The Orexin System in Addiction: Neuromodulatory Interactions and Therapeutic Potential
Abstract
1. Introduction
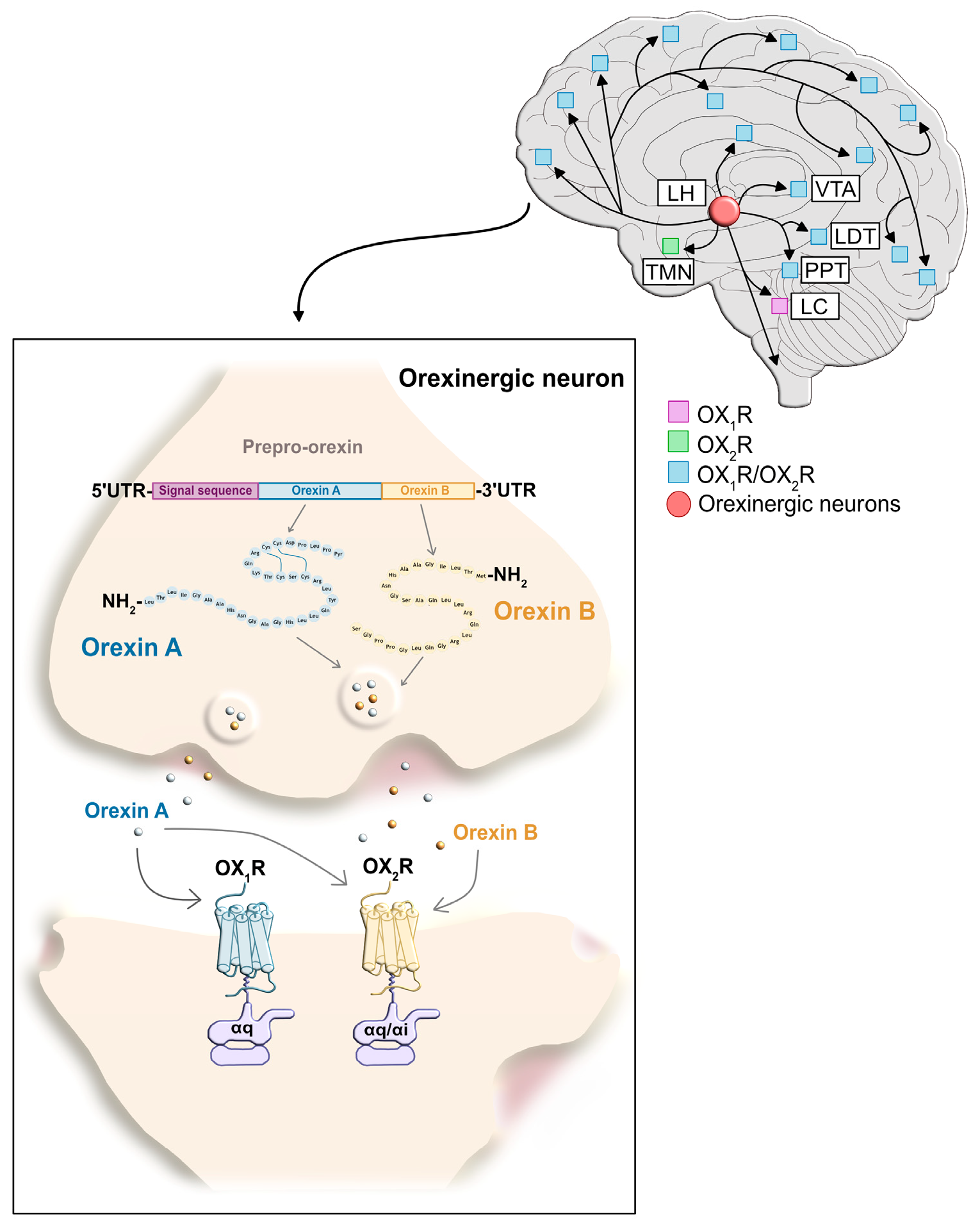
2. Orexin Receptors
2.1. The Orexin Receptor Type 1
2.2. The Orexin Receptor Type 2
2.3. Orexin Receptor Signaling: Regulation of Neuronal Excitability and Synaptic Plasticity
3. Different Roles of OX1 and OX2 Receptors in Addiction: Insights in Orexin Receptor Antagonists
4. Implication of Orexin Peptides in Addiction
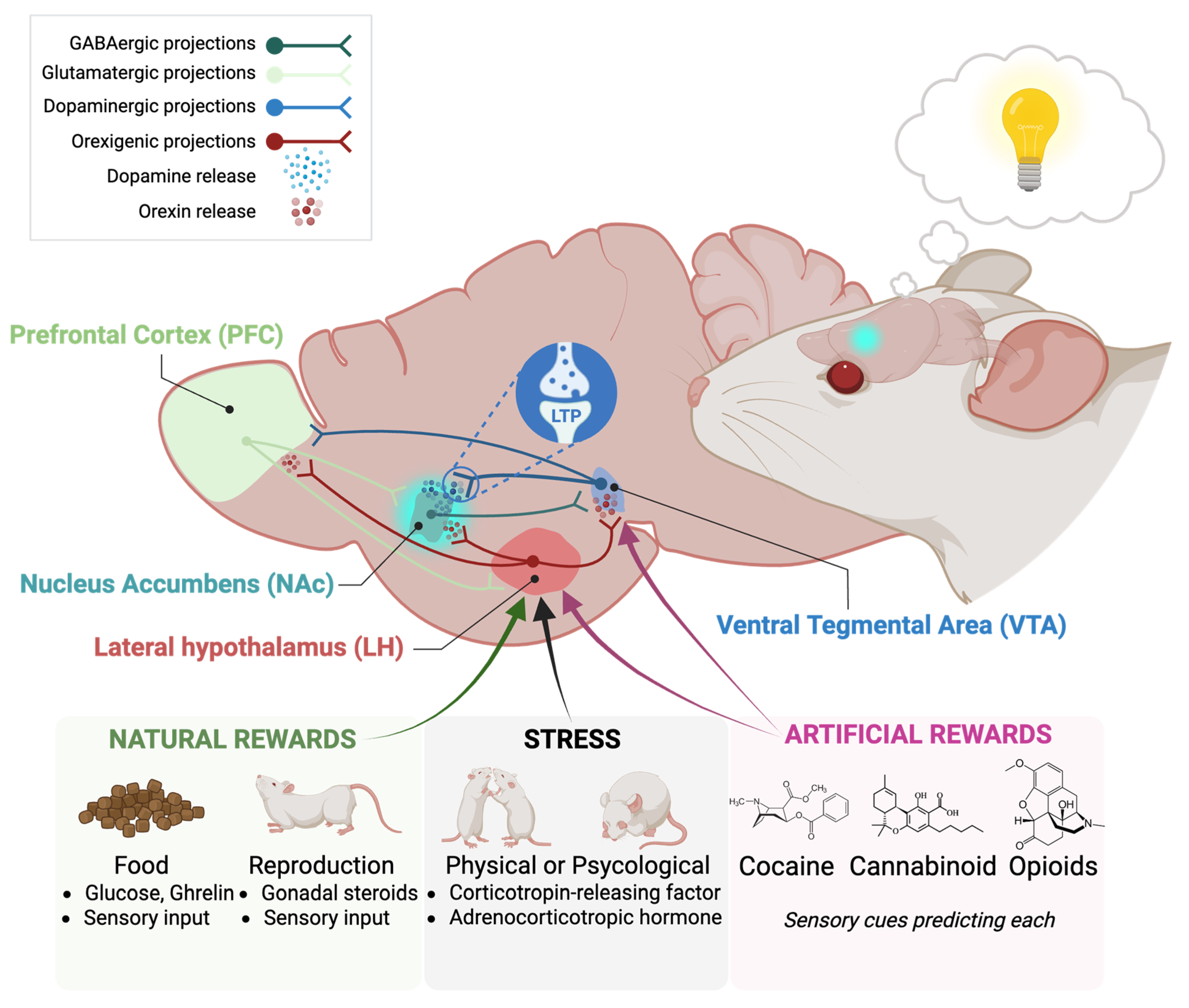
5. Orexin System Contribution to Relapse of Natural Rewards and Stress-Reward Circuitry
6. Orexin System Interactions with Other Systems in Addiction
6.1. Orexin-Opioid System Interaction
6.2. Orexin-Dopaminergic System Interaction
6.3. Orexin-Cannabinoid System Interaction
7. Orexin Receptor Heteromers as Therapeutic Targets in Addiction
8. Future Directions
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| δOR | δ-opioid receptor |
| κOR | κ-opioid receptor |
| μOR | μ-opioid receptor |
| σ1R | σ 1 receptor |
| 2-AG | 2-arachidonoylglycerol |
| A2AR | Adenosine receptor 2A |
| AUD | Alcohol use disorder |
| CB1R | Cannabinoid receptor 1 |
| CB2R | Cannabinoid receptor 2 |
| CNS | Central Nervous System |
| CPP | Conditioned place preference |
| CRF | Corticotropin-releasing factor |
| CRF1R | CRF receptor 1 |
| D1R | Dopamine receptor 1 |
| D2R | Dopamine receptor 2 |
| DG | Dentate gyrus |
| DORA | Dual orexin receptor antagonist |
| GPCRs | G protein-coupled receptors |
| HPA | Hypothalamic–pituitary–adrenal axis |
| iCa2+ | Intracellular calcium |
| IntA | Intermittent access |
| KO | Knockout |
| LC | Locus coeruleus |
| LDT | Laterodorsal tegmental nuclei |
| LH | Lateral hypothalamus |
| LTP | Long-term potentiation |
| MAPK | Mitogen-activated protein kinase |
| mTORC1 | Mammalian Target of Rapamycin complex 1 |
| NAc | Nucleus accumbens |
| NMDA | N-methyl-D-aspartate |
| OXA | Orexin A |
| OXB | Orexin B |
| OXRs | Orexin receptors |
| OX1R | Orexin receptor 1 |
| OX2R | Orexin receptor 2 |
| PKC | Protein kinase C |
| PLC | Phospolipase C |
| PFC | Prefrontal cortex |
| PPT | Pedunculopontine tegmental nuclei |
| pPVT | Paraventricular nucleus of the thalamus |
| TMN | Tuberomammillary nucleus |
| VGCC | Voltage-gated Ca2+ channel |
| VTA | Ventral tegmental area |
References
- United Nations Office on Drugs and Crime. World Drug Report 2025; United Nations Office on Drugs and Crime: Vienna, Austria, 2025; ISBN 9789211544084. [Google Scholar]
- Kelley, A.E. Memory and Addiction: Shared Neural Circuitry and Molecular Mechanisms. Neuron 2004, 44, 161–179. [Google Scholar] [CrossRef]
- NIDA. Drug Misuse and Addiction. 2020. Available online: https://nida.nih.gov/publications/drugs-brains-behavior-science-addiction/drug-misuse-addiction (accessed on 30 July 2025).
