Dopaminergic Degeneration Differentially Modulates Primary Motor Cortex Activity and Motor Behavior in Hemiparkinsonian Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
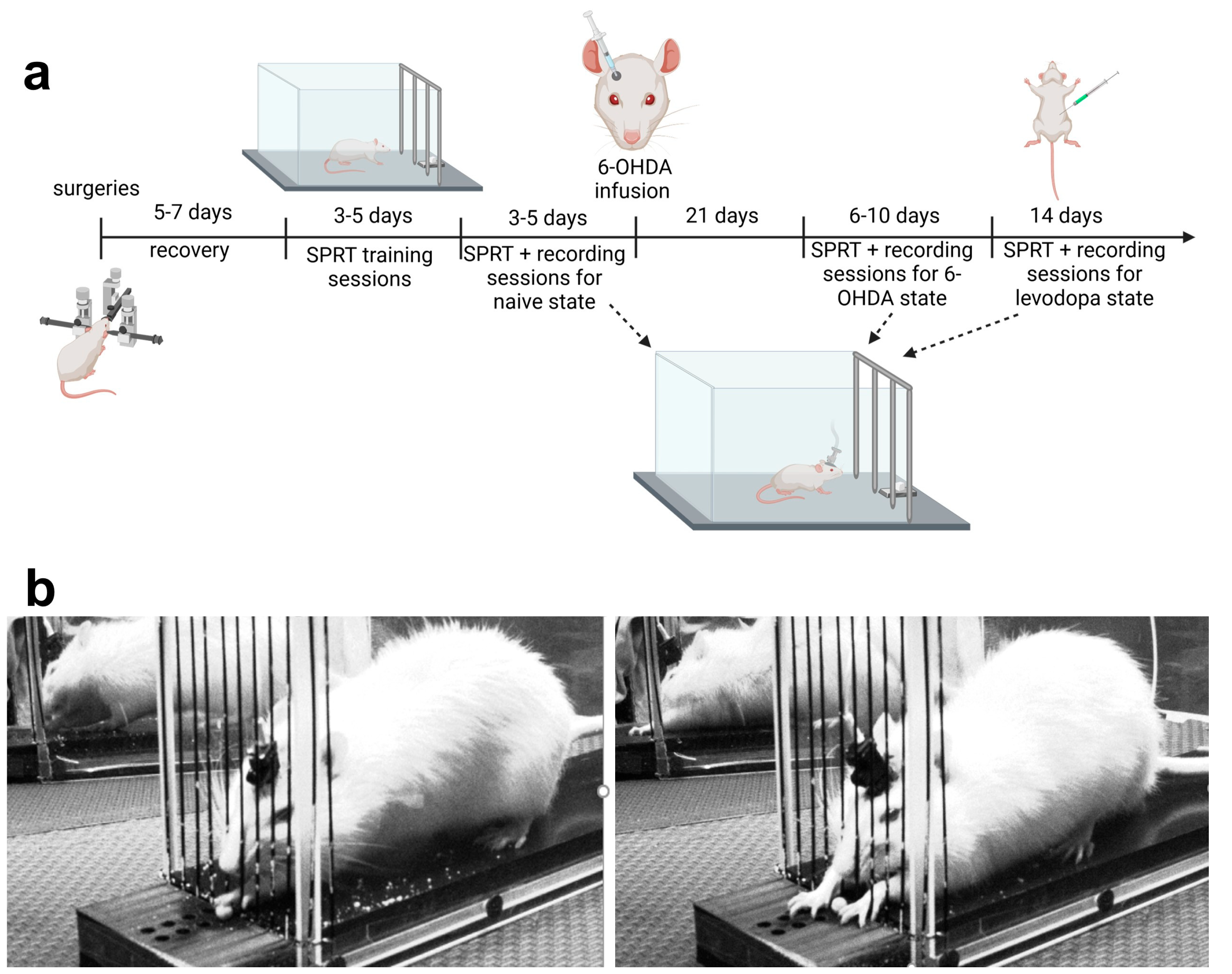
2.2. General Surgical Procedures
2.2.1. Viral Transduction Procedure
2.2.2. Guide Cannula and GRIN Lens Implantation Procedure
2.2.3. Baseplating Procedure
2.3. Food Restriction
2.4. Chamber for Assessment of Motor Function
2.5. SPRT Assessments of Motor Function
2.5.1. Habituation and Training Sessions
2.5.2. Testing Sessions
- •
- Full grasp: Extension of paw beyond the fence and grasping of a pellet. The success ratio was calculated based on categorization of full grasps as successful if the rat ate the pellet or failure if the rat did not eat or dropped the pellet after grasping it.
- •
- Reach without grasp: Extension of the paw beyond the fence allowing it to touch the pellet but not grasping it.
- •
- Grasp without pellet: Extension of paw beyond the fence to attempt to grasp a pellet in the absence of a sucrose pellet in the receptacle.
- •
- Number of attempts/trial (Performance): The total amount of full grasps, reaches without grasps, and grasps without pellets executed by the rat in each trial. The number of attempts per trial is an indication of the rat’s performance, i.e., the lower the number of attempts per trial, the better the performance.
- •
- Attempt duration: Starts when paw lifts from the floor, extends beyond the fence, and ends when pellet is brought to mouth (full grasp) or when paw is placed back on the floor (reach without grasp).
- •
- Reaching duration: Starts when paw lifts from the floor, extends beyond the fence, and ends when the pellet is grasped.
- •
- Grasping duration: Starts when pellet is grasped and ends when pellet is put in mouth or dropped.
2.6. 6-OHDA Lesion and Levodopa Treatment
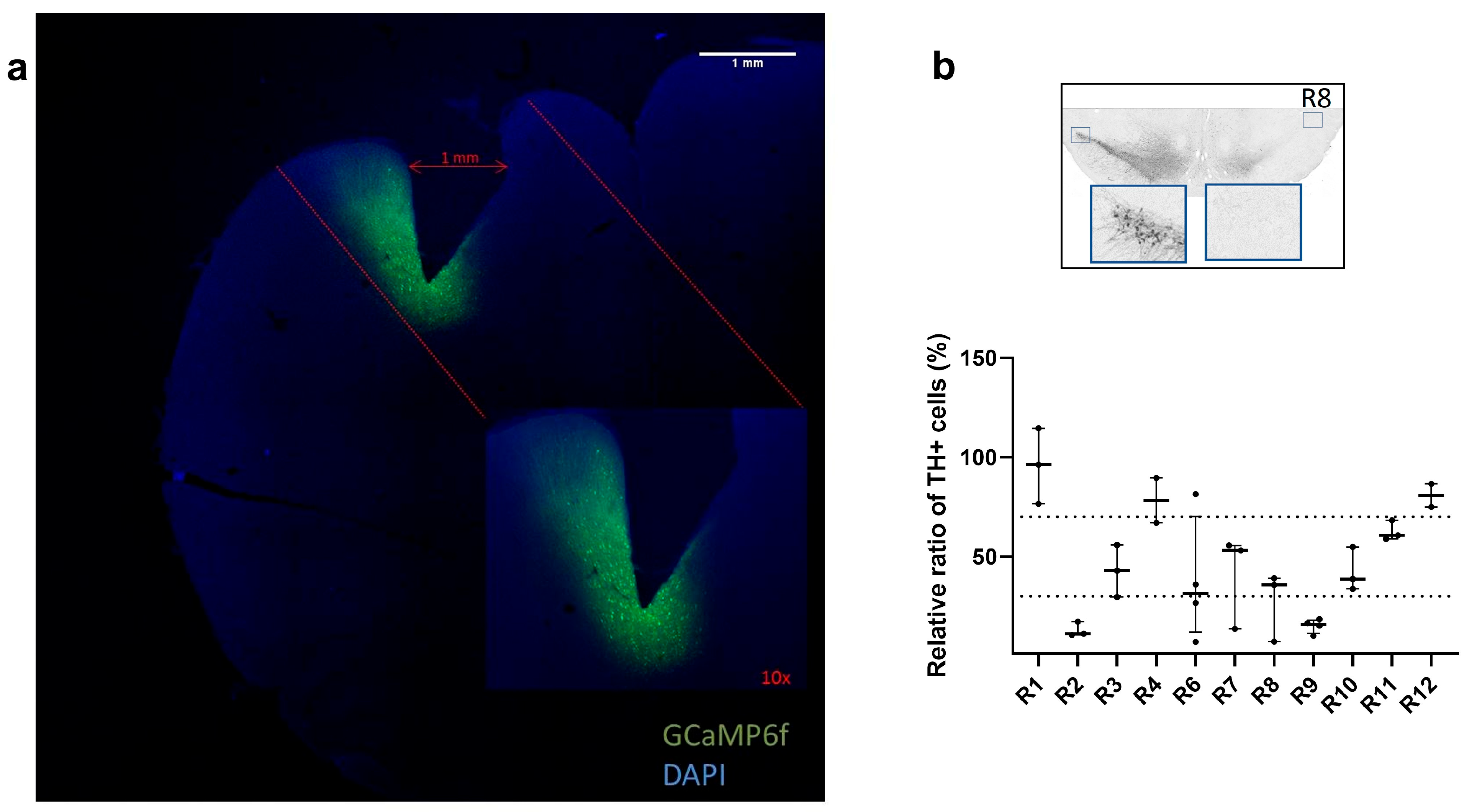
2.7. Histology
2.8. Characterization of Dopaminergic Lesion
2.9. Calcium Data Collection
2.10. Calcium Data Analysis
2.11. Statistical Analysis
3. Results
3.1. Confirmation of GCaMP6f Expression and PRISM Lens Placement
3.2. Characterization of Dopaminergic Lesion
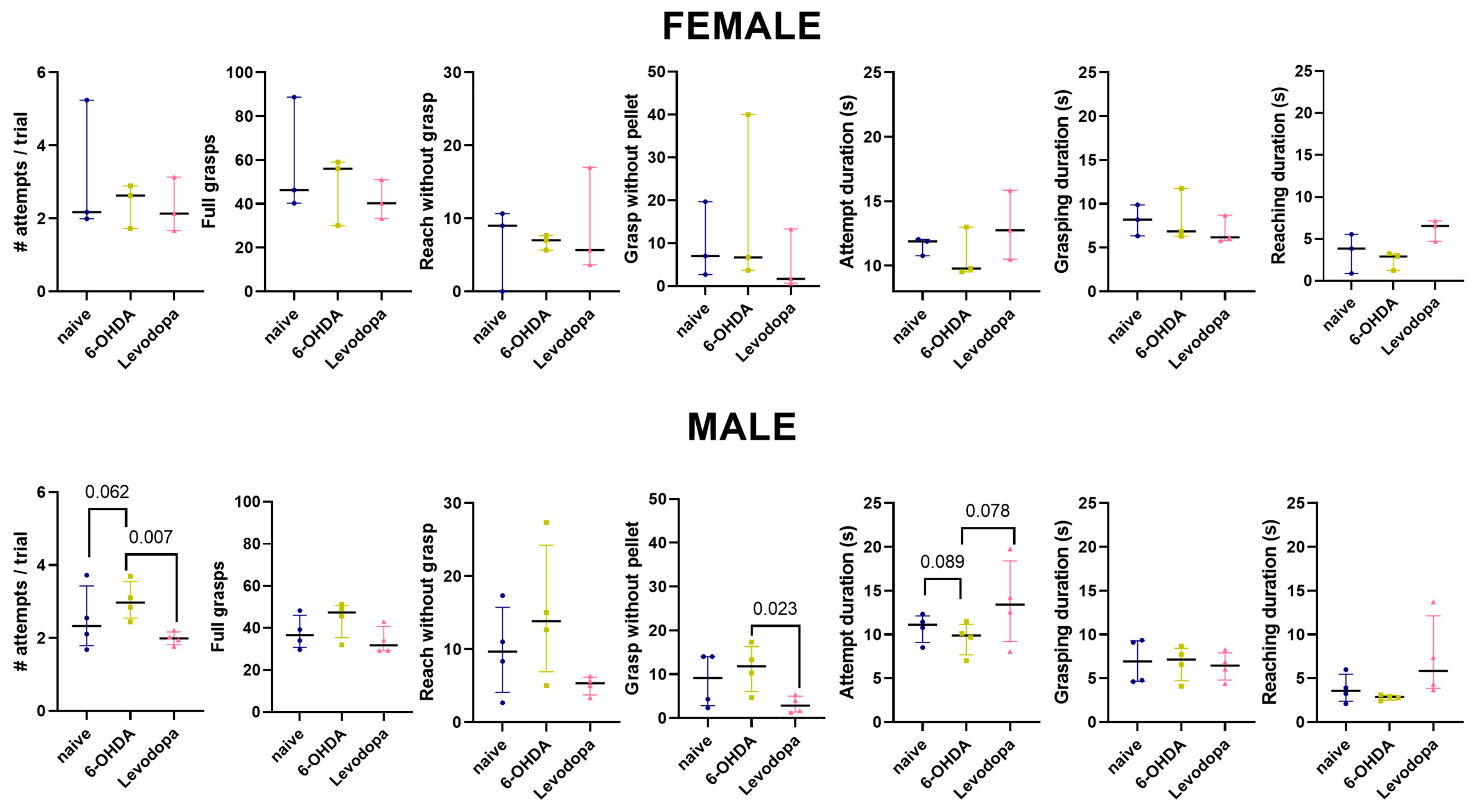
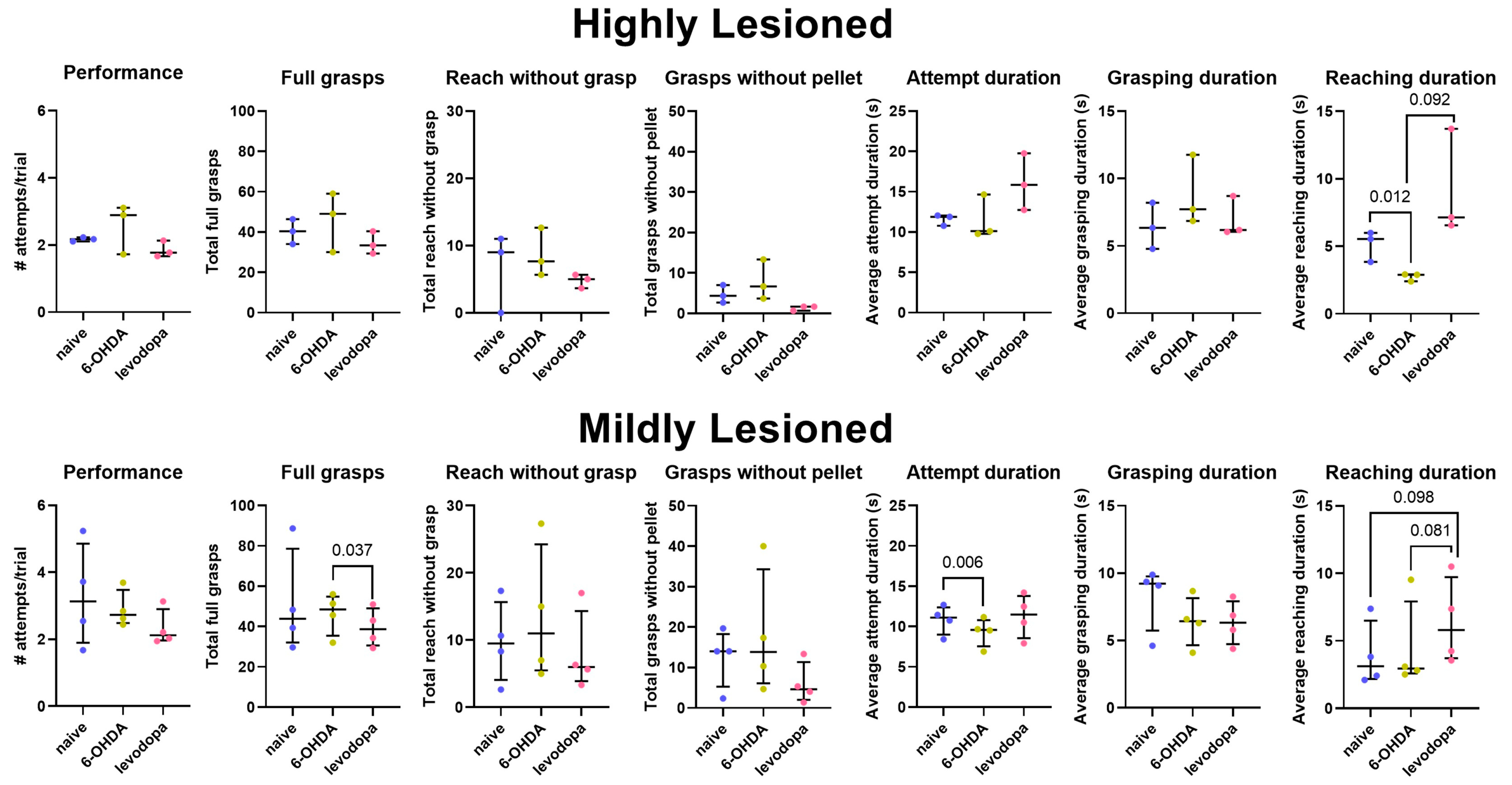
3.3. Levodopa Improves Fine Motor Abilities in Hemiparkinsonian Rats
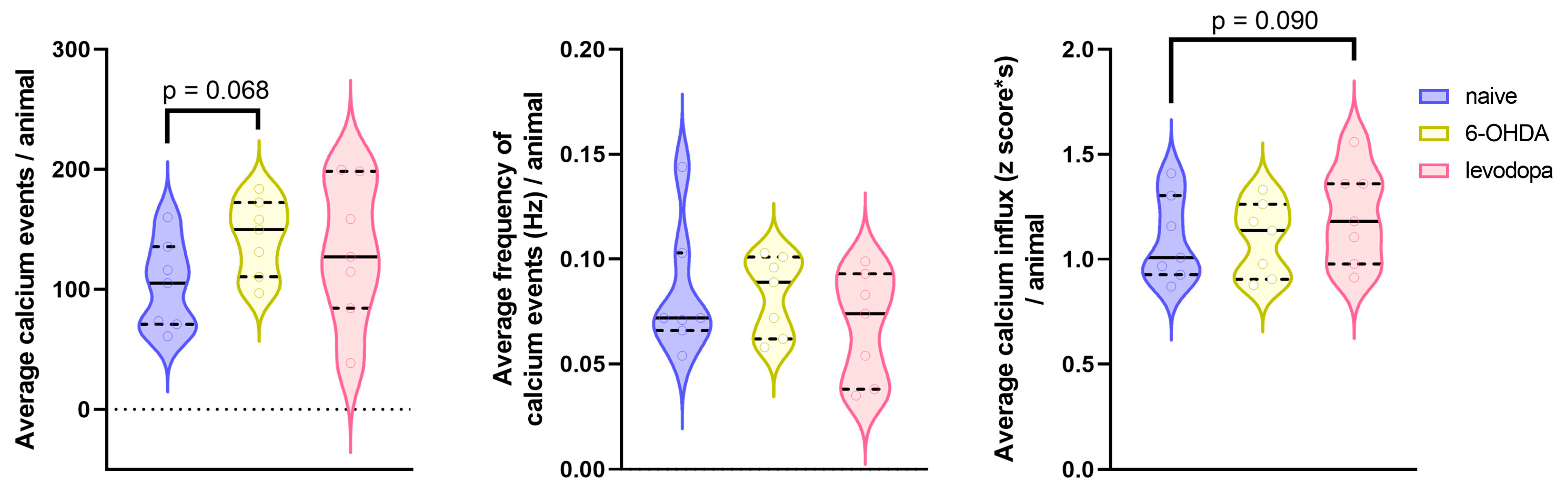
3.4. Changes in M1 Calcium Activity in Response to Dopaminergic Lesion and Levodopa Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 6-OHDA | 6-Hydroxydopamine |
| AAV9 | Adeno-Associated Virus Serotype 9 |
| ABC | Avidin–Biotin Complex |
| ARRIVE | Animal Research: Reporting of In Vivo Experiments (guidelines) |
| AUC | Area Under the Curve |
| CNMF | Constrained Non-negative Matrix Factorization |
| DA | Dopamine/Dopaminergic |
| DAB | 3,3′-Diaminobenzidine |
| DAPI | 4′,6-Diamidino-2-Phenylindole |
| DAQ | Data Acquisition |
| DAT | Dopamine Transporter |
| DBS | Deep Brain Stimulation |
| EMG | Electromyography |
| FDG | Fluorodeoxyglucose |
| GRIN | Gradient-Index (lens) |
| HRP | Horseradish Peroxidase |
| IACUC | Institutional Animal Care and Use Committee |
| IDPS | Inscopix Data Processing Software |
| ISXD | Inscopix Data File Format |
| L-DOPA | Levodopa (L-3,4-dihydroxyphenylalanine) |
| LID | Levodopa-Induced Dyskinesia |
| MFB | Medial Forebrain Bundle |
| MOSAIC | Modular Optical Imaging Analysis Center (Inscopix software module) |
| MPTP | 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| MRI | Magnetic Resonance Imaging |
| NGS | Normal Goat Serum |
| PBS | Phosphate-Buffered Saline |
| PRISM | Gradient-Index Prism Lens System |
| PVDF | Polyvinylidene Difluoride |
| SNc | Substantia Nigra Pars Compacta |
| SNR | Signal-to-Noise Ratio |
| SPRT | Single Pellet Reaching Test |
| STN | Subthalamic Nucleus |
| VTA | Ventral Tegmental Area |
| α-Syn | Alpha-Synuclein |
References
- Beitz, J.M. Parkinson’s disease: A review. Front. Biosci. 2014, 6, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, T.; Terzakis, K. Parkinson Disease. Home Healthc. Now 2016, 34, 300–307. [Google Scholar] [CrossRef]
- Halli-Tierney, A.D.; Luker, J.; Carroll, D.G. Parkinson Disease. Am. Fam. Physician 2020, 102, 679–691. [Google Scholar]
- Underwood, C.F.; Parr-Brownlie, L.C. Primary motor cortex in Parkinson’s disease: Functional changes and opportunities for neurostimulation. Neurobiol. Dis. 2021, 147, 105159. [Google Scholar] [CrossRef]
- Burciu, R.G.; Vaillancourt, D.E. Imaging of Motor Cortex Physiology in Parkinson’s Disease. Mov. Disord. 2018, 33, 1688–1699. [Google Scholar] [CrossRef]
- Cousineau, J.; Plateau, V.; Baufreton, J.; Le Bon-Jégo, M. Dopaminergic modulation of primary motor cortex: From cellular and synaptic mechanisms underlying motor learning to cognitive symptoms in Parkinson’s disease. Neurobiol. Dis. 2022, 167, 105674. [Google Scholar] [CrossRef] [PubMed]
- Molina-Luna, K.; Pekanovic, A.; Röhrich, S.; Hertler, B.; Schubring-Giese, M.; Rioult-Pedotti, M.S.; Luft, A.R. Dopamine in motor cortex is necessary for skill learning and synaptic plasticity. PLoS ONE 2009, 4, e7082. [Google Scholar] [CrossRef] [PubMed]
