Speech Markers of Parkinson’s Disease: Phonological Features and Acoustic Measures
Abstract
1. Introduction
2. Approaches to PD Speech Analyses
2.1. Overview of Analytical Approaches
2.2. Phonet
3. This Current Study
3.1. Research Questions and Hypotheses
3.2. Materials and Methods
3.2.1. Data
3.2.2. Measures
3.2.3. Analysis
4. Results
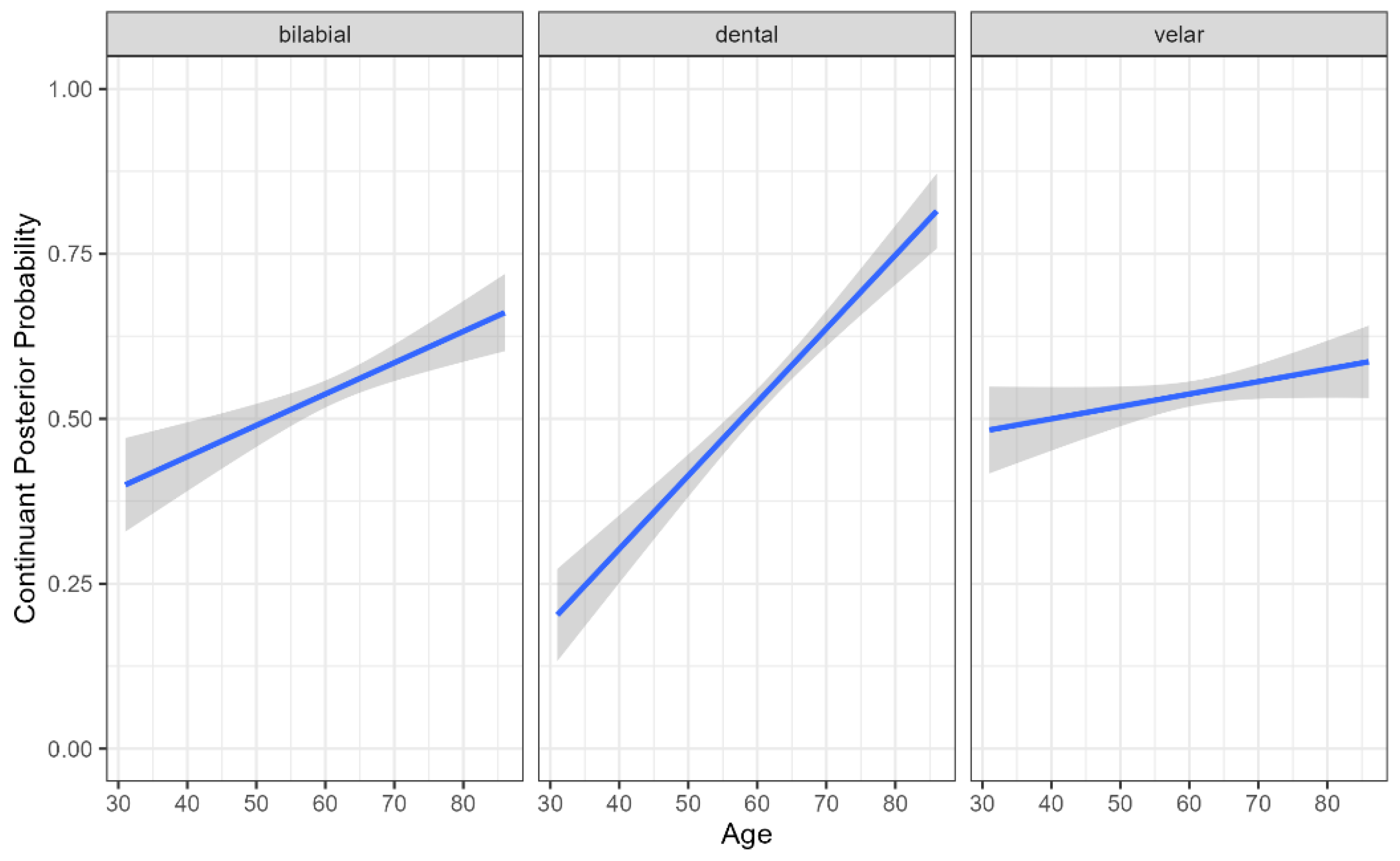
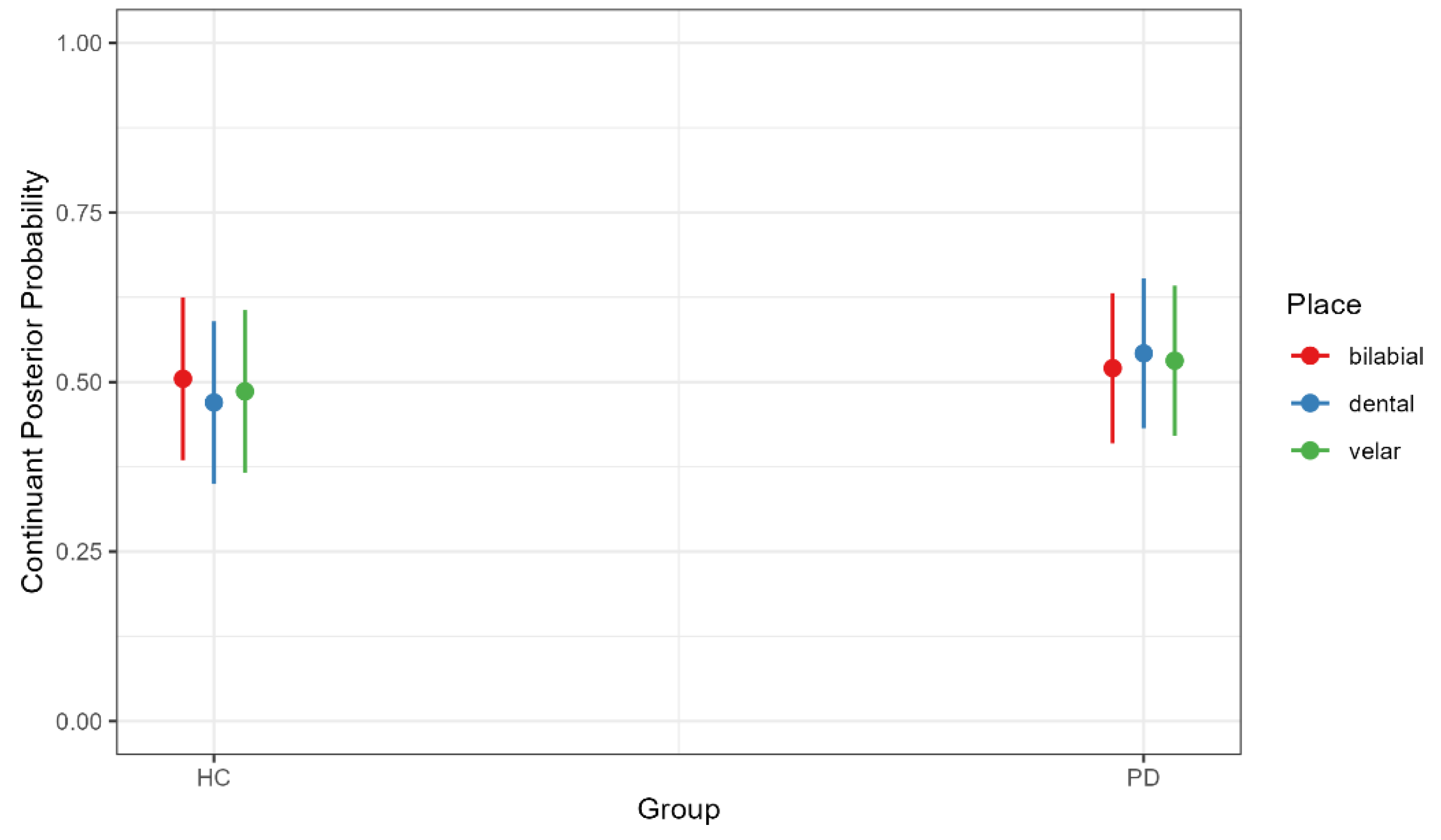
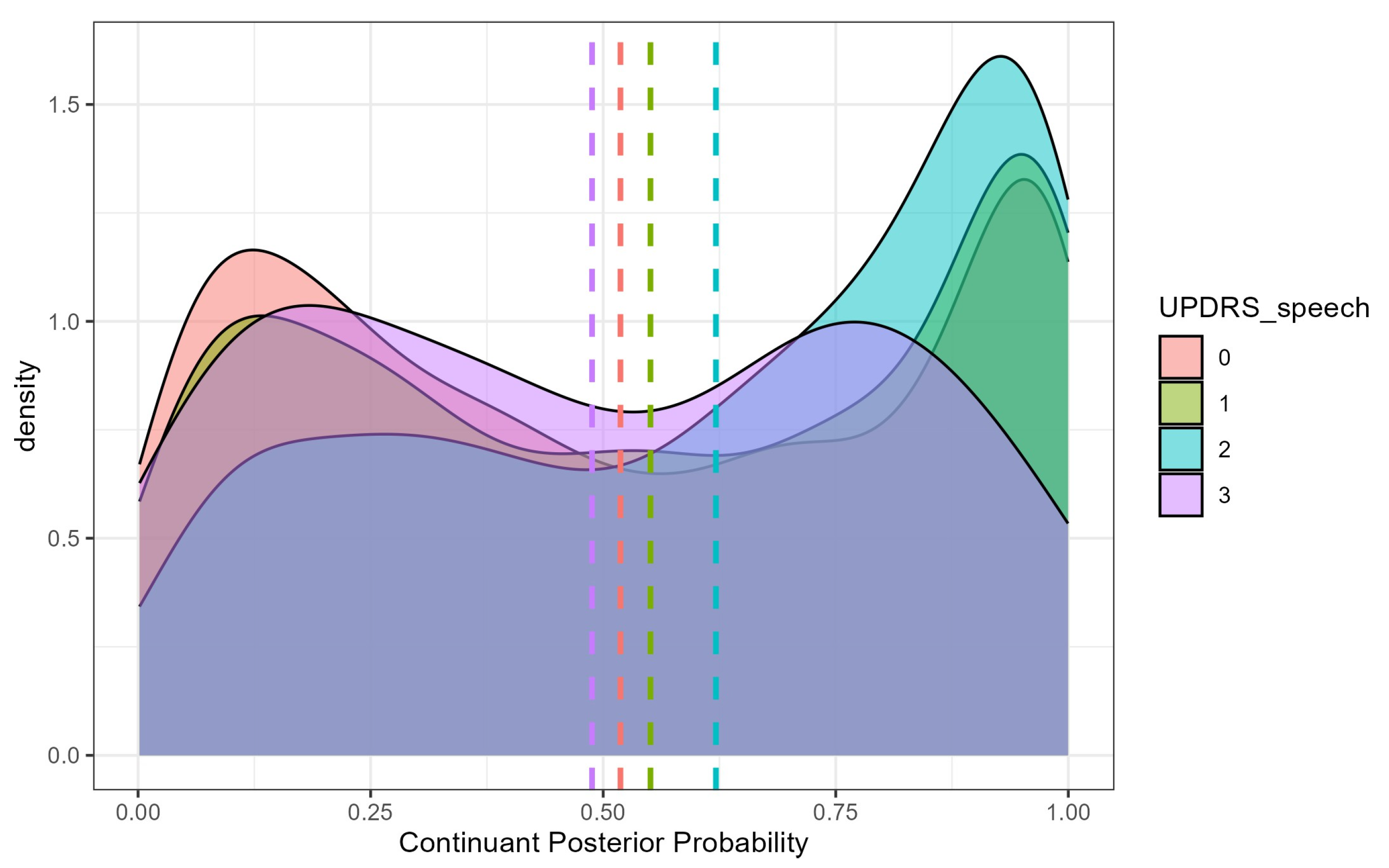
4.1. Continuant Posterior Probability
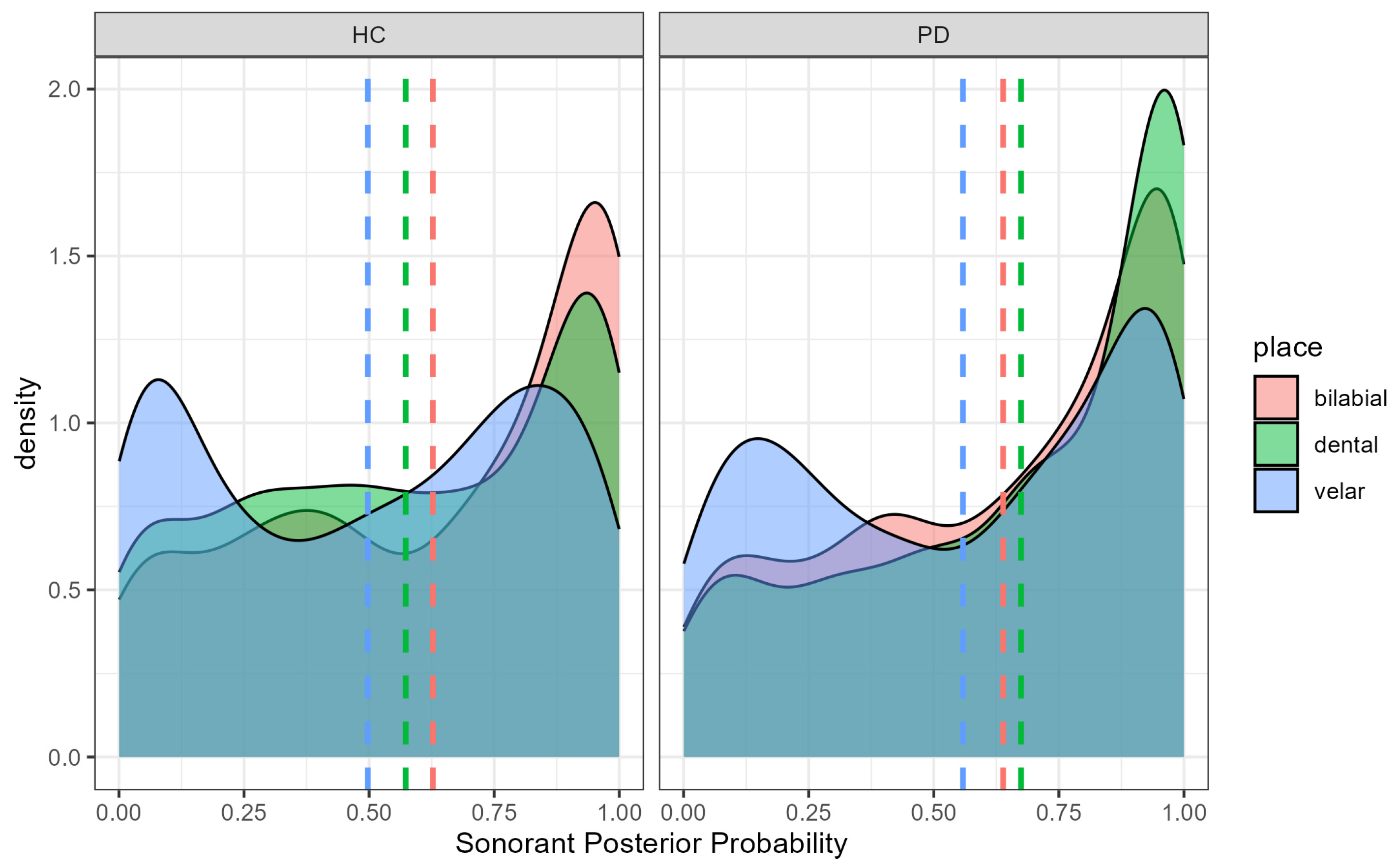
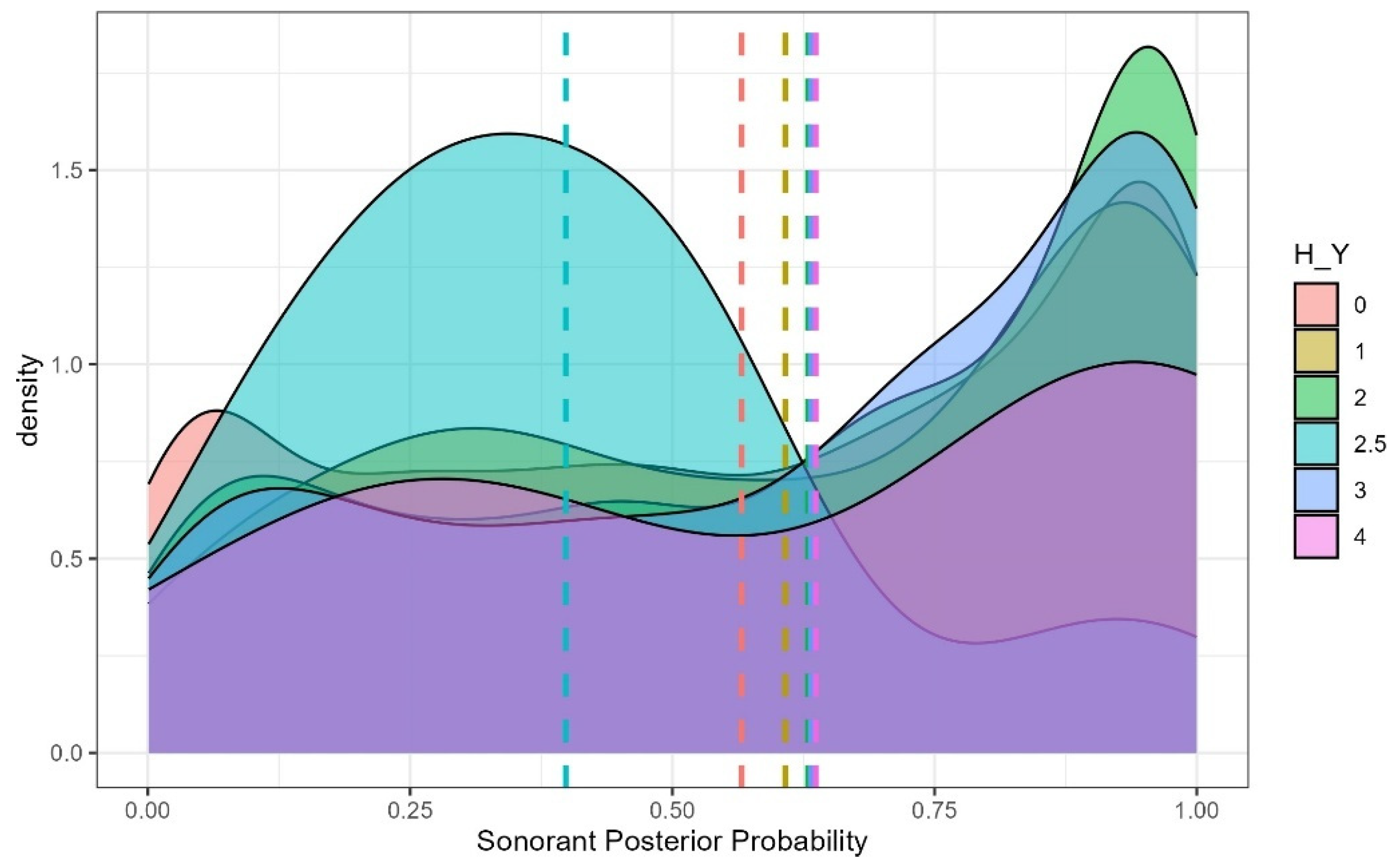
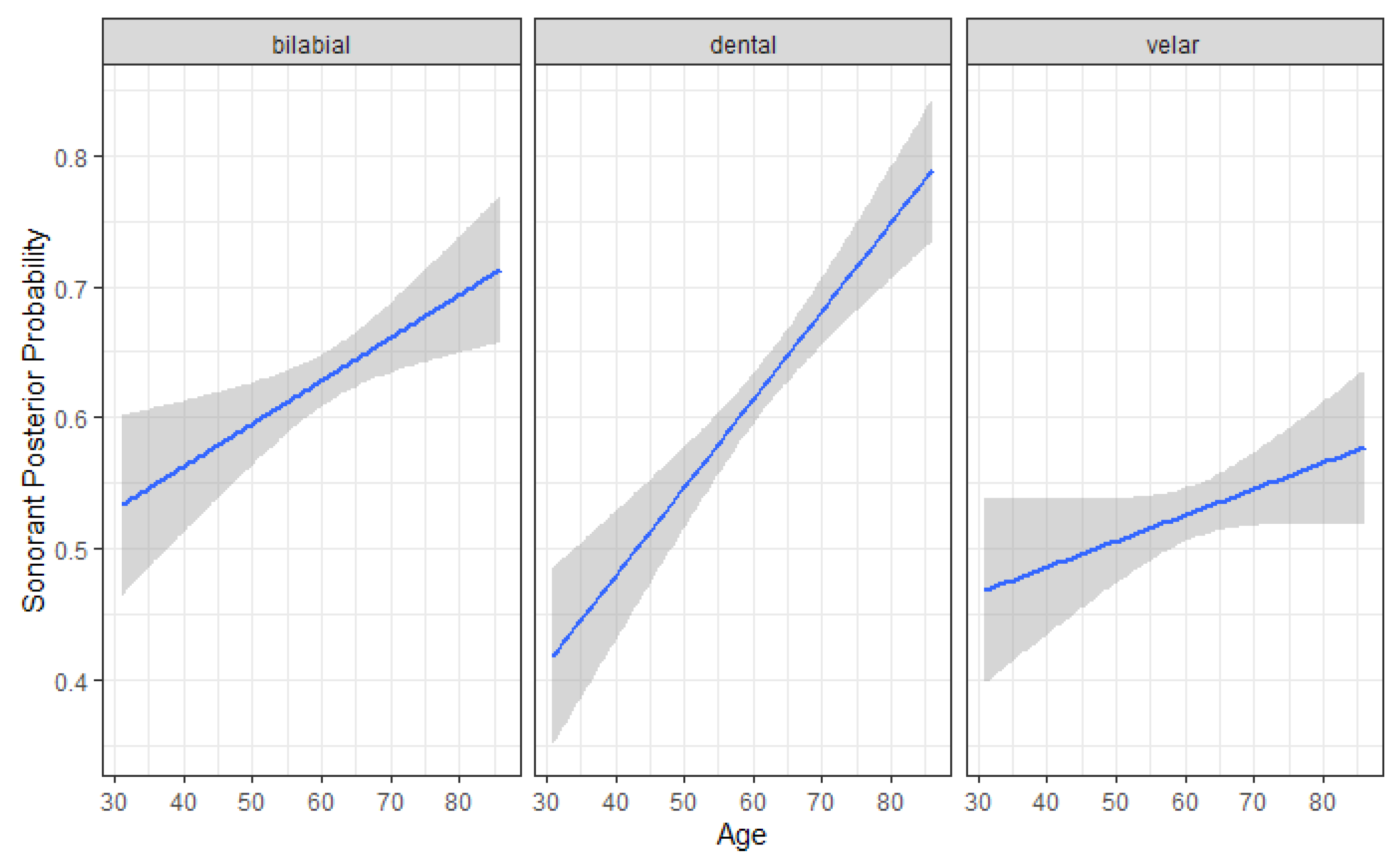
4.2. Sonorant Posterior Probability
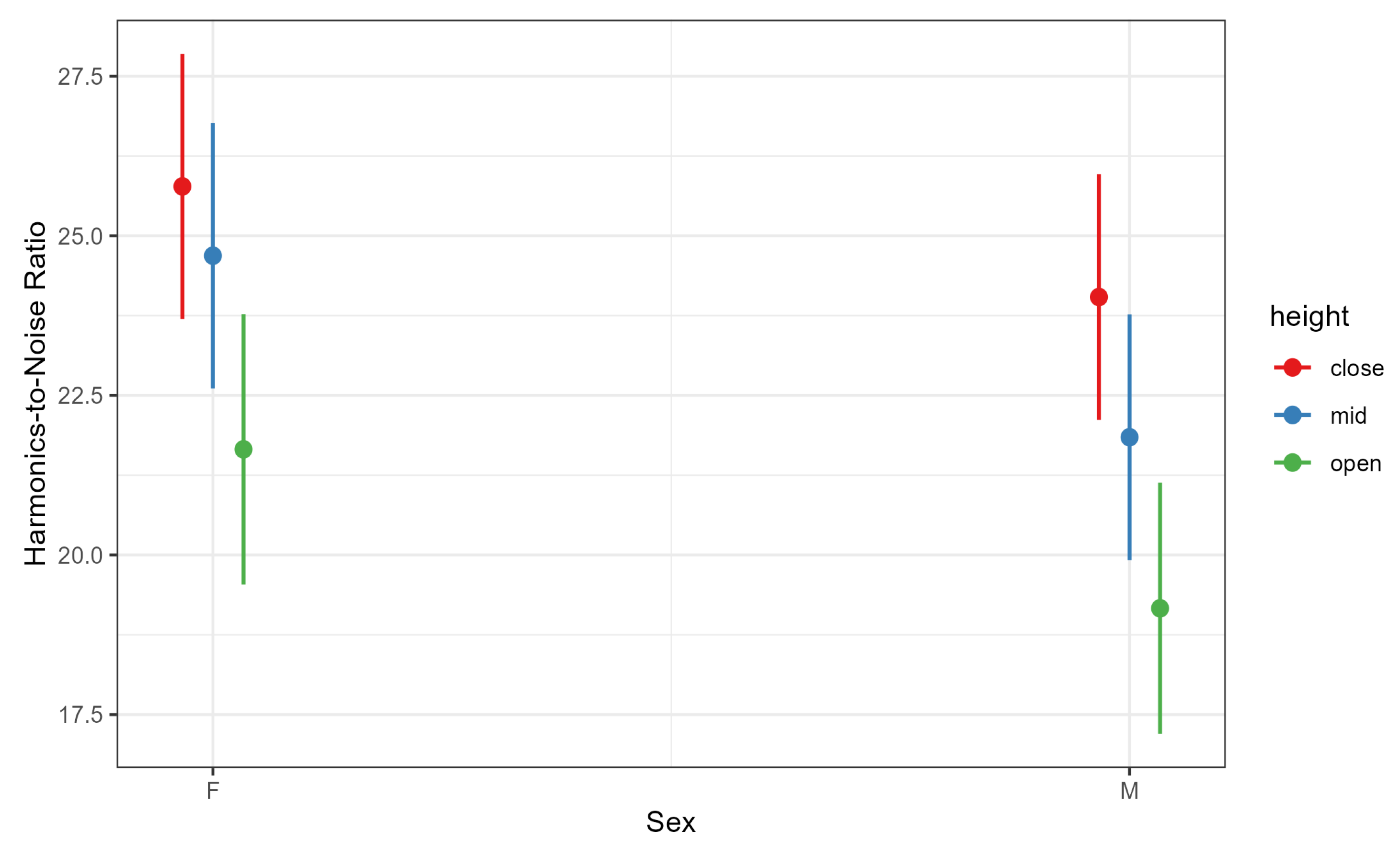
4.3. Harmonics-to-Noise Ratio
5. Discussion
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, F.; Vogel, A.P.; Gharahkhani, P.; Renteria, M.E. Speech and language biomarkers for Parkinson’s disease prediction, early diagnosis and progression. npj Park. Dis. 2025, 11, 57. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Elbaz, A.; Nichols, E.; Abbasi, N.; Abd-Allah, F.; Abdelalim, A.; Adsuar, J.C.; Ansha, M.G.; Brayne, C.; Choi, J.Y.J.; et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef]
- Moya-Galé, G.; Levy, E.S. Parkinson’s disease-associated dysarthria: Prevalence, impact and management strategies. Res. Rev. Park. 2019, 9, 9–16. [Google Scholar] [CrossRef]
- Ackermann, H.; Ziegler, W. Articulatory deficits in parkinsonian dysarthria: An acoustic analysis. J. Neurol. Neurosurg. Psychiatry 1991, 54, 1093–1098. [Google Scholar] [CrossRef]
- Weismer, G. Philosophy of research in motor speech disorders. Clin. Linguist. Phon. 2006, 20, 315–349. [Google Scholar] [CrossRef]
- Yunusova, Y.; Weismer, G.; Westbury, J.R.; Lindstrom, M.J. Articulatory movements during vowels in speakers with dysarthria and healthy controls. J. Speech Lang. Hear. Res. 2008, 51, 596–611. [Google Scholar] [CrossRef]
- Walsh, B.; Smith, A. Basic parameters of articulatory movements and acoustics in individuals with Parkinson’s disease. Mov. Disord. 2012, 27, 843–850. [Google Scholar] [CrossRef]
