computador |
| coffee | café |
| sweater | blusa |
| List 2 (English) | List 2 (Portuguese) |
| cherries | uvas |
| pants | calça |
| vitamins | vitamina |
| kite | pipa |
| hammer | furadeira |
| spoon | colher |
| banana | banana |
| drill | serrote |
| hat | chapéu |
| List 3 (English) | List 3 (Portuguese) |
| elbow | cotovelo |
| socks | meia |
| daisy | rosa |
| pliers | alicate |
| orange | laranja |
| belt | cinto |
| saw | prego |
| lemon | limão |
| gasoline | gasolina |
Appendix C
| Measure | Number Participants Minus Participants at Floor (% Participants at Floor) | Baseline Scores Arithmetic Mean (Standard Deviation) | Median (Min; L-Qtr; U-Qrt; Max) | Skew | Kurtosis |
|---|---|---|---|---|---|
| CVLT II Total Correct (1) | 190 − 5 = 185 (2.63%) | 14.12 (6.57) | 15.00 (1.00; 9.00; 20.00; 29.00) | −0.112 | −0.947 |
| CVLT-II Recall Discriminability (2) | 190 − 5 = 185 (2.63%) | 1.03 (0.83) | 1.10 (−1.5; 0.30; 1.70; 2.60) | −0.401 | 0.543 |
| DAS-II Digits Forward Total (3) | 190 − 10 = 180 (5.26%) | 8.96 (3.98) | 9 (1; 6; 11.50; 22) | 0.337 | −0.057 |
| PRM Total Correct (4) | 190 − 0 (0%) | 22.78 (6.14) | 23 (10; 18; 28; 36) | −0.015 | −0.955 |
| PAL 1st Memory Score (5) | 190 − 12 = 178 (6.32%) | 8.19 (3.90) | 8 (1; 5; 11; 17) | 0.059 | −0.689 |
| SWM Strategy (6) | 190 − 0 (0%) | 38.63 (3.55) | 39 (19; 37; 41; 47) | −1.378 ++ | 5.052 † |
| SWM Between Errors (7) | 190 − 0 (0%) | 70.83 (17.85) | 68 (16; 61; 79; 176) | 1.419 ++ | 6.528 † |
| SSP Span Length (8) | 150 − 26 = 124 (17.33%) | 3.59 (0.95) | 3.5 (2; 3; 4; 6) | 0.227 | −0.533 |
| SSP Usage Errors (9) | 150 − 26 = 124 (17.33%) | 2.49 (1.77) | 2 (0; 1; 3; 8) | 0.799+ | 0.575 |
| Go-No-Go Response Time (ms) (10) | 147 − 0 = 147 (0%) | 557(131) | 528 (326; 452; 646; 885) | 0.576 + | −0.499 |
| DAS-II Matrices Ability Score (11) | 185 − 6 = 179 (3.24%) | 52.33 (11.06) | 52 (18; 47; 57; 100) | 0.373 | 2.820 † |
| PPVT-4 Raw Score (12) | 150 − 0 = 150 (0%) | 108 (39.34) | 113 (8; 80; 135; 205) | −0.090 | −0.467 |
| TROG-2 Total Correct (13) | 150 − 0 (0%) | 18.67 (5.83) | 18 (8; 14; 23; 35) | 0.361 | −0.405 |
| SIB-R Broad Independence (14) | 189 − 0 (0%) | 41.53 (24.67) | 43 (−14; 25; 59; 98) | −0.157 | −0.489 |



| Pearson’s r Correlations Between Different Measures | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measure | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | (11) | (12) | (13) | (14) |
| CVLT Total (1) | 0.85 * | 0.48 * | 0.33 * | 0.21 * | 0.05 | −0.34 * | 0.41 * | −0.04 | −0.09 | 0.37 * | 0.50 * | 0.34 * | 0.29 * |
| CVLT Discrim. (2) | 0.50 * | 0.40 * | 0.30 * | 0.02 | −0.37 * | 0.42 * | −0.12 | −0.05 | 0.40 * | 0.58 * | 0.47 * | 0.38 * | |
| DAS-II Digits (3) | 0.36 * | 0.20 * | −0.16 | −0.53 * | 0.53 * | 0.09 | −0.19 * | 0.40 * | 0.61 * | 0.59 * | 0.43 * | ||
| PRM Total (4) | 0.35 * | −0.03 | −0.26 * | 0.40 * | 0.04 | −0.15 | 0.37 * | 0.53 * | 0.52 * | 0.33 * | |||
| PAL First (5) | 0.13 | −0.22 * | 0.28 * | −0.14 | −0.02 | 0.24 * | 0.30 * | 0.24 * | 0.17 | ||||
| SMW Strategy (6) | 0.29 * | −0.05 | 0.10 | −0.16 | −0.13 | −0.02 | −0.11 | −0.13 | |||||