- Vitrac, C.; Benoit-Marand, M. Monoaminergic Modulation of Motor Cortex Function. Front. Neural Circuits 2017, 11, 72. [Google Scholar] [CrossRef]
- Hosp, J.A.; Nolan, H.E.; Luft, A.R. Topography and collateralization of dopaminergic projections to primary motor cortex in rats. Exp. Brain Res. 2015, 233, 1365–1375. [Google Scholar] [CrossRef]
- Vitrac, C.; Péron, S.; Frappé, I.; Fernagut, P.-O.; Jaber, M.; Gaillard, A.; Benoit-Marand, M. Dopamine control of pyramidal neuron activity in the primary motor cortex via D2 receptors. Front. Neural Circuits 2014, 8, 13. [Google Scholar] [CrossRef]
- Lanciego, J.L.; Luquin, N.; Obeso, J.A. Functional neuroanatomy of the basal ganglia. Cold Spring Harb. Perspect. Med. 2012, 2, a009621. [Google Scholar] [CrossRef]
- Fazl, A.; Fleisher, J. Anatomy, Physiology, and Clinical Syndromes of the Basal Ganglia: A Brief Review. Semin. Pediatr. Neurol. 2018, 25, 2–9. [Google Scholar] [CrossRef]
- Groenewegen, H.J. The basal ganglia and motor control. Neural Plast. 2003, 10, 107–120. [Google Scholar] [CrossRef] [PubMed]
- DeLong, M.; Wichmann, T. Update on models of basal ganglia function and dysfunction. Park. Relat. Disord. 2009, 15 (Suppl 3), S237–S240. [Google Scholar] [CrossRef] [PubMed]
- Nambu, A. Seven problems on the basal ganglia. Curr. Opin. Neurobiol. 2008, 18, 595–604. [Google Scholar] [CrossRef]
- Obeso, J.A.; Marin, C.; Rodriguez-Oroz, C.; Blesa, J.; Benitez-Temiño, B.; Mena-Segovia, J.; Rodríguez, M.; Olanow, C.W. The basal ganglia in Parkinson’s disease: Current concepts and unexplained observations. Ann. Neurol. 2008, 64 (Suppl 2), S30–S46. [Google Scholar] [CrossRef]
- Mure, H.; Hirano, S.; Tang, C.C.; Isaias, I.U.; Antonini, A.; Ma, Y.; Dhawan, V.; Eidelberg, D. Parkinson’s disease tremor-related metabolic network: Characterization, progression, and treatment effects. Neuroimage 2011, 54, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Rascol, O.; Sabatini, U.; Brefel, C.; Fabre, N.; Rai, S.; Senard, J.M.; Celsis, P.; Viallard, G.; Montastruc, J.L.; Chollet, F. Cortical motor overactivation in parkinsonian patients with L-dopa-induced peak-dose dyskinesia. Brain 1998, 121, 527–533. [Google Scholar] [CrossRef]
- Mohl, B.; Berman, B.D.; Shelton, E.; Tanabe, J. Levodopa response differs in Parkinson’s motor subtypes: A task-based effective connectivity study. J. Comp. Neurol. 2017, 525, 2192–2201. [Google Scholar] [CrossRef]
- Buhmann, C.; Glauche, V.; Stürenburg, H.J.; Oechsner, M.; Weiller, C.; Büchel, C. Pharmacologically modulated fMRI--cortical responsiveness to levodopa in drug-naive hemiparkinsonian patients. Brain 2003, 126, 451–461. [Google Scholar] [CrossRef]
- Planetta, P.J.; Kurani, A.S.; Shukla, P.; Prodoehl, J.; Corcos, D.M.; Comella, C.L.; McFarland, N.R.; Okun, M.S.; Vaillancourt, D.E. Distinct functional and macrostructural brain changes in Parkinson’s disease and multiple system atrophy. Hum. Brain Mapp. 2015, 36, 1165–1179. [Google Scholar] [CrossRef]
- Wu, T.; Wang, L.; Hallett, M.; Chen, Y.; Li, K.; Chan, P. Effective connectivity of brain networks during self-initiated movement in Parkinson’s disease. Neuroimage 2011, 55, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Helmich, R.C.; Derikx, L.C.; Bakker, M.; Scheeringa, R.; Bloem, B.R.; Toni, I. Spatial remapping of cortico-striatal connectivity in Parkinson’s disease. Cereb. Cortex 2010, 20, 1175–1186. [Google Scholar] [CrossRef]
- Yu, H.; Sternad, D.; Corcos, D.M.; Vaillancourt, D.E. Role of hyperactive cerebellum and motor cortex in Parkinson’s disease. Neuroimage 2007, 35, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, U.; Boulanouar, K.; Fabre, N.; Martin, F.; Carel, C.; Colonnese, C.; Bozzao, L.; Berry, I.; Montastruc, J.L.; Chollet, F.; et al. Cortical motor reorganization in akinetic patients with Parkinson’s disease: A functional MRI study. Brain 2000, 123, 394–403. [Google Scholar] [CrossRef]
- Goldberg, J.A.; Boraud, T.; Maraton, S.; Haber, S.N.; Vaadia, E.; Bergman, H. Enhanced synchrony among primary motor cortex neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine primate model of Parkinson’s disease. J. Neurosci. 2002, 22, 4639–4653. [Google Scholar] [CrossRef] [PubMed]
- Doudet, D.J.; Gross, C.; Arluison, M.; Bioulac, B. Modifications of precentral cortex discharge and EMG activity in monkeys with MPTP-induced lesions of DA nigral neurons. Exp. Brain Res. 1990, 80, 177–188. [Google Scholar] [CrossRef]
- Pasquereau, B.; Turner, R.S. Primary motor cortex of the parkinsonian monkey: Differential effects on the spontaneous activity of pyramidal tract-type neurons. Cereb. Cortex 2011, 21, 1362–1378. [Google Scholar] [CrossRef]
- Costa, R.M.; Lin, S.C.; Sotnikova, T.D.; Cyr, M.; Gainetdinov, R.R.; Caron, M.G.; Nicolelis, M.A. Rapid alterations in corticostriatal ensemble coordination during acute dopamine-dependent motor dysfunction. Neuron 2006, 52, 359–369. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 7th ed.; Academic Press: London, UK, 2013; p. 480. [Google Scholar]
