Disability and Non-Motor Symptoms in Multiple Sclerosis: Exploring Associations and Predictive Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Procedure
2.2. Demographic Information and Disease-Related Variables
2.3. Participants
2.4. Questionnaires
2.4.1. Fatigue Severity Scale (FSS)
2.4.2. Hospital Anxiety and Depression Scale (HADS)
2.4.3. The Pittsburgh Sleep Quality Index (PSQI)
2.5. Statistical Analyses
3. Results
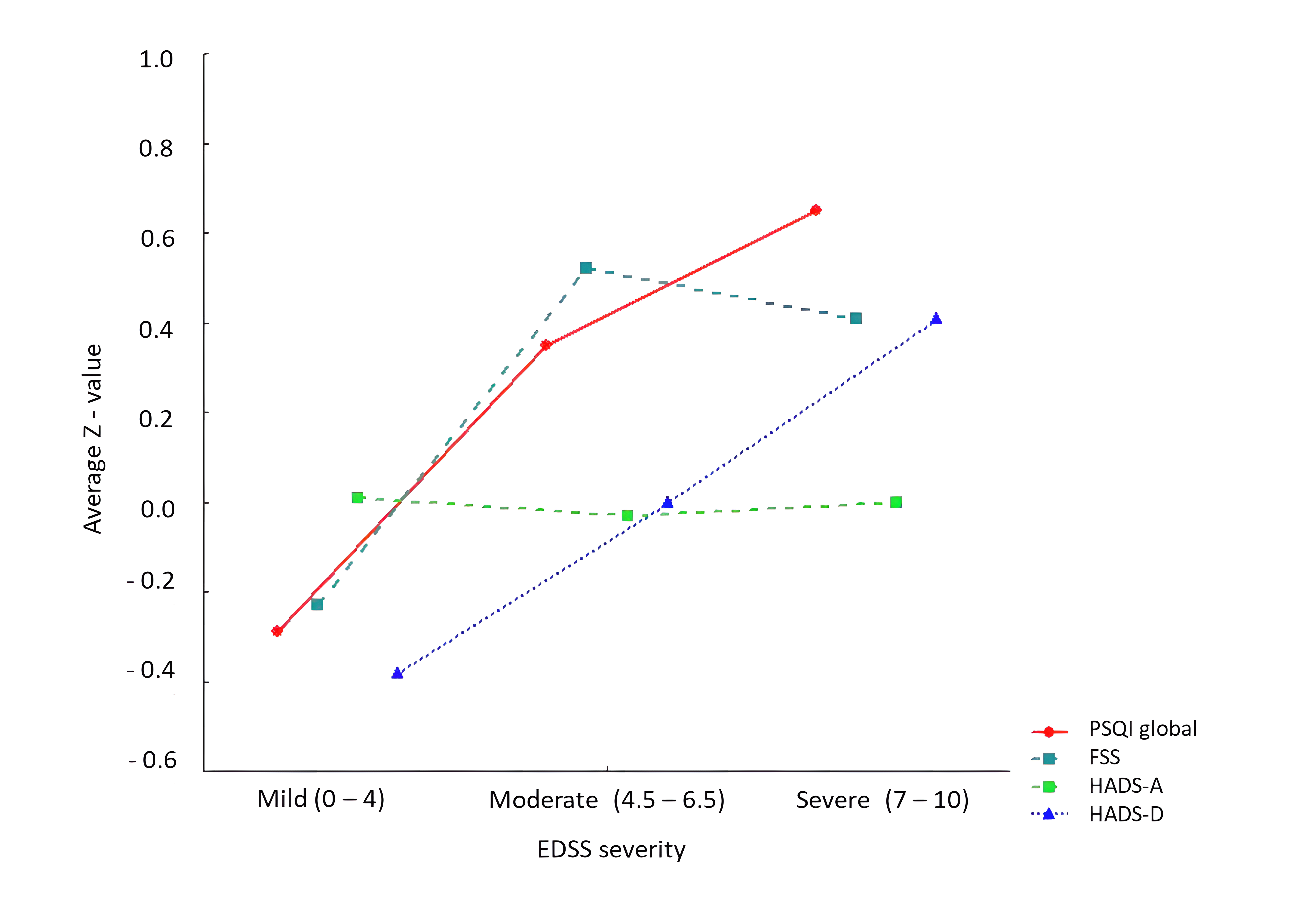
3.1. Group Differences and Symptom Interrelations
3.2. Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMSSC | Association of Multiple Sclerosis Societies of Croatia |
| CIS | Clinically Isolated Syndrome |
| CNS | Central Nervous System |
| EDSS | Expanded Disability Status Scale |
| FS | Functional Score |
| FSS | Fatigue Severity Scale |
| HADS | Hospital Anxiety and Depression Scale |
| HADS-A | Hospital Anxiety and Depression Scale (anxiety subscale) |
| HADS-D | Hospital Anxiety and Depression Scale (depression subscale) |
| MS | Multiple Sclerosis |
| PSQI | Pittsburgh Sleep Quality Index |
References
- Bjørklund, G.; Wallace, D.R.; Hangan, T.; Butnariu, M.; Gurgas, L.; Peana, M. Cerebral Iron Accumulation in Multiple Sclerosis: Pathophysiology and Therapeutic Implications. Autoimmun. Rev. 2025, 24, 103741. [Google Scholar] [CrossRef]
- Geurts, J.J.; Calabrese, M.; Fisher, E.; Rudick, R.A. Measurement and Clinical Effect of Grey Matter Pathology in Multiple Sclerosis. Lancet Neurol. 2012, 11, 1082–1092. [Google Scholar] [CrossRef]
- Lorenzut, S.; Negro, I.D.; Pauletto, G.; Verriello, L.; Spadea, L.; Salati, C.; Musa, M.; Gagliano, C.; Zeppieri, M. Exploring the Pathophysiology, Diagnosis, and Treatment Options of Multiple Sclerosis. J. Integr. Neurosci. 2025, 24, 25081. [Google Scholar] [CrossRef]
- Magliozzi, R.; Howell, O.W.; Calabrese, M.; Reynolds, R. Meningeal Inflammation as a Driver of Cortical Grey Matter Pathology and Clinical Progression in Multiple Sclerosis. Nat. Rev. Neurol. 2023, 19, 461–476. [Google Scholar] [CrossRef]
- Peruzzotti-Jametti, L.; Willis, C.M.; Krzak, G.; Hamel, R.; Pirvan, L.; Ionescu, R.-B.; Reisz, J.A.; Prag, H.A.; Garcia-Segura, M.E.; Wu, V.; et al. Mitochondrial Complex I Activity in Microglia Sustains Neuroinflammation. Nature 2024, 628, 195–203. [Google Scholar] [CrossRef]
- Woo, M.S.; Mayer, C.; Binkle-Ladisch, L.; Sonner, J.K.; Rosenkranz, S.C.; Shaposhnykov, A.; Rothammer, N.; Tsvilovskyy, V.; Lorenz, S.M.; Raich, L.; et al. STING Orchestrates the Neuronal Inflammatory Stress Response in Multiple Sclerosis. Cell 2024, 187, 4043–4060.e30. [Google Scholar] [CrossRef] [PubMed]
