Hereditary Sensory and Autonomic Neuropathy Type 2: A Case Report and a Review of the Literature
Abstract
1. Introduction
2. Case Report
- -
- Previously healthy, the patient reported no issues during the neonatal period or with psychomotor development.
- -
- Childhood-onset of symmetric distally predominant lower limb tactile, thermal, and pain hypo-anesthesia and tactile, thermal and pain hypoesthesia of the palms of the hands, with history of frequent burns and injuries especially to the lower extremities. He additionally described clumsiness in fine hands movements, particularly in the absence of visual feedback. Moreover, the subject reported dryness of the palms of the hands and soles of the feet, without sweating alterations and occasional gait instability. He did not experience positive sensory symptoms.
- -
- At the age of 25 the patient underwent amputation of the second toe of the left foot due to a cutaneous ulcer secondary to unrecognized traumas, which progressed to deep dermal infection and osteomyelitis. At the age of 32 the patient was diagnosed with squamous cell carcinoma of the third toe of the left foot, arising from a cutaneous ulcer, followed by amputation of the distal phalanx of the third toe of the left foot.
- -
- Previous electrophysiologic evaluation demonstrated sensitive axonal polyneuropathy. Previous genetic testing in MFN2, NEFL, and GDAP1 genes, associated with Charcot–Marie–Tooth neuropathy type 2 (CMT2) was negative.
- -
- Blood tests including autoimmune and microbiological screening, anti-neuronal antibodies, anti-ganglioside antibodies IgG and IgM, and autoimmune encephalitis panel, all of which were unremarkable.
- -
- Electromyography (EMG) with nerve conduction studies (NCS), which confirmed a severe predominantly sensitive polyneuropathy with non-elicitable sensory nerve action potential (SNAP) in the median, ulnar, and sural nerves using near-nerve technique. Despite a 24–28% reduction in motor conduction velocity of the right median and ulnar nerves, the compound muscle action potential (CMAP) amplitudes remained within normal limits, indicating preserved motor axon integrity. F-waves of the median and ulnar nerves, as well as bilateral tibial nerves, showed normal latency and persistence.
- -
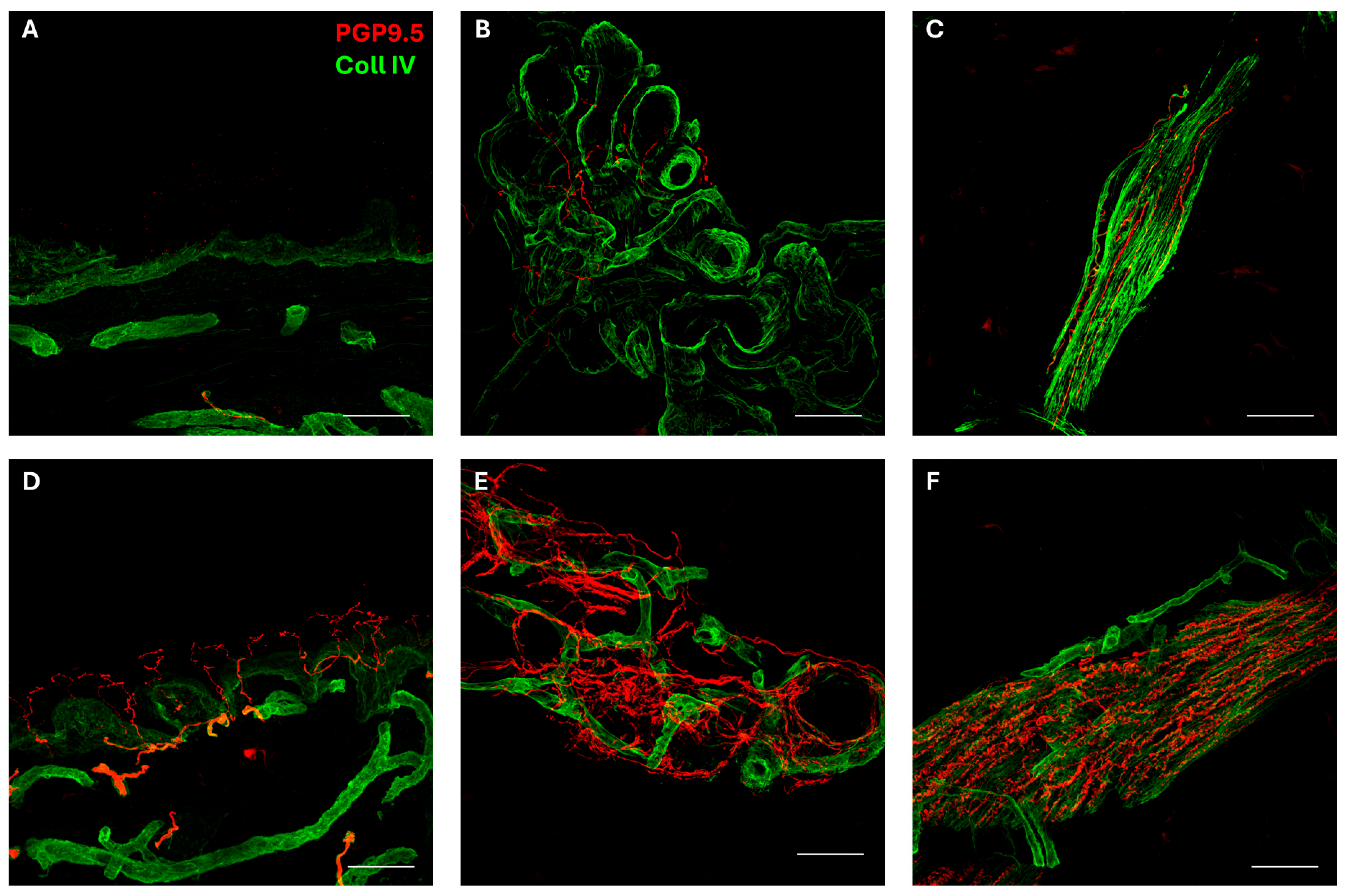
- Skin biopsy was performed according to a previously described method [25,26]. As shown in Figure 1, the biopsy disclosed a severe autonomic small-fiber neuropathy involving both sweat glands and muscle arrector pilorum compared to normal innervation [27]. In addition, epidermal somatic fibers were absent both in the proximal and distal skin sites of the patient [28].
- -
- The evaluation of autonomic control of cardiovascular reflexes reported normal cardiovagal modulation and sympathetic responses, excluding orthostatic hypotension.
- -
- The exome sequencing identified the presence of the homozygous pathogenic variant c.3526_3529del_p.Thr1176CysfsTer21 (NM_213655.5) in the WNK1 gene, already reported [29]. Biallelic pathogenic variants of the WNK1 gene (OMIM*605232) are associated with HSAN 2A. Thus, the result was considered consistent with the clinical picture.
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WNK1 | Protein kinase, lysine-deficient 1 |
| HSAN(s) | Hereditary sensory and autonomic neuropathy (neuropathies) |