| SMW Betw. Errors (7) | −0.53 * | 0.07 | 0.14 | −0.54 * | −0.46 * | −0.46 * | −0.45 * | ||||||
| SSP Span Length (8) | 0.16 | −0.34 * | 0.33 * | 0.62 * | 0.48 * | 0.48 * | |||||||
| SSP Errors (9) | −0.17 | −0.13 | 0.01 | 0.01 | −0.08 | ||||||||
| Go-No-Go Speed (10) | −0.01 | −0.20 * | −0.12 | −0.017 | |||||||||
| DAS-II Matrices (11) | 0.46 * | 0.39 * | 0.32 * | ||||||||||
| PPVT-IV (12) | 0.73 * | 0.50 * | |||||||||||
| TROG (13) | 0.43 * | ||||||||||||
| SIB-R Broad Independ. (14) | |||||||||||||
| Euclidian Distances Between Different Measures | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measure | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | (11) | (12) | (13) | (14) |
| CVLT Total (1) | 0.85 * | 0.48 * | 0.33 * | 0.21 * | 0.05 | −0.34 * | 0.41 * | −0.04 | −0.09 | 0.37 * | 0.50 * | 0.34 * | 0.29 * |
| CVLT Discrim. (2) | 0.50 * | 0.40 * | 0.30 * | 0.02 | −0.37 * | 0.42 * | −0.12 | −0.05 | 0.40 * | 0.58 * | 0.47 * | 0.38 * | |
| DAS-II Digits (3) | 0.36 * | 0.20 * | −0.16 | −0.53 * | 0.53 * | 0.09 | −0.19 * | 0.40 * | 0.61 * | 0.59 * | 0.43 * | ||
| PRM Total (4) | 0.35 * | −0.03 | −0.26 * | 0.40 * | 0.04 | −0.15 | 0.37 * | 0.53 * | 0.52 * | 0.33 * | |||
| PAL First (5) | 0.13 | −0.22 * | 0.28 * | −0.14 | −0.02 | 0.24 * | 0.30 * | 0.24 * | 0.17 | ||||
| SMW Strategy (6) | 0.29 * | −0.05 | 0.10 | −0.16 | −0.13 | −0.02 | −0.11 | −0.13 | |||||
| SMW Betw. Errors (7) | −0.53 * | 0.07 | 0.14 | −0.54 * | −0.46 * | −0.46 * | −0.45 * | ||||||
| SSP Span Length (8) | 0.16 | −0.34 * | 0.33 * | 0.62 * | 0.48 * | 0.48 * | |||||||
| SSP Errors (9) | −0.17 | −0.13 | 0.01 | 0.01 | −0.08 | ||||||||
| Go-No-Go Speed (10) | −0.01 | −0.20 * | −0.12 | −0.017 | |||||||||
| DAS-II Matrices (11) | 0.46 * | 0.39 * | 0.32 * | ||||||||||
| PPVT-IV (12) | 0.73 * | 0.50 * | |||||||||||
| TROG (13) | 0.43 * | ||||||||||||
| SIB-R Broad Independ. (14) | |||||||||||||
References
- Lejeune, J.; Gautier, M.; Turpin, R. Study of somatic chromosomes from 9 mongoloid children. Comptes Rendus Hebd. Seances Acad. Sci. 1959, 248, 1721–1722. [Google Scholar]
- Patterson, D.; Costa, A.C. Down syndrome and genetics—A case of linked histories. Nat. Rev. Genet. 2005, 6, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.; Collins, S. 50 years with Down syndrome: A longitudinal study. J. Appl. Res. Intellect. Disabil. 2018, 31, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Pennington, B.F.; Moon, J.; Edgin, J.; Stedron, J.; Nadel, L. The neuropsychology of Down syndrome: Evidence for hippocampal dysfunction. Child. Dev. 2003, 74, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Nadel, L. Down’s syndrome: A genetic disorder in biobehavioral perspective. Genes. Brain Behav. 2003, 2, 156–166. [Google Scholar] [CrossRef]
- Edgin, J.; Mason, G.M.; Allman, M.J.; Capone, G.T.; Deleon, I.; Maslen, C.; Reeves, R.H.; Sherman, S.L.; Nadel, L. Development and validation of the Arizona Cognitive Test Battery for Down syndrome. J. Neurodev. Disord. 2010, 2, 149–164. [Google Scholar] [CrossRef]
- Costa, A.C. An assessment of the vestibulo-ocular reflex (VOR) in persons with Down syndrome. Exp. Brain Res. 2011, 214, 199–213. [Google Scholar] [CrossRef]