- Glaser, A.; Stahmann, A.; Meissner, T.; Flachenecker, P.; Horáková, D.; Zaratin, P.; Brichetto, G.; Pugliatti, M.; Rienhoff, O.; Vukusic, S.; et al. Multiple Sclerosis Registries in Europe—An Updated Mapping Survey. Mult. Scler. Relat. Disord. 2019, 27, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Declemy, A.; Haddad, R.; Chesnel, C.; Charlanes, A.; Le Breton, F.; Sheikh Ismael, S.; Amarenco, G. Prevalence of Comorbidities in Multiple Sclerosis Patients with Neurogenic Bladder. Prog. Urol. 2021, 31, 732–738. [Google Scholar] [CrossRef]
- Rodrigues, P.; Da Silva, B.; Trevisan, G. A Systematic Review and Meta-Analysis of Neuropathic Pain in Multiple Sclerosis: Prevalence, Clinical Types, Sex Dimorphism, and Increased Depression and Anxiety Symptoms. Neurosci. Biobehav. Rev. 2023, 154, 105401. [Google Scholar] [CrossRef] [PubMed]
- Benedict, R.H.; Amato, M.P.; Boringa, J.; Brochet, B.; Foley, F.; Fredrikson, S.; Hamalainen, P.; Hartung, H.; Krupp, L.; Penner, I.; et al. Brief International Cognitive Assessment for MS (BICAMS): International Standards for Validation. BMC Neurol. 2012, 12, 55. [Google Scholar] [CrossRef]
- Benedict, R.H.B.; Amato, M.P.; DeLuca, J.; Geurts, J.J.G. Cognitive Impairment in Multiple Sclerosis: Clinical Management, MRI, and Therapeutic Avenues. Lancet Neurol. 2020, 19, 860–871. [Google Scholar] [CrossRef]
- Cordani, C.; Meani, A.; Esposito, F.; Valsasina, P.; Colombo, B.; Pagani, E.; Preziosa, P.; Comi, G.; Filippi, M.; Rocca, M.A. Imaging Correlates of Hand Motor Performance in Multiple Sclerosis: A Multiparametric Structural and Functional MRI Study. Mult. Scler. J. 2020, 26, 233–244. [Google Scholar] [CrossRef]
- Manjaly, Z.-M.; Harrison, N.A.; Critchley, H.D.; Do, C.T.; Stefanics, G.; Wenderoth, N.; Lutterotti, A.; Müller, A.; Stephan, K.E. Pathophysiological and Cognitive Mechanisms of Fatigue in Multiple Sclerosis. J. Neurol. Neurosurg. Psychiatry 2019, 90, 642–651. [Google Scholar] [CrossRef]
- Rocca, M.A.; Preziosa, P.; Barkhof, F.; Brownlee, W.; Calabrese, M.; De Stefano, N.; Granziera, C.; Ropele, S.; Toosy, A.T.; Vidal-Jordana, À.; et al. Current and Future Role of MRI in the Diagnosis and Prognosis of Multiple Sclerosis. Lancet Reg. Health—Eur. 2024, 44, 100978. [Google Scholar] [CrossRef]
- Jellinger, K.A. Depression and Anxiety in Multiple Sclerosis. Review of a Fatal Combination. J. Neural Transm. 2024, 131, 847–869. [Google Scholar] [CrossRef]
- Karimi, S.; Andayeshgar, B.; Khatony, A. Prevalence of Anxiety, Depression, and Stress in Patients with Multiple Sclerosis in Kermanshah-Iran: A Cross-Sectional Study. BMC Psychiatry 2020, 20, 166. [Google Scholar] [CrossRef]
- Oliva Ramirez, A.; Keenan, A.; Kalau, O.; Worthington, E.; Cohen, L.; Singh, S. Prevalence and Burden of Multiple Sclerosis-Related Fatigue: A Systematic Literature Review. BMC Neurol. 2021, 21, 468. [Google Scholar] [CrossRef] [PubMed]