| HSAN 1 | Hereditary sensory and autonomic neuropathy type 1 |
| HSAN 2 | Hereditary sensory and autonomic neuropathy type 2 |
| HSAN 3 | Hereditary sensory and autonomic neuropathy type 3 |
| HSAN 4 | Hereditary sensory and autonomic neuropathy type 4 |
| HSAN 5 | Hereditary sensory and autonomic neuropathy type 5 |
| HSAN 6 | Hereditary sensory and autonomic neuropathy type 6 |
| HSAN 7 | Hereditary sensory and autonomic neuropathy type 7 |
| HSAN 8 | Hereditary sensory and autonomic neuropathy type 8 |
| HSAN 9 | Hereditary sensory and autonomic neuropathy type 9 |
| HSAN 2A | Hereditary sensory and autonomic neuropathy type 2A |
| HSAN 2D | Hereditary sensory and autonomic neuropathy type 2D |
| TECPR2 | Tectonin beta-propeller repeat-containing protein 2 |
| HSP 49 | Hereditary spastic paraplegia 49 |
| IKBKAP/ELP1 | Inhibitor of kappa light polypeptide gene enhancer in B cells, kinase complex-associated protein/ Elongator protein 1 |
| SPTLC1 | Serine palmitoyltransferase, long-chain base subunit 1 |
| SPTLC2 | Serine palmitoyltransferase, long-chain base subunit 2 |
| RAB7A | Ras-Related Protein Rab-7A |
| ATL1 | Atlastin GTPase 1 |
| DNMT1 | DNA methyltransferase 1 |
| NTRK1 | Neurotrophic tyrosine kinase receptor type 1 |
| NGFB | Nerve growth factor beta |
| DST | Dystonin |
| SCN11A | Sodium voltage-gated channel alpha subunit 11 |
| SCN9A | Sodium voltage-gated channel alpha subunit 9 |
| KIF1A | Kinesin Family Member 1A |
| RETREG1 (FAM134B) | Reticulophagy regulator 1 (Family with Sequence Similarity 134, Member B) |
| MFN2 | Mitofusin 2 |
| NEFL | Neurofilament protein, light polypeptide |
| GDAP1 | Ganglioside-induced differentiation-associated protein 1 |
| AD | Autosomal dominant |
| AR | Autosomal recessive |
| CMT2 | Charcot–Marie–Tooth neuropathy type 2 |
| EMG | Electromyography |
| NCS | Nerve conduction studies |
| SNAPs | Sensory nerve action potentials |
| CMAP | Compound muscle action potential |
| DRG | Dorsal root ganglia |
| SNHL | Sensorineural hearing loss |
| TRPV4 | Transient receptor potential cation channel subfamily V member 4 |
| OH | Orthostatic hypotension |
| GERD | Gastroesophageal reflux disease |
| TF | Thermoregulatory failure |
References
- Rotthier, A.; Baets, J.; Timmerman, V.; Janssens, K. Mechanisms of disease in hereditary sensory and autonomic neuropathies. Nat. Rev. Neurol. 2012, 8, 73–85. [Google Scholar] [CrossRef]
- Auer-Grumbach, M. Hereditary sensory and autonomic neuropathies. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2013; pp. 893–906. [Google Scholar] [CrossRef]
- Dyck, P. Neuronal atrophy and degeneration predominantly affecting peripheral sensory and autonomic neurons. In Peripheral Neuropathy; Dyck, P.J., Griffin, P.K., Low, P.A., Poduslo, J.F., Eds.; WB Saunders: Philadelphia, PA, USA, 1993; pp. 1065–1093. [Google Scholar]
- Schwartzlow, C.; Kazamel, M. Hereditary Sensory and Autonomic Neuropathies: Adding More to the Classification. Curr. Neurol. Neurosci. Rep. 2019, 19, 52. [Google Scholar] [CrossRef]
- Lee, S.-S.; Lee, S.-H.; Han, S.-H. Terminal changes in hereditary sensory and autonomic neuropathy: A long-term follow-up of a sporadic case. Clin. Neurol. Neurosurg. 2003, 105, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Houlden, H.; King, R.; Blake, J.; Groves, M.; Love, S.; Woodward, C.; Hammans, S.; Nicoll, J.; Lennox, G.; O’Donovan, D.G.; et al. Clinical, pathological and genetic characterization of hereditary sensory and autonomic neuropathy type 1 (HSAN I). Brain 2006, 129, 411–425. [Google Scholar] [CrossRef]