- Costa, A.C. An assessment of optokinetic nystagmus (OKN) in persons with Down syndrome. Exp. Brain Res. 2011, 214, 381–391. [Google Scholar] [CrossRef]
- Sinai, A.; Mokrysz, C.; Bernal, J.; Bohnen, I.; Bonell, S.; Courtenay, K.; Dodd, K.; Gazizova, D.; Hassiotis, A.; Hillier, R.; et al. Predictors of Age of Diagnosis and Survival of Alzheimer’s Disease in Down Syndrome. J. Alzheimers Dis. 2018, 61, 717–728. [Google Scholar] [CrossRef]
- Rubenstein, E.; Tewolde, S.; Michals, A.; Weuve, J.; Fortea, J.; Fox, M.P.; Pescador Jimenez, M.; Scott, A.; Tripodis, Y.; Skotko, B.G. Alzheimer Dementia Among Individuals With Down Syndrome. JAMA Netw. Open 2024, 7, e2435018. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Andrews, S.J.; Beach, T.G.; Buracchio, T.; Dunn, B.; Graf, A.; Hansson, O.; Ho, C.; Jagust, W.; McDade, E.; et al. Revised criteria for the diagnosis and staging of Alzheimer’s disease. Nat. Med. 2024, 30, 2121–2124. [Google Scholar] [CrossRef]
- Hithersay, R.; Startin, C.M.; Hamburg, S.; Mok, K.Y.; Hardy, J.; Fisher, E.M.C.; Tybulewicz, V.L.J.; Nizetic, D.; Strydom, A. Association of Dementia with Mortality Among Adults With Down Syndrome Older Than 35 Years. JAMA Neurol. 2019, 76, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.C.S. On the Therapeutic Use of Monoclonal Antibodies Against Amyloid Plaques in Older Adults with Down Syndrome: A Narrative Review and Perspective. Brain Sci. 2024, 14, 1084. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.C. The glutamatergic hypothesis for Down syndrome: The potential use of N-methyl-D-aspartate receptor antagonists to enhance cognition and decelerate neurodegeneration. CNS Neurol. Disord. Drug Targets 2014, 13, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Boada, R.; Hutaff-Lee, C.; Schrader, A.; Weitzenkamp, D.; Benke, T.A.; Goldson, E.J.; Costa, A.C. Antagonism of NMDA receptors as a potential treatment for Down syndrome: A pilot randomized controlled trial. Transl. Psychiatry 2012, 2, e141. [Google Scholar] [CrossRef]
- Costa, A.C.; Brandao, A.C.; Boada, R.; Barrionuevo, V.L.; Taylor, H.G.; Roth, E.; Stasko, M.R.; Johnson, M.W.; Assir, F.F.; Roberto, M.P.; et al. Safety, efficacy, and tolerability of memantine for cognitive and adaptive outcome measures in adolescents and young adults with Down syndrome: A randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2022, 21, 31–41. [Google Scholar] [CrossRef]
- Hanney, M.; Prasher, V.; Williams, N.; Jones, E.L.; Aarsland, D.; Corbett, A.; Lawrence, D.; Yu, L.M.; Tyrer, S.; Francis, P.T.; et al. Memantine for dementia in adults older than 40 years with Down’s syndrome (MEADOWS): A randomised, double-blind, placebo-controlled trial. Lancet 2012, 379, 528–536. [Google Scholar] [CrossRef]
- Li, G.; Taljaard, M.; Van den Heuvel, E.R.; Levine, M.A.; Cook, D.J.; Wells, G.A.; Devereaux, P.J.; Thabane, L. An introduction to multiplicity issues in clinical trials: The what, why, when and how. Int. J. Epidemiol. 2017, 46, 746–755. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Delis, D.C.; Kramer, J.H.; Kaplan, E.; Ober, B.A. California Verbal Learning Test: Adult version (CVLT-II): Manual, 2nd ed.; Psychological Corporation: San Antonio, TX, USA, 2000. [Google Scholar] [CrossRef]
