How Swimming Modulates Inflammatory Pathways in Pain, Neurodegenerative, and Metabolic Disorders
Abstract
1. Introduction
1.1. Swimming
1.2. Swimming in Medical Sciences
1.3. Inflammation and Inflammatory Diseases
2. Methods
3. Results and Discussion
3.1. Neurological Disorders
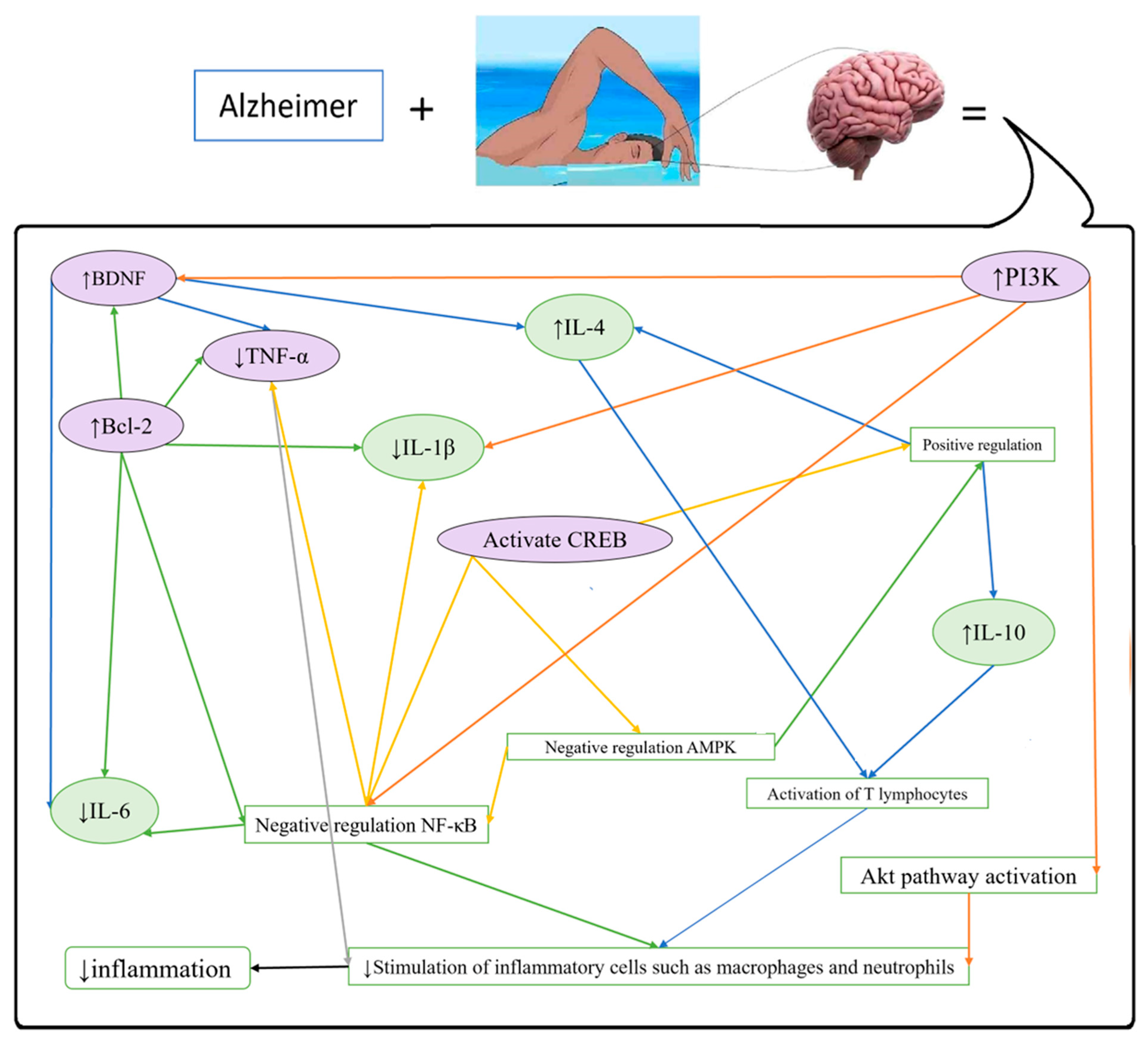
3.1.1. Alzheimer’s Disease (AD)
- There was an increase in SEMA3A and a decrease in PFN1 and NCAM in the AD-like control group. The opposite trend was observed in the healthy controls.
- There was a reduction in SEMA3A and an increase in NCAM and PFN1 in the healthy trained (HT) group compared to the healthy controls.
3.1.2. Parkinson’s Disease (PD)
| Row | Type of Disease | Inducing Agent | Intervention | Results | Reference |
|---|---|---|---|---|---|
| 1 | Alzheimer’s Disease (AD) | D-galactose intraperitoneally (i.p.) | Exercise (EXE) for 8 weeks |
| [23] |
| 2 | AD | Hippocampus injection of β-amyloid peptide (Aβ) | Swimming (20 sessions) |
| [21] |
| 3 | AD | Aβ (stereotaxic intracerebral injection) | Swimming training (ST) (30 min a day, 3 weeks) daily clove supplement (0.1 mg/kg) gavage |
| [24] |
| 4 | AD | Aβ oligomer | EXE (4 weeks) |
| [25] |
| 5 | AD | Intracerebroventricular (ICV) injection of streptozotocin (STZ) | ST (5 weeks) Oral carnosine (100 mg/kg/day) |
| [26] |
| 6 | AD | STZ (i.p.) | ST (4 weeks) |
| [27] |
| 7 | AD | Aluminium chloride (70 mg/kg, i.p.) | Vinpocetine (20 mg/kg, p.o.) Coenzyme Q10 (200 mg/kg, orally) Swimming and Y-maze tests once per week for four weeks |
| [33] |
| 8 | Neuropathies | Brachial plexus avulsion (BPA) surgery is performed on the unilateral brachial plexus. | After BPA surgery Cold-water swimming or sham training for 5 min twice a day for a period of two weeks |
| [34] |
| 9 | Neuropathies | Nerve compression for 30 s using hemostatic forceps | Sericin silk protein (hydrolyzed sericin is applied directly to the injury in the injury-sericin and injury-sericin-swimming groups). Swimming 5 days per week for 3 weeks, with a 10% overload of the body weight (weekly progressive elevation, lasting 15, 20, and 25 min/day). |
| [35] |
| 10 | Parkinson | 6-Hydroxydopamine (6-OHDA) was injected unilaterally | Melatonin ST at moderate intensity (30 min, 5 times a week, 6 weeks) |
| [29] |
| 11 | Parkinson | 6-OHDA (injected unilaterally into the medial forebrain bundle) | Daily ST (30 min, 6 weeks) |
| [32] |
| 12 | Parkinson | 6-OHDA (stereotaxic intracerebral injection) | ST (4 weeks) |
| [36] |
| 13 | Osteoarthritis | Mouse model of aged knee osteoarthritis (OA) through natural aging of mice | Adaptive swimming for 1 week and formal swimming for 8 weeks (15 min, once a day, 3 days a week, for a total period of 8 weeks) |
↓ Proteoglycan
| [37] |
| 14 | Osteoarthritis | 100 μL monosodium iodoacetate was injected intra-articularly into the right knee | Oral saline (4 weeks) ST (20 min) Oral curcumin 200 mg/kg till the end of the experimental period (35 days). |
↓ Pain and joint stiffness Development of histological and radiological osteoarthritis occurrence in the knee joints ↓ Serum C-reactive protein (CRP) and tissue cartilage oligomeric matrix protein levels Restoring the miR-130a and histone deacetylase 3
Increased peroxisome proliferator-activated receptor gamma (PPAR-γ) alongside ↓ nuclear factor kappa light-chain-enhancer of activated B lymphocytes (NF-κB) and its inflammatory cytokine targets TNF-α and IL-1β. ↓ Matrix metalloproteinase-1 and MMP-13 | [38] |
| 15 | Osteoarthritis | 100 mL of complete Freund’s adjuvant (CFA), inactivated M. butyricum (1 mg/mL), or 100 mL of saline (control group), was injected into the intra-articular space of the left ankle. | The stimulation of electroacupuncture (EA) was utilized at acupoints ST36 and GB30 on the left ankle (5 times a week, lasting for 30 min) The protocol consists of 10 min of swimming three times a week. The EA + swimming group received different EA sessions, three times a week swimming, and twice a week EA sessions. |
| [39] |
| 16 | Osteoarthritis | Median meniscectomy | Treadmill protocol: In weeks 1 and 2, the running time was 30 min with a 13 m/min speed, without incline. The execution time in week 3 was 30 min, and in weeks 4, 5, and 6 was 50 min. In weeks 3, 4, 5, and 6, the speed was 16 m/min. Training intensities and volumes were light to moderate (50% and 60% of maximal oxygen uptake [VO2max]). Swimming protocol: The adaptation period was 20 min per day for 1 week. A swimming program, consisting of 6 weeks and 20 min of daily sessions, has been conducted in alternating sessions on weekdays. |
| [40] |
| 17 | Osteoarthritis | Injection of papain in the knees: a total of 0.2 mL of 4% papain solution, 0.1 mL of 0.03 M cysteine (activator) was injected intra-articularly with a microsyringe into the right knee of the animal | All treatments were initiated 21 days after the final papain injection and were administered once daily, three times a week (every other day), for eight consecutive weeks, totaling 24 treatment sessions. Sodium diclofenac gel (10 mg∕knee applied) Swimming EXE (2 weeks adapt + 6 weeks training) PBMT: using a laser diode with a wavelength of 830 nm (infrared) continuously, spot area of 0.028 cm2, power of 100 mW, power density of 35.71 W∕cm2, energy density of 214.2 J∕cm2, energy of 6 J per point, 60 s per point, and 1 point on the OA joint. |
| [41] |
| 18 | Rheumatoid Arthritis | Age (young: 4 weeks; middle-aged: 14 months; rats) | ST for 9 weeks |
| [7] |
| 19 | Rheumatoid Arthritis | Bovine type II collagen (1 mg/mL) was injected into the right hind paw of each animal | ST (6 weeks, 5 days/week, 60 min/day) |
| [42] |
| 20 | Gout | Oxonic acid (1 mL/kg) and hypoxanthine (administered via diet) | Swimming in cold water at 10–12 °C, 10 min every day, for 12 days |