- Recasens, D.; Espinosa, A. An articulatory investigation of lingual coarticulatory resistance and aggressiveness for consonants and vowels in Catalan. J. Acoust. Soc. Am. 2009, 125, 2288–2298. [Google Scholar] [CrossRef] [PubMed]
- Recasens, D.; Rodríguez, C. A study on coarticulatory resistance and aggressiveness for front lingual consonants and vowels using ultrasound. J. Phon. 2016, 59, 58–75. [Google Scholar] [CrossRef]
- Chen, Y.W.; Watson, P.J. Speech production and sensory impairment in mild Parkinson’s disease. J. Acoust. Soc. Am. 2017, 141, 3030–3041. [Google Scholar] [CrossRef] [PubMed]
- Galaz, Z.; Mekyska, J.; Zvoncak, V.; Mucha, J.; Kiska, T.; Smekal, Z.; Eliasova, I.; Mrackova, M.; Kostalova, M.; Rektorova, I.; et al. Changes in phonation and their relations with progress of Parkinson’s disease. Appl. Sci. 2018, 8, 2339. [Google Scholar] [CrossRef]
- Whitfield, J.A.; Reif, A.; Goberman, A.M. Voicing contrast of stop consonant production in the speech of individuals with Parkinson disease ON and OFF dopaminergic medication. Clin. Linguist. Phon. 2018, 32, 587–594. [Google Scholar] [CrossRef]
- Forrest, K.; Weismer, G.; Turner, G.S. Kinematic, acoustic, and perceptual analyses of connected speech produced by Parkinsonian and normal geriatric adults. J. Acoust. Soc. Am. 1989, 85, 2608–2622. [Google Scholar] [CrossRef]
- Logemann, J.A.; Fisher, H.K.; Boshes, F.R.; Blonsky, E. Frequency and co-occurrence of vocal tract dysfunctions in the speech of a large sample of Parkinson patients. J. Speech Hear. Disord. 1978, 43, 47–57. [Google Scholar] [CrossRef]
- Condor, N.P.; Ludlow, C.L.; Schulz, G.M. Stop consonant production in isolated and repeated syllables in Parkinson’s disease. Neuropsychologia 1989, 27, 829–838. [Google Scholar] [CrossRef]
- Wayland, R.; Tang, K.; Wang, F.; Vellozzi, S.; Meyer, R.; Sengupta, R. Neural network-based measure of consonant lenition in Parkinson’s Disease. Proc. Meet. Acoust. 2023, 52, 060003. [Google Scholar]
- Skodda, S.; Grönheit, J.; Schlegel, U. Impairment of vowel articulation as a possible marker of disease progression in Parkinson’s disease. PLoS ONE 2012, 7, e32132. [Google Scholar] [CrossRef] [PubMed]