- Houlden, H.; Blake, J.; Reilly, M.M. Hereditary sensory neuropathies. Curr. Opin. Neurol. 2004, 17, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, K.; Timmerman, V.; Mauko, B.; Pieber, T.R.; De Jonghe, P.; Auer-Grumbach, M. Recent advances in hereditary sensory and autonomic neuropathies. Curr. Opin. Neurol. 2006, 19, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.-H.; Hashiguchi, A.; Yoshimura, A.; Sakai, N.; Takahashi, M.P.; Ueda, T.; Taniguchi, A.; Okamoto, S.; Kanazawa, N.; Yamamoto, Y.; et al. WNK1/HSN2 founder mutation in patients with hereditary sensory and autonomic neuropathy: A Japanese cohort study. Clin. Genet. 2017, 92, 659–663. [Google Scholar] [CrossRef]
- Jin, J.-Y.; Wu, P.-F.; He, J.-Q.; Fam, L.-L.; Yuan, Z.-Z.; Pang, X.-Y.; Tang, J.-Y.; Zhang, L.-Y. Novel Compound Heterozygous DST Variants Causing Hereditary Sensory and Autonomic Neuropathies VI in Twins of a Chinese Family. Front. Genet. 2020, 11, 492. [Google Scholar] [CrossRef]
- Reddi, R.; Horvath, G.A. Living without pain. Case series of patients with hereditary sensory and autonomic neuropathies in a Canadian tertiary care centre. Paediatr. Child. Health 2023, 28, 97–101. [Google Scholar] [CrossRef]
- Oz-Levi, D.; Ben-Zeev, B.; Ruzzo, E.K.; Hitomi, Y.; Gelman, A.; Pelak, K.; Anikster, Y.; Reznik-Wolf, H.; Bar-Joseph, I.; Olender, T.; et al. Mutation in TECPR2 Reveals a Role for Autophagy in Hereditary Spastic Paraparesis. Am. J. Hum. Genet. 2012, 91, 1065–1072. [Google Scholar] [CrossRef]
- Moeinafshar, A.; Tehrani Fateh, S.; Hashemi-Gorji, F.; Karimzadeh, P.; Gholibeglou, E.; Rostami, M.; Sadeghi, H.; Miryounesi, M.; Ghasemi, M.-R. Novel TECPR2 variant in two cases of hereditary sensory and autonomic neuropathy type 9: Insights from genetic characterization and comprehensive literature review. BMC Neurol. 2024, 24, 455. [Google Scholar] [CrossRef]
- Axelrod, F.B. Familial dysautonomia. Muscle Nerve 2004, 29, 352–363. [Google Scholar] [CrossRef]
- Axelrod, F.B. Hereditary sensory and autonomic neuropathies: Familial dysautonomia and other HSANs. Clin. Aut. Res. 2002, 12, I2–I14. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, F.B.; Gold-von Simson, G. Hereditary sensory and autonomic neuropathies: Types II, III, and IV. Orphanet J. Rare Dis. 2007, 2, 39. [Google Scholar] [CrossRef] [PubMed]
- Houlden, H.; King, R.H.M.; Hashemi-Nejad, A.; Wood, N.W.; Mathias, C.J.; Reilly, M.; Thomas, P.K. A novel TRK A (NTRK1) mutation associated with hereditary sensory and autonomic neuropathy type V. Ann. Neurol. 2001, 49, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Edvardson, S.; Cinnamon, Y.; Jalas, C.; Shaag, A.; Maayan, C.; Axelrod, F.B.; Elpeleg, O. Hereditary sensory autonomic neuropathy caused by a mutation in dystonin. Ann. Neurol. 2012, 71, 569–572. [Google Scholar] [CrossRef]
- Hilz, M. Assessment and evaluation of hereditary sensory and autonomic neuropathies with autonomic and neurophysiological examinations. Clin. Aut. Res. 2002, 12, I33–I43. [Google Scholar] [CrossRef]
- Kurth, I. Hereditary Sensory and Autonomic Neuropathy Type II; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; GeneReviews® [Internet]; University of Washington: Seattle, WA, USA, 2010. Available online: https://www.ncbi.nlm.nih.gov/books/NBK49247/ (accessed on 15 October 2025).