| [43] |
| 21 | Renal Disorders | Age (21 months old) | 12 weeks of moderate swimming exercise in aged rats (21 months old) |
| [44] |
| 22 | Renal Disorders | High-sodium diet (Addition of NaCl up to 2% w/w yielding a 0.90% w/w Na + chow) | 4-day adaptation period (15 min swimming on the first day and 15 min elevation in swimming time each day until reaching 60 min on the fourth day). From the second week on, training was done in the morning period, from 09:00 to 11:00 h, 5 days/week for 2 h straight and free of loading for 9 weeks |
| [45] |
| 23 | Renal Disorders | Doxorubicin injection (i.p.) | ST Garlic extract |
| [46] |
| 24 | Renal Disorders | Two-kidney, one-clip procedure using a silver clip (internal diameter 0.25 mm) to induce renovascular hypertension (RVH) | Nine weeks of swimming (Wistar albino rats) after RVH |
| [47] |
| 25 | Renal Disorders | Experimental autoimmune encephalomyelitis was induced in 4 weeks | Regular EXE (forced swimming) for 6 weeks |
| [48] |
| 26 | Liver Disorders | Subcutaneous injection of myelin oligodendrocyte glycoprotein emulsified in CFA. Intraperitoneal injection of pertussis toxin (twice, two days apart) | 6-week swimming EXE |
| [49] |
| 27 | Liver Disorders | HFD-induced non-alcoholic fatty liver disease | HFD and EXE were provided for 12 consecutive weeks |
| [50] |
| 28 | Liver Disorders | HFD (To induce a non-alcoholic fatty liver model in 6 weeks) | Swimming EXE (5 d/wk for 8 wk) + silymarin and vitamin C (supplemental gavage for 8 wk) |
| [6] |
| 29 | Liver Disorders | N/A | Group 1: sedentary (no EXE). Group 2: two weeks of adaptation (10 min swimming, followed by daily increments of 5 min until reaching 60 min) Group 3: two weeks of adaptation + one intense training session (intense EXE without any previous training). Group 4: two weeks of adaptation + 18 weeks of moderate ST (in which rats swam 60 min every other day, three times a week) + one session of intense swimming EXE. |
| [51] |
| 30 | Liver Disorders | Standard chow (10% lipid) or high-fat chow (60% lipid) for 22 weeks. | EXE protocol: 10 weeks of swimming, the first two weeks adaptation (6 min/day until 60 min/day, 5 times/week) without an increase in weight in the tail. Eight weeks with 40–60% of VO2max |
| [52] |
| 31 | Liver Disorders | Athymic BALB/c nude mice were implanted orthotopically with human liver cancer cells with high metastatic potential | Moderate swimming (8 min/day, 9 weeks) overload swimming (16 and 32 min/day, 9 weeks) |
↑ DA levels in the prefrontal cortex, serum, and tumor tissue suppressed growth, decreased lung metastasis of transplanted liver cancer, and prolonged survival suppressed the TGF-β1
| [53] |
| 32 | Liver Disorders | Young (4 weeks old) and middle-aged (14 months old) animals. 60–90 min of swimming EXE daily, 5 days per week for 9 weeks. Middle-aged (18-month-old) and old (28-month-old) male rats. Four times a week, 60–90 min/day of treadmill exercise for 9 weeks. |
↓ Protein carbonyl Proteasome activity was increased
↓ The binding of transcription factor NF-κB to the target DNA ↑ Reduced glutathione in the liver of old rats | [54] | |
| 33 | Liver Disorders | The normal control group had a normal diet, and the other groups were all fed a 60% kcal fat HFD for 16 weeks. | Chronic and acute swimming EXE training | Both long-term and short-term swimming EXE training
| [55] |
| 34 | Liver Disorders | The swimming protocol for exercise groups in the first week was as follows: 1st day, 10 min; 2nd day, 20 min; 3rd day, 30 min; 4th day, 40 min; and 5th day, 50 min. In the fructose-enriched diet (FED) group, 20% fructose (w/v) was mixed into the drinking water of the rats for 16 weeks. |
| [56] | |
| 35 | Liver Disorders | Obesity induced by HFD | Vitamin D and Swimming EXE (30 min, 5 days a week, 6 weeks) |
| [57] |
| 36 | Reproductive System Disorders | HFD-induced obesity | Variable EXE loads (5 days per week, 8 weeks) Gradually elevated training load to 2 h per day (moderate intensity group with obesity) and 2 h twice a day high-intensity group with obesity) |
| [58] |
| 37 | Pancreatic disorders | HFD, STZ (30 mg/kg, i.p.) Ovariectomized (OVX) | Swimming EXE (1 h/day, 8 weeks) |
| [59] |
| 38 | Pancreatic disorders | HFD (4 weeks) and STZ (35 mg/kg, i.p.) | 12 weeks of swimming |
| [60] |
| 39 | Pancreatic disorders | Control group, exercised group (3 weeks of swimming EXE), stressed group (3 weeks of immobilization stress), and stressed group practicing exercise (3 weeks of EXE, concomitant with 21 daily sessions of stress) |
↑ Pancreatic IL-10 and total antioxidant capacity ↓ Pancreatic TNF-α and malondialdehyde | [61] | |
| 40 | Pancreatic disorders | HFD and STZ (35 mg/kg, i.p.) | Swimming (60 min/5 days a week) for 10 weeks |
| [62] |
| 41 | Pancreatic disorders | Obese Zucker diabetic fatty (ZDF) rats were used | Swimming 1 h/day, 3 days/week, for 11 weeks |
| [63] |
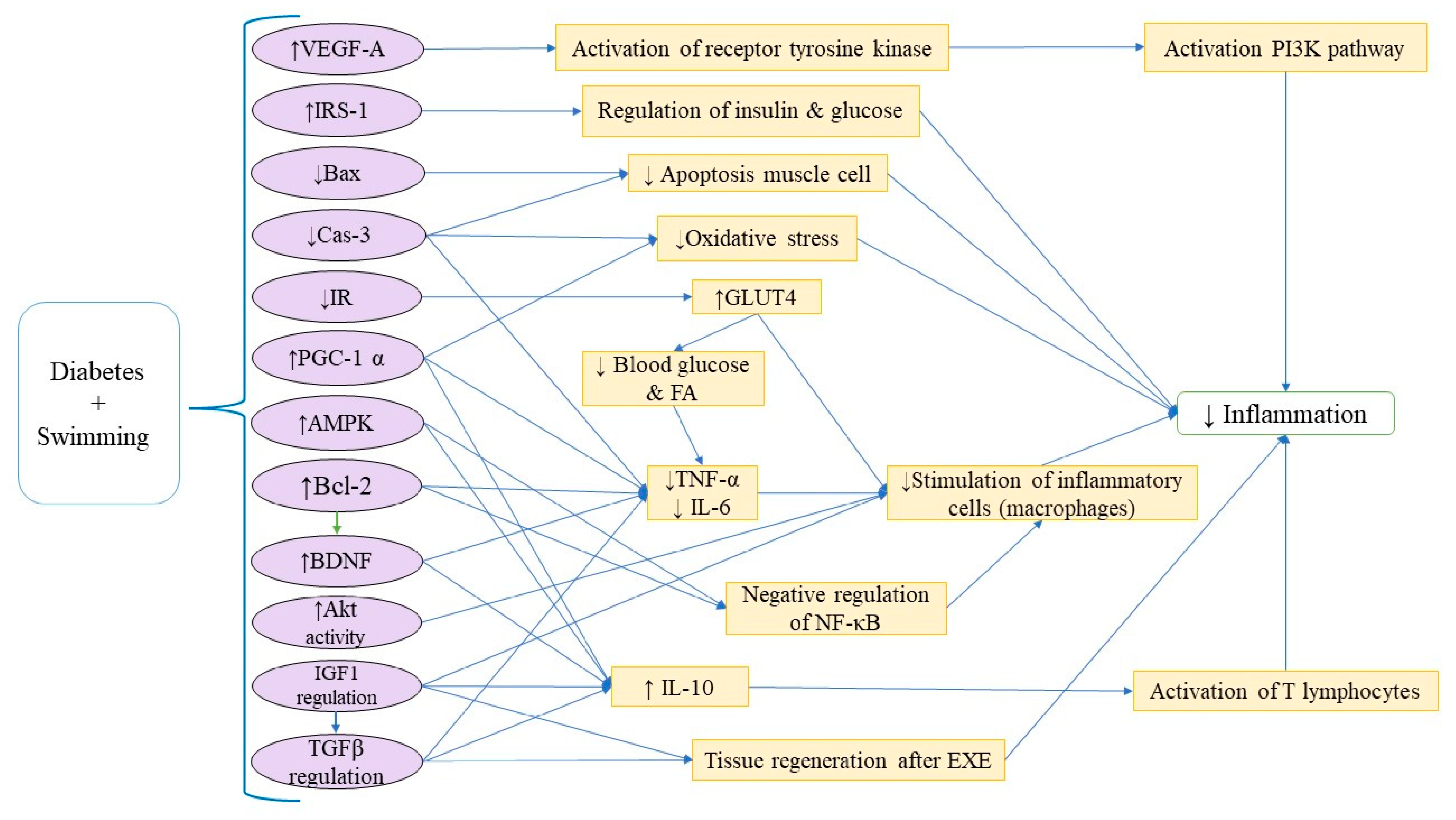
| 42 | Diabetes and Related Disorders | HFD for 2 weeks + STZ (35 mg/kg, i.p.) | ST for four weeks |
| [5] |
| 43 | Diabetes and Related Disorders | HFD (4 weeks) STZ (35 mg/kg, i.p.) | Swimming (30 min, 3 d/w, 8 weeks) Metformin (MET) (100 mg/kg) |
| [64] |
| 44 | Diabetes and Related Disorders | 12-week HFD STZ (i.p.) | Swimming: Gradually increased to 60 min/day without weights over 8 weeks. Resistance training: Ladder climbing with load progression from body weight to 100%, 60 min/day. Aerobic exercise: Treadmill running increased to 15 m/min for 60 min/day. HIIT (high-intensity interval training): Treadmill intervals (10 × 4 min high-intensity runs with 2 min rest), speed increased from 16 to 26 m/min. |
| [65] |
| 45 | Diabetes and Related Disorders | STZ injection (50 mg/kg, i.p.) | 4 weeks of swimming |