- Rusz, J.; Cmejla, R.; Tykalova, T.; Ruzickova, H.; Klempir, J.; Majerova, V.; Picmausova, J.; Roth, J.; Ruzicka, E. Imprecise vowel articulation as a potential early marker of Parkinson’s disease: Effect of speaking task. J. Acoust. Soc. Am. 2013, 134, 2171–2181. [Google Scholar] [CrossRef]
- Skodda, S.; Visser, W.; Schlegel, U. Vowel articulation in Parkinson’s disease. J. Voice 2011, 25, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Roland, V.; Huet, K.; Harmegnies, B.; Piccaluga, M.; Verhaegen, C.; Delvaux, V. Vowel production: A potential speech biomarker for early detection of dysarthria in Parkinson’s disease. Front. Psychol. 2023, 14, 1129830. [Google Scholar] [CrossRef]
- Tjaden, K. Anticipatory coarticulation in multiple sclerosis and Parkinson’s disease. J. Speech Lang. Hear. Res. 2003, 46, 990–1008. [Google Scholar] [CrossRef]
- Fischer, E.; Goberman, A.M. Voice onset time in Parkinson disease. J. Commun. Disord. 2010, 43, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Correa, J.; Klumpp, P.; Orozco-Arroyave, J.R.; Nöth, E. Phonet: A Tool Based on Gated Recurrent Neural Networks to Extract Phonological Posteriors from Speech. In Proceedings of the INTERSPEECH 2019, Graz, Austria, 15–19 September 2019; pp. 549–553. [Google Scholar] [CrossRef]
- van Gelderen, L.; Tejedor-García, C. Innovative Speech-Based Deep Learning Approaches for Parkinson’s Disease Classification: A Systematic Review. arXiv 2024, arXiv:2407.17844. [Google Scholar] [CrossRef]
- Naeem, I.; Ditta, A.; Mazhar, T.; Anwar, M.; Saeed, M.M.; Hamam, H. Voice biomarkers as prognostic indicators for Parkinson’s disease using machine learning techniques. Sci. Rep. 2025, 15, 12129. [Google Scholar] [CrossRef]
- Maryn, Y.; Roy, N.; De Bodt, M.; Van Cauwenberge, P.; Corthals, P. Acoustic measurement of overall voice quality: A meta-analysis. J. Acoust. Soc. Am. 2009, 126, 2619–2634. [Google Scholar] [CrossRef]
- Awan, S.N.; Roy, N.; Zhang, D.; Cohen, S.M. Validation of the Cepstral Spectral Index of Dysphonia (CSID) as a screening tool for voice disorders: Development of clinical cutoff scores. J. Voice 2016, 30, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Tsanas, A.; Little, M.; McSharry, P.; Ramig, L. Accurate telemonitoring of Parkinson’s disease progression by non-invasive speech tests. Nat. Preced. 2009, 1–10. [Google Scholar] [CrossRef]