- Laslett, L.L.; Honan, C.; Turner, J.A.; Dagnew, B.; Campbell, J.A.; Gill, T.K.; Appleton, S.; Blizzard, L.; Taylor, B.V.; Van Der Mei, I. Poor Sleep and Multiple Sclerosis: Associations with Symptoms of Multiple Sclerosis and Quality of Life. J. Neurol. Neurosurg. Psychiatry 2022, 93, 1162–1165. [Google Scholar] [CrossRef] [PubMed]
- Ozdogar, A.T.; Aldemir, E.; Yesiloglu, P.; Cilingir, V. Exploring the Relationship Between Sleep Quality and Fatigue, Quality of Life, Daytime Sleepiness, and Anxiety-Depression Levels in Patients with Multiple Sclerosis. J. Mult. Scler. Res. 2025, 4, 67–72. [Google Scholar] [CrossRef]
- Zhang, G.X.; Zhang, W.T.; Gao, S.S.; Zhao, R.Z.; Yu, W.J.; Izquierdo, G. Sleep Disorders in Patients with Multiple Sclerosis in Spain. Neurol. Engl. Ed. 2024, 39, 29–35. [Google Scholar] [CrossRef]
- Zekibakhsh Mohammadi, N.; Kianimoghadam, A.S.; Mikaeili, N.; Asgharian, S.S.; Jafari, M.; Masjedi-Arani, A. Sleep Disorders and Fatigue among Patients with MS: The Role of Depression, Stress, and Anxiety. Neurol. Res. Int. 2024, 2024, 6776758. [Google Scholar] [CrossRef]
- Curatoli, C.; Marcassoli, A.; Barbadoro, F.; Fornari, A.; Leonardi, M.; Raggi, A.; Schiavolin, S.; Terragni, R.; Antozzi, C.; Brambilla, L.; et al. Anxiety, Depression, and Expanded Disability Status Scale Independently Predict the Perception of Disability in Persons with Multiple Sclerosis: A Cross-Sectional Study. Behav. Neurol. 2025, 2025, 2744955. [Google Scholar] [CrossRef]
- D’Souza, M.; Heikkilä, A.; Lorscheider, J.; Haller, V.; Kravalis, K.; Gysin, S.; Fuertes, N.A.C.; Fricker, E.; Lam, E.; Higgins, P.; et al. Electronic Neurostatus-EDSS Increases the Quality of Expanded Disability Status Scale Assessments: Experience from Two Phase 3 Clinical Trials. Mult. Scler. J. 2020, 26, 993–996. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating Neurologic Impairment in Multiple Sclerosis: An Expanded Disability Status Scale (EDSS). Neurology 1983, 33, 1444. [Google Scholar] [CrossRef] [PubMed]
- Asadollahzadeh, E.; Ebadi, Z.; Owji, M.; Rezaeimanesh, N.; Sahraian, M.A.; Moghadasi, A.N. Exploring the Relationship between Disability Status, Depression, and Quality of Life in Individuals with Multiple Sclerosis. Mult. Scler. Relat. Disord. 2024, 87, 105629. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ginés, M.L.; Esquivel, A.; Hernández, Y.H.; Alvarez-Sala, L.A.; Benito-León, J. Investigating the Relationship between Multiple Sclerosis Disability and Driving Performance: A Comparative Study of the Multiple Sclerosis Functional Composite and Expanded Disability Status Scale. Clin. Neurol. Neurosurg. 2024, 244, 108431. [Google Scholar] [CrossRef] [PubMed]