- Kamińska, K.; Lenda, T.; Konieczny, J.; Wardas, J.; Lorenc-Koci, E. Interactions of the tricyclic antidepressant drug amitriptyline with L-DOPA in the striatum and substantia nigra of unilaterally 6-OHDA-lesioned rats. Relevance to motor dysfunction in Parkinson’s disease. Neurochem. Int. 2018, 121, 125–139. [Google Scholar] [CrossRef]
- Issy, A.C.; Padovan-Neto, F.E.; Lazzarini, M.; Bortolanza, M.; Del-Bel, E. Disturbance of sensorimotor filtering in the 6-OHDA rodent model of Parkinson’s disease. Life Sci. 2015, 125, 71–78. [Google Scholar] [CrossRef]
- Kaindlstorfer, C.; Stefanova, N.; Garcia, J.; Krismer, F.; Döbrössy, M.; Göbel, G.; Jellinger, K.; Granata, R.; Wenning, G.K. L-dopa response pattern in a rat model of mild striatonigral degeneration. PLoS ONE 2019, 14, e0218130. [Google Scholar] [CrossRef]
- Li, S.Y.; Wang, Y.L.; Liu, W.W.; Lyu, D.J.; Wang, F.; Mao, C.J.; Yang, Y.P.; Hu, L.F.; Liu, C.F. Long-term Levodopa Treatment Accelerates the Circadian Rhythm Dysfunction in a 6-hydroxydopamine Rat Model of Parkinson’s Disease. Chin. Med. J. 2017, 130, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Deng, M.; Zhang, S.; Lu, S.; Gui, X.; Fang, Y. β-asarone and levodopa coadministration increases striatal levels of dopamine and levodopa and improves behavioral competence in Parkinson’s rat by enhancing dopa decarboxylase activity. Biomed. Pharmacother. 2017, 94, 666–678. [Google Scholar] [CrossRef]
- Santiago, R.M.; Tonin, F.S.; Barbiero, J.; Zaminelli, T.; Boschen, S.L.; Andreatini, R.; Da Cunha, C.; Lima, M.M.S.; Vital, M.A.B.F. The nonsteroidal antiinflammatory drug piroxicam reverses the onset of depressive-like behavior in 6-OHDA animal model of Parkinson’s disease. Neuroscience 2015, 300, 246–253. [Google Scholar] [CrossRef]
- Bortolanza, M.; Wietzikoski, E.C.; Boschen, S.L.; Dombrowski, P.A.; Latimer, M.; MacLaren, D.A.A.; Winn, P.; Cunha, C.D. Functional disconnection of the substantia nigra pars compacta from the pedunculopontine nucleus impairs learning of a conditioned avoidance task. Neurobiol. Learn. Mem. 2010, 94, 229–239. [Google Scholar] [CrossRef]
- Bassani, T.B.; Gradowski, R.W.; Zaminelli, T.; Barbiero, J.K.; Santiago, R.M.; Boschen, S.L.; da Cunha, C.; Lima, M.M.S.; Andreatini, R.; Vital, M.A.B.F. Neuroprotective and antidepressant-like effects of melatonin in a rotenone-induced Parkinson’s disease model in rats. Brain Res. 2014, 1593, 95–105. [Google Scholar] [CrossRef]
- Santiago, R.M.; Barbiero, J.; Gradowski, R.W.; Bochen, S.; Lima, M.M.S.; Da Cunha, C.; Andreatini, R.; Vital, M.A.B.F. Induction of depressive-like behavior by intranigral 6-OHDA is directly correlated with deficits in striatal dopamine and hippocampal serotonin. Behav. Brain Res. 2014, 259, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Resendez, S.L.; Rodriguez-Romaguera, J.; Jimenez, J.C.; Neufeld, S.Q.; Giovannucci, A.; Friedrich, J.; Pnevmatikakis, E.A.; Stuber, G.D.; Hen, R.; et al. Efficient and accurate extraction of in vivo calcium signals from microendoscopic video data. eLife 2018, 7, e28728. [Google Scholar] [CrossRef] [PubMed]
- Hyland, B.I.; Seeger-Armbruster, S.; Smither, R.A.; Parr-Brownlie, L.C. Altered Recruitment of Motor Cortex Neuronal Activity During the Grasping Phase of Skilled Reaching in a Chronic Rat Model of Unilateral Parkinsonism. J. Neurosci. 2019, 39, 9660–9672. [Google Scholar] [CrossRef] [PubMed]
- Thobois, S.; Dominey, P.; Decety, J.; Pollak, P.; Gregoire, M.C.; Broussolle, E. Overactivation of primary motor cortex is asymmetrical in hemiparkinsonian patients. Neuroreport 2000, 11, 785–789. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vercruysse, S.; Spildooren, J.; Heremans, E.; Wenderoth, N.; Swinnen, S.P.; Vandenberghe, W.; Nieuwboer, A. The neural correlates of upper limb motor blocks in Parkinson’s disease and their relation to freezing of gait. Cereb. Cortex 2014, 24, 3154–3166. [Google Scholar] [CrossRef]
- Degos, B.; Deniau, J.-M.; Chavez, M.; Maurice, N. Subthalamic Nucleus High-Frequency Stimulation Restores Altered Electrophysiological Properties of Cortical Neurons in Parkinsonian Rat. PLoS ONE 2014, 8, e83608. [Google Scholar] [CrossRef]
- Lee, L.N.; Huang, C.S.; Wang, R.W.; Lai, H.J.; Chung, C.C.; Yang, Y.C.; Kuo, C.C. Deep brain stimulation rectifies the noisy cortex and irresponsive subthalamus to improve parkinsonian locomotor activities. NPJ Park. Dis. 2022, 8, 77. [Google Scholar] [CrossRef]
- Berding, G.; Odin, P.; Brooks, D.J.; Nikkhah, G.; Matthies, C.; Peschel, T.; Shing, M.; Kolbe, H.; van Den Hoff, J.; Fricke, H.; et al. Resting regional cerebral glucose metabolism in advanced Parkinson’s disease studied in the off and on conditions with [(18)F]FDG-PET. Mov. Disord. 2001, 16, 1014–1022. [Google Scholar] [CrossRef]
- Hilker, R.; Voges, J.; Weisenbach, S.; Kalbe, E.; Burghaus, L.; Ghaemi, M.; Lehrke, R.; Koulousakis, A.; Herholz, K.; Sturm, V.; et al. Subthalamic nucleus stimulation restores glucose metabolism in associative and limbic cortices and in cerebellum: Evidence from a FDG-PET study in advanced Parkinson’s disease. J. Cereb. Blood Flow Metab. 2004, 24, 7–16. [Google Scholar] [CrossRef]
- Pasquereau, B.; DeLong, M.R.; Turner, R.S. Primary motor cortex of the parkinsonian monkey: Altered encoding of active movement. Brain 2016, 139, 127–143. [Google Scholar] [CrossRef] [PubMed]