- Chiaramonte, R.; Bonfiglio, M. Acoustic analysis of voice in Parkinson’s disease: A systematic review of voice disability and meta-analysis of studies. Rev. Neurol. 2020, 70, 393–405. [Google Scholar] [CrossRef]
- Suppa, A.; Costantini, G.; Asci, F.; Di Leo, P.; Al-Wardat, M.S.; Di Lazzaro, G.; Scalise, S.; Pisani, A.; Saggio, G. Voice in Parkinson’s disease: A machine learning study. Front. Neurol. 2022, 13, 831428. [Google Scholar] [CrossRef] [PubMed]
- Patman, C.; Foulkes, P.; McDougall, K. Acoustic methods for analysing breathy and whispery voices: A systematic review. Phonetica 2025, 82, 271–299. [Google Scholar] [CrossRef]
- Gerratt, B.R.; Kreiman, J.; Garellek, M. Comparing measures of voice quality from sustained phonation and continuous speech. J. Speech Lang. Hear. Res. 2016, 59, 994–1001. [Google Scholar] [CrossRef]
- Wright, H.; Aharonson, V. Vocal Feature Changes for Monitoring Parkinson’s Disease Progression—A Systematic Review. Brain Sci. 2025, 15, 320. [Google Scholar] [CrossRef]
- Frota, S.; Cruz, M.; Cardoso, R.; Guimarães, I.; Ferreira, J.J.; Pinto, S.; Vigário, M. (Dys)Prosody in Parkinson’s Disease: Effects of Medication and Disease Duration on Intonation and Prosodic Phrasing. Brain Sci. 2021, 11, 1100. [Google Scholar] [CrossRef]
- Hlavnicka, J.; Tykalová, T.; Cmejla, R.; Klempír, J.; Ruzicka, E.; Rusz, J. Dysprosody Differentiate Between Parkinson’s Disease, Progressive Supranuclear Palsy, and Multiple System Atrophy. In Proceedings of the INTERSPEECH 2017, Stockholm, Sweden, 20–24 August 2017; pp. 1844–1848. [Google Scholar]
- Harris, R.; Leenders, K.L.; de Jong, B.M. Speech dysprosody but no music ‘dysprosody’in Parkinson’s disease. Brain Lang. 2016, 163, 1–9. [Google Scholar] [CrossRef]
- Moro-Velazquez, L.; Gomez-Garcia, J.A.; Arias-Londoño, J.D.; Dehak, N.; Godino-Llorente, J.I. Advances in Parkinson’s disease detection and assessment using voice and speech: A review of the articulatory and phonatory aspects. Biomed. Signal Process. Control 2021, 66, 102418. [Google Scholar] [CrossRef]