- Aparicio Castro, E.; Candeliere Merlicco, A.; María Santa, C.; Villaverde González, R. Utilidad de la escala de depresión de Beck para el diagnóstico de los trastornos depresivos en la esclerosis múltiple. Rev. Neurol. 2024, 78, 317. [Google Scholar] [CrossRef]
- Ezzeldin, M.Y.; Mahmoud, D.M.; Safwat, S.M.; Soliman, R.K.; Desoky, T.; Khedr, E.M. EDSS and Infratentorial White Matter Lesion Volume Are Considered Predictors of Fatigue Severity in RRMS. Sci. Rep. 2023, 13, 11404. [Google Scholar] [CrossRef]
- Riccitelli, G.C.; Disanto, G.; Sacco, R.; Sparasci, D.; Sacco, L.; Castelnovo, A.; Miano, S.; Manconi, M.; Gobbi, C.; Zecca, C. Contribution of Sleep Disturbances to Fatigue in Multiple Sclerosis: A Prospective Study Using Clinical and Polysomnographic Parameters. Eur. J. Neurol. 2021, 28, 3139–3146. [Google Scholar] [CrossRef]
- Alswat, A.M.; Altirkistani, B.A.; Alserihi, A.R.; Baeshen, O.K.; Alrushid, E.S.; Alkhudair, J.; Aldbas, A.A.; Wadaan, O.M.; Alsaleh, A.; Al Malik, Y.M.; et al. The Prevalence of Major Depression and Generalized Anxiety Disorder in Patients with Multiple Sclerosis in Saudi Arabia: A Cross-Sectional Multicentered Study. Front. Psychiatry 2023, 14, 1195101. [Google Scholar] [CrossRef]
- Kołtuniuk, A.; Kazimierska-Zając, M.; Pogłódek, D.; Chojdak-Łukasiewicz, J. Sleep Disturbances, Degree of Disability and the Quality of Life in Multiple Sclerosis Patients. Int. J. Environ. Res. Public Health 2022, 19, 3271. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A New Instrument for Psychiatric Practice and Research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Krupp, L.B. The Fatigue Severity Scale: Application to Patients with Multiple Sclerosis and Systemic Lupus Erythematosus. Arch. Neurol. 1989, 46, 1121. [Google Scholar] [CrossRef] [PubMed]
- McDonald, W.I.; Compston, A.; Edan, G.; Goodkin, D.; Hartung, H.; Lublin, F.D.; McFarland, H.F.; Paty, D.W.; Polman, C.H.; Reingold, S.C.; et al. Recommended Diagnostic Criteria for Multiple Sclerosis: Guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann. Neurol. 2001, 50, 121–127. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of Multiple Sclerosis: 2017 Revisions of the McDonald Criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Ayache, S.S.; Chalah, M.A. Fatigue in Multiple Sclerosis—Insights into Evaluation and Management. Neurophysiol. Clin. Neurophysiol. 2017, 47, 139–171. [Google Scholar] [CrossRef]
- Feldpausch, J.; Plummer, P.; Abou-Rass, Z.; Fritz, N. Characterizing Fatigue by Multiple Sclerosis Subtype and Determining Validity of a Fatigue Scale Specific to Persons with Progressive Multiple Sclerosis. Int. J. MS Care 2024, 26, 281–289. [Google Scholar] [CrossRef]