- Cabral Barata, P.; Oliveira, C.F.P.; Lima de Castro, S.; Rocha da Mota, A.M.P. A Systematic Review on Substance Addiction: Medical Diagnosis or Morality Flaw? Eur. J. Psychiatry 2019, 33, 143–151. [Google Scholar] [CrossRef]
- Volkow, N.D.; Michaelides, M.; Baler, R. The Neuroscience of Drug Reward and Addiction. Physiol. Rev. 2019, 99, 2115–2140. [Google Scholar] [CrossRef]
- Bevilacqua, L.; Goldman, D. Genes and Addictions. Clin. Pharmacol. Ther. 2009, 85, 359–361. [Google Scholar] [CrossRef]
- Murray, E.; Wise, S.; Rhodes, S. What Can Different Brains Do with Reward? In Neurobiology of Sensation and Reward; Gottfried, J.A., Ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Abingdon, UK, 2011. [Google Scholar]
- Lewis, R.G.; Florio, E.; Punzo, D.; Borrelli, E. The Brain’s Reward System in Health and Disease. Adv. Exp. Med. Biol. 2021, 1344, 57–69. [Google Scholar] [CrossRef]
- Saunders, B.T.; Richard, J.M.; Margolis, E.B.; Patricia, H.; Sciences, B. Dopamine Neurons Create Pavlovian Conditioned Stimuli with Circuit-Defined Motivational Properties. Nat. Neurosci. 2018, 21, 1072–1083. [Google Scholar] [CrossRef]
- Wise, R.A. Forebrain Substrates of Reward and Motivation. J. Comp. Neurol. 2005, 493, 115–121. [Google Scholar] [CrossRef]
- Lüscher, C.; Janak, P.H. Consolidating the Circuit Model for Addiction. Annu. Rev. Neurosci. 2021, 44, 173–195. [Google Scholar] [CrossRef]
- Wise, R.A.; Jordan, C.J. Dopamine, Behavior, and Addiction. J. Biomed. Sci. 2021, 28, 83. [Google Scholar] [CrossRef]
- Berke, J.D.; Hyman, S.E. Addiction, Dopamine, and the Molecular Mechanisms of Memory. Neuron 2000, 25, 515–532. [Google Scholar] [CrossRef]
- Messina, A.; De Fusco, C.; Monda, V.; Esposito, M.; Moscatelli, F.; Valenzano, A.; Carotenuto, M.; Viggiano, E.; Chieffi, S.; De Luca, V.; et al. Role of the Orexin System on the Hypothalamus-Pituitary-Thyroid Axis. Front. Neural Circuits 2016, 10, 66. [Google Scholar] [CrossRef]
- Brown, R.E.; Sergeeva, O.A.; Eriksson, K.S.; Haas, H.L. Convergent Excitation of Dorsal Raphe Serotonin Neurons by Multiple Arousal Systems (Orexin/Hypocretin, Histamine and Noradrenaline). J. Neurosci. 2002, 22, 8850–8859. [Google Scholar] [CrossRef]
- Chemelli, R.M.; Willie, J.T.; Sinton, C.M.; Elmquist, J.K.; Scammell, T.; Lee, C.; Richardson, J.A.; Clay Williams, S.; Xiong, Y.; Kisanuki, Y.; et al. Narcolepsy in Orexin Knockout Mice: Molecular Genetics of Sleep Regulation. Cell 1999, 98, 437–451. [Google Scholar] [CrossRef]
- Udana, P.K.S.; Dharmaratne, A. Facial Expression Generation in 3D Space. In Proceedings of the VINCI ’14: The 7th International Symposium on Visual Information Communication and Interaction, Sydney, Australia, 5–8 August 2014; pp. 236–237. [Google Scholar] [CrossRef]
- Mieda, M. The Roles of Orexins in Sleep/Wake Regulation. Neurosci. Res. 2017, 118, 56–65. [Google Scholar] [CrossRef]
- Shen, Y.C.; Sun, X.; Li, L.; Zhang, H.Y.; Huang, Z.L.; Wang, Y.Q. Funciones de Los Neuropéptidos En La Regulación Del Sueño y La Vigilia. Int. J. Mol. Sci. 2022, 23, 4599. [Google Scholar] [CrossRef]
- Weinhold, S.L.; Seeck-Hirschner, M.; Nowak, A.; Hallschmid, M.; Göder, R.; Baier, P.C. The Effect of Intranasal Orexin-A (Hypocretin-1) on Sleep, Wakefulness and Attention in Narcolepsy with Cataplexy. Behav. Brain Res. 2014, 262, 8–13. [Google Scholar] [CrossRef]
- Mieda, M.; Willie, J.T.; Hara, J.; Sinton, C.M.; Sakurai, T.; Yanagisawa, M. Orexin Peptides Prevent Cataplexy and Improve Wakefulness in an Orexin Neuron-Ablated Model of Narcolepsy in Mice. Proc. Natl. Acad. Sci. USA 2004, 101, 4649–4654. [Google Scholar] [CrossRef]
- Kayaba, Y.; Nakamura, A.; Kasuya, Y.; Ohuchi, T.; Yanagisawa, M.; Komuro, I.; Fukuda, Y.; Kuwaki, T. Attenuated Defense Response and Low Basal Blood Pressure in Orexin Knockout Mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R581–R593. [Google Scholar] [CrossRef]
- Ohnishi, H.; Pei, R.; Muto, Y.; Moriwaki, H.; Nagura, K. Sympathetic and Cardiovascular Actions of Orexins in Conscious Rats. Gastroenterol. Jpn. 1989, 24, 83. [Google Scholar] [CrossRef]
- Ida, T.; Nakahara, K.; Katayama, T.; Murakami, N.; Nakazato, M. Effect of Lateral Cerebroventricular Injection of the Appetite- Stimulating Neuropeptide, Orexin and Neuropeptide Y, on the Various Behavioral Activities of Rats. Brain Res. 1999, 821, 526–529. [Google Scholar] [CrossRef]
- Yaeger, J.D.W.; Krupp, K.T.; Jacobs, B.M.; Onserio, B.O.; Meyerink, B.L.; Cain, J.T.; Ronan, P.J.; Renner, K.J.; DiLeone, R.J.; Summers, C.H. Orexin 1 Receptor Antagonism in the Basolateral Amygdala Shifts the Balance from Pro- to Antistress Signaling and Behavior. Biol. Psychiatry 2022, 91, 841–852. [Google Scholar] [CrossRef]
- Gorka, S.M.; Khorrami, K.J.; Manzler, C.A.; Phan, K.L. Acute Orexin Antagonism Selectively Modulates Anticipatory Anxiety in Humans: Implications for Addiction and Anxiety. Transl. Psychiatry 2022, 12, 308. [Google Scholar] [CrossRef]
- Li, B.; Chang, L.; Peng, X. Orexin 2 Receptor in the Nucleus Accumbens Is Critical for the Modulation of Acute Stress-Induced Anxiety. Psychoneuroendocrinology 2021, 131, 105317. [Google Scholar] [CrossRef]
- Palotai, M.; Telegdy, G.; Jászberényi, M. Orexin A-Induced Anxiety-like Behavior Is Mediated through GABA-Ergic, α- and β-Adrenergic Neurotransmissions in Mice. Peptides 2014, 57, 129–134. [Google Scholar] [CrossRef]
- Suzuki, M.; Beuckmann, C.T.; Shikata, K.; Ogura, H.; Sawai, T. Orexin-A (Hypocretin-1) Is Possibly Involved in Generation of Anxiety-like Behavior. Brain Res. 2005, 1044, 116–121. [Google Scholar] [CrossRef]
- Vanderhaven, M.W.; Cornish, J.L.; Staples, L.G. The Orexin-1 Receptor Antagonist SB-334867 Decreases Anxiety-like Behavior and c-Fos Expression in the Hypothalamus of Rats Exposed to Cat Odor. Behav. Brain Res. 2015, 278, 563–568. [Google Scholar] [CrossRef]
- Peyron, C.; Faraco, J.; Rogers, W.; Ripley, B.; Overeem, S.; Charnay, Y.; Nevsimalova, S.; Aldrich, M.; Raynolds, D.; Albin, R.; et al. A Mutation in a Case of Early Onset Narcolepsy and a Generalized Absence of Hypocretin Peptides in Human Narcoleptic Brains. Nat. Med. 2000, 6, 991–997. [Google Scholar] [CrossRef]
- Soya, S.; Shoji, H.; Hasegawa, E.; Hondo, M.; Miyakawa, T.; Yanagisawa, M.; Mieda, M.; Sakurai, T. Orexin Receptor-1 in the Locus Coeruleus Plays an Important Role in Cue-Dependent Fear Memory Consolidation. J. Neurosci. 2013, 33, 14549–14557. [Google Scholar] [CrossRef]
- Strawn, J.R.; Pyne-Geithman, G.J.; Ekhator, N.N.; Horn, P.S.; Uhde, T.W.; Shutter, L.A.; Baker, D.G.; Geracioti, T.D. Low Cerebrospinal Fluid and Plasma Orexin-A (Hypocretin-1) Concentrations in Combat-Related Posttraumatic Stress Disorder. Psychoneuroendocrinology 2010, 35, 1001–1007. [Google Scholar] [CrossRef]
- Wang, H.; Li, S.; Kirouac, G.J. Role of the Orexin (Hypocretin) System in Contextual Fear Conditioning in Rats. Behav. Brain Res. 2017, 316, 47–53. [Google Scholar] [CrossRef]
- Flores, Á.; Saravia, R.; Maldonado, R.; Berrendero, F. Orexins and Fear: Implications for the Treatment of Anxiety Disorders. Trends Neurosci. 2015, 38, 550–559. [Google Scholar] [CrossRef]