- Tsanas, A.; Little, M.A.; McSharry, P.E.; Spielman, J.; Ramig, L.O. Novel speech signal processing algorithms for high-accuracy classification of Parkinson’s disease. IEEE Trans. Biomed. Eng. 2012, 59, 1264–1271. [Google Scholar] [CrossRef]
- Orozco-Arroyave, J.R.; Hönig, F.; Arias-Londoño, J.; Vargas-Bonilla, J.; Daqrouq, K.; Skodda, S.; Rusz, J.; Nöth, E. Automatic detection of Parkinson’s disease in running speech spoken in three different languages. J. Acoust. Soc. Am. 2016, 139, 481–500. [Google Scholar] [CrossRef]
- Vásquez-Correa, J.C.; Orozco-Arroyave, J.; Bocklet, T.; Nöth, E. Towards an automatic evaluation of the dysarthria level of patients with Parkinson’s disease. J. Commun. Disord. 2018, 76, 21–36. [Google Scholar] [CrossRef]
- Ngo, Q.C.; Motin, M.A.; Pah, N.D.; Drotár, P.; Kempster, P.; Kumar, D. Computerized analysis of speech and voice for Parkinson’s disease: A systematic review. Comput. Methods Programs Biomed. 2022, 226, 107133. [Google Scholar] [CrossRef]
- Saravanan, S.; Ramkumar, K.; Adalarasu, K.; Sivanandam, V.; Kumar, S.R.; Stalin, S.; Amirthara-jan, R. A systematic review of artificial intelligence (AI) based approaches for the diagnosis of Parkinson’s disease. Arch. Comput. Methods Eng. 2022, 29, 3639–3653. [Google Scholar] [CrossRef]
- Adnan, T.; Abdelkader, A.; Liu, Z.; Hossain, E.; Park, S.; Islam, M.S.; Hoque, E. A novel fusion architecture for detecting Parkinson’s Disease using semi-supervised speech embeddings. npj Park. Dis. 2025, 11, 176. [Google Scholar] [CrossRef]
- Dudek, M.; Hemmerling, D.; Kaczmarska, M.; Stepien, J.; Daniol, M.; Wodzinski, M.; Wojcik-Pedziwiatr, M. Analysis of voice, speech, and language biomarkers of parkinson’s disease collected in a mixed reality setting. Sensors 2025, 25, 2405. [Google Scholar] [CrossRef] [PubMed]
- Gimeno-Gómez, D.; Botelho, C.; Pompili, A.; Abad, A.; Martínez-Hinarejos, C.D. Unveiling Interpretability in Self-Supervised Speech Representations for Parkinson’s Diagnosis. IEEE J. Sel. Top. Signal Process. 2025, 1–14. [Google Scholar] [CrossRef]