- Jerković, A.; Mikac, U.; Matijaca, M.; Košta, V.; Ćurković Katić, A.; Dolić, K.; Vujović, I.; Šoda, J.; Đogaš, Z.; Pavelin, S.; et al. Psychometric Properties of the Pittsburgh Sleep Quality Index (PSQI) in Patients with Multiple Sclerosis: Factor Structure, Reliability, Correlates, and Discrimination. J. Clin. Med. 2022, 11, 2037. [Google Scholar] [CrossRef]
- Armutlu, K.; Cetisli Korkmaz, N.; Keser, I.; Sumbuloglu, V.; Irem Akbiyik, D.; Guney, Z.; Karabudak, R. The Validity and Reliability of the Fatigue Severity Scale in Turkish Multiple Sclerosis Patients. Int. J. Rehabil. Res. 2007, 30, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Lerdal, A.; Johansson, S.; Kottorp, A.; Von Koch, L. Psychometric Properties of the Fatigue Severity Scale: Rasch Analyses of Responses in a Norwegian and a Swedish MS Cohort. Mult. Scler. J. 2010, 16, 733–741. [Google Scholar] [CrossRef]
- Valko, P.O.; Bassetti, C.L.; Bloch, K.E.; Held, U.; Baumann, C.R. Validation of the Fatigue Severity Scale in a Swiss Cohort. Sleep 2008, 31, 1601–1607. [Google Scholar] [CrossRef]
- Honarmand, K.; Feinstein, A. Validation of the Hospital Anxiety and Depression Scale for Use with Multiple Sclerosis Patients. Mult. Scler. J. 2009, 15, 1518–1524. [Google Scholar] [CrossRef]
- Marrie, R.A.; Zhang, L.; Lix, L.M.; Graff, L.A.; Walker, J.R.; Fisk, J.D.; Patten, S.B.; Hitchon, C.A.; Bolton, J.M.; Sareen, J.; et al. The Validity and Reliability of Screening Measures for Depression and Anxiety Disorders in Multiple Sclerosis. Mult. Scler. Relat. Disord. 2018, 20, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Pais Ribeiro, J.L.; Martins Da Silva, A.; Vilhena, E.; Moreira, I.; Santos, E.; Mendonça, D. The Hospital Anxiety and Depression Scale, in Patients with Multiple Sclerosis. Neuropsychiatr. Dis. Treat. 2018, 14, 3193–3197. [Google Scholar] [CrossRef] [PubMed]
- Watson, T.M.; Ford, E.; Worthington, E.; Lincoln, N.B. Validation of Mood Measures for People with Multiple Sclerosis. Int. J. MS Care 2014, 16, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Jerković, A.; Proroković, A.; Matijaca, M.; Vuko, J.; Poljičanin, A.; Mastelić, A.; Ćurković Katić, A.; Košta, V.; Kustura, L.; Dolić, K.; et al. Psychometric Properties of the HADS Measure of Anxiety and Depression Among Multiple Sclerosis Patients in Croatia. Front. Psychol. 2021, 12, 794353. [Google Scholar] [CrossRef]
- Curcio, G.; Tempesta, D.; Scarlata, S.; Marzano, C.; Moroni, F.; Rossini, P.M.; Ferrara, M.; De Gennaro, L. Validity of the Italian Version of the Pittsburgh Sleep Quality Index (PSQI). Neurol. Sci. 2013, 34, 511–519. [Google Scholar] [CrossRef]
- Team, R.C. R Foundation for Statistical Computing.