- Taheri, S.; Gardiner, J.; Hafizi, S.; Murphy, K.; Dakin, C.; Seal, L.; Small, C.; Ghatei, M.; Bloom, S. Orexin A Immunoreactivity and Prepro-Orexin MRNA in the Brain of Zucker and WKY Rats. NeuroReport 2001, 2, 459–464. [Google Scholar] [CrossRef]
- Hsu, C.W.; Wang, S. Changes in the Orexin System in Rats Exhibiting Learned Helplessness Behaviors. Brain Sci. 2021, 11, 1634. [Google Scholar] [CrossRef]
- Scott, M.M.; Marcus, J.N.; Pettersen, A.; Birnbaum, S.G.; Mochizuki, T.; Scammell, T.E.; Nestler, E.J.; Elmquist, J.K.; Lutter, M. Hcrtr1 and 2 Signaling Differentially Regulates Depression-like Behaviors. Behav. Brain Res. 2011, 222, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Vollmayr, B.; Gass, P. Learned Helplessness: Unique Features and Translational Value of a Cognitive Depression Model. Cell Tissue Res. 2013, 354, 171–178. [Google Scholar] [CrossRef]
- Allard, J.S.; Tizabi, Y.; Shaffery, J.P.; Ovid Trouth, C.; Manaye, K. Stereological Analysis of the Hypothalamic Hypocretin/Orexin Neurons in an Animal Model of Depression. Neuropeptides 2004, 38, 311–315. [Google Scholar] [CrossRef]
- Chung, H.S.; Kim, J.G.; Kim, J.W.; Kim, H.W.; Yoon, B.J. Orexin Administration to Mice That Underwent Chronic Stress Produces Bimodal Effects on Emotion-Related Behaviors. Regul. Pept. 2014, 194–195, 16–22. [Google Scholar] [CrossRef]
- Mikrouli, E.; Wörtwein, G.; Soylu, R.; Mathé, A.A.; Petersén, Å. Increased Numbers of Orexin/Hypocretin Neurons in a Genetic Rat Depression Model. Neuropeptides 2011, 45, 401–406. [Google Scholar] [CrossRef]
- Lin, C.C.; Huang, T.L. Orexin/Hypocretin and Major Psychiatric Disorders. Adv. Clin. Chem. 2022, 109, 185–212. [Google Scholar] [CrossRef]
- Lu, J.; Huang, M.L.; Li, J.H.; Jin, K.Y.; Li, H.M.; Mou, T.T.; Fronczek, R.; Duan, J.F.; Xu, W.J.; Swaab, D.; et al. Changes of Hypocretin (Orexin) System in Schizophrenia: From Plasma to Brain. Schizophr. Bull. 2021, 47, 1310–1319. [Google Scholar] [CrossRef]
- Perez, S.M.; Lodge, D.J. Orexin Modulation of VTA Dopamine Neuron Activity: Relevance to Schizophrenia. Int. J. Neuropsychopharmacol. 2021, 24, 344–353. [Google Scholar] [CrossRef]
- Tsuchimine, S.; Hattori, K.; Ota, M.; Hidese, S.; Teraishi, T.; Sasayama, D.; Hori, H.; Noda, T.; Yoshida, S.; Yoshida, F.; et al. Reduced Plasma Orexin-A Levels in Patients with Bipolar Disorder. Neuropsychiatr. Dis. Treat. 2019, 15, 2221–2230. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.L.; Liu, C.M.; Shan, J.C.; Lee, H.J.; Hsieh, M.H.; Hwu, H.G.; Chiou, L.C. Elevated Plasma Orexin A Levels in a Subgroup of Patients with Schizophrenia Associated with Fewer Negative and Disorganized Symptoms. Psychoneuroendocrinology 2015, 53, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; White, R.E.; Xu, L.; Yang, L.; Sun, X.; Zou, B.; Pascual, C.; Sakurai, T.; Giffard, R.G.; Xie, X. Mitigation of Murine Focal Cerebral Ischemia by the Hypocretin/Orexin System is Associated With Reduced Inflammation. Stroke 2013, 44, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Li, X.; Guo, J.; Zhang, Z.; Guo, X.; Li, Z.; Lin, J.; Li, P.; Jiang, Z.; Zhu, Y. Orexin A protects against cerebral ischemia-reperfusion injury by enhancing reperfusion in ischemic cortex via HIF-1α-ET-1/eNOS pathway. Brain Res. Bull. 2024, 218, 111105. [Google Scholar] [CrossRef]
- Dohi, K.; Ripley, B.; Fujiki, N.; Ohtaki, H.; Shioda, S.; Aruga, T.; Nishino, S. CSF Hypocretin-1/Orexin-A Concentrations in Patients with Subarachnoid Hemorrhage (SAH). Peptides 2005, 26, 2339–2343. [Google Scholar] [CrossRef]
- Nishino, S.; Mignot, E. Pharmacological Aspects of Human and Canine Narcolepsy. Prog. Neurobiol. 1997, 52, 27–78. [Google Scholar] [CrossRef]
- Kitamura, E.; Hamada, J.; Kanazawa, N.; Yonekura, J.; Masuda, R.; Sakai, F.; Mochizuki, H. The Effect of Orexin-A on the Pathological Mechanism in the Rat Focal Cerebral Ischemia. Neurosci. Res. 2010, 68, 154–157. [Google Scholar] [CrossRef]
- Liguori, C.; Romigi, A.; Nuccetelli, M.; Zannino, S.; Sancesario, G.; Martorana, A.; Albanese, M.; Mercuri, N.B.; Izzi, F.; Bernardini, S.; et al. Orexinergic System Dysregulation, Sleep Impairment, and Cognitive Decline in Alzheimer Disease. JAMA Neurol. 2014, 71, 1498. [Google Scholar] [CrossRef]
- Liguori, C.; Nuccetelli, M.; Izzi, F.; Sancesario, G.; Romigi, A.; Martorana, A.; Amoroso, C.; Bernardini, S.; Marciani, M.G.; Mercuri, N.B.; et al. Rapid Eye Movement Sleep Disruption and Sleep Fragmentation Are Associated with Increased Orexin-A Cerebrospinal-Fluid Levels in Mild Cognitive Impairment Due to Alzheimer’s Disease. Neurobiol. Aging 2016, 40, 120–126. [Google Scholar] [CrossRef]
- Liguori, C.; Spanetta, M.; Izzi, F.; Franchini, F.; Nuccetelli, M.; Sancesario, G.M.; Di Santo, S.; Bernardini, S.; Mercuri, N.B.; Placidi, F. Sleep-Wake Cycle in Alzheimer’s Disease Is Associated with Tau Pathology and Orexin Dysregulation. J. Alzheimer’s Dis. 2020, 74, 501–508. [Google Scholar] [CrossRef]
- Li, M.; Meng, Y.; Chu, B.; Shen, Y.; Xue, X.; Song, C.; Liu, X.; Ding, M.; Cao, X.; Wang, P.; et al. Orexin-A Exacerbates Alzheimer’s Disease by Inducing Mitochondrial Impairment. Neurosci. Lett. 2020, 718, 134741. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.H.; Jiang, H.; Finn, M.B.; Stewart, F.R.; Mahan, T.E.; Cirrito, J.R.; Heda, A.; Joy Snider, B.; Li, M.; Yanagisawa, M.; et al. Correction to Potential Role of Orexin and Sleep Modulation in the Pathogenesis of Alzheimer’s Disease. J. Exp. Med. 2014, 211, 2487–2496. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.M.; Kratzsch, J.; Gertz, H.J.; Tittmann, M.; Jahn, I.; Pietsch, U.C.; Kaisers, U.X.; Thiery, J.; Hegerl, U.; Schönknecht, P. Cerebrospinal Fluid Melanin-Concentrating Hormone (MCH) and Hypocretin-1 (HCRT-1, Orexin-A) in Alzheimer’s Disease. PLoS ONE 2013, 8, e63136. [Google Scholar] [CrossRef]
- Wang, C.; Holtzman, D.M. Bidirectional Relationship between Sleep and Alzheimer’s Disease: Role of Amyloid, Tau, and Other Factors. Neuropsychopharmacology 2020, 45, 104–120. [Google Scholar] [CrossRef]
- Pasban-Aliabadi, H.; Esmaeili-Mahani, S.; Abbasnejad, M. Orexin-A Protects Human Neuroblastoma SH-SY5Y Cells Against 6-Hydroxydopamine-Induced Neurotoxicity: Involvement of PKC and PI3K Signaling Pathways. Rejuvenation Res. 2017, 20, 125–133. [Google Scholar] [CrossRef]
- Liu, C.; Xue, Y.; Liu, M.F.; Wang, Y.; Liu, Z.R.; Diao, H.L.; Chen, L. Orexins Increase the Firing Activity of Nigral Dopaminergic Neurons and Participate in Motor Control in Rats. J. Neurochem. 2018, 147, 380–394. [Google Scholar] [CrossRef]
- Liu, M.F.; Xue, Y.; Liu, C.; Liu, Y.H.; Diao, H.L.; Wang, Y.; Pan, Y.P.; Chen, L. Orexin—A Exerts Neuroprotective Effects via OX1R in Parkinson⇔s Disease. Front. Neurosci. 2018, 12, 835. [Google Scholar] [CrossRef]
- Kotz, C.M.; Wang, C.; Teske, J.A.; Thorpe, A.J.; Novak, C.M.; Kiwaki, K.; Levine, J.A. Orexin A Mediation of Time Spent Moving in Rats: Neural Mechanisms. Neuroscience 2006, 142, 29–36. [Google Scholar] [CrossRef]