- Lafrenière, R.G.; MacDonald, M.L.E.; Dubé, M.-P.; MacFarlane, J.; O’Driscoll, M.; Brais, B.; Meilleur, S.; Brinkman, R.R.; Dadivas, O.; Pape, T.; et al. Identification of a Novel Gene (HSN2) Causing Hereditary Sensory and Autonomic Neuropathy Type II through the Study of Canadian Genetic Isolates. Am. J. Hum. Genet. 2004, 74, 1064–1073. [Google Scholar] [CrossRef]
- Roddier, K.; Thomas, T.; Marleau, G.; Gagnon, A.M.; Dicaire, M.J.; St-Denis, A.; Gosselin, I.; Sarrazin, A.M.; Larbrisseau, A.; Lambert, M.; et al. Two mutations in the HSN2 gene explain the high prevalence of HSAN2 in French Canadians. Neurology 2005, 64, 1762–1767. [Google Scholar] [CrossRef]
- Sapio, M.R.; King, D.M.; Staedtler, E.S.; Maric, D.; Jahanipour, J.; Kurochkina, N.A.; Manalo, A.P.; Ghetti, A.; Mannes, A.J.; Iadarola, M.J. Expression pattern analysis and characterization of the hereditary sensory and autonomic neuropathy 2 A (HSAN2A) gene with no lysine kinase (WNK1) in human dorsal root ganglion. Exp. Neurol. 2023, 370, 114552. [Google Scholar] [CrossRef]
- Klein, C.J.; Duan, X.; Shy, M.E. Inherited neuropathies: Clinical overview and update. Muscle Nerve 2013, 48, 604–622. [Google Scholar] [CrossRef]
- Donadio, V.; Incensi, A.; Vacchiano, V.; Infante, R.; Magnani, M.; Liguori, R. The autonomic innervation of hairy skin in humans: An in vivo confocal study. Sci. Rep. 2019, 9, 16982. [Google Scholar] [CrossRef]
- Donadio, V.; Incensi, A.; Rizzo, G.; Scaglione, C.; Capellari, S.; Fileccia, E.; Avoni, P.; Liguori, R. Spine Topographical Distribution of Skin α-Synuclein Deposits in Idiopathic Parkinson Disease. J. Neuropathol. Exp. Neurol. 2017, 76, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Donadio, V.; Incensi, A.; Giannoccaro, M.P.; Cortelli, P.; Stasi, V.D.; Pizza, F.; Jaber, M.A.; Baruzzi, A.; Liguori, R. Peripheral Autonomic Neuropathy: Diagnostic Contribution of Skin Biopsy. J. Neuropathol. Exp. Neurol. 2012, 71, 1000–1008. [Google Scholar] [CrossRef]
- Provitera, V.; Gibbons, C.H.; Wendelschafer-Crabb, G.; Donadio, V.; Vitale, D.V.; Loavenbruck, A.; Stancanelli, A.; Caporaso, G.; Liguori, R.; Wang, N.; et al. The role of skin biopsy in differentiating small-fiber neuropathy from ganglionopathy. Eur. J. Neurol. 2018, 25, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Davidson, G.L.; Murphy, S.M.; Polke, J.M.; Laura, M.; Salih, M.A.M.; Muntoni, F.; Blake, J.; Brandner, S.; Davies, N.; Horvath, R.; et al. Frequency of mutations in the genes associated with hereditary sensory and autonomic neuropathy in a UK cohort. J. Neurol. 2012, 259, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Rivière, J.B.; Verlaan, D.J.; Shekarabi, M.; Lafrenière, R.G.; Bénard, M.; Kaloustian, V.M.D.; Shbaklo, Z.; Rouleau, G.A. A mutation in the HSN2 gene causes sensory neuropathy type II in a Lebanese family. Ann. Neurol. 2004, 56, 572–575. [Google Scholar] [CrossRef]
- Wang, J.J.; Yu, B.; Li, Z. The coexistence of a novel WNK1 variant and a copy number variation causes hereditary sensory and autonomic neuropathy type IIA. BMC Med. Genet. 2019, 20, 91. [Google Scholar] [CrossRef]