- Basten, I.A.; Boada, R.; Taylor, H.G.; Koenig, K.; Barrionuevo, V.L.; Brandao, A.C.; Costa, A.C.S. On the Design of Broad-Based Neuropsychological Test Batteries to Assess the Cognitive Abilities of Individuals with Down Syndrome in the Context of Clinical Trials. Brain Sci. 2018, 8, 205. [Google Scholar] [CrossRef]
- Elliott, C.D. Differential Ability Scales–Second Edition: Administration and Scoring Manual; Harcourt Assessment, Inc.: San Antonio, TX, USA, 2007. [Google Scholar]
- Robbins, T.W.; James, M.; Owen, A.M.; Sahakian, B.J.; McInnes, L.; Rabbitt, P. Cambridge Neuropsychological Test Automated Battery (CANTAB): A factor analytic study of a large sample of normal elderly volunteers. Dementia 1994, 5, 266–281. [Google Scholar] [CrossRef]
- Owen, A.M.; Sahakian, B.J.; Semple, J.; Polkey, C.E.; Robbins, T.W. Visuo-spatial short-term recognition memory and learning after temporal lobe excisions, frontal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia 1995, 33, 1–24. [Google Scholar] [CrossRef]
- Weniger, G.; Boucsein, K.; Irle, E. Impaired associative memory in temporal lobe epilepsy subjects after lesions of hippocampus, parahippocampal gyrus, and amygdala. Hippocampus 2004, 14, 785–796. [Google Scholar] [CrossRef]
- Petrides, M.; Milner, B. Deficits on subject-ordered tasks after frontal- and temporal-lobe lesions in man. Neuropsychologia 1982, 20, 249–262. [Google Scholar] [CrossRef]
- Fillmore, M.T.; Rush, C.R.; Hays, L. Acute effects of cocaine in two models of inhibitory control: Implications of non-linear dose effects. Addiction 2006, 101, 1323–1332. [Google Scholar] [CrossRef]
- Dunn, L.M.; Dunn, D.M. Peabody Picture Vocabulary Test, Fourth Edition: Manual; Pearson Assessments: San Antonio, TX, USA, 2007. [Google Scholar]
- Bishop, D.V.M. Test for Reception of Grammar: Trog-2; Psychological Corporation: San Antonio, TX, USA, 2003. [Google Scholar]
- Unsworth, N.; Spillers, G.J.; Brewer, G.A. Working memory capacity and retrieval limitations from long-term memory: An examination of differences in accessibility. Q. J. Exp. Psychol. 2012, 65, 2397–2410. [Google Scholar] [CrossRef] [PubMed]
- Murdock, B.B., Jr. Serial order effects in short-term memory. J. Exp. Psychol. 1968, 76, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Glanzer, M.; Peters, S.C. Re-examination of the serial position effect. J. Exp. Psychol. 1962, 64, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.C.; Shiffrin, R.M. Human Memory: A Proposed System and its Control Processes. In Psychology of Learning and Motivation; Spence, K.W., Spence, J.T., Eds.; Academic Press: Cambridge, MA, USA, 1968; Volume 2, pp. 89–195. [Google Scholar]
- Griffin, J.W.; John, S.E.; Adams, J.W.; Bussell, C.A.; Saurman, J.L.; Gavett, B.E. The effects of age on the learning and forgetting of primacy, middle, and recency components of a multi-trial word list. J. Clin. Exp. Neuropsychol. 2017, 39, 900–912. [Google Scholar] [CrossRef]