- Rios, A.; Soma, S.; Yoshida, J.; Nonomura, S.; Kawabata, M.; Sakai, Y.; Isomura, Y. Differential Changes in the Lateralized Activity of Identified Projection Neurons of Motor Cortex in Hemiparkinsonian Rats. eNeuro 2019, 6, e01-19. [Google Scholar] [CrossRef]
- Parr-Brownlie, L.C.; Hyland, B.I. Bradykinesia induced by dopamine D2 receptor blockade is associated with reduced motor cortex activity in the rat. J. Neurosci. 2005, 25, 5700–5709. [Google Scholar] [CrossRef]
- Li, Q.; Ke, Y.; Chan, D.C.; Qian, Z.M.; Yung, K.K.; Ko, H.; Arbuthnott, G.W.; Yung, W.H. Therapeutic deep brain stimulation in Parkinsonian rats directly influences motor cortex. Neuron 2012, 76, 1030–1041. [Google Scholar] [CrossRef] [PubMed]
- Swanson, O.K.; Yevoo, P.E.; Richard, D.; Maffei, A. Altered Thalamocortical Signaling in a Mouse Model of Parkinson’s Disease. J. Neurosci. 2023, 43, 6021–6034. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.-Y.; Smith, Y.; Lytton, W.W.; Grafton, S.; Villalba, R.; Masilamoni, G.; Wichmann, T. Dysfunction of motor cortices in Parkinson’s disease. Cereb. Cortex 2024, 34, bhae294. [Google Scholar] [CrossRef]
- Wiesman, A.I.; Vinding, M.C.; Tsitsi, P.; Svenningsson, P.; Waldthaler, J.; Lundqvist, D. Cortical Effects of Dopamine Replacement Account for Clinical Response Variability in Parkinson’s Disease. Mov. Disord. 2025, 40, 1551–1560. [Google Scholar] [CrossRef]
- Johansson, M.E.; Toni, I.; Kessels, R.P.C.; Bloem, B.R.; Helmich, R.C. Clinical severity in Parkinson’s disease is determined by decline in cortical compensation. Brain 2023, 147, 871–886. [Google Scholar] [CrossRef]
- Rioult-Pedotti, M.-S.; Pekanovic, A.; Atiemo, C.O.; Marshall, J.; Luft, A.R. Dopamine Promotes Motor Cortex Plasticity and Motor Skill Learning via PLC Activation. PLoS ONE 2015, 10, e0124986. [Google Scholar] [CrossRef] [PubMed]
- Ferro, M.M.; Bellissimo, M.I.; Anselmo-Franci, J.A.; Angellucci, M.E.M.; Canteras, N.S.; Da Cunha, C. Comparison of bilaterally 6-OHDA- and MPTP-lesioned rats as models of the early phase of Parkinson’s disease: Histological, neurochemical, motor and memory alterations. J. Neurosci. Methods 2005, 148, 78–87. [Google Scholar] [CrossRef]
- Cui, J.; Zhao, D.; Xu, M.; Li, Z.; Qian, J.; Song, N.; Wang, J.; Xie, J. Characterization of graded 6-Hydroxydopamine unilateral lesion in medial forebrain bundle of mice. Sci. Rep. 2024, 14, 3721. [Google Scholar] [CrossRef]
- Cousineau, J.; Lescouzères, L.; Taupignon, A.; Delgado-Zabalza, L.; Valjent, E.; Baufreton, J.; Le Bon-Jégo, M. Dopamine D2-Like Receptors Modulate Intrinsic Properties and Synaptic Transmission of Parvalbumin Interneurons in the Mouse Primary Motor Cortex. eNeuro 2020, 7, ENEURO.0081-20.2020. [Google Scholar] [CrossRef]
- Swanson, O.K.; Evinger, C.; Plotkin, J.; Role, L.; Bishop, C. Primary Motor Cortex Circuitry in a Mouse Model of Parkinson’s Disease; State University of New York at Stony Brook: Stony Brook, NY, USA, 2020. [Google Scholar]
- McGregor, M.M.; Nelson, A.B. Circuit Mechanisms of Parkinson’s Disease. Neuron 2019, 101, 1042–1056. [Google Scholar] [CrossRef]
- Valverde, S.; Vandecasteele, M.; Piette, C.; Derousseaux, W.; Gangarossa, G.; Aristieta Arbelaiz, A.; Touboul, J.; Degos, B.; Venance, L. Deep brain stimulation-guided optogenetic rescue of parkinsonian symptoms. Nat. Commun. 2020, 11, 2388. [Google Scholar] [CrossRef]
- Däuper, J.; Peschel, T.; Schrader, C.; Kohlmetz, C.; Joppich, G.; Nager, W.; Dengler, R.; Rollnik, J.D. Effects of subthalamic nucleus (STN) stimulation on motor cortex excitability. Neurology 2002, 59, 700–706. [Google Scholar] [CrossRef]
- Pierantozzi, M.; Palmieri, M.G.; Mazzone, P.; Marciani, M.G.; Rossini, P.M.; Stefani, A.; Giacomini, P.; Peppe, A.; Stanzione, P. Deep brain stimulation of both subthalamic nucleus and internal globus pallidus restores intracortical inhibition in Parkinson’s disease paralleling apomorphine effects: A paired magnetic stimulation study. Clin. Neurophysiol. 2002, 113, 108–113. [Google Scholar] [CrossRef]
- Siegel, G.J.; Chauhan, N.B. Neurotrophic factors in Alzheimer’s and Parkinson’s disease brain. Brain Res. Rev. 2000, 33, 199–227. [Google Scholar] [CrossRef]
- Åkerud, P.; Holm, P.C.; Castelo-Branco, G.; Sousa, K.; Rodriguez, F.J.; Arenas, E. Persephin-Overexpressing Neural Stem Cells Regulate the Function of Nigral Dopaminergic Neurons and Prevent Their Degeneration in a Model of Parkinson’s Disease. Mol. Cell. Neurosci. 2002, 21, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Alster, P.; Otto-Ślusarczyk, D.; Szlufik, S.; Duszyńska-Wąs, K.; Drzewińska, A.; Wiercińska-Drapało, A.; Struga, M.; Kutyłowski, M.; Friedman, A.; Madetko-Alster, N. The significance of glial cell line-derived neurotrophic factor analysis in Progressive Supranuclear Palsy. Sci. Rep. 2024, 14, 2805. [Google Scholar] [CrossRef]