- Yamada, K.; Yuan, J.; Mano, T.; Takashima, H.; Shibata, M. Arthropathy-related pain in a patient with congenital impairment of pain sensation due to hereditary sensory and autonomic neuropathy type II with a rare mutation in the WNK1/HSN2 gene: A case report. BMC Neurol. 2016, 16, 201. [Google Scholar] [CrossRef]
- Shima, T.; Yamamoto, Y.; Kanazawa, N.; Murata, K.-Y.; Ito, H.; Kondo, T.; Yuan, J.; Hashiguchi, A.; Takashima, H. Repeated hyperhidrosis and chilblain-like swelling with ulceration of the fingers and toes in hereditary sensory and autonomic neuropathy type II. J. Dermatol. 2018, 45, e308–e309. [Google Scholar] [CrossRef]
- Pacheco-Cuellar, G.; González-Huerta, L.M.; Valdés-Miranda, J.M.; Peláez-González, H.; Zenteno-Bacheron, S.; Cazarin-Barrientos, J.; Cuevas-Covarrubias, S.A. Hereditary sensory and autonomic neuropathy II due to novel mutation in the HSN2 gene in Mexican families. J. Neurol. 2011, 258, 1890–1892. [Google Scholar] [CrossRef]
- Rahmani, B.; Fekrmandi, F.; Ahadi, K.; Ahadi, T.; Alavi, A.; Ahmadiani, A.; Asadi, S. A novel nonsense mutation in WNK1/HSN2 associated with sensory neuropathy and limb destruction in four siblings of a large Iranian pedigree. BMC Neurol. 2018, 18, 195. [Google Scholar] [CrossRef]
- Pastore, S.; Harripaul, R.; Azam, M.; Vincent, J.B. A novel biallelic single base insertion in WNK1 in a Pakistani family with congenital insensitivity to pain. J. Hum. Genet. 2020, 65, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Coen, K.; Pareyson, D.; Auer-Grumbach, M.; Buyse, G.; Goemans, N.; Claeys, K.G.; Verpoorten, N.; Laurà, M.; Scaioli, V.; Salmhofer, W.; et al. Novel mutations in the HSN2 gene causing hereditary sensory and autonomic neuropathy type II. Neurology 2006, 66, 748–751. [Google Scholar] [CrossRef]
- Potulska-Chromik, A.; Kabzińska, D.; Lipowska, M.; Kostera-Pruszczyk, A.; Kochański, A. A novel homozygous mutation in the WNK1/HSN2 gene causing hereditary sensory neuropathy type 2. Acta Biochim. Pol. 2012, 59, 413–415. [Google Scholar] [CrossRef] [PubMed]
- de Filette, J.; Hasaerts, D.; Seneca, S.; Gheldof, A.; Stouffs, K.; Keymolen, K.; Velkeniers, B. Polyneuropathy in a young Belgian patient: A novel heterozygous mutation in the WNK1/HSN2 gene. Neurol. Genet. 2016, 2, e42. [Google Scholar] [CrossRef]
- Ma, S.; Ji, C.; Li, J.; Zhou, J.; Zhu, J.; Yang, P. A novel mutation in the WNK1/HSN2 gene causing hereditary sensory and autonomic neuropathy type 2 in Chinese patient. J. Hum. Genet. 2025, 70, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Naghinejad, M.; Ebrahimi, A.; Shekari Khaniani, M.; Mansoori Derakhshan, S. A Novel Pathogenic Mutation in WNK1 Gene Causing HSAN Type II in Three Siblings. J. Mol. Neurosci. 2024, 74, 99. [Google Scholar] [CrossRef]
- Shekarabi, M.; Girard, N.; Rivière, J.-B.; Dion, P.; Houle, M.; Toulouse, A.; Lafrenière, R.G.; Vercauteren, F.; Hince, P.; Laganiere, J.; et al. Mutations in the nervous system–specific HSN2 exon of WNK1 cause hereditary sensory neuropathy type II. J. Clin. Investig. 2008, 118, 2496–2505. [Google Scholar] [CrossRef]
- Cho, H.-J.; Kim, B.J.; Suh, Y.-L.; An, J.-Y.; Ki, C.-S. Novel mutation in the HSN2 gene in a Korean patient with hereditary sensory and autonomic neuropathy type 2. J. Hum. Genet. 2006, 51, 905–908. [Google Scholar] [CrossRef]