- Rosser, T.C.; Edgin, J.O.; Capone, G.T.; Hamilton, D.R.; Allen, E.G.; Dooley, K.J.; Anand, P.; Strang, J.F.; Armour, A.C.; Frank-Crawford, M.A.; et al. Associations Between Medical History, Cognition, and Behavior in Youth With Down Syndrome: A Report From the Down Syndrome Cognition Project. Am. J. Intellect. Dev. Disabil. 2018, 123, 514–528. [Google Scholar] [CrossRef]
- de Sola, S.; de la Torre, R.; Sanchez-Benavides, G.; Benejam, B.; Cuenca-Royo, A.; Del Hoyo, L.; Rodriguez, J.; Catuara-Solarz, S.; Sanchez-Gutierrez, J.; Duenas-Espin, I.; et al. A new cognitive evaluation battery for Down syndrome and its relevance for clinical trials. Front. Psychol. 2015, 6, 708. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, R.; de Sola, S.; Hernandez, G.; Farre, M.; Pujol, J.; Rodriguez, J.; Espadaler, J.M.; Langohr, K.; Cuenca-Royo, A.; Principe, A.; et al. Safety and efficacy of cognitive training plus epigallocatechin-3-gallate in young adults with Down’s syndrome (TESDAD): A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2016, 15, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.H.; Kaat, A.; Sansone, S.M.; Michalak, C.; Coleman, J.; Thompson, T.; McKenzie, F.J.; Dakopolos, A.; Riley, K.; Berry-Kravis, E.; et al. Sensitivity of the NIH Toolbox to Detect Cognitive Change in Individuals With Intellectual and Developmental Disability. Neurology 2023, 100, e778–e789. [Google Scholar] [CrossRef] [PubMed]
- Myrelid, A.; Bergman, S.; Elfvik Stromberg, M.; Jonsson, B.; Nyberg, F.; Gustafsson, J.; Anneren, G. Late effects of early growth hormone treatment in Down syndrome. Acta Paediatr. 2010, 99, 763–769. [Google Scholar] [CrossRef]
- Nicham, R.; Weitzdorfer, R.; Hauser, E.; Freidl, M.; Schubert, M.; Wurst, E.; Lubec, G.; Seidl, R. Spectrum of cognitive, behavioural and emotional problems in children and young adults with Down syndrome. J. Neural Transm. Suppl. 2003, 67, 173–191. [Google Scholar] [CrossRef]
- Schworer, E.K.; Voth, K.; Hoffman, E.K.; Esbensen, A.J. Short-term memory outcome measures: Psychometric evaluation and performance in youth with Down syndrome. Res. Dev. Disabil. 2022, 120, 104147. [Google Scholar] [CrossRef]
- Woods, S.P.; Delis, D.C.; Scott, J.C.; Kramer, J.H.; Holdnack, J.A. The California Verbal Learning Test--second edition: Test-retest reliability, practice effects, and reliable change indices for the standard and alternate forms. Arch. Clin. Neuropsychol. 2006, 21, 413–420. [Google Scholar] [CrossRef]
- Schneider, W.; Niklas, F. Intelligence and Verbal Short-Term Memory/Working Memory: Their Interrelationships from Childhood to Young Adulthood and Their Impact on Academic Achievement. J. Intell. 2017, 5, 26. [Google Scholar] [CrossRef]
- Daunhauer, L.A.; Will, E.; Schworer, E.; Fidler, D.J. Young students with Down syndrome: Early longitudinal academic achievement and neuropsychological predictors. J. Intellect. Dev. Dis. 2020, 45, 211–221. [Google Scholar] [CrossRef]
- Tomaszewski, B.; Fidler, D.; Talapatra, D.; Riley, K. Adaptive behaviour, executive function and employment in adults with Down syndrome. J. Intellect. Disabil. Res. 2018, 62, 41–52. [Google Scholar] [CrossRef]
- Swanson, H.L. Developmental recall lag in learning-disabled children: Perceptual deficit or verbal mediation deficiency? J. Abnorm. Child. Psychol. 1979, 7, 199–210. [Google Scholar] [CrossRef]