- Dogan, S.; Yildiz, S.; Kazgan Kılıçaslan, A.; Sirlier Emir, B.; Kurt, O.; Sehlikoğlu, S. Does Anxiety, Depression, and Sleep Levels Affect the Quality of Life in Patients Diagnosed with Multiple Sclerosis? Eur. Rev. Med. Pharmacol. Sci. 2024, 28, 1306–1313. [Google Scholar] [CrossRef]
- Beiske, A.G.; Svensson, E.; Sandanger, I.; Czujko, B.; Pedersen, E.D.; Aarseth, J.H.; Myhr, K.M. Depression and Anxiety amongst Multiple Sclerosis Patients. Eur. J. Neurol. 2008, 15, 239–245. [Google Scholar] [CrossRef]
- Dahl, O.-P.; Stordal, E.; Lydersen, S.; Midgard, R. Anxiety and Depression in Multiple Sclerosis. A Comparative Population-Based Study in Nord-Trøndelag County, Norway. Mult. Scler. J. 2009, 15, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
| MS (n = 469) | Control (n = 369) | |
|---|---|---|
| Age in years, mean (SD) | 42.7 (10.8) | 42.3 (11.7) |
| Age, range | 18–78 | 18–75 |
| Sex, n (%) | ||
| Women | 398 (84.8) | 304 (82.4) |
| Men | 71 (15.2) | 65 (17.6) |
| Right-hand dominance, n (%) | 438 (93.4) | 344 (93.2) |
| Education, n (%) | ||
| Primary school | 8 (1.7) | 0 |
| Secondary school | 252 (53.7) | 85 (23.3) |
| Professional study | 31 (6.6) | 24 (6.3) |
| Undergraduate study | 32 (6.9) | 34 (9.2) |
| Graduate study | 131 (27.9) | 189 (51.2) |
| Postgraduate study | 15 (3.2) | 37 (10.0) |
| Comorbidity, n (%) | 136 (29.0) | 95 (25.7) |
| FSS, mean (SD) | 5.01 (1.68) | 4.02 (1.37) |
| HADS-A, mean (SD) | 8.18 (4.49) | 8.19 (2.97) |
| HADS-D, mean (SD) | 7.02 (4.31) | 5.48 (3.51) |
| PSQI global, mean (SD) | 7.75 (4.04) | 5.97 (3.14) |
| MS type, n (%) | - | |
| RRMS | 353 (75.3) | |
| PPMS | 60 (12.8) | |
| SPMS | 19 (7.8) | |
| CIS | 2 (0.4) | |
| EDSS, median (Q1–Q3) | 2 (1–4) | - |
| Duration of MS, mean (SD) | 8.39 (7.74) | - |
| Immunomodulatory drug, n (%) | 342 (72.9) | - |
| MS Mean (SD) | Control Mean (SD) | p-Value | |
|---|---|---|---|
| FSS | 5.01 (1.68) | 4.02 (1.37) | <2.2 × 10−16 |
| HADS-A | 8.18 (4.49) | 8.19 (2.97) | 0.961 |
| HADS-D | 7.02 (4.31) | 5.48 (3.51) | 1.83 × 10−8 |
| PSQI global | 7.75 (4.04) | 5.97 (3.14) | 1.717 × 10−12 |
| HADS-A | HADS-D | PSQI Global | EDSS | |
|---|---|---|---|---|
| FSS | 0.517 (<0.001) | 0.577 (<0.001) | 0.399 (<0.001) | 0.266 (<0.001) |
| HADS-A | - | 0.665 (<0.001) | 0.474 (<0.001) | 0.095 (0.056) |
| HADS-D | - | - | 0.466 (<0.001) | 0.294 (<0.001) |
| PSQI global | - | - | - | 0.153 (0.004) |
| Predictor Variables | Dependent Variable: HADS-A | ||
|---|---|---|---|
| (1) | (2) | (3) | |
| PSQI global | 0.528 (<0.001) | 0.519 (<0.001) | 0.527 (<0.001) |
| Age | −0.022 (0.227) | −0.026 (0.193) | |
| Sex (Male) | −1.072 (0.05) | −1.033 (0.069) | |
| EDSS | 0.122 (0.249) | ||
| Observations | 461 | 441 | 386 |
| Adjusted R2 | 0.223 | 0.220 | 0.240 |
| F-statistic | 132.812 (df = 1; 459) | 42.362 (df = 3; 437) | 31.467 (df = 4; 381) |
| Predictor Variables | Dependent Variable: HADS-D | ||
|---|---|---|---|
| (1) | (2) | (3) | |
| PSQI global | 0.499 (<0.001) | 0.489 (<0.001) | 0.480 (<0.001) |