- Takagi, M.; Ozawa, T.; Hara, K.; Naruse, S.; Ishihara, T.; Shimbo, J.; Igarashi, S.; Tanaka, K.; Onodera, O.; Nishizawa, M. New HSN2 mutation in Japanese patient with hereditary sensory and autonomic neuropathy type 2. Neurology 2006, 66, 1251–1252. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Li, H.; Luo, X.; Li, H.; Gao, Q.; Zhang, L.; Teng, Y.; Zhao, Q.; Zuo, Z.; Ren, J. IBS 2.0: An upgraded illustrator for the visualization of biological sequences. Nucleic Acids Res. 2022, 50, W420–W426. [Google Scholar] [CrossRef]
- Van den Bergh, P.Y.K.; van Doorn, P.A.; Hadden, R.D.M.; Avau, B.; Vankrunkelsven, P.; Allen, J.A.; Attarian, S.; Blomkwist-Markens, P.-H.; Cornblath, D.R.; Eftimov, F.; et al. European Academy of Neurology/Peripheral Nerve Society guideline on diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy: Report of a joint Task Force—Second revision. Eur. J. Neurol. 2021, 28, 3556–3583. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; English, J.M.; Wilsbacher, J.L.; Stippec, S.; Goldsmith, E.J.; Cobb, M.H. WNK1, a Novel Mammalian Serine/Threonine Protein Kinase Lacking the Catalytic Lysine in Subdomain II. J. Biol. Chem. 2000, 275, 16795–16801. [Google Scholar] [CrossRef] [PubMed]
- Nolano, M.; Crisci, C.; Santoro, L.; Barbieri, F.; Casale, R.; Kennedy, W.R.; Wendelschafer-Crabb, G.; Provitera, V.; Lorenzo, L.D.; Caruso, G. Absent innervation of skin and sweat glands in congenital insensitivity to pain with anhidrosis. Clin. Neurophysiol. 2000, 111, 1596–1601. [Google Scholar] [CrossRef]
- Verzé, L.; Viglietti–Panzica, C.; Plumari, L.; Calcagni, M.; Stella, M.; Schrama, L.H.; Panzica, G.C. Cutaneous innervation in hereditary sensory and autonomic neuropathy type IV. Neurology 2000, 55, 126–128. [Google Scholar] [CrossRef]
- Sztriha, L.; Lestringant, G.G.; Hertecant, J.; Frossard, P.M.; Masouyé, I. Congenital insensitivity to pain with anhidrosis. Pediatr. Neurol. 2001, 25, 63–66. [Google Scholar] [CrossRef]
- Ismail, E.A.R.; Al-Shammari, N.; Anim, J.T.; Moosa, A. Congenital Insensitivity to Pain with Anhidrosis: Lack of Eccrine Sweat Gland Innervation Confirmed. J. Child. Neurol. 1998, 13, 243–246. [Google Scholar] [CrossRef]
- Myers, M.I.; Peltier, A.C. Uses of Skin Biopsy for Sensory and Autonomic Nerve Assessment. Curr. Neurol. Neurosci. Rep. 2013, 13, 323. [Google Scholar] [CrossRef]
- Furia, A.; Liguori, R.; Donadio, V. Small-Fiber Neuropathy: An Etiology-Oriented Review. Brain Sci. 2025, 15, 158. [Google Scholar] [CrossRef]
- Provitera, V.; Gibbons, C.H.; Wendelschafer-Crabb, G.; Donadio, V.; Vitale, D.F.; Stancanelli, A.; Caporaso, G.; Liguori, R.; Wang, N.; Santoro, L.; et al. A multi-center, multinational age- and gender-adjusted normative dataset for immunofluorescent intraepidermal nerve fiber density at the distal leg. Eur. J. Neurol. 2016, 23, 333–338. [Google Scholar] [CrossRef] [PubMed]

| Population [Reference] Family/Patient | WNK1/HSN2 Variant (NM_213655.5) [Type of Mutation] | Onset Age (Years) | First Symptom/Clinical Presentation | Autonomic Involvement | NCS |
|---|---|---|---|---|---|
| China Ma S. et al., 2025 [40] A single case | Homozygous c.2689G>T; p.(Glu897*) [Nonsense] | 12 | Bilateral toe ulceration and infections | None | Severe sensory nerve damage |
| Turkey Naghinejad M. et al., 2024 [41] 3 offspring of Azari Turkish descent | Homozygous c.3226C>T; p.Arg1076* [Nonsense] | I—Childhood II—U III—8 | I, II, III—Dysphagia, hypoesthesia and recurrent distal wounds, self-mutilating behavior, hyperkeratosis. III—Distal paresthesia | I, II—Constipation III—None | I—Generalized axonal sensory neuropathy II, III—Mild nerve and muscle involvement |