- Wang, Q.; Cao, F.; Wu, Y. Orexinergic System in Neurodegenerative Diseases. Front. Aging Neurosci. 2021, 13, 713201. [Google Scholar] [CrossRef]
- Gabery, S.; Murphy, K.; Schultz, K.; Loy, C.T.; McCusker, E.; Kirik, D.; Halliday, G.; Petersén, Å. Changes in Key Hypothalamic Neuropeptide Populations in Huntington Disease Revealed by Neuropathological Analyses. Acta Neuropathol. 2010, 120, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Gabery, S.; Sajjad, M.U.; Hult, S.; Soylu, R.; Kirik, D.; Petersén, Å. Characterization of a Rat Model of Huntington’s Disease Based on Targeted Expression of Mutant Huntingtin in the Forebrain Using Adeno-Associated Viral Vectors. Eur. J. Neurosci. 2012, 36, 2789–2800. [Google Scholar] [CrossRef] [PubMed]
- Cabanas, M.; Pistono, C.; Puygrenier, L.; Rakesh, D.; Jeantet, Y.; Garret, M.; Cho, Y.H. Neurophysiological and Behavioral Effects of Anti-Orexinergic Treatments in a Mouse Model of Huntington’s Disease. Neurotherapeutics 2019, 16, 784–796. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.H.; Morton, A.J.; Burdakov, D. Paradoxical Function of Orexin/Hypocretin Circuits in a Mouse Model of Huntington’s Disease. Neurobiol. Dis. 2011, 42, 438–445. [Google Scholar] [CrossRef]
- Ten-Blanco, M.; Flores, Á.; Cristino, L.; Pereda-Pérez, I.; Berrendero, F. Targeting the Orexin/Hypocretin System for the Treatment of Neuropsychiatric and Neurodegenerative Diseases: From Animal to Clinical Studies. Front. Neuroendocrinol. 2023, 69, 101066. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Q.; Ji, B.; Pan, Y.; Xu, C.; Cheng, B.; Bai, B.; Chen, J. The Orexin/Receptor System: Molecular Mechanism and Therapeutic Potential for Neurological Diseases. Front. Mol. Neurosci. 2018, 11, 220. [Google Scholar] [CrossRef]
- Ferris, M.J.; España, R.A.; Locke, J.L.; Konstantopoulos, J.K.; Rose, J.H.; Chen, R.; Jones, S.R. Dopamine Transporters Govern Diurnal Variation in Extracellular Dopamine Tone. Proc. Natl. Acad. Sci. USA 2014, 111, E2751–E2759. [Google Scholar] [CrossRef]
- Bicker, J.; Alves, G.; Falc, A.; Fortuna, A. Timing in Drug Absorption and Disposition: The Past, Present, and Future of Chronopharmacokinetics. Br. J. Pharmacol. 2020, 177, 2215–2239. [Google Scholar] [CrossRef]
- Matzeu, A.; Martin-Fardon, R. Blockade of Orexin Receptors in the Posterior Paraventricular Nucleus of the Thalamus Prevents Stress-Induced Reinstatement of Reward-Seeking Behavior in Rats with a History of Ethanol Dependence. Front. Integr. Neurosci. 2020, 14, 599710. [Google Scholar] [CrossRef]
- Alcaraz-Iborra, M.; Navarrete, F.; Rodríguez-Ortega, E.; de la Fuente, L.; Manzanares, J.; Cubero, I. Different Molecular/Behavioral Endophenotypes in C57BL/6J Mice Predict the Impact of OX1 Receptor Blockade on Binge-Like Ethanol Intake. Front. Behav. Neurosci. 2017, 11, 186. [Google Scholar] [CrossRef]
- Bonifazi, A.; Del Bello, F.; Giorgioni, G.; Piergentili, A.; Saab, E.; Botticelli, L.; Cifani, C.; Micioni Di Bonaventura, E.; Micioni Di Bonaventura, M.V.; Quaglia, W. Targeting Orexin Receptors: Recent Advances in the Development of Subtype Selective or Dual Ligands for the Treatment of Neuropsychiatric Disorders. Med. Res. Rev. 2023, 43, 1607–1667. [Google Scholar] [CrossRef]
- Couvineau, A.; Nicole, P.; Gratio, V.; Voisin, T. The Orexin Receptors: Structural and Anti-Tumoral Properties. Front. Endocrinol. 2022, 13, 931970. [Google Scholar] [CrossRef] [PubMed]
- Tsujino, N.; Sakurai, T. Orexin/Hypocretin: A Neuropeptide at the Interface of Sleep, Energy Homeostasis, and Reward System. Pharmacol. Rev. 2009, 61, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Scammel, T.E.; Winrow, C.J. Orexin Receptors: Pharmacology and Therapeutic Opportunities. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 243. [Google Scholar] [CrossRef] [PubMed]
- Fronczek, R.; Lammers, G.J.; Balesar, R.; Unmehopa, U.A.; Swaab, D.F. The Number of Hypothalamic Hypocretin (Orexin) Neurons Is Not Affected in Prader-Willi Syndrome. J. Clin. Endocrinol. Metab. 2005, 90, 5466–5470. [Google Scholar] [CrossRef]
- Zhou, L.; Smith, R.J.; Do, P.H.; Aston-Jones, G.; See, R.E. Repeated Orexin 1 Receptor Antagonism Effects on Cocaine Seeking in Rats. Neuropharmacology 2012, 63, 1201–1207. [Google Scholar] [CrossRef]
- Haynes, A.C.; Chapman, H.; Taylor, C.; Moore, G.B.T.; Cawthorne, M.A.; Tadayyon, M.; Clapham, J.C.; Arch, J.R.S. Anorectic, Thermogenic and Anti-Obesity Activity of a Selective Orexin-1 Receptor Antagonist in Ob/Ob Mice. Regul. Pept. 2002, 104, 153–159. [Google Scholar] [CrossRef]
- Johnstone, L.E.; Fong, T.M.; Leng, G. Neuronal Activation in the Hypothalamus and Brainstem during Feeding in Rats. Cell Metab. 2006, 4, 313–321. [Google Scholar] [CrossRef]
- Yamanaka, A.; Beuckmann, C.T.; Willie, J.T.; Hara, J.; Tsujino, N.; Mieda, M.; Tominaga, M.; Yagami, K.I.; Sugiyama, F.; Goto, K.; et al. Hypothalamic Orexin Neurons Regulate Arousal According to Energy Balance in Mice. Neuron 2003, 38, 701–713. [Google Scholar] [CrossRef]
- Moriguchi, T.; Sakurai, T.; Nambu, T.; Yanagisawa, M.; Goto, K. Neurons Containing Orexin in the Lateral Hypothalamic Area of the Adult Rat Brain Are Activated by Insulin-Induced Acute Hypoglycemia. Neurosci. Lett. 1999, 264, 101–104. [Google Scholar] [CrossRef]
- Xia, L.B.; Liu, H.Y.; Wang, B.Y.; Lin, H.N.; Wang, M.C.; Ren, J.X. A Review of Physiological Functions of Orexin: From Instinctive Responses to Subjective Cognition. Medicine 2023, 102, e34206. [Google Scholar] [CrossRef]
- Ohno, K.; Sakurai, T. Orexin Neuronal Circuitry: Role in the Regulation of Sleep and Wakefulness. Front. Neuroendocrinol. 2008, 29, 70–87. [Google Scholar] [CrossRef] [PubMed]
- Willie, J.T.; Chemelli, R.M.; Sinton, C.M.; Tokita, S.; Williams, S.C.; Kisanuki, Y.Y.; Marcus, J.N.; Lee, C.; Elmquist, J.K.; Kohlmeier, K.A.; et al. Distinct Narcolepsy Syndromes in Orexin Receptor-2 and Orexin Null Mice: Molecular Genetic Dissection of Non-REM and REM Sleep Regulatory Processes. Neuron 2003, 38, 715–730. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T. The Neural Circuit of Orexin (Hypocretin): Maintaining Sleep and Wakefulness. Nat. Rev. Neurosci. 2007, 8, 171–181. [Google Scholar] [CrossRef]
- Fujiki, N.; Yoshida, Y.; Ripley, B.; Mignot, E.; Nishino, S. Effects of IV and ICV Hypocretin-1 (Orexin A) in Hypocretin Receptor-2 Gene Mutated Narcoleptic Dogs and IV Hypocretin-1 Replacement Therapy in a Hypocretin-Ligand-Deficient Narcoleptic Dog. Sleep 2003, 26, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Kukkonen, J.P.; Leonard, C.S. Orexin/Hypocretin Receptor Signalling Cascades. Br. J. Pharmacol. 2014, 171, 314–331. [Google Scholar] [CrossRef]
- Leonard, C.S.; Kukkonen, J.P. Orexin/Hypocretin Receptor Signalling: A Functional Perspective. Br. J. Pharmacol. 2014, 171, 294–313. [Google Scholar] [CrossRef]
- Urbańska, A.; Sokołowska, P.; Woldan-Tambor, A.; Biegańska, K.; Brix, B.; Jöhren, O.; Namiecińska, M.; Zawilska, J.B. Orexins/Hypocretins Acting at Gi Protein-Coupled OX2 Receptors Inhibit Cyclic AMP Synthesis in the Primary Neuronal Cultures. J. Mol. Neurosci. 2012, 46, 10–17. [Google Scholar] [CrossRef]