- Boschen, S.L.; Andreatini, R.; da Cunha, C. Activation of postsynaptic D2 dopamine receptors in the rat dorsolateral striatum prevents the amnestic effect of systemically administered neuroleptics. Behav. Brain Res. 2015, 281, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Wietzikoski, E.C.; Boschen, S.L.; Miyoshi, E.; Bortolanza, M.; dos Santos, L.M.; Frank, M.; Brandão, M.L.; Winn, P.; Da Cunha, C. Roles of D1-like dopamine receptors in the nucleus accumbens and dorsolateral striatum in conditioned avoidance responses. Psychopharmacology 2012, 219, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Boschen, S.L.; Wietzikoski, E.C.; Winn, P.; Cunha, C.D. The role of nucleus accumbens and dorsolateral striatal D2 receptors in active avoidance conditioning. Neurobiol. Learn. Mem. 2011, 96, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Metz, G.A.S.; Farr, T.; Ballermann, M.; Whishaw, I.Q. Chronic levodopa therapy does not improve skilled reach accuracy or reach range on a pasta matrix reaching task in 6-OHDA dopamine-depleted (hemi-Parkinson analogue) rats. Eur. J. Neurosci. 2001, 14, 27–37. [Google Scholar] [CrossRef]
- Seeger-Armbruster, S.; Bosch-Bouju, C.; Little, S.T.C.; Smither, R.A.; Hughes, S.M.; Hyland, B.I.; Parr-Brownlie, L.C. Patterned, But Not Tonic, Optogenetic Stimulation in Motor Thalamus Improves Reaching in Acute Drug-Induced Parkinsonian Rats. J. Neurosci. 2015, 35, 1211–1216. [Google Scholar] [CrossRef]
- Ohno, Y.; Horikoshi, A.; Imamura, K. Reaching Task in Rats: Quantitative Evaluation and Effects of 6-OHDA into the Striatum. J. Mot. Behav. 2022, 54, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.P.; Santos, D.B.; Colle, D.; Naime, A.A.; Gonçalves, C.L.; Ghizoni, H.; Hort, M.A.; Godoi, M.; Dias, P.F.; Braga, A.L.; et al. Decreased forelimb ability in mice intracerebroventricularly injected with low dose 6-hydroxidopamine: A model on the dissociation of bradykinesia from hypokinesia. Behav. Brain Res. 2016, 305, 30–36. [Google Scholar] [CrossRef]
- Mazzoni, P.; Shabbott, B.; Cortés, J.C. Motor control abnormalities in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009282. [Google Scholar] [CrossRef] [PubMed]
- Whishaw, I.Q.; Gorny, B.; Tran-Nguyen, L.T.; Castañeda, E.; Miklyaeva, E.I.; Pellis, S.M. Making two movements at once: Impairments of movement, posture, and their integration underlie the adult skilled reaching deficit of neonatally dopamine-depleted rats. Behav. Brain Res. 1994, 61, 65–77. [Google Scholar] [CrossRef]
- Lange, F.; Guarin, D.L.; Ademola, E.; Mahdy, D.; Acevedo, G.; Odorfer, T.; Wong, J.K.; Volkmann, J.; Peach, R.; Reich, M. Computer vision uncovers three fundamental dimensions of levodopa-responsive motor improvement in Parkinson’s disease. NPJ Park. Dis. 2025, 11, 140. [Google Scholar] [CrossRef]
- Xie, Y.; Gao, C.; Wu, B.; Peng, L.; Wu, J.; Lang, L. Morphologic brain network predicts levodopa responsiveness in Parkinson disease. Front. Aging Neurosci. 2023, 14, 990913. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, S.; Liang, Y.; Cai, D.; Zhou, X.; He, W.; Xia, J. Predictive Value of Perivascular Space Network and Choroid Plexus for Levodopa Responsiveness in Parkinson’s Disease. Eur. J. Neurol. 2025, 32, e70290. [Google Scholar] [CrossRef]
- Riederer, P.; Strobel, S.; Nagatsu, T.; Watanabe, H.; Chen, X.; Löschmann, P.-A.; Sian-Hulsmann, J.; Jost, W.H.; Müller, T.; Dijkstra, J.M.; et al. Levodopa treatment: Impacts and mechanisms throughout Parkinson’s disease progression. J. Neural Transm. 2025, 132, 743–779. [Google Scholar] [CrossRef]
- Peng, Q.; Zhong, S.; Tan, Y.; Zeng, W.; Wang, J.; Cheng, C.; Yang, X.; Wu, Y.; Cao, X.; Xu, Y. The Rodent Models of Dyskinesia and Their Behavioral Assessment. Front. Neurol. 2019, 10, 1016. [Google Scholar] [CrossRef]
- Lindgren, H.S.; Lane, E.L. Lane, E.L., Dunnett, S.B., Eds.; Rodent Models of l-DOPA-Induced Dyskinesia. In Animal Models of Movement Disorders; Humana Press: Totowa, NJ, USA, 2012; Volume I, pp. 337–351. [Google Scholar]
- Cattaneo, C.; Pagonabarraga, J. Sex Differences in Parkinson’s Disease: A Narrative Review. Neurol. Ther. 2025, 14, 57–70. [Google Scholar] [CrossRef]
- Reekes, T.H.; Higginson, C.I.; Ledbetter, C.R.; Sathivadivel, N.; Zweig, R.M.; Disbrow, E.A. Sex specific cognitive differences in Parkinson disease. NPJ Park. Dis. 2020, 6, 7. [Google Scholar] [CrossRef]
- Tremblay, C.; Abbasi, N.; Zeighami, Y.; Yau, Y.; Dadar, M.; Rahayel, S.; Dagher, A. Sex effects on brain structure in de novo Parkinson’s disease: A multimodal neuroimaging study. Brain 2020, 143, 3052–3066. [Google Scholar] [CrossRef] [PubMed]
- Tullo, S.; Park, J.S.H.; Gallino, D.; Park, M.; Mar, K.; Novikov, V.; Sandoval Contreras, R.; Patel, R.; del Cid-Pellitero, E.; Fon, E.A.; et al. Female mice exhibit resistance to disease progression despite early pathology in a transgenic mouse model inoculated with alpha-synuclein fibrils. Commun. Biol. 2025, 8, 288. [Google Scholar] [CrossRef]
- Nordengen, K.; Cappelletti, C.; Bahrami, S.; Frei, O.; Pihlstrøm, L.; Henriksen, S.P.; Geut, H.; Rozemuller, A.J.M.; van de Berg, W.D.J.; Andreassen, O.A.; et al. Pleiotropy with sex-specific traits reveals genetic aspects of sex differences in Parkinson’s disease. Brain 2023, 147, 858–870. [Google Scholar] [CrossRef]
- McArthur, S.; Murray, H.E.; Dhankot, A.; Dexter, D.T.; Gillies, G.E. Striatal susceptibility to a dopaminergic neurotoxin is independent of sex hormone effects on cell survival and DAT expression but is exacerbated by central aromatase inhibition. J. Neurochem. 2007, 100, 678–692. [Google Scholar] [CrossRef]