- Plantinga, P.; Chen, J.K.; Sattari, R.; Ravanelli, M.; Klein, D. From Black Box to Biomarker: Sparse Autoencoders for Interpreting Speech Models of Parkinson’s Disease. arXiv 2025, arXiv:2507.16836. [Google Scholar]
- Frankford, S.A.; Estrada, A.; Stepp, C.E. Contributions of Speech Timing and Articulatory Precision to Listener Perceptions of Intelligibility and Naturalness in Parkinson’s Disease. J. Speech Lang. Hear. Res. 2024, 67, 2951–2963. [Google Scholar] [CrossRef]
- Cernak, M.; Orozco-Arroyave, J.R.; Rudzicz, F.; Christensen, H.; Vásquez-Correa, J.C.; Nöth, E. Characterisation of voice quality of Parkinson’s disease using differential phonological posterior features. Comput. Speech Lang. 2017, 46, 196–208. [Google Scholar] [CrossRef]
- Hosseini-Kivanani, N.; Vásquez-Correa, J.C.; Schommer, C.; Nöth, E. Exploring the use of phonological features for Parkinson’s disease detection. In Proceedings of the 20th International Congress of the Phonetic Sciences (ICPhS 2023), Prague, Czech Republic, 7–11 August 2023; pp. 3897–3901. [Google Scholar]
- Tang, K.; Wayland, R.; Wang, F.; Vellozzi, S.; Sengupta, R.; Altmann, L. From sonority hierarchy to posterior probability as a measure of lenition: The case of Spanish stops. J. Acoust. Soc. Am. 2023, 153, 1191–1203. [Google Scholar] [CrossRef]
- Tang, K.; Wayland, R.; Wang, F.; Vellozzi, S.; Sengupta, R. Evaluating the consistency of lenition measures: Neural networks’ posterior probability, intensity velocity, and duration. J. Acoust. Soc. Am. 2024, 156, 1367–1379. [Google Scholar] [CrossRef]
- Wayland, R.; Tang, K.; Wang, F.; Vellozzi, S.; Sengupta, R. Quantitative acoustic versus deep learning metrics of lenition. Languages 2023, 8, 98. [Google Scholar] [CrossRef]
- Wayland, R.; Meyer, R.; Reddy, R.; Tang, K.; Hegland, K.W. Quantifying Lenition as a Diagnostic Marker for Parkinson’s Disease and Atypical Parkinsonism. BioMedInformatics 2024, 4, 2287–2305. [Google Scholar] [CrossRef]
- Wayland, R.; Tang, K.; Vellozzi, S.; Wang, F.; Sengupta, R. Measuring gradient effects of alcohol on speech with neural networks’ posterior probability of phonological features. In Proceedings of the 20th International Congress of Phonetic Sciences, Prague, Czech Republic, 7–11 August 2023. [Google Scholar]