- Woldan-Tambor, A.; Biegańska, K.; Wiktorowska-Owczarek, A.; Zawilska, J.B. Activation of Orexin/Hypocretin Type 1 Receptors Stimulates CAMP Synthesis in Primary Cultures of Rat Astrocytes. Pharmacol. Rep. 2011, 63, 717–723. [Google Scholar] [CrossRef]
- Turunen, P.M.; Jäntti, M.H.; Kukkonen, J.P. OX 1 Orexin/Hypocretin Receptor Signaling through Arachidonic Acid and Endocannabinoid Release. Mol. Pharmacol. 2012, 82, 156–167. [Google Scholar] [CrossRef]
- Johansson, L.; Ekholm, M.E.; Kukkonen, J.P. Multiple Phospholipase Activation by OX1 Orexin/Hypocretin Receptors. Cell. Mol. Life Sci. 2008, 65, 1948–1956. [Google Scholar] [CrossRef]
- Peltonen, H.M.; Magga, J.M.; Bart, G.; Turunen, P.M.; Antikainen, M.S.H.; Kukkonen, J.P.; Åkerman, K.E. Involvement of TRPC3 Channels in Calcium Oscillations Mediated by OX1 Orexin Receptors. Biochem. Biophys. Res. Commun. 2009, 385, 408–412. [Google Scholar] [CrossRef]
- Hoang, Q.V.; Bajic, D.; Yanagisawa, M.; Nakajima, S.; Nakajima, Y. Effects of Orexin (Hypocretin) on GIRK Channels. J. Neurophysiol. 2003, 90, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, K.S.; Sergeeva, O.; Brown, R.E.; Haas, H.L. Orexin/Hypocretin Excites the Histaminergic Neurons of the Tuberomammillary Nucleus. J. Neurosci. 2001, 21, 9273–9279. [Google Scholar] [CrossRef] [PubMed]
- Uramura, K.; Funahashi, H.; Muroya, S.; Shioda, S.; Takigawa, M.; Yada, T. Orexin-a Activates Phospholipase C- and Protein Kinase C-Mediated Ca2+ Signaling in Dopamine Neurons of the Ventral Tegmental Area. NeuroReport 2001, 12, 1885–1889. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.X.; Fan, S.Y.; Yan, J.; Chen, F.; Li, Y.; Yu, Z.P.; Hu, Z.A. Orexin A-Induced Extracellular Calcium Influx in Prefrontal Cortex Neurons Involves L-Type Calcium Channels. J. Physiol. Biochem. 2009, 65, 125–136. [Google Scholar] [CrossRef]
- Wu, W.-N.; Wu, P.-F.; Zhou, J.; Guan, X.-L.; Zhang, Z.; Yang, Y.-J.; Long, L.-H.; Xie, N.; Chen, J.-G.; Wang, F. Orexin-A Activates Hypothalamic AMP-Activated Protein Kinase Signaling through a Ca2+-Dependent Mechanism Involving Voltage-Gated L-Type Calcium Channel. Mol. Pharmacol. 2013, 84, 876–887. [Google Scholar] [CrossRef]
- Milasta, S.; Evans, N.A.; Ormiston, L.; Wilson, S.; Lefkowitz, R.J.; Milligan, G. The Sustainability of Interactions between the Orexin-1 Receptor and β-Arrestin-2 Is Defined by a Single C-Terminal Cluster of Hydroxy Amino Acids and Modulates the Kinetics of ERK MAPK Regulation. Biochem. J. 2005, 387, 573–584. [Google Scholar] [CrossRef]
- Ammoun, S.; Lindholm, D.; Wootz, H.; Åkerman, K.E.O.; Kukkonen, J.P. G-Protein-Coupled OX1 Orexin/Hcrtr-1 Hypocretin Receptors Induce Caspase-Dependent and -Independent Cell Death through P38 Mitogen-/Stress-Activated Protein Kinase. J. Biol. Chem. 2006, 281, 834–842. [Google Scholar] [CrossRef]
- Ramanjaneya, M.; Conner, A.C.; Chen, J.; Kumar, P.; Brown, J.E.P.; Jöhren, O.; Lehnert, H.; Stanfield, P.R.; Randeva, H.S. Orexin-Stimulated MAP Kinase Cascades Are Activated through Multiple G-Protein Signalling Pathways in Human H295R Adrenocortical Cells: Diverse Roles for Orexins A and B. J. Endocrinol. 2009, 202, 249–261. [Google Scholar] [CrossRef]
- Wenzel, J.; Grabinski, N.; Knopp, C.A.; Dendorfer, A.; Ramanjaneya, M.; Randeva, H.S.; Ehrhart-Bornstein, M.; Dominiak, P.; Jöhren, O. Hypocretin/Orexin Increases the Expression of Steroidogenic Enzymes in Human Adrenocortical NCI H295R Cells. Am. J. Physiol. Integr. Comp. Physiol. 2009, 297, R1601–R1609. [Google Scholar] [CrossRef] [PubMed]
- Sokołowska, P.; Urbańska, A.; Biegańska, K.; Wagner, W.; Ciszewski, W.; Namiecińska, M.; Zawilska, J.B. Orexins Protect Neuronal Cell Cultures Against Hypoxic Stress: An Involvement of Akt Signaling. J. Mol. Neurosci. 2014, 52, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, S.; Kakizaki, M.; Hirose, Y.; Ishikawa, Y.; Funato, H.; Yanagisawa, M.; Yu, Y.; Liu, Q. Orexin/Hypocretin Activates MTOR Complex 1 (MTORC1) via an Erk/Akt-Independent and Calcium-Stimulated Lysosome v-ATPase Pathway. J. Biol. Chem. 2014, 289, 31950–31959. [Google Scholar] [CrossRef] [PubMed]
- Borgland, S.L.; Taha, S.A.; Sarti, F.; Fields, H.L.; Bonci, A. Orexin A in the VTA Is Critical for the Induction of Synaptic Plasticity and Behavioral Sensitization to Cocaine. Neuron 2006, 49, 589–601. [Google Scholar] [CrossRef]
- Chen, X.-W.; Mu, Y.; Huang, H.-P.; Guo, N.; Zhang, B.; Fan, S.-Y.; Xiong, J.-X.; Wang, S.-R.; Xiong, W.; Huang, W.; et al. Hypocretin-1 Potentiates NMDA Receptor-Mediated Somatodendritic Secretion from Locus Ceruleus Neurons. J. Neurosci. 2008, 28, 3202–3208. [Google Scholar] [CrossRef]
- Borgland, S.L.; Storm, E.; Bonci, A. Orexin B/Hypocretin 2 Increases Glutamatergic Transmission to Ventral Tegmental Area Neurons. Eur. J. Neurosci. 2008, 28, 1545–1556. [Google Scholar] [CrossRef]
- Katzman, M.A.; Katzman, M.P. Neurobiology of the Orexin System and Its Potential Role in the Regulation of Hedonic Tone. Brain Sci. 2022, 12, 150. [Google Scholar] [CrossRef]
- Fragale, J.E.; James, M.H.; Avila, J.A.; Spaeth, A.M.; Aurora, R.N.; Langleben, D.; Aston-Jones, G. The Insomnia-Addiction Positive Feedback Loop: Role of the Orexin System. Front. Neurol. Neurosci. 2021, 45, 117–127. [Google Scholar]
- Moorman, D.E.; James, M.H.; Kilroy, E.A.; Aston-Jones, G. Orexin/Hypocretin-1 Receptor Antagonism Reduces Ethanol Self-Administration and Reinstatement Selectively in Highly-Motivated Rats. Brain Res. 2017, 1654, 34–42. [Google Scholar] [CrossRef]
- Dehkordi, O.; Rose, J.E.; Dávila-García, M.I.; Millis, R.M.; Mirzaei, S.A.; Manaye, K.F.; Jayam Trouth, A. Neuroanatomical Relationships between Orexin/Hypocretin-Containing Neurons/Nerve Fibers and Nicotine-Induced c-Fos-Activated Cells of the Reward-Addiction Neurocircuitry. J. Alcohol. Drug Depend. 2017, 5, 273. [Google Scholar] [CrossRef]
- Smith, R.J.; See, R.E.; Aston-Jones, G. Orexin/Hypocretin Signaling at the Orexin 1 Receptor Regulates Cue-Elicited Cocaine-Seeking. Eur. J. Neurosci. 2009, 30, 493–503. [Google Scholar] [CrossRef]
- Esmaili-Shahzade-Ali-Akbari, P.; Ghaderi, A.; Sadeghi, A.; Nejat, F.; Mehramiz, A. The Role of Orexin Receptor Antagonists in Inhibiting Drug Addiction: A Review Article. Addict. Health 2024, 16, 130–139. [Google Scholar] [CrossRef]
- Plaza-Zabala, A.; Flores, Á.; Martín-García, E.; Saravia, R.; Maldonado, R.; Berrendero, F. A Role for Hypocretin/Orexin Receptor-1 in Cue-Induced Reinstatement of Nicotine-Seeking Behavior. Neuropsychopharmacology 2013, 38, 1724–1736. [Google Scholar] [CrossRef] [PubMed]