| [66] |
| 46 | Diabetes and Related Disorders | High-sugar and high-fat diet A single of 1% STZ | Non-weight-bearing swimming, 60 min per day, 6 days per week for 8 weeks | In the diabetic exercise group:
| [67] |
| 47 | Diabetes and Related Disorders | 6-week HFD and STZ (i.p.) | Sodium-glucose transport-2 inhibitor dapagliflozin swimming |
| [68] |
| 48 | Diabetes and Related Disorders | Bilateral ovariectomy, HFD feeding, single dose of STZ | 8 weeks of swimming | There was a major difference in the protein expression between the exercise and OVX diabetic groups | [69] |
| 49 | Diabetes and Related Disorders | STZ (i.p.) | DIG group (pregnant diabetic rats): insulin 5 U/day, i.p (2 U at 10 am and 3 U at 7 pm) DIEG group (pregnant diabetic rats treated with insulin and subjected to swimming): one dose of 2 U/day at 7 pm |
| [70] |
| 50 | Diabetes and Related Disorders | OVX | Genistein (1 mg/kg, eight weeks; daily subcutaneous [s.c.]) 8 weeks of swimming |
| [71] |
| 51 | Diabetes and Related Disorders | High-fat diet (60%) for 45 days, then STZ (40 mg/kg body) and nicotinamide (200 mg/kg body) i.p. injection | Swimming EXE (40 min, 5 days per week) or oral melatonin (10 mg/kg bwt per day) alone or in combination. |
| [72] |
| 52 | Diabetes and Related Disorders | N-acetylcysteine (50 mg/kg daily, for 21 days, i.p.) anti-CD4/CD8 25 μg/mL on days 0, 7, 14, and 21 | Daily 30 min of swimming |
| [73] |
| 53 | Diabetes and Related Disorders | HFD (58% fat) STZ (35 mg/kg, i.p.) | Swimming (5 days per week for 4 weeks) |
| [74] |
| 54 | Diabetes and Related Disorders | Swimming (60 min/day, 5 days/week for 8 weeks) |
| [75] | |
| 55 | Diabetes and Related Disorders | A 5 × 5 mm fragment of the right uterine horn was sutured to the peritoneum to induce endometriosis. | Light EXE (swimming once a week) Moderate EXE (swimming 3 times a week) Intense EXE (swimming 5 times a week) |
| [76] |
| 56 | Diabetes and Related Disorders | HFD (45 days, 60% fat), followed by i.p. injection of STZ (40 mg/kg) and nicotinamide injection after 15 min | Melatonin supplement (5 mg/kg twice daily) Swimming (40 min/day, 5 days/week) |
| [77] |
| 57 | Diabetes and Related Disorders | HFD (12 weeks) | HIIT |
| [78] |
| 58 | Diabetes and Related Disorders | HFD STZ (35 mg/kg, i.p.) | Swimming (60 min/5 days a week) for 10 weeks |
| [62] |
| 59 | Diabetes and Related Disorders | HFD | Swimming (1 h/day for 5 days/week for 8 weeks) |
| [79] |
| 60 | Diabetes and Related Disorders | Genetic model- ZDF rats | Acute EXE: 1 session of swimming until exhaustion Long-term EXE: Initially, rats swam 15 min/d (5 d/wk) gradually increased in 1 week, 1 h/d, 3 d/wk, for 11 wks |
| [80] |
| 61 | Diabetes and Related Disorders | Genetic model- ZDF rats | Swimming (1 h/day 3 days/week for 12 weeks) |
| [81] |
| 62 | Cardiovascular Disorders | Isoproterenol (ISO) i.p. | Copper nanoparticle (CuNP) (1 mg/kg/day, orally, 4 weeks) Wortmannin (1 mg/kg/day, i.p.) for 4 weeks Swimming (90 min, 5 days/4 weeks) |
| [82] |
| 63 | Cardiovascular Disorders | Fed a 0.9% Na+ (equivalent to 2% NaCl) | ST (22 weeks) |
| [45] |
| 64 | Cardiovascular Disorders | D-galactose injection (i.p.) | ST in warm water 60 min/day, five days/week |
| [83] |
| 65 | Cardiovascular Disorders | RVH surgery | ST (9 weeks) |
| [47] |
| 66 | Cardiovascular Disorders | OVX rats | ST (4 weeks) Saline, an estrogen receptor beta agonist (diarylpropionitrile [DPN]), an estrogen receptor alpha agonist (propyl pyrazole triol [PPT]), or oxytocin |
| [84] |
| 67 | Cardiovascular Disorders | HFD (75 days) | Four groups: 1: HL, sedentary, subjected to swimming stress (5 min per day, 5 times per week); 2: NAT submitted to a swimming protocol (1 h per day, 5 times per week) from the 16th day of the experiment; 3: PRO, sedentary, submitted to swimming stress and received oral propolis extract from the 16th day of the experiment; 4: HL + NAT + PRO were swum and received propolis | Swimming and propolis alone and in combination:
| [85] |
| 68 | Cardiovascular Disorders | mice with chronic Chagas disease | 30 min/daily, 5 times a week, 8 weeks |
| [86] |
| 69 | Cardiovascular Disorders | ISO (S.c.) | Flaxseed supplementation (6 weeks) ST | Flaxseed supplementation + ST:
| [87] |
| 70 | Cardiovascular Disorders | Treadmill running/ST |
| [88] | |
| 71 | Cardiovascular Disorders | Surgical ligation of the left coronary artery. | ST (60 min/day, 5 days/week, for 8 weeks) |
| [89] |
3.1.3. Neuropathies
3.2. Rheumatological Disorders
3.2.1. Osteoarthritis
3.2.2. Rheumatoid Arthritis (RA)
3.2.3. Gout
3.3. Renal Disorders
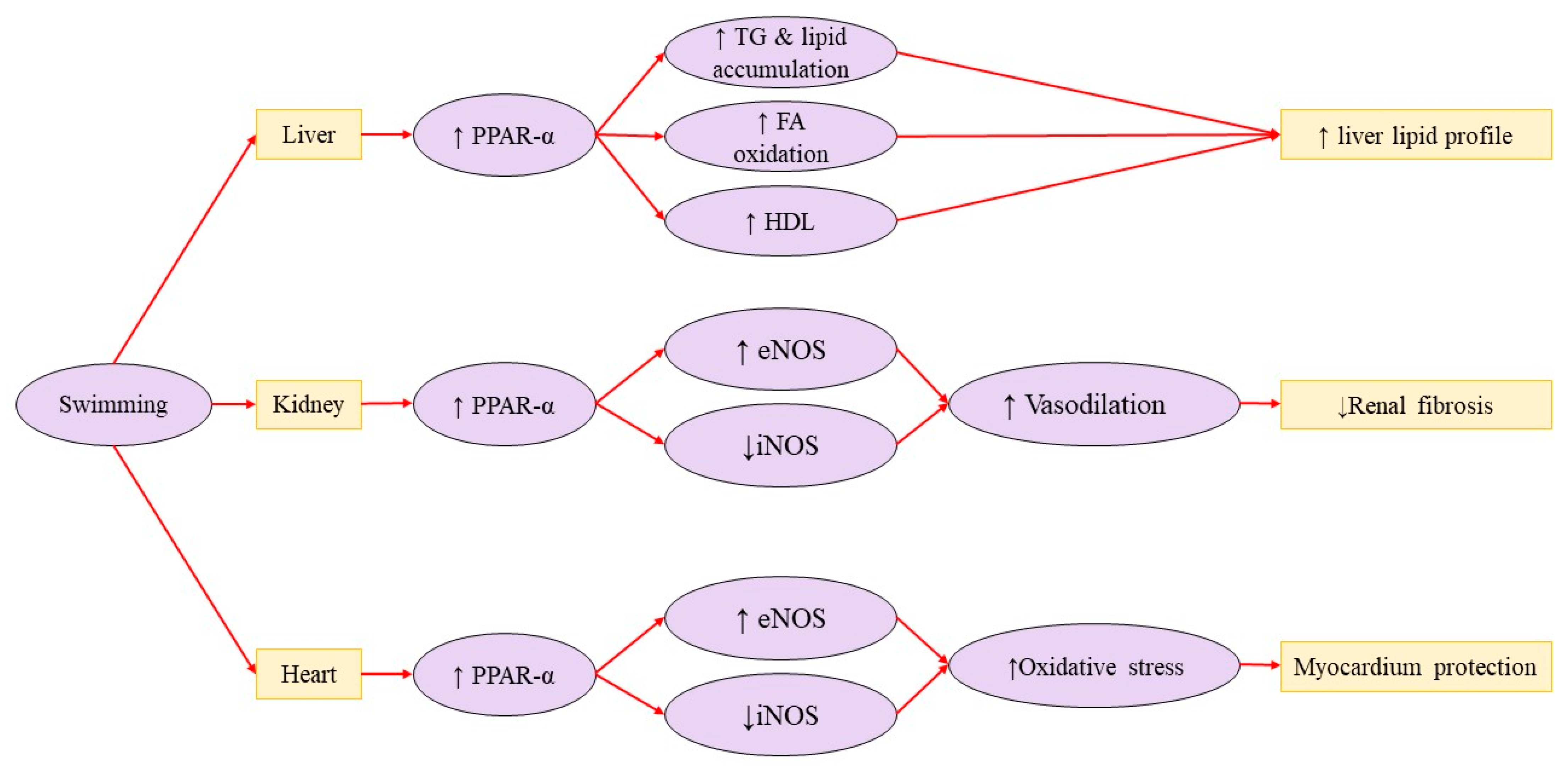
3.4. Liver Disorders
3.5. Reproductive System Disorders
3.6. Pancreatic Disorders
3.7. Respiratory Disorders
3.8. Diabetes and Related Disorders
3.9. Cardiovascular Disorders
3.10. Negative Effects of Swimming
4. Current and Future Perspectives
- Reduces inflammation, activates the TAN1/PI3K/CREB signaling pathway, decreases miR-34a levels, and preserves neuronal function and survival by preventing extra demyelination and inflammatory infiltration in the CNS [23].
- Significantly enhances axon regeneration and neuronal creation in motor neurons [21].
- Improves AD-induced alterations in α7nAChR, NLRP1, memory, and dark cells [24].
- Improves the neurogenesis and behavioral performance in adult neurogenesis mouse models of AD [25].
- Normalizes the hippocampal FNDC5/irisin expression (related to the reduced soluble β-amyloid peptide and phosphorylated tau protein, improved BDNF and insulin signaling proteins, and corresponding mitigation of cognitive impairments) [26].
- Increases BDNF, lowers both glutamate hippocampal concentration and TNF-α, and decreases neurobehavioral dysfunctions in patients with AD [27].
5. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 6-OHDA | 6-Hydroxydopamine |
| HSP70 | 70 kilodalton heat shock proteins |
| AD | Alzheimer’s disease |
| AMPK | AMP-activated protein kinase |
| AS | Ankylosing spondylitis |
| ADB | Anxiety and depression-like behavior |
| ApoE | Apolipoprotein E |
| Bcl-2 | B-cell lymphoma-2 |
| Bax | Bcl-2-associated X protein |
| BPA | Brachial plexus avulsion |
| BP | Blood pressure |
| BDNF | Brain-derived neurotrophic factor |
| BrdU | Bromodeoxyuridine |
| CREB | cAMP response element-binding protein |
| cTnI | Cardiac troponin I |
| CV | Cardiovascular |
| Cas-3 | Caspase-3 |
| CSF | Cerebrospinal fluid |
| CHF | Chronic heart failure |
| CC16 | Clara cell protein |
| CWS | Cold-water swimming |
| CIA | Collagen-induced arthritis |
| CuNP | Copper nanoparticles |
| CRP | C-reactive protein |
| CKMB | Creatine kinase MB |
| cAMP | Cyclic adenosine monophosphate |
| DCM | Diabetic cardiomyopathy |
| DPN | Diarylpropionitrile |
| DBPs | Disinfection by-products |
| DA | Dopamine |
| DR2 | Dopamine type 2 receptor |
| EA | Electroacupuncture |
| eNOS | Endothelial nitric oxide synthase |
| eWAT | Epididymal white adipose tissue |
| EMT | Epithelial-mesenchymal transition |
| EXE | Exercise |
| EAE | Experimental autoimmune encephalomyelitis |
| FBG | Fasting blood glucose |
| FATP-4 | Fatty acid transport protein 4 |
| FNDC5 | Fibronectin type III domain-containing protein 5 |
| FeNO | Fractional exhaled nitric oxide |
| FED | Fructose-enriched diet |
| GFAP | Glial fibrillary acidic protein |
| GLUT4 | Glucose transporter type 4 |
| GR | Glutathione reductase |
| GSH | Glutathione |
| GSK-3β | Glycogen synthase kinase-3 beta |
| HRR | Heart rate reserve |
| HRV | Heart rate variability |
| HDL | High-density lipoprotein |
| HFD | High-fat diet |
| HIIT | High-intensity interval training |
| HS | High-sodium |
| HDAC | Histone deacetylase |
| HDAC3 | Histone deacetylase 3 |
| HOMA-IR | Homeostatic model assessment of insulin resistance index |
| IgA | Immunoglobulin A |
| iNOS | Inducible nitric oxide synthase |
| I-κB | Inhibitor of kappa B |
| IR | Insulin resistance |
| IGF-1 | Insulin-like Growth Factor-1 |
| IFN-γ | Interferon gamma |
| IL-1β | Interleukin-1 beta |
| IRAK1 | Interleukin-1 receptor-associated kinase 1 |
| IL-10 | Interleukin-10 |
| IL-2 | Interleukin-2 |
| IL-6 | Interleukin-6 |
| IL-4 | interleukin-4 |
| ICV | Intracerebroventricular |
| ISO | Isoproterenol |
| LDH | Lactate dehydrogenase |
| LDL | Low-density lipoprotein |
| MΦ | Macrophages |
| MDA | Malondialdehyde |
| MMP-9 | Matrix metalloproteinase-9 |
| MMP-13 | Matrix metalloproteinase-13 |
| VO2max | Maximal oxygen uptake |
| MET | Metformin |
| MPTP | Mitochondrial permeability transition pore |
| MCP-1 | Monocyte chemotactic protein-1 |
| MS | Multiple sclerosis |
| MI | Myocardial infarction |
| NAC | N-acetylcysteine |
| NCAM | Neural cell adhesion molecule |
| NeuN | Neuronal nuclei |
| NAD | Nicotinamide adenine dinucleotide |
| NO | Nitric oxide |
| NAFLD | Nonalcoholic fatty liver disease |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| ND | Normal diet |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| NF-κB | Nuclear factor kappa light-chain-enhancer of activated B lymphocytes |
| NOD | Non-obese diabetic |
| NLRP1 | NLR family pyrin domain containing 1 |
| NLRP3 | NLR family pyrin domain containing 3 |
| OA | Osteoarthritis |
| OVX | Ovariectomized |
| OS | Oxidative stress |
| PD | Parkinson’s disease |
| PLIN2 | Perilipin-2 |
| PPAR-γ | Peroxisome proliferator-activated receptor gamma |
| PPAR-α | Peroxisome proliferator-activated receptor alpha |
| PTX3 | Pentraxin 3 |
| PI3K | Phosphoinositide 3-kinase |
| PBMT | Photobiomodulation therapy |
| pIgR | Polymeric immunoglobulin receptor |
| PSD95 | Postsynaptic density 95 |
| PFN1 | Profilin-1 |
| PGC-1α | Proliferator-activated receptor gamma coactivator-1 alpha |
| PPT | Propyl pyrazole triol |
| Akt | Protein kinase B |
| RBC | Red blood cell |
| ROS | Reactive oxygen species |
| RANKL | Receptor activator of nuclear factor kappa-Β ligand |
| RVH | Renovascular hypertension |
| RA | Rheumatoid arthritis |
| sIgA | Secretory immunoglobulin A |
| SCD1 | Severe combined immunodeficiency 1 |
| SG | Sebaceous gland |
| SEMA3A | Semaphorin 3A |
| SS | Serotonergic system |
| SIRT1 | Sirtuin 1 |
| SCRI | Skibinski’s cardio-respiratory index |
| STZ | Streptozotocin |
| SOD | Superoxide dismutase |
| ST | Swimming training |
| SNS | Sympathetic nervous system |
| sWAT | Substance white adipose tissue |
| SP-D | Surfactant protein D |
| SBP | Systolic blood pressure |
| TRAF6 | TNF receptor-associated factor 6 |
| TLR-4 | Toll-like receptor 4 |
| TCA | Total antioxidant capacity |
| TNF-α | Tumor necrosis factor alpha |
| T1D | Type 1 diabetes |
| T2D | Type 2 diabetes |
| T2DM | Type 2 diabetes mellitus |
| VEGF | Vascular endothelial growth factor |
| ZDF | Zucker diabetic fatty |
| α7nAChR | α7nicotinic acetylcholine receptor |
| Aβ | β-Amyloid peptide |
References
- Wei, W.; Lin, Z.; Xu, P.; Lv, X.; Lin, L.; Li, Y.; Zhou, Y.; Lu, T.; Xue, X. Diet Control and Swimming Exercise Ameliorate HFD-Induced Cognitive Impairment Related to the SIRT1-NF-κB/PGC-1α Pathways in ApoE−/− Mice. Neural Plast. 2023, 2023, 9206875. [Google Scholar] [CrossRef]
- Oja, P.; Memon, A.R.; Titze, S.; Jurakic, D.; Chen, S.-T.; Shrestha, N.; Em, S.; Matolic, T.; Vasankari, T.; Heinonen, A.; et al. Health Benefits of Different Sports: A Systematic Review and Meta-Analysis of Longitudinal and Intervention Studies Including 2.6 Million Adult Participants. Sports Med.-Open 2024, 10, 46. [Google Scholar] [CrossRef]
- Trinidad, A.; González-Garcia, H.; López-Valenciano, A. An Updated Review of the Epidemiology of Swimming Injuries. PMR 2021, 13, 1005–1020. [Google Scholar] [CrossRef]
- Mahdirejei, H.A.; Peeri, M.; Azarbayjani, M.A.; Fattahi Masrour, F. Fluoxetine combined with swimming exercise synergistically reduces lipopolysaccharide-induced depressive-like behavior by normalizing the HPA axis and brain inflammation in mice. Pharmacol. Biochem. Behav. 2023, 232, 173640. [Google Scholar] [CrossRef]
- Shekarchian, M.; Peeri, M.; Azarbayjani, M.A. Physical activity in a swimming pool attenuates memory impairment by reducing glutamate and inflammatory cytokines and increasing BDNF in the brain of mice with type 2 diabetes. Brain Res. Bull. 2023, 201, 110725. [Google Scholar] [CrossRef]
- Aghaei, F.; Wong, A.; Zargani, M.; Sarshin, A.; Feizolahi, F.; Derakhshan, Z.; Hashemi, M.; Arabzadeh, E. Effects of swimming exercise combined with silymarin and vitamin C supplementation on hepatic inflammation, oxidative stress, and histopathology in elderly rats with high-fat diet-induced liver damage. Nutrition 2023, 115, 112167. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Radák, Z.; Nyakas, C.; Hae, Y.C.; Naito, H.; Takahashi, R.; Nakamoto, H.; Abe, R. Regular exercise: An effective means to reduce oxidative stress in old rats. Ann. N. Y. Acad. Sci. 2004, 1019, 471–474. [Google Scholar] [CrossRef]
- Rahmanian-Devin, P.; Rakhshandeh, H.; Baradaran Rahimi, V.; Sanei-Far, Z.; Hasanpour, M.; Memarzia, A.; Iranshahi, M.; Askari, V.R. Intraperitoneal Lavage with Crocus sativus Prevents Postoperative-Induced Peritoneal Adhesion in a Rat Model: Evidence from Animal and Cellular Studies. Oxid. Med. Cell. Longev. 2021, 2021, 5945101. [Google Scholar] [CrossRef] [PubMed]
- Yahyazadeh, R.; Baradaran Rahimi, V.; Yahyazadeh, A.; Mohajeri, S.A.; Askari, V.R. Promising effects of gingerol against toxins: A review article. Biofactors 2021, 47, 885–913. [Google Scholar] [CrossRef] [PubMed]
- Bosak, F.; Baradaran Rahimi, V.; Sobhani, B.; Dabbaghi, M.M.; Soukhtanloo, M.; Zahedi Avval, F.; Askari, V.R. Evaluation of the Protective Effects of Noscapine on Paraquat-Induced Parkinson’s Disease in Rats. Mol. Neurobiol. 2025, 62, 11848–11876. [Google Scholar] [CrossRef]
- Baradaran Rahimi, V.; Askari, V.R.; Hosseini, M.; Yousefsani, B.S.; Sadeghnia, H.R. Anticonvulsant Activity of Viola tricolor against Seizures Induced by Pentylenetetrazol and Maximal Electroshock in Mice. Iran. J. Med. Sci. 2019, 44, 220–226. [Google Scholar] [CrossRef]