| Age | 0.051 (0.003) | 0.028 (0.147) | |
| Sex (Male) | 0.505 (0.326) | 0.148 (0.788) | |
| EDSS | 0.435 (<0.001) | ||
| Observations | 461 | 441 | 386 |
| Adjusted R2 | 0.215 | 0.221 | 0.263 |
| F-statistic | 127.094 (df = 1; 459) | 42.593 (df = 3; 437) | 35.434 (df = 4; 381) |
| Predictor Variables | Dependent Variable: FSS | ||
|---|---|---|---|
| (1) | (2) | (3) | |
| PSQI global | 0.166 (<0.001) | 0.153 (<0.001) | 0.151 (<0.001) |
| Age | 0.017 (0.018) | 0.009 (0.227) | |
| Sex (Male) | −0.266 (0.195) | − 0.484 (0.024) | |
| EDSS | 0.170 (<0.001) | ||
| Observations | 464 | 445 | 390 |
| Adjusted R2 | 0.157 | 0.150 | 0.212 |
| F-statistic | 87.288 (df = 1; 462) | 27.030 (df = 3; 441) | 27.114 (df = 4; 385) |
| Predictor Variables | Dependent Variable | ||
|---|---|---|---|
| HADS-A | HADS-D | FSS | |
| PSQI1 (Subjective quality) | 0.545 (0.066) | 0.487 (0.081) | 0.129 (0.267) |
| PSQI2 (Sleep latency) | 0.064 (0.775) | 0.026 (0.903) | 0.003 (0.969) |
| PSQI3 (Sleep duration) | 0.106 (0.715) | −0.353 (0.198) | −0.140 (0.218) |
| PSQI4 (Sleep efficiency) | 0.044 (0.845) | 0.256 (0.228) | 0.067 (0.445) |
| PSQI5 (Sleep disturbances) | 1.161 (0.002) | 0.833 (0.017) | 0.426 (0.003) |
| PSQI6 (Use of sleep medications) | 0.658 (0.001) | 0.668 (0.001) | 0.169 (0.035) |
| PSQI7 (Daytime dysfunction) | 1.990 (<0.001) | 2.220 (<0.001) | 0.675 (<0.001) |
| Age | −0.015 (0.414) | 0.036 (0.044) | 0.010 (0.163) |
| Sex (Male) | −0.792 (0.130) | 0.287 (0.561) | −0.388 (0.057) |
| EDSS | 0.101 (0.302) | 0.383 (<0.001) | 0.161 (<0.001) |
| Observations | 373 | 373 | 377 |
| Adjusted R2 | 0.372 | 0.427 | 0.326 |
| F-statistic | 23.081 (df = 10; 362) | 28.726 (df = 10; 362) | 19.183 (df = 10; 366) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jerković, A.; Safić, I.S.; Pavelin, S.; Pleić, N.; Duka Glavor, K.; Vujović, I.; Šoda, J.; Duranović, J.; Rogić Vidaković, M. Disability and Non-Motor Symptoms in Multiple Sclerosis: Exploring Associations and Predictive Factors. Brain Sci. 2025, 15, 1122. https://doi.org/10.3390/brainsci15101122
Jerković A, Safić IS, Pavelin S, Pleić N, Duka Glavor K, Vujović I, Šoda J, Duranović J, Rogić Vidaković M. Disability and Non-Motor Symptoms in Multiple Sclerosis: Exploring Associations and Predictive Factors. Brain Sciences. 2025; 15(10):1122. https://doi.org/10.3390/brainsci15101122
Chicago/Turabian StyleJerković, Ana, Ivona Stipica Safić, Sanda Pavelin, Nikolina Pleić, Klaudia Duka Glavor, Igor Vujović, Joško Šoda, Jasna Duranović, and Maja Rogić Vidaković. 2025. "Disability and Non-Motor Symptoms in Multiple Sclerosis: Exploring Associations and Predictive Factors" Brain Sciences 15, no. 10: 1122. https://doi.org/10.3390/brainsci15101122
APA StyleJerković, A., Safić, I. S., Pavelin, S., Pleić, N., Duka Glavor, K., Vujović, I., Šoda, J., Duranović, J., & Rogić Vidaković, M. (2025). Disability and Non-Motor Symptoms in Multiple Sclerosis: Exploring Associations and Predictive Factors. Brain Sciences, 15(10), 1122. https://doi.org/10.3390/brainsci15101122