- Quarta, D.; Valerio, E.; Hutcheson, D.M.; Hedou, G.; Heidbreder, C. The Orexin-1 Receptor Antagonist SB-334867 Reduces Amphetamine-Evoked Dopamine Outflow in the Shell of the Nucleus Accumbens and Decreases the Expression of Amphetamine Sensitization. Neurochem. Int. 2010, 56, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Hutcheson, D.M.; Quarta, D.; Halbout, B.; Rigal, A.; Valerio, E.; Heidbreder, C. Orexin-1 Receptor Antagonist SB-334867 Reduces the Acquisition and Expression of Cocaine-Conditioned Reinforcement and the Expression of Amphetamine-Conditioned Reward. Behav. Pharmacol. 2011, 22, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Matzeu, A.; Martin-Fardon, R. Cocaine-Seeking Behavior Induced by Orexin A Administration in the Posterior Paraventricular Nucleus of the Thalamus Is Not Long-Lasting: Neuroadaptation of the Orexin System During Cocaine Abstinence. Front. Behav. Neurosci. 2021, 15, 620868. [Google Scholar] [CrossRef]
- Black, E.M.; Samels, S.B.; Xu, W.; Barson, J.R.; Bass, C.E.; Kortagere, S.; España, R.A. Hypocretin/Orexin Receptor 1 Knockdown in GABA or Dopamine Neurons in the Ventral Tegmental Area Differentially Impact Mesolimbic Dopamine and Motivation for Cocaine. Addict. Neurosci. 2023, 7, 100104. [Google Scholar] [CrossRef]
- Bentzley, B.S.; Aston-Jones, G. Orexin-1 Receptor Signaling Increases Motivation for Cocaine-associated Cues. Eur. J. Neurosci. 2015, 41, 1149–1156. [Google Scholar] [CrossRef]
- James, M.H.; Stopper, C.M.; Zimmer, B.A.; Koll, N.E.; Bowrey, H.E.; Aston-Jones, G. Increased Number and Activity of a Lateral Subpopulation of Hypothalamic Orexin/Hypocretin Neurons Underlies the Expression of an Addicted State in Rats. Biol. Psychiatry 2019, 85, 925–935. [Google Scholar] [CrossRef]
- Moorman, D.E. The Hypocretin/Orexin System as a Target for Excessive Motivation in Alcohol Use Disorders. Psychopharmacology 2018, 235, 1663–1680. [Google Scholar] [CrossRef]
- Morganstern, I.; Chang, G.; Barson, J.R.; Ye, Z.; Karatayev, O.; Leibowitz, S.F. Differential Effects of Acute and Chronic Ethanol Exposure on Orexin Expression in the Perifornical Lateral Hypothalamus. Alcohol. Clin. Exp. Res. 2010, 34, 886–896. [Google Scholar] [CrossRef]
- Amodeo, L.R.; Liu, W.; Wills, D.N.; Vetreno, R.P.; Crews, F.T.; Ehlers, C.L. Adolescent Alcohol Exposure Increases Orexin-A/Hypocretin-1 in the Anterior Hypothalamus. Alcohol 2020, 88, 65–72. [Google Scholar] [CrossRef]
- Barson, J.R.; Fagan, S.E.; Chang, G.; Leibowitz, S.F. Neurochemical Heterogeneity of Rats Predicted by Different Measures to Be High Ethanol Consumers. Alcohol. Clin. Exp. Res. 2013, 37, E141–E151. [Google Scholar] [CrossRef]
- Collier, A.D.; Min, S.S.; Campbell, S.D.; Roberts, M.Y.; Camidge, K.; Leibowitz, S.F. Maternal Ethanol Consumption before Paternal Fertilization: Stimulation of Hypocretin Neurogenesis and Ethanol Intake in Zebrafish Offspring. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 96, 109728. [Google Scholar] [CrossRef] [PubMed]
- Kastman, H.E.; Blasiak, A.; Walker, L.; Siwiec, M.; Krstew, E.V.; Gundlach, A.L.; Lawrence, A.J. Nucleus incertus Orexin2 receptors mediate alcohol seeking in rats. Neuropharmacology 2016, 110, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Olney, J.J.; Navarro, M.; Thiele, T.E. Binge-Like Consumption of Ethanol and Other Salient Reinforcers is Blocked by Orexin-1 Receptor Inhibition and Leads to a Reduction of Hypothalamic Orexin Immunoreactivity. Alcohol. Clin. Exp. Res. 2015, 39, 21–29. [Google Scholar] [CrossRef]
- Olney, J.J.; Navarro, M.; Thiele, T.E. The Role of Orexin Signaling in the Ventral Tegmental Area and Central Amygdala in Modulating Binge-Like Ethanol Drinking Behavior. Alcohol. Clin. Exp. Res. 2017, 41, 551–561. [Google Scholar] [CrossRef]
- Shoblock, J.R.; Welty, N.; Aluisio, L.; Fraser, I.; Motley, S.T.; Morton, K.; Palmer, J.; Bonaventure, P.; Carruthers, N.I.; Lovenberg, T.W.; et al. Selective Blockade of the Orexin-2 Receptor Attenuates Ethanol Self-Administration, Place Preference, and Reinstatement. Psychopharmacology 2011, 215, 191–203. [Google Scholar] [CrossRef]
- Anderson, R.I.; Becker, H.C.; Adams, B.L.; Jesudason, C.D.; Rorick-Kehn, L.M. Orexin-1 and Orexin-2 Receptor Antagonists Reduce Ethanol Self-Administration in High-Drinking Rodent Models. Front. Neurosci. 2014, 8, 33. [Google Scholar] [CrossRef]
- Srinivasan, S.; Simms, J.A.; Nielsen, C.K.; Lieske, S.P.; Bito-Onon, J.J.; Yi, H.; Hopf, F.W.; Bonci, A.; Bartlett, S.E. The Dual Orexin/Hypocretin Receptor Antagonist, Almorexant, in the Ventral Tegmental Area Attenuates Ethanol Self-Administration. PLoS ONE 2012, 7, e44726. [Google Scholar] [CrossRef]
- Campbell, E.J.; Bonomo, Y.; Collins, L.; Norman, A.; O’Neill, H.; Streitberg, A.; Galloway, K.; Kyoong, A.; Perkins, A.; Pastor, A.; et al. The Dual Orexin Receptor Antagonist Suvorexant in Alcohol Use Disorder and Comorbid Insomnia: A Case Report. Clin. Case Rep. 2024, 12, e8740. [Google Scholar] [CrossRef]
- Harris, G.C.; Wimmer, M.; Aston-Jones, G. A Role for Lateral Hypothalamic Orexin Neurons in Reward Seeking. Nature 2005, 437, 556–559. [Google Scholar] [CrossRef]
- España, R.A.; Melchior, J.R.; Roberts, D.C.S.; Jones, S.R. Hypocretin 1/Orexin A in the Ventral Tegmental Area Enhances Dopamine Responses to Cocaine and Promotes Cocaine Self-Administration. Psychopharmacology 2011, 214, 415–426. [Google Scholar] [CrossRef]
- Boutrel, B.; Kenny, P.J.; Specio, S.E.; Martin-Fardon, R.; Markou, A.; Koob, G.F.; de Lecea, L. Role for Hypocretin in Mediating Stress-Induced Reinstatement of Cocaine-Seeking Behavior. Proc. Natl. Acad. Sci. USA 2005, 102, 19168–19173. [Google Scholar] [CrossRef]
- Flores-Ramirez, F.J.; Varodayan, F.P.; Patel, R.R.; Illenberger, J.M.; Di Ottavio, F.; Roberto, M.; Martin-Fardon, R. Blockade of Orexin Receptors in the Infralimbic Cortex Prevents Stress-induced Reinstatement of Alcohol-seeking Behaviour in Alcohol-dependent Rats. Br. J. Pharmacol. 2023, 180, 1500–1515. [Google Scholar] [CrossRef]
- Thannickal, T.C.; John, J.; Shan, L.; Swaab, D.F.; Wu, M.-F.; Ramanathan, L.; McGregor, R.; Chew, K.-T.; Cornford, M.; Yamanaka, A.; et al. Opiates Increase the Number of Hypocretin-Producing Cells in Human and Mouse Brain and Reverse Cataplexy in a Mouse Model of Narcolepsy. Sci. Transl. Med. 2018, 10, eaao4953. [Google Scholar] [CrossRef]
- Zhou, Y.; Bendor, J.; Hofmann, L.; Randesi, M.; Ho, A.; Kreek, M.J. Mu Opioid Receptor and Orexin/Hypocretin MRNA Levels in the Lateral Hypothalamus and Striatum Are Enhanced by Morphine Withdrawal. J. Endocrinol. 2006, 191, 137–145. [Google Scholar] [CrossRef]
- Prince, C.D.; Rau, A.R.; Yorgason, J.T.; España, R.A. Hypocretin/Orexin Regulation of Dopamine Signaling and Cocaine Self-Administration Is Mediated Predominantly by Hypocretin Receptor 1. ACS Chem. Neurosci. 2015, 6, 138–146. [Google Scholar] [CrossRef]
- Gentile, T.A.; Simmons, S.J.; Watson, M.N.; Connelly, K.L.; Brailoiu, E.; Zhang, Y.; Muschamp, J.W. Effects of Suvorexant, a Dual Orexin/Hypocretin Receptor Antagonist, on Impulsive Behavior Associated with Cocaine. Neuropsychopharmacology 2018, 43, 1001–1009. [Google Scholar] [CrossRef]
- Study Details | NCT03897062 | Suvorexant in the Management Comorbid Sleep Disorder and Alcohol Dependence | ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/study/NCT03897062 (accessed on 3 October 2025).