- Montoliu-Gaya, L.; Strydom, A.; Blennow, K.; Zetterberg, H.; Ashton, N.J. Blood Biomarkers for Alzheimer’s Disease in Down Syndrome. J. Clin. Med. 2021, 10, 3639. [Google Scholar] [CrossRef]
- Hendrix, J.A.; Airey, D.C.; Britton, A.; Burke, A.D.; Capone, G.T.; Chavez, R.; Chen, J.; Chicoine, B.; Costa, A.C.S.; Dage, J.L.; et al. Cross-Sectional Exploration of Plasma Biomarkers of Alzheimer’s Disease in Down Syndrome: Early Data from the Longitudinal Investigation for Enhancing Down Syndrome Research (LIFE-DSR) Study. J. Clin. Med. 2021, 10, 1907. [Google Scholar] [CrossRef]

| Measure | Site Comparisons (3 Sites ANOVA; 2 Sites t-Test) | Sample Mean Value | Standard Deviation s (95% CI) | Goodness of Fit R2 Value | Shapiro–Wilk W; “p” Value |
|---|---|---|---|---|---|
| CVLT II Total Correct (1) | F(2, 181) = 1.2474; p = 0.2897 | 14.99 (12.67 to 17.15) | 7.89 (6.07 to 10.75 | 0.6906 | 0.9599; 0.6596 |
| CVLT-II Recall Discriminability (2) | F(2, 181) = 3.0259; p = 0.051 | 1.26 (1.04 to 1.49) | 0.97 (0.76 to 1.27) | 0.7577 | 0.9765; 0.8531 |
| DAS-II Digits Forward Total (3) | F(2, 177) = 1.7044; p = 0.1849 | 9.25 (8.41 to 10.09) | 4.36 (3.65 to 5.24) | 0.9243 | 0.9717; 0.9280 |
| PRM Total Correct (4) | F(2, 186) = 0.536; p = 0.5862 | 23.45 (21.11 to 25.85) | 7.70 (5.88 to 11.13) | 0.6577 | 0.8922; 0.0871 |
| PAL 1st Memory Score (5) | F(2, 186) = 0.669; p = 0.5132 | 8.02 (7.26 to 8.71) | 4.63 (3.91 to 5.63) | 0.8449 | 0.9705; 0.8275 |
| SWM Strategy (6) | F(2, 187) = 1.59; p = 0.2065 | 39.80 (39.71 to 39.89) | 2.67 (2.57 to 2.77) | 0.9979 | 0.9119; 0.1448 |
| SWM Between Errors (7) | F(2, 187) = 2.377; p = 0.0957 | 68.29 (66.73 to 69.83) | 10.48 (8.85 to 12.43) | 0.9701 | 0.9391; 0.4870 |
| SSP Span Length (8) | p = 0.5487 (Cleveland n = 53; São Paulo n = 71) | 3.49 (2.74 to 4.11) | 1.04 (0 to 2.21) | 0.9529 | 0.8678; 0.2578 |
| SSP Usage Errors (9) | p = 0.0395 * (Cleveland n = 53; São Paulo n = 71) | 2.58 (1.11 to 3.04) | 1.63 (1.24 to 2.26) | 0.9055 | 0.9672; 0.8698 |
| 1.73 (0.59 to 2.94) | 1.64 (1.14 to 2.94) | 0.9055 | 0.9672; 0.8698 | ||
| Go-No-Go Response Time (ms) (10) | p = 0.0082 ** (Cleveland n = 53; São Paulo n = 71) | Cleveland: 481 (422 to 549) | 118 (54.8 to 237) | 0.6294 | 0.9369; 0.44589 |
| São Paulo: 546 (503 to 588) | 140 (100 to 211) | 0.7644 | 0.9578; 0.7517 | ||
| DAS-II Matrices Ability Score (11) | F(2, 180) = 0.003; p = 0.9966 | 49.31 (47.65 to 50.86) | 7.94 (6.67 to 9.49) | 0.9231 | 0.9411; 0.3320 |
| PPVT-4 Raw Score (12) | p = 0.2606 (t test; Cleveland vs. São Paulo) | 112.6 (101.1 to 123.2) | 40.86 (30.20 to 53.87) | 0.8709 | 0.9205; 0.2898 |
| TROG-2 Total Correct (13) | F(2, 187) = 6.501; p = 0.0019 ** | 18.43 (17.15 to 19.68) | 6.65 (5.49 to 8.22) | 0.8689 | 0.9756; 0.9306 |
| SIB-R Broad Independence (14) | F(2, 185) = 3.058; p = 0.049 * (no post hoc differences) | 44.10 (37.68 to 50.23) | 26.14 (20.38 to 33.77) | 0.8472 | 0.8926; 0.1059 |
| Measure | Placebo Baseline Scores | Placebo Retest Scores | Mean Differences | Cohen’s d | ICC * |
|---|---|---|---|---|---|