- Alamri, H.S.; Mufti, R.; Sabir, D.K.; Abuderman, A.A.; Dawood, A.F.; ShamsEldeen, A.M.; Haidara, M.A.; Isenovic, E.R.; El-Bidawy, M.H. Forced Swimming-Induced Depressive-like Behavior and Anxiety Are Reduced by Chlorpheniramine via Suppression of Oxidative and Inflammatory Mediators and Activating the Nrf2-BDNF Signaling Pathway. Curr. Issues Mol. Biol. 2023, 45, 6449–6465. [Google Scholar] [CrossRef]
- Askari, V.R.; Baradaran Rahimi, V.; Assaran, A.; Iranshahi, M.; Boskabady, M.H. Evaluation of the anti-oxidant and anti-inflammatory effects of the methanolic extract of Ferula szowitsiana root on PHA-induced inflammation in human lymphocytes. Drug Chem. Toxicol. 2020, 43, 353–360. [Google Scholar] [CrossRef]
- Akhlaghipour, I.; Shad, A.N.; Askari, V.R.; Maharati, A.; Rahimi, V.B. How caffeic acid and its derivatives combat diabetes and its complications: A systematic review. J. Funct. Foods 2023, 110, 105862. [Google Scholar] [CrossRef]
- Yoshida, S.; Hagiwara, Y.; Tsuchiya, M.; Shinoda, M.; Koide, M.; Hatakeyama, H.; Chaweewannakorn, C.; Suzuki, K.; Yano, T.; Sogi, Y. Involvement of inflammasome activation via elevation of uric acid level in nociception in a mouse model of muscle pain. Mol. Pain 2019, 15, 1744806919858797. [Google Scholar] [CrossRef]
- Askari, V.R.; Khosravi, K.; Baradaran Rahimi, V.; Garzoli, S. A Mechanistic Review on How Berberine Use Combats Diabetes and Related Complications: Molecular, Cellular, and Metabolic Effects. Pharmaceuticals 2024, 17, 7. [Google Scholar] [CrossRef]
- Rakhshandeh, H.; Rajabi Khasevan, H.; Saviano, A.; Mahdinezhad, M.R.; Baradaran Rahimi, V.; Ehtiati, S.; Etemad, L.; Ebrahimzadeh-Bideskan, A.; Maione, F.; Askari, V.R. Protective Effect of Portulaca oleracea on Streptozotocin-Induced Type I Diabetes-Associated Reproductive System Dysfunction and Inflammation. Molecules 2022, 27, 6075. [Google Scholar] [CrossRef]
- Salem, H.A.; Abu-Elfotuh, K.; Alzahrani, S.; Rizk, N.I.; Ali, H.S.; Elsherbiny, N.; Aljohani, A.; Hamdan, A.M.E.; Chellasamy, P.; Abdou, N.S.; et al. Punicalagin’s Protective Effects on Parkinson’s Progression in Socially Isolated and Socialized Rats: Insights into Multifaceted Pathway. Pharmaceutics 2023, 15, 2420. [Google Scholar] [CrossRef] [PubMed]
- Che Ramli, M.D.B.; Nizam, N.B.B.; Uzid, M.B.M.; Md Nazrey, N.A.B.; Mazlan, N.B.; Mohd Mizan, N.B.; Muhammad, H.; Hasan, M.K.N. The therapeutic effect of bacopa monnieri in treating parkinson’s disease. Int. J. Med. Toxicol. Leg. Med. 2022, 24, 206–211. [Google Scholar] [CrossRef]
- Ding, Z.; Du, L. Swimming exercise ameliorates depressive-like behavior by anti-inflammation activity, rebalancing gut Escherichia coli and Lactobacilli. Brain Res. 2022, 1797, 148113. [Google Scholar] [CrossRef] [PubMed]
- Farsani, M.S.; Fathi, M.; Farsani, Z.H.; Gourgin Karaji, Z. Swimming alters some proteins of skeletal muscle tissue in rats with Alzheimer-like phenotype. Arch. Gerontol. Geriatr. 2024, 117, 105260. [Google Scholar] [CrossRef]
- Xie, Y.; Wu, Z.; Sun, L.; Zhou, L.; Xiao, L.; Wang, H.; Wang, G. Swimming exercise reverses chronic unpredictable mild stress-induced depression-like behaviors and alleviates neuroinflammation and collapsing response mediator protein-2-mediated neuroplasticity injury in adult male mice. NeuroReport 2022, 33, 272–280. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, X.K.; Shao, W.Z.; Liu, Y.Q.; Tang, C.; Deng, S.S.; Tang, C.F.; Zheng, L.; Guo, W. miR-34a/TAN1/CREB Axis Engages in Alleviating Oligodendrocyte Trophic Factor-Induced Myelin Repair Function and Astrocyte-Dependent Neuroinflammation in the Early Stages of Alzheimer’s Disease: The Anti-Neurodegenerative Effect of Treadmill Exercise. Neurochem. Res. 2024, 49, 1105–1120. [Google Scholar] [CrossRef]
- Karaji, Z.G.; Fathi, M.; Mirnasori, R.; van der Zee, E.A. Swimming exercise and clove oil can improve memory by molecular responses modification and reduce dark cells in rat model of Alzheimer’s disease. Exp. Gerontol. 2023, 177, 112192. [Google Scholar] [CrossRef]
- Liu, Z.T.; Ma, Y.T.; Pan, S.T.; Xie, K.; Shen, W.; Lin, S.Y.; Gao, J.Y.; Li, W.Y.; Li, G.Y.; Wang, Q.W.; et al. Effects of involuntary treadmill running in combination with swimming on adult neurogenesis in an Alzheimer’s mouse model. Neurochem. Int. 2022, 155, 105309. [Google Scholar] [CrossRef]
- Hegazy, M.A.; Abdelmonsif, D.A.; Zeitoun, T.M.; El-Sayed, N.S.; Samy, D.M. Swimming exercise versus L-carnosine supplementation for Alzheimer’s dementia in rats: Implication of circulating and hippocampal FNDC5/irisin. J. Physiol. Biochem. 2022, 78, 109–124. [Google Scholar] [CrossRef]
- Bashiri, H.; Enayati, M.; Bashiri, A.; Salari, A.A. Swimming exercise improves cognitive and behavioral disorders in male NMRI mice with sporadic Alzheimer-like disease. Physiol. Behav. 2020, 223, 113003. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Yang, L.; Tucker, D.; Dong, Y.; Zhu, L.; Duan, R.; Liu, T.C.; Zhang, Q. Beneficial Effects of Exercise Pretreatment in a Sporadic Alzheimer’s Rat Model. Med. Sci. Sports Exerc. 2018, 50, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Gergin, S.; Kirazlı, Ö.; Boracı, H.; Yıldız, S.D.; Yananlı, H.R.; Şehirli, Ü.S. The effects of regular swimming exercise and melatonin on the neurons localized in the striatum of hemiparkinsonian rats. Anat. Sci. Int. 2023, 98, 204–219. [Google Scholar] [CrossRef] [PubMed]
- Skripkina, N.A.; Smolentseva, I.G.; Kuzmina, A.V.; Levin, O.S. Unusual failure of swimming skills in patients with Parkinson’s disease. Zhurnal Nevrologii i Psikhiatrii Imeni SS Korsakova 2021, 121, 76–79. [Google Scholar] [CrossRef]
- Neves, M.A.; Bouça-Machado, R.; Guerreiro, D.; Caniça, V.; Pona-Ferreira, F.; Ferreira, J.J. Swimming is compromised in Parkinson’s disease patients. Mov. Disord. 2020, 35, 365–369. [Google Scholar] [CrossRef]
- Boracı, H.; Kirazlı, Ö.; Gülhan, R.; Yıldız Sercan, D.; Şehirli, Ü.S. Neuroprotective effect of regular swimming exercise on calretinin-positive striatal neurons of Parkinsonian rats. Anat. Sci. Int. 2020, 95, 429–439. [Google Scholar] [CrossRef]
- Ali, A.A.; Abo El-Ella, D.M.; El-Emam, S.Z.; Shahat, A.S.; El-Sayed, R.M. Physical & mental activities enhance the neuroprotective effect of vinpocetine & coenzyme Q10 combination against Alzheimer & bone remodeling in rats. Life Sci. 2019, 229, 21–35. [Google Scholar] [CrossRef]
- Hsieh, Y.L.; Yang, N.P.; Chen, S.F.; Lu, Y.L.; Yang, C.C. Early Intervention of Cold-Water Swimming on Functional Recovery and Spinal Pain Modulation Following Brachial Plexus Avulsion in Rats. Int. J. Mol. Sci. 2022, 23, 1178. [Google Scholar] [CrossRef]
- Debastiani, J.C.; Santana, A.J.; Ribeiro, L.D.F.C.; Brancalhão, R.M.C.; Bertolini, G.R.F. Sericin silk protein in peripheral nervous repair associated with the physical exercise of swimming in Wistar rats. Neurol. Res. 2019, 41, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Goes, A.T.R.; Souza, L.C.; Filho, C.B.; Del Fabbro, L.; De Gomes, M.G.; Boeira, S.P.; Jesse, C.R. Neuroprotective effects of swimming training in a mouse model of Parkinson’s disease induced by 6-hydroxydopamine. Neuroscience 2014, 256, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.J.; Yang, Y.T.; Cao, Y.T.; Zheng, L.D.; Lin, K.L.; Zhu, R. Cartilage protective effect of swimming exercise in aged mice with knee osteoarthritis. Chin. J. Tissue Eng. Res. 2024, 28, 3170–3175. [Google Scholar] [CrossRef]
- Saber, M.M.; Mahmoud, M.M.; Amin, H.M.; Essam, R.M. Therapeutic effects of combining curcumin and swimming in osteoarthritis using a rat model. Biomed. Pharmacother. 2023, 166, 115309. [Google Scholar] [CrossRef]
- Martins, G.A.; Degen, A.N.; Antunes, F.T.T.; da Rosa, L.G.; Ferraz, A.G.; Wiilland, E.; Vieira, L.B.; de Souza, A.H. Benefits of electroacupuncture and a swimming association when compared with isolated protocols in an osteoarthritis model. J. Tradit. Complement. Med. 2022, 12, 375–383. [Google Scholar] [CrossRef]
- da Silva, L.A.; Thirupathi, A.; Colares, M.C.; Haupenthal, D.P.D.S.; Venturini, L.M.; Corrêa, M.E.A.B.; Silveira, G.D.B.; Haupenthal, A.; do Bomfim, F.R.C.; de Andrade, T.A.M.; et al. The effectiveness of treadmill and swimming exercise in an animal model of osteoarthritis. Front. Physiol. 2023, 14, 1101159. [Google Scholar] [CrossRef]
- Tomazoni, S.S.; Leal-Junior, E.C.; Frigo, L.; Pallotta, R.C.; Teixeira, S.; de Almeida, P.; Bjordal, J.M.; Lopes-Martins, R. Isolated and combined effects of photobiomodulation therapy, topical nonsteroidal anti-inflammatory drugs, and physical activity in the treatment of osteoarthritis induced by papain. J. Biomed. Opt. 2016, 21, 108001. [Google Scholar] [CrossRef]