| Pakistan Pastore et al., 2020 [36] 2 offspring of Punjabi Pakistan descent | Homozygous c.3463dup; p.Thr1155Asnfs*11 [Frameshift] | I—Puberal age II—U | I—Deformities II—U, milder phenotype | None | U |
| China Wang et al., 2019 [31] 2 siblings in a Han family | Heterozygous c.3002T>G; p.Leu1001* [Nonsense] c.3352del; p.Ser1118Leufs*3 [Frameshift] | Infancy | Analgesia, ulcers and neurogenic osteolysis, burning acroparesthesias. | Sweating disorders | U |
| Iran Rahmani et al., 2018 [35] 4 affected siblings | Homozygous c.3718C>A; p.Gln1240Lys [Nonsense] | I—6 months II—2 III—7 IV—10 | Distal limb multimodal reduced sensory function with amputations | None | III, IV –Symmetric peripheral sensory axonal neuropathy |
| Japan Shima et al., 2018 [33] A single case | Homozygous c.3492dup; p.Asp1165* [Frameshift] | 17 | Autonomic symptoms with fingers and toes ulceration | Hyperidrosis and extremities chilblain like edema | U |
| Japan Yuan et al., 2017 [9] 33 unrelated patients | Homozygous c.3492dup; p.Asp1165* [Frameshift] Heterozygous c.2870C>G; p.Ser957* in Case II [Nonsense] | I, II, III, V—Infancy IV—17 | I, II, III, V—Analgesia IV—Hyperhidrosis | I—OH, TF, defecation disorder II—Dyshidrosis, urination disorder III, IV, V—Dyshidrosis | SNAPs could not be evoked, markedly reduced in Case IV |
| Japan Yamada et al., 2016 [32] A single case | Homozygous c.3492dup; p.Asp1165* [Frameshift] | Infancy | Multimodal sensory loss and taste disorder | OH, fluctuation in body temperature, and absence of defecatory urge | Absent SNAPs of the median, ulnar, and sural nerves. |
| Belgium De Filette et al., 2016 [39] A single case | Compound heterozygous c.3550_3554del; p.Phe1184Leufs*39 [Nonsense] c.3076A>T | p.Lys1026* [Frameshift] | 3 | Ecchymoses of the toes | OH, GERD, hand hyperhidrosis with cold-triggered purple discoloration | Absent SNAPs in upper and lower limbs |
| East Europe, Poland Potulska-Chromik A. et al., 2012 [38] A single case | Homozygous c.2898_2899del; p.Gln966Hisfs*3 [Frameshift] | 1 | Dysphagia and loss of nociception | None | Absent SNAPs |
| Malta Davidson et al., 2012 [29] 2 unrelated cases | Homozygous c.3526_3529del; p.Thr1176Cysfs*21 # [Frameshift] Compound heterozygous c.2418_2419del; p.Cys806Trpfs*18 [Frameshift] | Congenital | Ulcers, distal amputations | None | Sensory motor axonal neuropathy |
| Chiapas, Southeast of Mexico Pacheco-Cuellar G. et al., 2011 [34] 4 patients belonging to 2 families | Homozygous c.3577_3584del; p.Ser1193Glyfs*29 [Frameshift] | I—19 II—20 III—10 IV—9 | Sensory loss, osteolysis and Charcot joints, amputations | None | I and IV—U II and III—absent SNAPs |
| France Shekarabi M. et al., 2008 [42] A single case | Compound heterozygous c.2998del; p.Arg1000Aspfs*2 [Frameshift] c.1591_1592del; p.Asp531Cysfs*17 [Frameshift] | U | U | Hand hyperhidrosis | U |
| Korea Cho H.J. et al., 2006 [43] A single case | Compound heterozygous c.3492dup; p.Asp1165* c.2575C>T; p.Gln859* [Nonsense] | 11 | Multimodal limb sensory loss | Dry hands | Distal sensory dominant poly neuropathy |
| Japan Takagi M. et al., 2006 [44] A single case | Homozygous c.3492dup; p.Asp1165* [Frameshift] | Teenage years. | Pain insensitivity | None | Absence of SNAPs in the median and sural nerves of both sides |