| CVLT II Total Correct (1) | 14.18 ± 7.03 (95) | 17.33 ± 7.06 (95) | 3.15 ± 5.17 (95) | 0.447 + | 0.67 |
| CVLT-II Recall Discriminability (2) | 0.96 ± 0.88 (95) | 1.37 ± 0.81 (95) | 0.41 ± 0.62 (95) | 0.485 + | 0.66 |
| DAS-II Digits Forward Total (3) | 8.49 ± 4.46 (95) | 8.12 ± 4.45 (95) | −0.07 ± 2.48 (95) | −0.083 | 0.85 |
| PRM Total Correct (4) | 21.40 ± 6.23 (94) | 21.62 ± 6.31 (94) | 0.22 ± 4.16 (94) | 0.035 | 0.79 |
| PAL 1st Memory Score (5) | 7.68 ± 4.40 (94) | 8.62 ± 4.73 (94) | 0.91 ± 3.71 (94) | 0.206 | 0.66 |
| SWM Strategy (6) | 38.49 ± 3.78 (95) | 38.02 ± 3.83 (95) | −0.63 ± 3.64 (95) | −0.124 | 0.51 |
| SWM Between Errors (7) | 71.74 ± 16.78 (95) | 73.16 ± 23.74 (95) | 0.14 ± 12.82 (95) | 0.069 | 0.83 |
| SSP Span Length (8) | 2.78 ± 1.77 (76) | 2.91 ± 1.57 (76) | 0.13 ± 1.30 (76) | 0.078 | 0.70 |
| SSP Usage Errors (9) | 2.36 ± 1.85 (76) | 2.20 ± 1.76 (76) | −0.16 ± 1.96 (76) | −0.089 | 0.41 † |
| Go-No-Go Response Time (ms) (10) | 557.1 ± 135.4 (72) | 559.5 ± 147.1 (72) | 2.40 ± 96.8 (72) | 0.017 | 0.77 |
| DAS-II Matrices Ability Score (11) | 51.68 ± 12.51 (95) | 52.13 ± 13.15 (95) | 0.54 ± 8.89 (95) | 0.035 | 0.75 |
| PPVT-4 Raw Score (12) | 109.12 ± 37.78 (76) | 113.58 ± 38.73 (76) | 4.46 ± 12.96 (76) | 0.117 | 0.94 |
| TROG-2 Total Correct (13) | 18.75 ± 5.90 (95) | 19.48 ± 7.49 (95) | 0.31 ± 4.97 (95) | 0.108 | 0.79 |
| SIB-R Broad Independence (14) | 44.30 ± 25.67 (91) | 50.42 ± 27.29 (91) | 6.12 ± 18.09 (91) | 0.231 + | 0.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, A.C.S.; Brandão, A.C.; Leiva, V.; Taylor, H.G.; Johnson, M.W.; Salmona, P.; Abreu-Silveira, G.; Scheidemantel, T.; Roizen, N.J.; Ruedrich, S.; et al. Baseline Neuropsychological Characteristics of Adolescents and Young Adults with Down Syndrome Who Participated in Two Clinical Trials of the Drug Memantine. Brain Sci. 2025, 15, 1164. https://doi.org/10.3390/brainsci15111164
Costa ACS, Brandão AC, Leiva V, Taylor HG, Johnson MW, Salmona P, Abreu-Silveira G, Scheidemantel T, Roizen NJ, Ruedrich S, et al. Baseline Neuropsychological Characteristics of Adolescents and Young Adults with Down Syndrome Who Participated in Two Clinical Trials of the Drug Memantine. Brain Sciences. 2025; 15(11):1164. https://doi.org/10.3390/brainsci15111164
Chicago/Turabian StyleCosta, Alberto C. S., Ana C. Brandão, Veridiana Leiva, H. Gerry Taylor, Mark W. Johnson, Patrícia Salmona, Guilherme Abreu-Silveira, Thomas Scheidemantel, Nancy J. Roizen, Stephen Ruedrich, and et al. 2025. "Baseline Neuropsychological Characteristics of Adolescents and Young Adults with Down Syndrome Who Participated in Two Clinical Trials of the Drug Memantine" Brain Sciences 15, no. 11: 1164. https://doi.org/10.3390/brainsci15111164
APA StyleCosta, A. C. S., Brandão, A. C., Leiva, V., Taylor, H. G., Johnson, M. W., Salmona, P., Abreu-Silveira, G., Scheidemantel, T., Roizen, N. J., Ruedrich, S., & Boada, R. (2025). Baseline Neuropsychological Characteristics of Adolescents and Young Adults with Down Syndrome Who Participated in Two Clinical Trials of the Drug Memantine. Brain Sciences, 15(11), 1164. https://doi.org/10.3390/brainsci15111164