- Navarro, F.; Bacurau, A.V.; Almeida, S.S.; Barros, C.C.; Moraes, M.R.; Pesquero, J.L.; Ribeiro, S.M.; Araújo, R.C.; Costa Rosa, L.F.; Bacurau, R.F. Exercise prevents the effects of experimental arthritis on the metabolism and function of immune cells. Cell Biochem. Funct. 2010, 28, 266–273. [Google Scholar] [CrossRef]
- Shi, L.; Xu, L.; Yin, L.; Zeng, F.W.; Mei, Q. Effect of swimming in cold water on gouty arthritis of rats with hyperuricemia. Chin. J. Pharmacol. Toxicol. 2012, 26, 25–29. [Google Scholar] [CrossRef]
- Zhao, H.X.; Zhang, Z.; Hu, F.; Wei, Q.F.; Yu, Y.S.; Zhao, H.D. Swimming exercise activates peroxisome proliferator-activated receptor-alpha and mitigates age-related renal fibrosis in rats. Mol. Cell. Biochem. 2023, 478, 1109–1116. [Google Scholar] [CrossRef]
- de Souza, J.A.; Becker, L.K.; Batista, M.A.C.; de Assis Braga, D.C.; Gomes, P.M.; Alzamora, A.C.; Vieira, M.A.R.; de Lima, W.G.; Andrade, M.G.C.; de Lima Sanches, B.; et al. Swimming training improves cardiovascular autonomic dysfunctions and prevents renal damage in rats fed a high-sodium diet from weaning. Exp. Physiol. 2021, 106, 412–426. [Google Scholar] [CrossRef]
- Farzanegi, P.; Asadi, M.; Abdi, A.; Etemadian, M.; Amani, M.; Amrollah, V.; Shahri, F.; Gholami, V.; Abdi, Z.; Moradi, L.; et al. Swimming exercise in combination with garlic extract administration as a therapy against doxorubicin-induced hepatic, heart and renal toxicity to rats. Toxin Rev. 2020, 39, 434–443. [Google Scholar] [CrossRef]
- Kumral, Z.N.; Sener, G.; Ozgur, S.; Koc, M.; Suleymanoglu, S.; Hurdag, C.; Yegen, B.C. Regular exercise alleviates renovascular hypertension-induced cardiac/endothelial dysfunction and oxidative injury in rats. J. Physiol. Pharmacol. 2016, 67, 45–55. [Google Scholar] [PubMed]
- Bernardes, D.; Oliveira-Lima, O.C.; da Silva, T.V.; Juliano, M.A.; dos Santos, D.M.; Carvalho-Tavares, J. Metabolic Alterations in Experimental Autoimmune Encephalomyelitis in Mice: Effects of Prior Physical Exercise. Neurophysiology 2016, 48, 117–121. [Google Scholar] [CrossRef]
- Nazari, M.; Kordi, M.R.; Minasian, V.; Quchan, A.H.S.K. Ameliorating effect of 6-week swimming exercise on mice with experimental autoimmune encephalomyelitis (EAE) by reducing fetuin-A and increasing AMPK & NAD+ levels in liver tissue. Iran. J. Basic Med. Sci. 2022, 26, 1016–1020. [Google Scholar] [CrossRef]
- Huang, W.C.; Xu, J.W.; Li, S.; Ng, X.E.; Tung, Y.T. Effects of exercise on high-fat diet-induced non-alcoholic fatty liver disease and lipid metabolism in ApoE knockout mice. Nutr. Metab. 2022, 19, 10. [Google Scholar] [CrossRef]
- Godínez-Victoria, M.; Drago-Serrano, M.E.; Reyna-Garfias, H.; Viloria, M.; Lara-Padilla, E.; Resendiz-Albor, A.A.; Sánchez-Torres, L.E.; Cruz-Hernández, T.R.; Campos-Rodriguez, R. Effects on secretory IgA levels in small intestine of mice that underwent moderate exercise training followed by a bout of strenuous swimming exercise. Brain Behav. Immun. 2012, 26, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Schultz, A.; Mendonca, L.S.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Swimming training beneficial effects in a mice model of nonalcoholic fatty liver disease. Exp. Toxicol. Pathol. 2012, 64, 273–282. [Google Scholar] [CrossRef]
- Zhang, Q.B.; Zhang, B.H.; Zhang, K.Z.; Meng, X.T.; Jia, Q.A.; Zhang, Q.B.; Bu, Y.; Zhu, X.D.; Ma, D.N.; Ye, B.G.; et al. Moderate swimming suppressed the growth and metastasis of the transplanted liver cancer in mice model: With reference to nervous system. Oncogene 2016, 35, 4122–4131. [Google Scholar] [CrossRef]
- Goto, S.; Radák, Z. Regular exercise attenuates oxidative stress in aging rat tissues: A possible mechanism toward anti-aging medicine. J. Exerc. Sci. Fit. 2007, 5, 1–6. [Google Scholar]
- Zhang, Y.; Xu, J.; Zhou, D.; Ye, T.; Zhou, P.; Liu, Z.; Liu, X.; Wang, Z.; Hua, T.; Zhang, Z.; et al. Swimming exercise ameliorates insulin resistance and nonalcoholic fatty liver by negatively regulating PPARγ transcriptional network in mice fed high fat diet. Mol. Med. 2023, 29, 150. [Google Scholar] [CrossRef] [PubMed]
- Altintas, F.; Caliskan, S.; Ozmen, O.; Kilic-Toprak, E. Swimming exercise restores damaging effects of fructose-enriched diet on the liver in rats. Tissue Cell 2022, 78, 101894. [Google Scholar] [CrossRef]
- Kolieb, E.; Maher, S.A.; Shalaby, M.N.; Alsuhaibani, A.M.; Alharthi, A.; Hassan, W.A.; El-Sayed, K. Vitamin D and Swimming Exercise Prevent Obesity in Rats under a High-Fat Diet via Targeting FATP4 and TLR4 in the Liver and Adipose Tissue. Int. J. Environ. Res. Public Health 2022, 19, 13740. [Google Scholar] [CrossRef]
- Yi, X.; Tang, D.; Cao, S.; Li, T.; Gao, H.; Ma, T.; Yao, T.; Li, J.; Chang, B. Effect of different exercise loads on testicular oxidative stress and reproductive function in obese male mice. Oxid. Med. Cell. Longev. 2020, 2020, 3071658. [Google Scholar] [CrossRef]
- Habibi, P.; Ahmadiasl, N.; Nourazarian, A.; Yousefi, H. Swimming exercise improves SIRT1, NF-κB, and IL-1β protein levels and pancreatic tissue injury in ovariectomized diabetic rats. Horm. Mol. Biol. Clin. Investig. 2022, 43, 345–352. [Google Scholar] [CrossRef]
- Alipour, M.R.; Yousefzade, N.; Bavil, F.M.; Naderi, R.; Ghiasi, R. Swimming impacts on pancreatic inflammatory cytokines, mir-146a and NF-κB expression levels in type-2 diabetic rats. Curr. Diabetes Rev. 2020, 16, 889–894. [Google Scholar] [CrossRef]
- Elbassuoni, E.A.; Abdel Hafez, S.M. Impact of chronic exercise on counteracting chronic stress-induced functional and morphological pancreatic changes in male albino rats. Cell Stress Chaperones 2019, 24, 567–580. [Google Scholar] [CrossRef]
- Ghiasi, R.; Soufi, F.G.; Mohaddes, G.; Alihemmati, A.; Somi, M.H.; Ebrahimi, H.; Bavil, F.M.; Alipour, M.R. Influance of regular swimming on serum levels of CRP, IL-6, TNF-α in high-fat diet-induced type 2 diabetic rats. Gen. Physiol. Biophys. 2016, 35, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Teixeira de Lemos, E.; Reis, F.; Baptista, S.; Pinto, R.; Sepodes, B.; Vala, H.; Rocha-Pereira, P.; Correia da Silva, G.; Teixeira, N.; Silva, A.S.; et al. Exercise training decreases proinflammatory profile in Zucker diabetic (type 2) fatty rats. Nutrition 2009, 25, 330–339. [Google Scholar] [CrossRef]
- Özüdoğru, E.; Atay, E.; Savran, M.; Aşci, H.; Özmen, Ö.; Topsakal, Ş. Protective effects of swimming exercises and metformin on cardiac and aortic damage caused by a high-fat diet in obese rats with type 2 diabetes, by regulating the Bcl2/Bax signaling pathway. Turk. J. Med. Sci. 2023, 53, 1582–1592. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Q.; Zheng, L.; Shou, J.; Zhuang, S.; Xiao, W.; Chen, P. Depot-specific adaption of adipose tissue for different exercise approaches in high-fat diet/streptozocin-induced diabetic mice. Front. Physiol. 2023, 14, 1189528. [Google Scholar] [CrossRef]
- Azizi, N.; Rahbarghazi, A.; Bavil, F.M.; Rahbarghazi, R.; Ghaffari-Nasab, A.; Rezaie, J.; Delkhosh, A.; Ahmadi, M. Swimming training reduced inflammation and apoptotic changes in pulmonary tissue in type 1 diabetic mice. J. Diabetes Metab. Disord. 2023, 22, 793–800. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ya, L.; Xia, L.; Penghui, D.; Wei, J.; Jianping, L. Exercise effects on myocardial type I, III collagen and angiotensin II/transforming growth factor beta1/Smad2 pathway in diabetic myocardial fibrosis rats. Chin. J. Tissue Eng. Res. 2022, 26, 4173–4179. [Google Scholar] [CrossRef]
- Eldesoqui, M.; Eldken, Z.H.; Mostafa, S.A.; Al-Serwi, R.H.; El-Sherbiny, M.; Elsherbiny, N.; Mohammedsaleh, Z.M.; Sakr, N.H. Exercise Augments the Effect of SGLT2 Inhibitor Dapagliflozin on Experimentally Induced Diabetic Cardiomyopathy, Possible Underlying Mechanisms. Metabolites 2022, 12, 635. [Google Scholar] [CrossRef] [PubMed]
- Daghigh, F.; Karimi, P.; Alihemmati, A.; Majidi Zolbin, M.; Ahmadiasl, N. Swimming training modulates lung injury induced by ovariectomy in diabetic rats: Involvement of inflammatory and fibrotic biomarkers. Arch. Physiol. Biochem. 2022, 128, 514–520. [Google Scholar] [CrossRef] [PubMed]