- Study Details | NCT04262193 | Dual-Orexin Antagonism As a Mechanism for Improving Sleep and Drug Abstinence in Opioid Use Disorder | ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/study/NCT04262193 (accessed on 3 October 2025).
- Martin, C.E.; Bjork, J.M.; Keyser-Marcus, L.; Sabo, R.T.; Pignatello, T.; Simmons, K.; La Rosa, C.; Arias, A.J.; Ramey, T.; Moeller, F.G. Phase 1b/2a Safety Study of Lemborexant as an Adjunctive Treatment for Insomnia to Buprenorphine-Naloxone for Opioid Use Disorder: A Randomized Controlled Trial. Drug Alcohol Depend. Reports 2025, 14, 100304. [Google Scholar] [CrossRef]
- Ufer, M.; Kelsh, D.; Schoedel, K.A.; Dingemanse, J. Abuse Potential Assessment of the New Dual Orexin Receptor Antagonist Daridorexant in Recreational Sedative Drug Users as Compared to Suvorexant and Zolpidem. Sleep 2022, 45, zsab224. [Google Scholar] [CrossRef]
- Han, Y.; Yuan, K.; Zheng, Y.; Lu, L. Orexin Receptor Antagonists as Emerging Treatments for Psychiatric Disorders. Neurosci. Bull. 2020, 36, 432–448. [Google Scholar] [CrossRef]
- Carpi, M.; Palagini, L.; Fernandes, M.; Calvello, C.; Geoffroy, P.A.; Miniati, M.; Pini, S.; Gemignani, A.; Mercuri, N.B.; Liguori, C. Clinical Usefulness of Dual Orexin Receptor Antagonism beyond Insomnia: Neurological and Psychiatric Comorbidities. Neuropharmacology 2024, 245, 109815. [Google Scholar] [CrossRef]
- Shigetsura, Y.; Imai, S.; Endo, H.; Shimizu, Y.; Ueda, K.; Murai, T.; Itohara, K.; Nakagawa, S.; Yonezawa, A.; Ikemi, Y.; et al. Assessment of Suvorexant and Eszopiclone as Alternatives to Benzodiazepines for Treating Insomnia in Patients with Major Depressive Disorder. Clin. Neuropharmacol. 2022, 45, 52–60. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Krishnamurthy, S. Non-Selective Orexin-Receptor Antagonist Attenuates Stress-Re-Stress-Induced Core PTSD-like Symptoms in Rats: Behavioural and Neurochemical Analyses. Behav. Brain Res. 2021, 399, 113015. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Ahmed, S.; Rai, V.; Gupta, S.C.; Krishnamurthy, S. Suvorexant Improves Mitochondrial Dynamics with the Regulation of Orexinergic and MTOR Activation in Rats Exhibiting PTSD-like Symptoms. J. Affect. Disord. 2024, 350, 24–38. [Google Scholar] [CrossRef]
- Suzuki, H.; Hibino, H.; Inoue, Y.; Mikami, A.; Matsumoto, H.; Mikami, K. Reduced Insomnia Following Short-Term Administration of Suvorexant during Aripiprazole Once-Monthly Treatment in a Patient with Schizophrenia. Asian J. Psychiatr. 2017, 28, 165–166. [Google Scholar] [CrossRef]
- Steiner, M.A.; Gatfield, J.; Brisbare-Roch, C.; Dietrich, H.; Treiber, A.; Jenck, F.; Boss, C. Discovery and Characterization of ACT-335827, an Orally Available, Brain Penetrant Orexin Receptor Type 1 Selective Antagonist. ChemMedChem 2013, 8, 898–903. [Google Scholar] [CrossRef]
- Georgescu, D.; Zachariou, V.; Barrot, M.; Mieda, M.; Willie, J.T.; Eisch, A.J.; Yanagisawa, M.; Nestler, E.J.; DiLeone, R.J. Involvement of the Lateral Hypothalamic Peptide Orexin in Morphine Dependence and Withdrawal. J. Neurosci. 2003, 23, 3106–3111. [Google Scholar] [CrossRef]
- Narita, M.; Nagumo, Y.; Hashimoto, S.; Narita, M.; Khotib, J.; Miyatake, M.; Sakurai, T.; Yanagisawa, M.; Nakamachi, T.; Shioda, S.; et al. Direct Involvement of Orexinergic Systems in the Activation of the Mesolimbic Dopamine Pathway and Related Behaviors Induced by Morphine. J. Neurosci. 2006, 26, 398–405. [Google Scholar] [CrossRef]
- Schmeichel, B.E.; Barbier, E.; Misra, K.K.; Contet, C.; Schlosburg, J.E.; Grigoriadis, D.; Williams, J.P.; Karlsson, C.; Pitcairn, C.; Heilig, M.; et al. Hypocretin Receptor 2 Antagonism Dose-Dependently Reduces Escalated Heroin Self-Administration in Rats. Neuropsychopharmacology 2015, 40, 1123–1129. [Google Scholar] [CrossRef]
- Fragale, J.E.; Pantazis, C.B.; James, M.H.; Aston-Jones, G. The Role of Orexin-1 Receptor Signaling in Demand for the Opioid Fentanyl. Neuropsychopharmacology 2019, 44, 1690–1697. [Google Scholar] [CrossRef]
- Mohammadkhani, A.; Fragale, J.E.; Pantazis, C.B.; Bowrey, H.E.; James, M.H.; Aston-Jones, G. Orexin-1 Receptor Signaling in Ventral Pallidum Regulates Motivation for the Opioid Remifentanil. J. Neurosci. 2019, 39, 9831–9840. [Google Scholar] [CrossRef]
- Matzeu, A.; Martin-Fardon, R. Targeting the Orexin System for Prescription Opioid Use Disorder: Orexin-1 Receptor Blockade Prevents Oxycodone Taking and Seeking in Rats. Neuropharmacology 2020, 164, 107906. [Google Scholar] [CrossRef]
- Fragale, J.E.; James, M.H.; Aston-Jones, G. Intermittent Self-Administration of Fentanyl Induces a Multifaceted Addiction State Associated with Persistent Changes in the Orexin System. Addict. Biol. 2021, 26, e12946. [Google Scholar] [CrossRef]
- McGregor, R.; Wu, M.F.; Holmes, B.; Lam, H.A.; Maidment, N.T.; Gera, J.; Yamanaka, A.; Siegel, J.M. Hypocretin/Orexin Interactions with Norepinephrine Contribute to the Opiate Withdrawal Syndrome. J. Neurosci. 2022, 42, 255–263. [Google Scholar] [CrossRef]
- McKendrick, G.; Clapham, C.; Zipunnikov, V.; Ellis, J.D.; Finan, P.; Dunn, K.E.; Strain, E.C.; Wolinsky, D.; Huhn, A.S. Effects of Suvorexant on Diurnal Cortisol and Patient-Reported Stress during Opioid Withdrawal in a Randomized Trial. Psychoneuroendocrinology 2025, 180, 107570. [Google Scholar] [CrossRef]
- Esmaili-Shahzade-Ali-Akbari, P.; Hosseinzadeh, H.; Mehri, S. Effect of Suvorexant on Morphine Tolerance and Dependence in Mice: Role of NMDA, AMPA, ERK and CREB Proteins. Neurotoxicology 2021, 84, 64–72. [Google Scholar] [CrossRef]
- Łupina, M.; Tarnowski, M.; Baranowska-Bosiacka, I.; Talarek, S.; Listos, P.; Kotlińska, J.; Gutowska, I.; Listos, J. SB-334867 (an Orexin-1 Receptor Antagonist) Effects on Morphine-Induced Sensitization in Mice—A View on Receptor Mechanisms. Mol. Neurobiol. 2018, 55, 8473–8485. [Google Scholar] [CrossRef]
- Davoudi, M.; Azizi, H.; Mirnajafi-Zadeh, J.; Semnanian, S. The Blockade of GABAA Receptors Attenuates the Inhibitory Effect of Orexin Type 1 Receptors Antagonist on Morphine Withdrawal Syndrome in Rats. Neurosci. Lett. 2016, 617, 201–206. [Google Scholar] [CrossRef]
- Hooshmand, B.; Azizi, H.; Javan, M.; Semnanian, S. Intra-LC Microinjection of Orexin Type-1 Receptor Antagonist SB-334867 Attenuates the Expression of Glutamate-Induced Opiate Withdrawal like Signs during the Active Phase in Rats. Neurosci. Lett. 2017, 636, 276–281. [Google Scholar] [CrossRef]
- Sharf, R.; Sarhan, M.; DiLeone, R.J. Orexin Mediates the Expression of Precipitated Morphine Withdrawal and Concurrent Activation of the Nucleus Accumbens Shell. Biol. Psychiatry 2008, 64, 175–183. [Google Scholar] [CrossRef]
- Farahimanesh, S.; Zarrabian, S.; Haghparast, A. Role of Orexin Receptors in the Ventral Tegmental Area on Acquisition and Expression of Morphine-Induced Conditioned Place Preference in the Rats. Neuropeptides 2017, 66, 45–51. [Google Scholar] [CrossRef]
- Ebrahimian, F.; Naghavi, F.S.; Yazdi, F.; Sadeghzadeh, F.; Taslimi, Z.; Haghparast, A. Differential Roles of Orexin Receptors within the Dentate Gyrus in Stress- and Drug Priming-Induced Reinstatement of Conditioned Place Preference in Rats. Behav. Neurosci. 2016, 130, 91–102. [Google Scholar] [CrossRef]