- Wayland, R.; Meyer, R.; Vellozzi, S.; Tang, K. Lenition in L2 Spanish: The Impact of Study Abroad on Phonological Acquisition. Brain Sci. 2024, 14, 946. [Google Scholar] [CrossRef]
- McAuliffe, M.J.; Ward, E.C.; Murdoch, B.E.; Farrell, A.M. A nonspeech investigation of tongue function in Parkinson’s disease. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2005, 60, 667–674. [Google Scholar] [CrossRef]
- Teixeira, J.P.; Fernandes, P.O. Acoustic analysis of vocal dysphonia. Procedia Comput. Sci. 2015, 64, 466–473. [Google Scholar] [CrossRef]
- Fernandes, J.; Teixeira, F.; Guedes, V.; Junior, A.; Teixeira, J.P. Harmonic to noise ratio measurement-selection of window and length. Procedia Comput. Sci. 2018, 138, 280–285. [Google Scholar] [CrossRef]
- Skodda, S.; Grönheit, W.; Mancinelli, N.; Schlegel, U. Progression of voice and speech impairment in the course of Parkinson’s disease: A longitudinal study. Park. Dis. 2013, 2013, 389195. [Google Scholar] [CrossRef]
- Liu, C.T.; Chu, S.W.; Chen, Y.S. On the Relationship between Speech Intelligibility and Fluency Indicators among English-Speaking Individuals with Parkinson’s Diseases. Behav. Neurol. 2022, 2022, 1224680. [Google Scholar] [CrossRef]
- Orozco-Arroyave, J.R.; Arias-Londoño, J.D.; Vargas-Bonilla, J.F.; Gonzalez-Rátiva, M.C.; Nöth, E. New Spanish speech corpus database for the analysis of people suffering from Parkinson’s disease. In Proceedings of the Ninth International Conference on Language Resources and Evaluation (LREC’14), Reykjavik, Iceland, 26–31 May 2014; pp. 342–347. [Google Scholar]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. Off. J. Mov. Disord. Soc. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Rabey, J.; Korczyn, A. The Hoehn and Yahr rating scale for Parkinson’s disease. In Instrumental Methods and Scoring in Extrapyramidal Disorders; Springer: Berlin/Heidelberg, Germany, 1995; pp. 7–17. [Google Scholar]
- McAuliffe, M.; Sonderegger, M. Spanish MFA Acoustic Model v2.0.0. Available online: https://mfa-models.readthedocs.io/en/latest/acoustic/Spanish/Spanish%20MFA%20acoustic%20model%20v2_0_0.html (accessed on 26 October 2025).