- Pinizzotto, C.C.; Patwardhan, A.; Aldarondo, D.; Kritzer, M.F. Task-specific effects of biological sex and sex hormones on object recognition memories in a 6-hydroxydopamine-lesion model of Parkinson’s disease in adult male and female rats. Horm. Behav. 2022, 144, 105206. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Lam, R.K.; Blumenfeld, S.E.; Tan, W.; Ciari, P.; Chu, E.K.; Saw, N.L.; Rijsketic, D.R.; Lin, J.S.; Heifets, B.D.; et al. Mapping of catecholaminergic denervation, neurodegeneration, and inflammation in 6-OHDA-treated Parkinson’s disease mice. NPJ Park. Dis. 2025, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Kwan, A.C. Interpreting in vivo calcium signals from neuronal cell bodies, axons, and dendrites: A review. Neurophotonics 2019, 7, 011402. [Google Scholar] [CrossRef] [PubMed]
- Harding, E.K.; Boivin, B.; Salter, M.W. Intracellular Calcium Responses Encode Action Potential Firing in Spinal Cord Lamina I Neurons. J. Neurosci. 2020, 40, 4439–4456. [Google Scholar] [CrossRef] [PubMed]
- Rondoni, N.A.; Lu, F.; Turner-Evans, D.B.; Gomez, M. Predicting neuronal firing from calcium imaging using a control theoretic approach. PLOS Comput. Biol. 2025, 21, e1012603. [Google Scholar] [CrossRef]
- Shannonhouse, J.; Zhang, Y.; Son, H.; Kim, E.; Han, D.; Park, J.T.; Kim, Y.S. Lessons from the use of in vivo cellular calcium imaging in primary sensory neurons and spinal cord. Neuroscientist 2025, 10738584251360724. [Google Scholar] [CrossRef]
- Guillamón-Vivancos, T.; Vandael, D.; Torres, D.; López-Bendito, G.; Martini, F.J. Mesoscale calcium imaging in vivo: Evolution and contribution to developmental neuroscience. Front. Neurosci. 2023, 17, 1210199. [Google Scholar] [CrossRef] [PubMed]

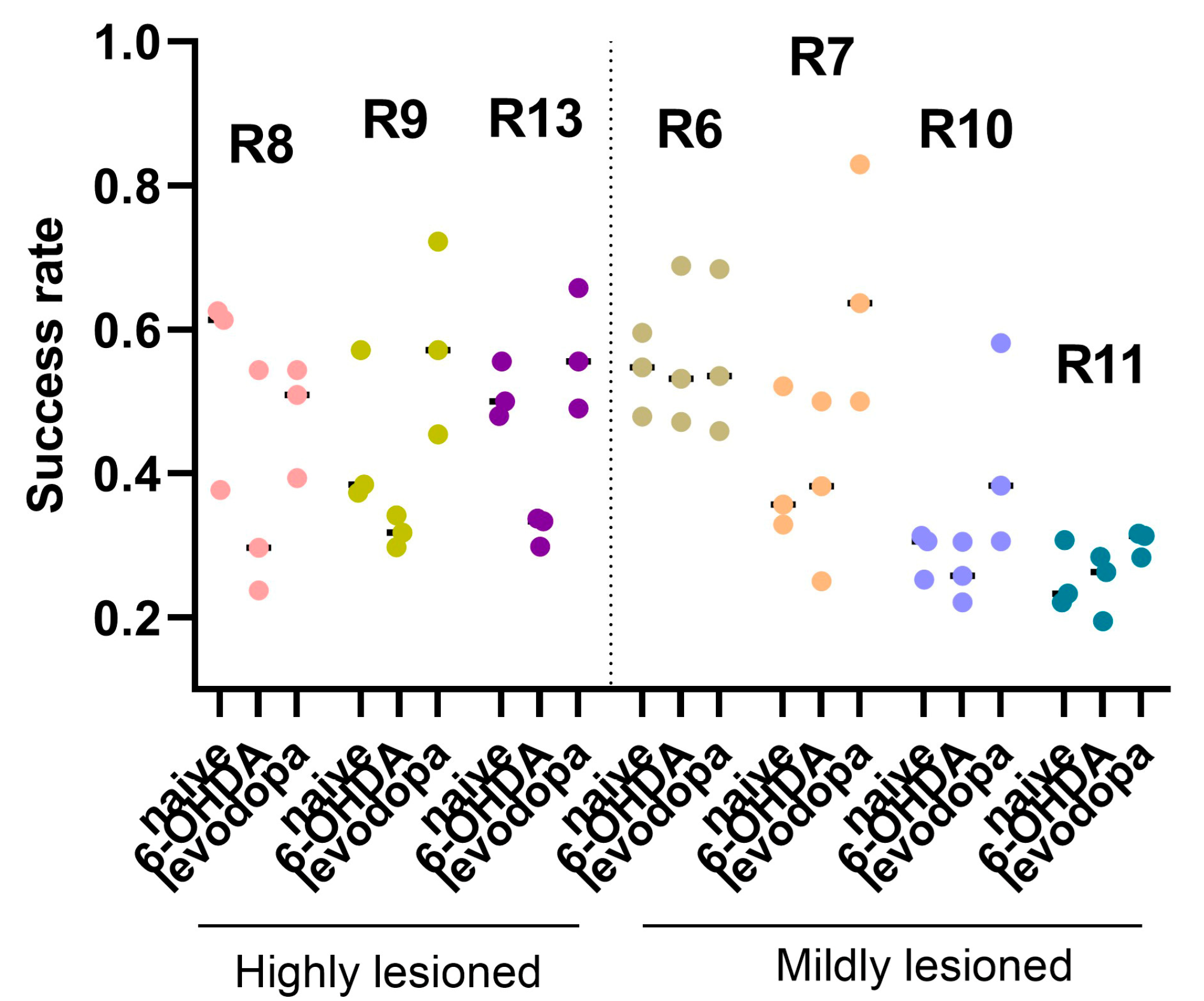



| Subject ID/Sex | Histology | SPRT | Ca2+ Activity | Reason for Removal from SPRT or Ca2+ Imaging Analysis |
|---|---|---|---|---|
| R1/male | Low TH+ loss | Yes: 15 trials | Yes | SPRT protocol different from final analysis |
| R2/male | High TH+ loss | Yes: 15 trials | Yes | SPRT protocol different from final analysis |
| R3/male | Mild TH+ loss | Yes: 15 trials | Yes | SPRT protocol different from final analysis |
| R4/male | Low TH+ loss | Yes: 15 trials | Yes | SPRT protocol different from final analysis |
| R5/female | Not assessed | Yes: 15 trials | No | SPRT protocol different from final analysis; Baseplate detached from headcap |
| R6/male | Mild TH+ loss | Yes: 25 trials | Yes | N/A |
| R7/male | Mild TH+ loss | Yes: 25 trials | Yes | N/A |
| R8/female | High TH+ loss | Yes: 25 trials | Yes | N/A |
| R9/female | High TH+ loss | Yes: 25 trials | No | No calcium activity during levodopa treatment state |
| R10/male | Mild TH+ loss | Yes: 25 trials | No | No calcium activity during 6-OHDA lesion and levodopa treatment states |
| R11/female | Mild TH+ loss | Yes: 25 trials | No | No calcium activity during 6-OHDA lesion and levodopa treatment states |
| R12/female | Low TH+ loss | No | No | No calcium activity in any state, inflammation in M1 |
| R13/male | High TH+ loss in the STR | Yes: 25 trials | No | No calcium activity during levodopa treatment state |
| Naïve vs. 6-OHDA | Naïve vs. Levodopa | 6-OHDA vs. Levodopa | ||||
|---|---|---|---|---|---|---|
| Variable | Mean Difference (95% CI) | p-Value | Mean Difference (95% CI) | p-Value | Mean Difference (95% CI) | p-Value |
| Attempts/Trial (Performance) | −0.02 (−1.16, 1.12) | 0.970 | −0.65 (−1.47, 0.17) | 0.100 | −0.63 (−1.31, 0.05) | 0.063 |
| Full Grasps | −0.52 (−17.67, 16.62) | 0.940 | −9.43 (−22.49, 3.63) | 0.130 | −8.91 (−16.66, −1.16) | 0.031 |
| Reach without Grasp | 3.05 (−1.89, 7.99) | 0.180 | −1.76 (−7.77, 4.25) | 0.500 | −4.81 (−13.31, 3.69) | 0.220 |
| Grasp without Pellet | 1.94 (−3.10, 6.98) | 0.370 | −4.94 (−10.90, 1.01) | 0.086 | −6.89 (−12.79, −0.98) | 0.030 |
| Attempt Duration | −1.03 (−1.96, −0.10) | 0.036 | 2.28 (−0.83, 5.38) | 0.120 | 3.31 (0.45, 6.17) | 0.030 |
| Reaching Duration | −0.99 (−2.77, 0.79) | 0.220 | 3.14 (0.58, 5.70) | 0.024 | 4.13 (0.94, 7.32) | 0.019 |
| Grasping Duration | −0.04 (−2.50, 2.42) | 0.970 | −0.86 (−2.57, 0.84) | 0.2600 | −0.82 (−1.92, 0.27) | 0.120 |
| Female | ||||||
| Naïve vs. 6-OHDA | Naïve vs. Levodopa | 6-OHDA vs. Levodopa | ||||
| Variable | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value |
| Attempts/Trial (Performance) | −0.80 (−1.08, 2.68) | 0.476 | 0.90 (−0.46, 2.27) | 0.204 | 0.10 (−0.67, 0.88) | 0.925 |
| Full Grasps | 10.11 (−31.95, 52.17) | 0.777 | 16.89 (−14.49, 48.26) | 0.325 | 6.78 (−15.17, 28.73) | 0.666 |
| Reach without Grasp | −0.22 (−17.07, 16.62) | 0.999 | −2.22 (−13.66, 9.21) | 0.847 | −2.00 (−11.64, 7.64) | 0.828 |
| Grasp without Pellet | −0.33 (−11.47, 10.80) | 0.995 | 3.67 (−2.77, 10.10) | 0.245 | 4.00 (−0.97, 8.97) | 0.101 |
| Attempt Duration | 0.80 (−2.09, 3.69) | 0.718 | −1.47 (−4.52, 1.58) | 0.398 | −2.27 (−6.77, 2.23) | 0.367 |
| Reaching Duration | 0.48 (−2.22, 3.18) | 0.869 | −2.48 (−6.98, 2.02) | 0.310 | −2.96 (−6.56, 0.64) | 0.106 |