| Europe (Italy, Austria, and Belgium) Coen K. et al., 2006 [37] 3 unrelated families (CMT-451, CMT-260, and CMT-178) | Family CMT-451 Patient II-2: compound heterozygous c.2612del; p.Pro871Hisfs*14 [Frameshift] c.3447dup; p.Gln1150Serfs*16 [Frameshift]. Family CMT-260 Patient II-6: Homozygous c.2908C>T; p.Gln970* [Nonsense]. Family CMT-178 Patient III-1: Homozygous c.3422_3423del; p.Ile1141Asnfs*7 [Frameshift] | II-2—6 months II-6—early childhood III-1—2 | II-2: Difficulties in hand manipulation II-6: Clumsiness of the hands, recurrent osteomyelitis. III-1: Poor wound healing and recurrent hand and foot ulcers | None | III-1: Sensory neuropathy with absent SNAPs in all limbs |
| Quebec, Newfoundland and Nova Scotia Lafreniere R.G. et al., 2004 [21] Five families from the two population clusters in Canada: Newfoundland F1 (8 A), F2 (2 A); French Canada F3 (2 A), F4 (1 A); Nova Scotia F5 (2 A) | Patient F1-70 from Newfoundland: Homozygous c.2952del; p.Glu984Aspfs*10 [Frameshift]; Patient F5-301 from Nova Scotia: Homozygous c.3276dup; p.Ser1093Ilefs*13 [Frameshift]. | Early childhood | Reduced nociception and cold-induced numbness in hands and feet | None | U |
| French Canadian from Southern Quebec (Lanaudière region) Roddier et al., 2005 [22] 18 patients belonging to 13 families and one Canadian patients of Lebanese origin | Mutation 1: c.3301C>T; p.Gln1101* [Nonsense] Mutation 2: c.3276dup; p.Ser1093Ilefs*13 [Frameshift] 56% Homozygous c.3301C>T 6% Homozygous c.3276dup 38% Compound heterozygotes The Canadian child of Lebanese origin resulted homozygote for a novel mutation: c.3226C>T | p.Arg1076* [Nonsense]. | Infancy or early childhood | Paronychia, ulcers and Charcot joints with multimodal sensory loss | Minimal dysautonomia (U) | Absence of SNAPs |
| Lebanon Rivière J.B. et al., 2004 [30] A family with 4 affected individuals | Homozygous c.3305del; p.Pro1102Leufs*2 [Frameshift] | First decade | Loss of sensation and insensitivity to pain causing ulcers and amputations | U | U |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ragucci, C.; Furia, A.; Palombo, F.; Giannoccaro, M.P.; Vacchiano, V.; Incensi, A.; Di Stasi, V.; Rizzo, G.; Liguori, R.; Donadio, V.A. Hereditary Sensory and Autonomic Neuropathy Type 2: A Case Report and a Review of the Literature. Brain Sci. 2025, 15, 1163. https://doi.org/10.3390/brainsci15111163
Ragucci C, Furia A, Palombo F, Giannoccaro MP, Vacchiano V, Incensi A, Di Stasi V, Rizzo G, Liguori R, Donadio VA. Hereditary Sensory and Autonomic Neuropathy Type 2: A Case Report and a Review of the Literature. Brain Sciences. 2025; 15(11):1163. https://doi.org/10.3390/brainsci15111163
Chicago/Turabian StyleRagucci, Cosmanna, Alessandro Furia, Flavia Palombo, Maria Pia Giannoccaro, Veria Vacchiano, Alex Incensi, Vitantonio Di Stasi, Giovanni Rizzo, Rocco Liguori, and Vincenzo Angelo Donadio. 2025. "Hereditary Sensory and Autonomic Neuropathy Type 2: A Case Report and a Review of the Literature" Brain Sciences 15, no. 11: 1163. https://doi.org/10.3390/brainsci15111163
APA StyleRagucci, C., Furia, A., Palombo, F., Giannoccaro, M. P., Vacchiano, V., Incensi, A., Di Stasi, V., Rizzo, G., Liguori, R., & Donadio, V. A. (2025). Hereditary Sensory and Autonomic Neuropathy Type 2: A Case Report and a Review of the Literature. Brain Sciences, 15(11), 1163. https://doi.org/10.3390/brainsci15111163