- DiCanio, C. Acoustic Measures of Obstruent Lenition; Technical Report; University of Buffalo: Buffalo, NY, USA, 2020. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Teixeira, J.P.; Fernandes, P.O. Jitter, shimmer and HNR classification within gender, tones and vowels in healthy voices. Procedia Technol. 2014, 16, 1228–1237. [Google Scholar] [CrossRef]
- Titze, I.R. Physiologic and acoustic differences between male and female voices. J. Acoust. Soc. Am. 1989, 85, 1699–1707. [Google Scholar] [CrossRef]
- Tykalova, T.; Novotny, M.; Ruzicka, E.; Dusek, P.; Rusz, J. Short-term effect of dopaminergic medication on speech in early-stage Parkinson’s disease. npj Park. Dis. 2022, 8, 22. [Google Scholar] [CrossRef]
- Fabbri, M.; Guimarães, I.; Cardoso, R.; Coelho, M.; Guedes, L.C.; Rosa, M.M.; Godinho, C.; Abreu, D.; Gonçalves, N.; Antonini, A.; et al. Speech and voice response to a levodopa challenge in late-stage Parkinson’s disease. Front. Neurol. 2017, 8, 432. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.; Krýže, P.; Amstutz, D.; Petermann, K.; Averna, A.; Castelli, M.; Magalhães, A.; Jorge, A.; Švihlík, J.; Tykalová, T.; et al. Digital speech biomarkers can measure acute effects of levodopa in Parkinson’s disease. npj Park. Dis. 2025, 11, 184. [Google Scholar] [CrossRef] [PubMed]
- Pah, N.D.; Motin, M.A.; Kempster, P.; Kumar, D.K. Detecting effect of levodopa in Parkinson’s disease patients using sustained phonemes. IEEE J. Transl. Eng. Health Med. 2021, 9, 4900409. [Google Scholar] [CrossRef]
- Hlavnička, J.; Čmejla, R.; Tykalová, T.; Šonka, K.; Růžička, E.; Rusz, J. Automated analysis of connected speech reveals early biomarkers of Parkinson’s disease in patients with rapid eye movement sleep behaviour disorder. Sci. Rep. 2017, 7, 12. [Google Scholar] [CrossRef]
- Rusz, J.; Hlavnička, J.; Čmejla, R.; Růžička, E. Automatic evaluation of speech rhythm instability and acceleration in dysarthrias associated with basal ganglia dysfunction. Front. Bioeng. Biotechnol. 2015, 3, 104. [Google Scholar] [CrossRef]
- Marek, K.; Jennings, D.; Lasch, S.; Siderowf, A.; Tanner, C.; Simuni, T.; Coffey, C.; Kieburtz, K.; Flagg, E.; Chowdhury, S.; et al. The Parkinson progression marker initiative (PPMI). Prog. Neurobiol. 2011, 95, 629–635. [Google Scholar] [CrossRef] [PubMed]

| Phone | Healthy Controls | Parkinson’s Patients |
|---|---|---|
| p | 307 | 293 |
| b | 207 | 255 |
| t | 382 | 395 |
| d | 162 | 184 |
| k | 492 | 526 |
| g | 22 | 21 |
| Total | 1572 | 1674 |
| Scale | Average Score | Standard Deviation | Range | Scale Range |
|---|---|---|---|---|
| UPDRS | 37.660 | 18.315 | 6–93 | 0–200 |
| UPDRS-speech | 1.34 | 0.823 | 0–2 | 0–4 |
| H–Y | 2.19 | 0.662 | 0–4 | 0–5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wayland, R.; Meyer, R.; Tang, K. Speech Markers of Parkinson’s Disease: Phonological Features and Acoustic Measures. Brain Sci. 2025, 15, 1162. https://doi.org/10.3390/brainsci15111162
Wayland R, Meyer R, Tang K. Speech Markers of Parkinson’s Disease: Phonological Features and Acoustic Measures. Brain Sciences. 2025; 15(11):1162. https://doi.org/10.3390/brainsci15111162
Chicago/Turabian StyleWayland, Ratree, Rachel Meyer, and Kevin Tang. 2025. "Speech Markers of Parkinson’s Disease: Phonological Features and Acoustic Measures" Brain Sciences 15, no. 11: 1162. https://doi.org/10.3390/brainsci15111162
APA StyleWayland, R., Meyer, R., & Tang, K. (2025). Speech Markers of Parkinson’s Disease: Phonological Features and Acoustic Measures. Brain Sciences, 15(11), 1162. https://doi.org/10.3390/brainsci15111162