- da Silva Pereira, M.M.; de Melo, I.M.F.; Braga, V.A.Á.; Teixeira, Á.A.C.; Wanderley-Teixeira, V. Effect of swimming exercise, insulin-associated or not, on inflammatory cytokines, apoptosis, and collagen in diabetic rat placentas. Histochem. Cell Biol. 2022, 157, 467–479. [Google Scholar] [CrossRef]
- Sadeghian, R.; Shahidi, S.; Komaki, A.; Habibi, P.; Ahmadiasl, N.; Yousefi, H.; Daghigh, F. Synergism effect of swimming exercise and genistein on the inflammation, oxidative stress, and VEGF expression in the retina of diabetic-ovariectomized rats. Life Sci. 2021, 284, 119931. [Google Scholar] [CrossRef]
- Rahman, M.M.; Park, S.J.; Jeon, H.Y.; Kim, S. Exercise and oral melatonin attenuate anxiety and depression like behavior in type 2 diabetic rats. J. Adv. Biotechnol. Exp. Ther. 2021, 4, 238–247. [Google Scholar] [CrossRef]
- Netto, R.O.R.F.; Moura, E.G.; Ferretti, R.; de Andrade Araújo, M.J.; Mâncio, R.D.; da Silva, R.E.; Cajazeiro, D.C.; Fernandes, V.A.R.; Bortolato, R.S.; Col, L.O.; et al. Combine treatment with N-acetylcysteine, anti-CD4/CD8 antibodies and physical exercise reduces histopathological damage in salivary glands of spontaneously diabetic mice. Rom. J. Diabetes Nutr. Metab. Dis. 2021, 28, 242–254. [Google Scholar] [CrossRef]
- Gilak-Dalasm, M.; Peeri, M.; Azarbayjani, M.A. Swimming exercise decreases depression-like behaviour and inflammatory cytokines in a mouse model of type 2 diabetes. Exp. Physiol. 2021, 106, 1981–1991. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Qin, X.; Zhang, X.; Liu, B.; Chen, M. Upregulation of IL-4 signaling contributes to aerobic exercise-induced insulin sensitivity. Biochem. Biophys. Res. Commun. 2020, 525, 662–667. [Google Scholar] [CrossRef]
- Montenegro, M.L.; Bonocher, C.M.; Meola, J.; Portella, R.L.; Ribeiro-Silva, A.; Brunaldi, M.O.; Ferriani, R.A.; Rosa-e-Silva, J.C. Effect of Physical Exercise on Endometriosis Experimentally Induced in Rats. Reprod. Sci. 2019, 26, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Kwon, H.S.; Kim, M.J.; Go, H.K.; Oak, M.H.; Kim, D.H. Melatonin supplementation plus exercise behavior ameliorate insulin resistance, hypertension and fatigue in a rat model of type 2 diabetes mellitus. Biomed. Pharmacother. 2017, 92, 606–614. [Google Scholar] [CrossRef]
- Motta, V.F.; Aguila, M.B.; Mandarim-De-lacerda, C.A. High-intensity interval training (swimming) significantly improves the adverse metabolism and comorbidities in diet-induced obese mice. J. Sports Med. Phys. Fit. 2016, 56, 655–663. [Google Scholar]
- Kesherwani, V.; Chavali, V.; Hackfort, B.T.; Tyagi, S.C.; Mishra, P.K. Exercise ameliorates high fat diet induced cardiac dysfunction by increasing interleukin 10. Front. Physiol. 2015, 6, 124. [Google Scholar] [CrossRef]
- Teixeira De Lemos, E.; Pinto, R.; Oliveira, J.; Garrido, P.; Sereno, J.; Mascarenhas-Melo, F.; Páscoa-Pinheiro, J.; Teixeira, F.; Reis, F. Differential effects of acute (extenuating) and chronic (training) exercise on inflammation and oxidative stress status in an animal model of type 2 diabetes mellitus. Mediat. Inflamm. 2011, 2011, 253061. [Google Scholar] [CrossRef]
- Teixeira De Lemos, E.T.; Reis, F.; Baptista, S.; Pinto, R.; Sepodes, B.; Vala, H.; Rocha-Pereira, P.; Silva, A.S.; Teixeira, F. Exercise training is associated with improved levels of C-reactive protein and adiponectin in ZDF (type 2) diabetic rats. Med. Sci. Monit. 2007, 13, BR168–BR174. [Google Scholar]
- Sharma, A.K.; Kumar, A.; Taneja, G.; Nagaich, U.; Deep, A.; Datusalia, A.K.; Rajput, S.K. Combined and individual strategy of exercise generated preconditioning and low dose copper nanoparticles serve as superlative approach to ameliorate ISO-induced myocardial infarction in rats. Pharmacol. Rep. 2018, 70, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.K.; Tsai, Y.L.; Shibu, M.A.; Shen, C.Y.; Chang-Lee, S.N.; Chen, R.J.; Yao, C.H.; Ban, B.; Kuo, W.W.; Huang, C.Y. Exercise training augments Sirt1-signaling and attenuates cardiac inflammation in D-galactose induced-aging rats. Aging 2018, 10, 4166–4174. [Google Scholar] [CrossRef]
- Bulut, E.C.; Abueid, L.; Ercan, F.; Süleymanoğlu, S.; Ağırbaşlı, M.; Yeğen, B. Treatment with oestrogen-receptor agonists or oxytocin in conjunction with exercise protects against myocardial infarction in ovariectomized rats. Exp. Physiol. 2016, 101, 612–627. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.B.; Miranda, A.P.; Silva, D.B.; D’Angelo, L.R.; Rosa, B.B.; Soares, E.A.; Ramalho, J.G.; Boriollo, M.F.; Garcia, J.A. Propolis and swimming in the prevention of atherogenesis and left ventricular hypertrophy in hypercholesterolemic mice. Braz. J. Biol. 2015, 75, 414–422. [Google Scholar] [CrossRef]
- Preto, E.; Lima, N.E.; Simardi, L.; Fonseca, F.L.; Filho, A.A.; Maifrino, L.B. Effect of mild aerobic training on the myocardium of mice with chronic Chagas disease. Biol. Targets Ther. 2015, 9, 87–92. [Google Scholar] [CrossRef]
- Nounou, H.A.; Deif, M.M.; Shalaby, M.A. Effect of flaxseed supplementation and exercise training on lipid profile, oxidative stress and inflammation in rats with myocardial ischemia. Lipids Health Dis. 2012, 11, 129. [Google Scholar] [CrossRef]
- Baptista, S.; Piloto, N.; Reis, F.; Teixeira-De-Lemos, E.; Garrido, A.P.; Dias, A.; Lourenço, M.; Palmeiro, A.; Ferrer-Antunes, C.; Teixeira, F. Treadmill running and swimming imposes distinct cardiovascular physiological adaptations in the rat: Focus on serotonergic and sympathetic nervous systems modulation. Acta Physiol. Hung. 2008, 95, 365–381. [Google Scholar] [CrossRef]
- Nunes, R.B.; Tonetto, M.; Machado, N.; Chazan, M.; Heck, T.G.; Veiga, A.B.; Dall’Ago, P. Physical exercise improves plasmatic levels of IL-10, left ventricular end-diastolic pressure, and muscle lipid peroxidation in chronic heart failure rats. J. Appl. Physiol. 2008, 104, 1641–1647. [Google Scholar] [CrossRef]
- Kuzmina, A.V.; Smolentseva, I.G.; Levin, O.S. Swimming disorders in Parkinson’s disease. Zhurnal Nevrologii i Psikhiatrii Imeni SS Korsakova 2022, 122, 30–33. [Google Scholar] [CrossRef]
- Yázigi, F.; Espanha, M.; Vieira, F.; Messier, S.P.; Monteiro, C.; Veloso, A.P. The PICO project: Aquatic exercise for knee osteoarthritis in overweight and obese individuals. BMC Musculoskelet. Disord. 2013, 14, 320. [Google Scholar] [CrossRef]
- Alkatan, M.; Machin, D.R.; Baker, J.R.; Akkari, A.S.; Park, W.; Tanaka, H. Effects of Swimming and Cycling Exercise Intervention on Vascular Function in Patients With Osteoarthritis. Am. J. Cardiol. 2016, 117, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, Y.M.A.E.; Othman, G. Challenge of diabetes control in patients with rheumatic diseases. Kuwait Med. J. 2014, 46, 287–299. [Google Scholar]
- Siqueira, U.S.; Orsini Valente, L.G.; de Mello, M.T.; Szejnfeld, V.L.; Pinheiro, M.M. Effectiveness of Aquatic Exercises in Women With Rheumatoid Arthritis: A Randomized, Controlled, 16-Week Intervention-The HydRA Trial. Am. J. Phys. Med. Rehabil. 2017, 96, 167–175. [Google Scholar] [CrossRef]
- Zaccarin, M.; Zanni, S.; Gallè, F.; Protano, C.; Valeriani, F.; Liguori, G.; Romano Spica, V.; Vitali, M. Studying Respiratory Symptoms Related to Swimming Pools Attendance in Young Athletes: The SPHeRA Study. Toxics 2022, 10, 759. [Google Scholar] [CrossRef] [PubMed]
- Bougault, V.; Loubaki, L.; Joubert, P.; Turmel, J.; Couture, C.; Laviolette, M.; Chakir, J.; Boulet, L.P. Airway remodeling and inflammation in competitive swimmers training in indoor chlorinated swimming pools. J. Allergy Clin. Immunol. 2012, 129, 351–358.e351. [Google Scholar] [CrossRef] [PubMed]
- Font-Ribera, L.; Kogevinas, M.; Zock, J.; Gómez, F.P.; Barreiro, E.; Nieuwenhuijsen, M.J.; Fernandez, P.; Lourencetti, C.; Pérez-Olabarría, M.; Bustamante, M.; et al. Short-term changes in respiratory biomarkers after swimming in a chlorinated pool. Environ. Health Perspect. 2010, 118, 1538–1544. [Google Scholar] [CrossRef]
- Pachalski, A.; Mekarski, T. Effect of swimming on increasing of cardio-respiratory capacity in paraplegics. Paraplegia 1980, 18, 190–196. [Google Scholar] [CrossRef]
- Korb, A.; Bertoldi, K.; Lovatel, G.A.; Delevatti, R.S.; Elsner, V.R.; Meireles, L.C.F.; Kruel, L.F.M.; Siqueira, I.R. Acute exercise and periodized training in different environments affect histone deacetylase activity and interleukin-10 levels in peripheral blood of patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2018, 141, 132–139. [Google Scholar] [CrossRef]