| Grasping Duration | −0.16 (−3.43, 3.10) | 0.989 | 1.24 (−2.17, 4.66) | 0.574 | 1.41 (−0.75, 3.56) | 0.209 |
| Male | ||||||
| Naïve vs. 6-OHDA | Naïve vs. Levodopa | 6-OHDA vs. Levodopa | ||||
| Variable | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value |
| Attempts/Trial (Performance) | −0.51 (−1.04, 0.03) | 0.062 | 0.52 (−0.16, 1.21) | 0.143 | 1.03 (0.31, 1.75) | 0.007 |
| Full Grasps | −6.67 (−27.28, 13.95) | 0.667 | 3.83 (−10.79, 18.46) | 0.764 | 10.50 (−3.9, 24.9) | 0.166 |
| Reach without Grasp | −5.17 (−21.82, 11.49) | 0.688 | 4.75 (−6.80, 16.30) | 0.527 | 9.92 (−1.80, 21.64) | 0.100 |
| Grasp without Pellet | −2.75 (−10.13, 4.63) | 0.588 | 5.58 (−1.02, 12.19) | 0.100 | 8.33 (1.2, 15.47) | 0.023 |
| Attempt Duration | 1.26 (−0.18, 2.69) | 0.089 | −2.88 (−7.45, 1.7) | 0.249 | −4.13 (−8.70, 0.44) | 0.078 |
| Reaching Duration | 0.88 (−1.72, 3.48) | 0.645 | −3.63 (−8.02, 0.75) | 0.108 | −4.51 (−10.26, 1.23) | 0.131 |
| Grasping Duration | 0.08 (−2.18, 2.34) | 0.995 | 0.46 (−1.33, 2.25) | 0.773 | 0.38 (−1.51, 2.28) | 0.852 |
| Highly Lesioned (R8, R9, R13) | ||||||
| Naïve vs. 6-OHDA | Naïve vs. Levodopa | 6-OHDA vs. Levodopa | ||||
| Variable | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value |
| Attempts/Trial (Performance) | 0.48 (−1.17, 2.14) | 0.340 | −0.23 (−0.65, 0.18) | 0.140 | −0.72 (−2.31, 0.88) | 0.190 |
| Full Grasps | 5.78 (−33.89, 45.45) | 0.590 | −5.89 (−22.25, 10.47) | 0.260 | −11.67 (−43.96, 20.62) | 0.260 |
| Reach without Grasp | 2.00 (−11.68, 15.68) | 0.590 | −1.89 (−14.29, 10.51) | 0.580 | −3.89 (−13.20, 5.42) | 0.210 |
| Grasp without Pellet | 3.22 (−10.21, 16.65) | 0.410 | −3.33 (−8.95, 2.28) | 0.130 | −6.55 (−17.82, 4.71) | 0.130 |
| Attempt Duration | −0.61 (−4.41, 3.20) | 0.560 | 4.55 (−4.35, 13.45) | 0.160 | 5.16 (−4.50, 14.81) | 0.150 |
| Reaching Duration | −2.95 (−4.34, −1.55) | 0.012 | 4.01 (−4.45, 12.47) | 0.180 | 6.95 (−2.82, 16.73) | 0.092 |
| Grasping Duration | 2.34 (−1.65, 6.33) | 0.130 | 0.54 (−1.23, 2.31) | 0.320 | −1.80 (−4.77, 1.18) | 0.120 |
| Mildly Lesioned (R6, R7, R10, R11) | ||||||
| Naïve vs. 6-OHDA | Naïve vs. Levodopa | 6-OHDA vs. Levodopa | ||||
| Variable | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value |
| Attempts/Trial (Performance) | −0.40 (−2.80, 2.01) | 0.640 | −0.97 (−2.75, 0.81) | 0.180 | −0.57 (−1.81, 0.67) | 0.240 |
| Full Grasps | −5.25 (−35.90, 25.41) | 0.620 | −12.08 (−41.77, 17.60) | 0.290 | −6.84 (−12.88, −0.79) | 0.037 |
| Reach without Grasp | 3.83 (−5.56, 13.23) | 0.280 | −1.67 (−14.77, 11.44) | 0.710 | −5.50 (−24.30, 13.31) | 0.420 |
| Grasp without Pellet | 0.66 (−8.74, 10.07) | 0.790 | −6.56 (−24.04, 10.93) | 0.250 | −7.22 (−23.77, 9.33) | 0.200 |
| Attempt Duration | −1.34 (−1.97, −0.72) | 0.006 | 0.58 (−1.80, 2.96) | 0.500 | 1.92 (−0.88, 4.72) | 0.120 |
| Reaching Duration | 0.48 (−1.65, 2.61) | 0.530 | 2.49 (−0.85, 5.83) | 0.098 | 2.01 (−0.46, 4.48) | 0.081 |
| Grasping Duration | −1.82 (−4.38, 0.73) | 0.110 | −1.91 (−4.68, 0.85) | 0.120 | −0.09 (−0.77, 0.59) | 0.710 |
| Naïve vs. 6-OHDA | Naïve vs. Levodopa | 6-OHDA vs. Levodopa | ||||
|---|---|---|---|---|---|---|
| Variable | Mean Difference (95% CI) | p-Value | Mean Difference (95% CI) | p-Value | Mean Difference (95% CI) | p-Value |
| Calcium events | −38.92 (−81.3 to 3.42) | 0.068 | −27.4 (−69.7 to 14.99) | 0.185 | 11.6 (−30.8 to 53.91) | 0.563 |
| Frequency of calcium events | 0.0001 (−0.02 to 0.02) | 0.992 | 0.01 (−0.01 to 0.04) | 0.222 | 0.01 (−0.01 to 0.04) | 0.225 |
| Calcium influx magnitude | −0.004 (−0.14 to 0.13) | 0.948 | −0.12 (−0.25 to 0.02) | 0.090 | −0.11 (−0.25 to 0.02) | 0.101 |
| Low Lesion (R1, R4) | ||||||
| Naïve vs. 6-OHDA | Naïve vs. Levodopa | 6-OHDA vs. Levodopa | ||||
| Variable | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value |
| Average Calcium Events (Hz) | 0.04 (0.001, 0.08) | 0.045 | 0.04 (−0.001, 0.08) | 0.056 | −0.002 (−0.04, 0.04) | 0.897 |
| Average Calcium Influx (z score*s) | −0.20 (−0.38, −0.02) | 0.038 | −0.12 (−0.31, 0.06) | 0.164 | −0.08 (−0.11, 0.26) | 0.367 |
| Mild Lesion (R3, R6, R7) | ||||||
| Naïve vs. 6−OHDA | Naïve vs. Levodopa | 6−OHDA vs. Levodopa | ||||
| Variable | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value |
| Average Calcium Events (Hz) | −0.02 (−0.05, 0.01) | 0.238 | −0.007 (−0.04, 0.03) | 0.639 | 0.01 (−0.02, 0.04) | 0.453 |
| Average Calcium Influx (z score*s) | 0.06 (−0.09, 0.21) | 0.356 | −0.02 (−0.17, 0.13) | 0.749 | −0.09 (−0.24, 0.06) | 0.226 |
| High Lesion (R2, R8) | ||||||
| Naïve vs. 6−OHDA | Naïve vs. Levodopa | 6−OHDA vs. Levodopa | ||||
| Variable | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value |
| Average Calcium Events (Hz) | −0.01 (−0.10, 0.03) | 0.457 | 0.02 (−0.02, 0.06) | 0.227 | 0.04 (−0.004, 0.08) | 0.069 |
| Average Calcium Influx (z score*s) | 0.09 (−0.10, 0.27) | 0.301 | −0.25 (−0.43, −0.07) | 0.014 | −0.34 (−0.52, −0.16) | 0.003 |
| Frequency of Calcium Events (Hz) | ||||||
| Low Lesion (R1, R4) | ||||||
| Success X Success | Failed X Failed | Success X Failed | ||||
| Variable | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value |
| Naïve vs. Naïve | - | - | - | - | 0.04 (−0.11, 0.12) | >0.999 |