- Saki, H.; Nazem, F.; Fariba, F.; Sheikhsharbafan, R. A High intensity Interval training (running and swimming) and resistance training intervention on heart rate variability and the selected biochemical factors in boys with type 1 diabetes. Diabetes Res. Clin. Pract. 2023, 204, 110915. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Santos, G.R.; Cunha, M.R.; Caldeira, E.J.; Galdeano, E.A.; Prudente, R.C.S.; Pinto, C.A.L. Effect of antioxidant treatment with n-acetylcysteine and swimming on lipid expression of sebaceous glands in diabetic mice. Sci. Rep. 2021, 11, 11924. [Google Scholar] [CrossRef]
- Tso, J.; Hollowed, C.; Liu, C.; Alkhoder, A.; Dommisse, M.; Gowani, Z.; Miller, A.; Nguyen, G.; Nguyen, P.; Prabakaran, G.; et al. Nonsteroidal Anti-inflammatory Drugs and Cardiovascular Risk in American Football. Med. Sci. Sports Exerc. 2020, 52, 2522–2528. [Google Scholar] [CrossRef]
- Santos, M.H.; Higuchi Mde, L.; Tucci, P.J.; Garavelo, S.M.; Reis, M.M.; Antonio, E.L.; Serra, A.J.; Maranhão, R.C. Previous exercise training increases levels of PPAR-α in long-term post-myocardial infarction in rats, which is correlated with better inflammatory response. Clinics 2016, 71, 163–168. [Google Scholar] [CrossRef]
- Shapoval, L.N.; Pobegailo, L.S.; Stepanenko, L.G.; Dmytrenko, O.V.; Bouryi, V.A.; Sagach, V.F. Impact of swimming exercise training on the effects of modulation of mitochondrial permeability transition and NOS-1 activation in medullary cardiovascular neurons of rats. Neurophysiology 2011, 43, 299–308. [Google Scholar] [CrossRef]
- Manolis, A.S.; Manolis, S.A.; Manolis, A.A.; Manolis, T.A.; Apostolaki, N.; Melita, H. Winter swimming: Body hardening and cardiorespiratory protection via sustainable acclimation. Curr. Sports Med. Rep. 2019, 18, 401–415. [Google Scholar] [CrossRef]
- Llorens-Martín, M.; Jurado-Arjona, J.; Bolós, M.; Pallas-Bazarra, N.; Ávila, J. Forced swimming sabotages the morphological and synaptic maturation of newborn granule neurons and triggers a unique pro-inflammatory milieu in the hippocampus. Brain Behav. Immun. 2016, 53, 242–254. [Google Scholar] [CrossRef]
- Gatmaitan, B.G.; Chason, J.L.; Lerner, A.M. Augmentation of the virulence of murine coxsackie-virus B-3 myocardiopathy by exercise. J. Exp. Med. 1970, 131, 1121–1136. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; Sanchis-Gomar, F.; Perego, S.; Sansoni, V.; Banfi, G. Implications of exercise-induced adipo-myokines in bone metabolism. Endocrine 2016, 54, 284–305. [Google Scholar] [CrossRef] [PubMed]
- Koop, M.A.; Sleijser-Koehorst, M.L.S.; Hooijmans, C.R.; Tdlohreg, P.Q.; Lutke Schipholt, I.J.; Scholten-Peeters, G.G.M.; Coppieters, M.W. The potential protective effects of pre-injury exercise on neuroimmune responses following experimentally-induced traumatic neuropathy: A systematic review with meta-analysis. Front. Immunol. 2023, 14, 1215566. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Santos, R.; Galdino, G. Endogenous systems involved in exercise-induced analgesia. J. Physiol. Pharmacol. 2018, 69, 3–13. [Google Scholar] [CrossRef]
- Oh, S.J.; Ahn, H.; Jung, K.H.; Han, S.J.; Nam, K.R.; Kang, K.J.; Park, J.A.; Lee, K.C.; Lee, Y.J.; Choi, J.Y. Evaluation of the Neuroprotective Effect of Microglial Depletion by CSF-1R Inhibition in a Parkinson’s Animal Model. Mol. Imaging Biol. 2020, 22, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Becker, B.E. Aquatic therapy: Scientific foundations and clinical rehabilitation applications. PM&R 2009, 1, 859–872. [Google Scholar] [CrossRef]
- Faíl, L.B.; Marinho, D.A.; Marques, E.A.; Costa, M.J.; Santos, C.C.; Marques, M.C.; Izquierdo, M.; Neiva, H.P. Benefits of aquatic exercise in adults with and without chronic disease-A systematic review with meta-analysis. Scand. J. Med. Sci. Sports 2022, 32, 465–486. [Google Scholar] [CrossRef]
- Mooventhan, A.; Nivethitha, L. Scientific evidence-based effects of hydrotherapy on various systems of the body. N. Am. J. Med. Sci. 2014, 6, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.J.; Becker, B.E. (Eds.) Comprehensive Aquatic Therapy; Butterworth-Heinemann: Oxford, UK, 2004. [Google Scholar]
- Font-Ribera, L.; Villanueva, C.M.; Nieuwenhuijsen, M.J.; Zock, J.P.; Kogevinas, M.; Henderson, J. Swimming pool attendance, asthma, allergies, and lung function in the Avon Longitudinal Study of Parents and Children cohort. Am. J. Respir. Crit. Care Med. 2011, 183, 582–588. [Google Scholar] [CrossRef]
- Bougault, V.; Turmel, J.; St-Laurent, J.; Bertrand, M.; Boulet, L.P. Asthma, airway inflammation and epithelial damage in swimmers and cold-air athletes. Eur. Respir. J. 2009, 33, 740–746. [Google Scholar] [CrossRef] [PubMed]
| Searched Words + Swimming + Inflammatory | Databases | Total | Duplicate | Unrelated/Unavailable | Related/Citable | |
|---|---|---|---|---|---|---|
| 1 | Alzheimer’s Disease | PubMed | 36 | 22 | 98 | 7 |
| Scopus | 91 | |||||
| 2 | Parkinson | PubMed | 9 | 7 | 39 | 6 |
| Scopus | 43 | |||||
| 3 | Neuropathies | PubMed | 2 | 2 | 9 | 2 |
| Scopus | 11 | |||||
| 4 | Rheumatoid Arthritis | PubMed | 6 | 5 | 11 | 3 |
| Scopus | 13 | |||||
| 5 | Gout | PubMed | 1 | 1 | 2 | 1 |
| Scopus | 3 | |||||
| 6 | Osteoarthritis | PubMed | 6 | 6 | 14 | 6 |
| Scopus | 20 | |||||
| 7 | Renal/kidney Disorders | PubMed | 12 | 0 | 16 | 5 |
| Scopus | 9 | |||||
| 8 | Liver Disorders | PubMed | 13 | 0 | 30 | 10 |
| Scopus | 27 | |||||
| 9 | Reproductive Disorders | PubMed | 18 | 0 | 19 | 1 |
| Scopus | 2 | |||||
| 10 | Pancreatic Disorders | PubMed | 2 | 1 | 5 | 5 |
| Scopus | 9 | |||||
| 11 | Respiratory Disorders | PubMed | 8 | 1 | 21 | 4 |
| Scopus | 18 | |||||
| 12 | Diabetes | PubMed | 24 | 10 | 74 | 26 |
| Scopus | 86 | |||||
| 13 | Cardiovascular Disorders | PubMed | 24 | 3 | 10 | 22 |
| Scopus | 11 | |||||
| 14 | Negative effects of swimming | 5 | - | 1 | 4 |
| Row | Type of Disease | Subjects or Participants | Intervention | Results | Reference |
|---|---|---|---|---|---|
| 1 | Rheumatoid Arthritis | Women with Rheumatoid Arthritis | Swimming (3 times per week, 5 min warm-up, and 15 to 30 min specific exercises for lower limbs, which gradually increased) for 16 weeks. |
| [94] |
| 2 | Respiratory Disorders | Competitive swimmers | Winter training Summer training |
| [95] |
| 3 | Respiratory Disorders | Intense swimming training (ST) |
| [96] | |
| 4 | Respiratory Disorders | Healthy nonsmoking adults aged 18–50, with no history of asthma or recent respiratory infection (past 3 weeks) | Swimming for 40 min |
| [97] |
| 5 | Respiratory Disorders | Three years of swimming training |
| [98] | |
| 6 | Diabetes and Related Disorders | Diabetes mellitus type 2 patients | 12 weeks of walking or running in a swimming pool (swimming group) or on a track (dry land group). |
| [99] |
| 7 | Diabetes and Related Disorders | Boys with type 1 diabetes | High-intensity Interval training (running and swimming) and resistance training 12 weeks, 3 days weekly |
| [100] |
| 8 | Cardiovascular Disorders | Middle-aged and older patients with osteoarthritis | swimming and cycling exercise Initially, 20 to 30 min/day and 3 days/week, 40% to 50% of the heart rate reserve intensity, which is elevated gradually to 40 to 45 min/day and 3 days/week at an intensity of 60% to 70% of heart rate |
| [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kooshki, M.; Rezeai-Farimani, R.; Moradpour, A.; Baradaran Rahimi, V.; Askari, V.R. How Swimming Modulates Inflammatory Pathways in Pain, Neurodegenerative, and Metabolic Disorders. Brain Sci. 2025, 15, 1121. https://doi.org/10.3390/brainsci15101121
Kooshki M, Rezeai-Farimani R, Moradpour A, Baradaran Rahimi V, Askari VR. How Swimming Modulates Inflammatory Pathways in Pain, Neurodegenerative, and Metabolic Disorders. Brain Sciences. 2025; 15(10):1121. https://doi.org/10.3390/brainsci15101121
Chicago/Turabian StyleKooshki, Mahdiyeh, Rozhin Rezeai-Farimani, Amirmohammad Moradpour, Vafa Baradaran Rahimi, and Vahid Reza Askari. 2025. "How Swimming Modulates Inflammatory Pathways in Pain, Neurodegenerative, and Metabolic Disorders" Brain Sciences 15, no. 10: 1121. https://doi.org/10.3390/brainsci15101121
APA StyleKooshki, M., Rezeai-Farimani, R., Moradpour, A., Baradaran Rahimi, V., & Askari, V. R. (2025). How Swimming Modulates Inflammatory Pathways in Pain, Neurodegenerative, and Metabolic Disorders. Brain Sciences, 15(10), 1121. https://doi.org/10.3390/brainsci15101121