| Naïve vs. 6-OHDA | 0.12 (0.006, 0.23) | 0.031 | 0.10 (−0.002, 0.21) | 0.057 | - | - |
| Naïve vs. Levodopa | 0.02 (−0.13, 0.17) | 0.100 | −0.03 (−0.17, 0.10) | 0.986 | - | - |
| 6-OHDA vs. 6-OHDA | - | - | - | - | −0.006 (−0.08, 0.07) | 0.100 |
| 6-OHDA vs. Levodopa | −0.10 (−0.22, 0.02) | 0.184 | −0.14 (−0.27, −0.00) | 0.048 | - | - |
| Levodopa vs. Levodopa | - | - | - | - | −0.04 (−0.19, 0.11) | 0.962 |
| Mild Lesion (R3, R6, R7) | ||||||
| Success X Success | Failed X Failed | Success X Failed | ||||
| Variable | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value |
| Naïve vs. Naïve | - | - | - | - | -0.03 (-0.15, 0.09) | 0.100 |
| Naïve vs. 6-OHDA | −0.41 (−0.56,−0.25) | <0.001 | −0.30 (−0.47, −0.12) | <0.001 | - | - |
| Naïve vs. Levodopa | −0.08 (−0.20, 0.04) | 0.465 | −0.07 (−0.21, 0.07) | 0.762 | - | - |
| 6-OHDA vs. 6-OHDA | - | - | - | - | 0.08 (−0.04, 0.20) | 0.353 |
| 6-OHDA vs. Levodopa | 0.33 (0.23, 0.43) | <0.001 | 0.23 (0.13, 0.32) | <0.001 | - | - |
| Levodopa vs. Levodopa | - | - | - | - | −0.01 (−0.12, 0.09) | 0.998 |
| High Lesion (R2, R8) | ||||||
| Success X Success | Failed X Failed | Success X Failed | ||||
| Variable | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value |
| Naïve vs. Naïve | - | - | - | - | −0.01 (−0.10, 0.09) | >0.999 |
| Naïve vs. 6-OHDA | −0.13 (−0.32, 0.07) | 0.415 | −0.10 (−0.28, 0.08) | 0.589 | - | - |
| Naïve vs. Levodopa | 0.11 (0.01, 0.21) | 0.018 | 0.09 (−0.01, 0.20) | 0.138 | - | - |
| 6-OHDA vs. 6-OHDA | - | - | - | - | 0.02 (−0.18, 0.22) | 0.100 |
| 6-OHDA vs. Levodopa | 0.24 (0.05, 0.42) | 0.003 | 0.20 (0.01, 0.38) | 0.032 | - | - |
| Levodopa vs. Levodopa | - | - | - | - | −0.02 (−0.09, 0.05) | 0.969 |
| Average Calcium Influx | ||||||
| Low Lesion (R1, R4) | ||||||
| Success X Success | Failed X Failed | Success X Failed | ||||
| Variable | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value |
| Naïve vs. Naïve | - | - | - | - | 0.11 (−0.52, 0.74) | 0.995 |
| Naïve vs. 6-OHDA | −0.78 (−1.18, −0.39) | <0.001 | −0.91 (−1.52, −0.30) | <0.001 | - | - |
| Naïve vs. Levodopa | −0.24 (−0.71, 0.22) | 0.660 | −0.33 (−0.10, 0.33) | 0.713 | - | - |
| 6-OHDA vs. 6-OHDA | - | - | - | - | −0.01 (−0.30, 0.27) | >0.999 |
| 6-OHDA vs. Levodopa | 0.54 (0.24, 0.84) | <0.001 | 0.58 (0.09, 1.08) | 0.011 | - | - |
| Levodopa vs. Levodopa | - | - | - | - | 0.03 (−0.38, 0.43) | >0.999 |
| Mild Lesion (R3, R6, R7) | ||||||
| Success X Success | Failed X Failed | Success X Failed | ||||
| Variable | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value |
| Naïve vs. Naïve | - | - | - | - | 0.07 (−0.14, 0.27) | 0.940 |
| Naïve vs. 6-OHDA | −0.08 (−0.30, 0.15) | 0.929 | −0.09 (−0.38, −0.19) | 0.944 | - | - |
| Naïve vs. Levodopa | −0.07 (−0.34, 0.21) | 0.984 | 0.12 (−0.22, 0.46) | 0.922 | - | - |
| 6-OHDA vs. 6-OHDA | - | - | - | - | 0.05 (−0.23, 0.33) | 0.996 |
| 6-OHDA vs. Levodopa | 0.01 (−0.31, 0.33) | >0.999 | 0.21 (−0.17, 0.58) | 0.608 | - | - |
| Levodopa vs. Levodopa | - | - | - | - | 0.25 (−0.09, 0.59) | 0.284 |
| High Lesion (R2, R8) | ||||||
| Success X Success | Failed X Failed | Success X Failed | ||||
| Variable | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value |
| Naïve vs. Naïve | - | - | - | - | −0.43 (−0.84, -0.02) | 0.034 |
| Naïve vs. 6-OHDA | 0.22 (−0.20, 0.64) | 0.649 | 0.44 (−0.18, 1.06) | 0.329 | - | - |
| Naïve vs. Levodopa | 0.15 (−0.10, 0.40) | 0.556 | 0.60 (0.10, 1.10) | 0.009 | - | - |
| 6-OHDA vs. 6-OHDA | - | - | - | - | −0.21 (−0.70, 0.29) | 0.826 |
| 6-OHDA vs. Levodopa | −0.08 (−0.42, 0.27) | 0.988 | 0.16 (−0.40, 0.72) | 0.968 | - | - |
| Levodopa vs. Levodopa | - | - | - | - | 0.02 (−0.31, 0.35) | >0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boschen, S.L.; Seethaler, J.; Wang, S.; Lujan, W.D.; Silvernail, J.L.; White, L.J.; Heckman, M.G.; Carter, R.E.; Chang, S.-Y.; Lujan, J.L. Dopaminergic Degeneration Differentially Modulates Primary Motor Cortex Activity and Motor Behavior in Hemiparkinsonian Rats. Brain Sci. 2025, 15, 1123. https://doi.org/10.3390/brainsci15101123
Boschen SL, Seethaler J, Wang S, Lujan WD, Silvernail JL, White LJ, Heckman MG, Carter RE, Chang S-Y, Lujan JL. Dopaminergic Degeneration Differentially Modulates Primary Motor Cortex Activity and Motor Behavior in Hemiparkinsonian Rats. Brain Sciences. 2025; 15(10):1123. https://doi.org/10.3390/brainsci15101123
Chicago/Turabian StyleBoschen, Suelen L., Julian Seethaler, Shaohua Wang, Wendy D. Lujan, Jodi L. Silvernail, Launia J. White, Michael G. Heckman, Rickey E. Carter, Su-Youne Chang, and J. Luis Lujan. 2025. "Dopaminergic Degeneration Differentially Modulates Primary Motor Cortex Activity and Motor Behavior in Hemiparkinsonian Rats" Brain Sciences 15, no. 10: 1123. https://doi.org/10.3390/brainsci15101123
APA StyleBoschen, S. L., Seethaler, J., Wang, S., Lujan, W. D., Silvernail, J. L., White, L. J., Heckman, M. G., Carter, R. E., Chang, S.-Y., & Lujan, J. L. (2025). Dopaminergic Degeneration Differentially Modulates Primary Motor Cortex Activity and Motor Behavior in Hemiparkinsonian Rats. Brain Sciences, 15(10), 1123. https://doi.org/10.3390/brainsci15101123