- Porter-Stransky, K.A.; Bentzley, B.S.; Aston-Jones, G. Individual Differences in Orexin-I Receptor Modulation of Motivation for the Opioid Remifentanil. Addict. Biol. 2017, 22, 303–317. [Google Scholar] [CrossRef]
- Aghajani, N.; Pourhamzeh, M.; Azizi, H.; Semnanian, S. Central Blockade of Orexin Type 1 Receptors Reduces Naloxone Induced Activation of Locus Coeruleus Neurons in Morphine Dependent Rats. Neurosci. Lett. 2021, 755, 135909. [Google Scholar] [CrossRef]
- Fakhari, M.; Azizi, H.; Semnanian, S. Central Antagonism of Orexin Type-1 Receptors Attenuates the Development of Morphine Dependence in Rat Locus Coeruleus Neurons. Neuroscience 2017, 363, 1–10. [Google Scholar] [CrossRef]
- Mousavi, Y.; Azizi, H.; Mirnajafi-Zadeh, J.; Javan, M.; Semnanian, S. Blockade of Orexin Type-1 Receptors in Locus Coeruleus Nucleus Attenuates the Development of Morphine Dependency in Rats. Neurosci. Lett. 2014, 578, 90–94. [Google Scholar] [CrossRef]
- Sanchez-Alavez, M.; Benedict, J.; Wills, D.N.; Ehlers, C.L. Effect of Suvorexant on Event-Related Oscillations and EEG Sleep in Rats Exposed to Chronic Intermittent Ethanol Vapor and Protracted Withdrawal. Sleep 2019, 42, zsz020. [Google Scholar] [CrossRef]
- Hoch, M.; Hay, J.L.; Hoever, P.; de Kam, M.L.; te Beek, E.T.; van Gerven, J.M.A.; Dingemanse, J. Dual Orexin Receptor Antagonism by Almorexant Does Not Potentiate Impairing Effects of Alcohol in Humans. Eur. Neuropsychopharmacol. 2013, 23, 107–117. [Google Scholar] [CrossRef]
- Steiner, M.A.; Lecourt, H.; Strasser, D.S.; Brisbare-Roch, C.; Jenck, F. Differential Effects of the Dual Orexin Receptor Antagonist Almorexant and the GABAA-A1 Receptor Modulator Zolpidem, Alone or Combined with Ethanol, on Motor Performance in the Rat. Neuropsychopharmacology 2011, 36, 848–856. [Google Scholar] [CrossRef]
- Mayannavar, S.; Rashmi, K.; Rao, Y.; Yadav, S.; Ganaraja, B. Effect of Orexin A Antagonist (SB-334867) Infusion into the Nucleus Accumbens on Consummatory Behavior and Alcohol Preference in Wistar Rats. Indian J. Pharmacol. 2016, 48, 53. [Google Scholar] [CrossRef]
- Dhaher, R.; Hauser, S.R.; Getachew, B.; Bell, R.L.; McBride, W.J.; McKinzie, D.L.; Rodd, Z.A. The Orexin-1 Receptor Antagonist SB-334867 Reduces Alcohol Relapse Drinking, but Not Alcohol-Seeking, in Alcohol-Preferring (P) Rats. J. Addict. Med. 2010, 4, 153–159. [Google Scholar] [CrossRef]
- Lopez, M.F.; Moorman, D.E.; Aston-Jones, G.; Becker, H.C. The Highly Selective Orexin/Hypocretin 1 Receptor Antagonist GSK1059865 Potently Reduces Ethanol Drinking in Ethanol Dependent Mice. Brain Res. 2016, 1636, 74–80. [Google Scholar] [CrossRef]
- Barson, J.R.; Ho, H.T.; Leibowitz, S.F. Anterior Thalamic Paraventricular Nucleus Is Involved in Intermittent Access Ethanol Drinking: Role of Orexin Receptor 2. Addict. Biol. 2015, 20, 469–481. [Google Scholar] [CrossRef]
- Borgland, S.L.; Chang, S.-J.; Bowers, M.S.; Thompson, J.L.; Vittoz, N.; Floresco, S.B.; Chou, J.; Chen, B.T.; Bonci, A. Orexin A/Hypocretin-1 Selectively Promotes Motivation for Positive Reinforcers. J. Neurosci. 2009, 29, 11215–11225. [Google Scholar] [CrossRef]
- Tung, L.-W.; Lu, G.-L.; Lee, Y.-H.; Yu, L.; Lee, H.-J.; Leishman, E.; Bradshaw, H.; Hwang, L.-L.; Hung, M.-S.; Mackie, K.; et al. Orexins Contribute to Restraint Stress-Induced Cocaine Relapse by Endocannabinoid-Mediated Disinhibition of Dopaminergic Neurons. Nat. Commun. 2016, 7, 12199. [Google Scholar] [CrossRef]
- Gentile, T.A.; Simmons, S.J.; Barker, D.J.; Shaw, J.K.; España, R.A.; Muschamp, J.W. Suvorexant, an Orexin/Hypocretin Receptor Antagonist, Attenuates Motivational and Hedonic Properties of Cocaine. Addict. Biol. 2018, 23, 247–255. [Google Scholar] [CrossRef]
- Schmeichel, B.E.; Herman, M.A.; Roberto, M.; Koob, G.F. Hypocretin Neurotransmission Within the Central Amygdala Mediates Escalated Cocaine Self-Administration and Stress-Induced Reinstatement in Rats. Biol. Psychiatry 2017, 81, 606–615. [Google Scholar] [CrossRef]
- Muschamp, J.W.; Hollander, J.A.; Thompson, J.L.; Voren, G.; Hassinger, L.C.; Onvani, S.; Kamenecka, T.M.; Borgland, S.L.; Kenny, P.J.; Carlezon, W.A. Hypocretin (Orexin) Facilitates Reward by Attenuating the Antireward Effects of Its Cotransmitter Dynorphin in Ventral Tegmental Area. Proc. Natl. Acad. Sci. USA 2014, 111, E1648–E1655. [Google Scholar] [CrossRef]
- Foltin, R.W.; Evans, S.M. Hypocretin/Orexin Antagonists Decrease Cocaine Self-Administration by Female Rhesus Monkeys. Drug Alcohol Depend. 2018, 188, 318–327. [Google Scholar] [CrossRef]
- Levy, K.A.; Brodnik, Z.D.; Shaw, J.K.; Perrey, D.A.; Zhang, Y.; España, R.A. Hypocretin Receptor 1 Blockade Produces Bimodal Modulation of Cocaine-Associated Mesolimbic Dopamine Signaling. Psychopharmacology 2017, 234, 2761–2776. [Google Scholar] [CrossRef]
- Steiner, M.A.; Lecourt, H.; Jenck, F. The Dual Orexin Receptor Antagonist Almorexant, Alone and in Combination with Morphine, Cocaine and Amphetamine, on Conditioned Place Preference and Locomotor Sensitization in the Rat. Int. J. Neuropsychopharmacol. 2013, 16, 417–432. [Google Scholar] [CrossRef]
- Flores, Á.; Maldonado, R.; Berrendero, F. The Hypocretin/Orexin Receptor-1 as a Novel Target to Modulate Cannabinoid Reward. Biol. Psychiatry 2014, 75, 499–507. [Google Scholar] [CrossRef]
- Khoo, S.Y.-S.; McNally, G.P.; Clemens, K.J. The Dual Orexin Receptor Antagonist TCS1102 Does Not Affect Reinstatement of Nicotine-Seeking. PLoS ONE 2017, 12, e0173967. [Google Scholar] [CrossRef]
- James, M.H.; Aston-Jones, G. Orexin Reserve: A Mechanistic Framework for the Role of Orexins (Hypocretins) in Addiction. Biol. Psychiatry 2022, 92, 836–844. [Google Scholar] [CrossRef]
- Rao, Y.; Mineur, Y.S.; Gan, G.; Wang, A.H.; Liu, Z.; Wu, X.; Suyama, S.; de Lecea, L.; Horvath, T.L.; Picciotto, M.R.; et al. Repeated in Vivo Exposure of Cocaine Induces Long-lasting Synaptic Plasticity in Hypocretin/Orexin-producing Neurons in the Lateral Hypothalamus in Mice. J. Physiol. 2013, 591, 1951–1966. [Google Scholar] [CrossRef]
- Yeoh, J.W.; James, M.H.; Adams, C.D.; Bains, J.S.; Sakurai, T.; Aston-Jones, G.; Graham, B.A.; Dayas, C.V. Activation of Lateral Hypothalamic Group III Metabotropic Glutamate Receptors Suppresses Cocaine-Seeking Following Abstinence and Normalizes Drug-Associated Increases in Excitatory Drive to Orexin/Hypocretin Cells. Neuropharmacology 2019, 154, 22–33. [Google Scholar] [CrossRef]
- Tunisi, L.; D’Angelo, L.; Fernández-Rilo, A.C.; Forte, N.; Piscitelli, F.; Imperatore, R.; de Girolamo, P.; Di Marzo, V.; Cristino, L. Orexin-A/Hypocretin-1 Controls the VTA-NAc Mesolimbic Pathway via Endocannabinoid-Mediated Disinhibition of Dopaminergic Neurons in Obese Mice. Front. Synaptic Neurosci. 2021, 13, 622405. [Google Scholar] [CrossRef]
- Mehr, J.B.; Mitchison, D.; Bowrey, H.E.; James, M.H. Sleep Dysregulation in Binge Eating Disorder and “Food Addiction”: The Orexin (Hypocretin) System as a Potential Neurobiological Link. Neuropsychopharmacology 2021, 46, 2051–2061. [Google Scholar] [CrossRef]
- Chou, Y.; Hor, C.C.; Lee, M.T.; Lee, H.; Guerrini, R.; Calo, G.; Chiou, L. Stress Induces Reinstatement of Extinguished Cocaine Conditioned Place Preference by a Sequential Signaling via Neuropeptide S, Orexin, and Endocannabinoid. Addict. Biol. 2021, 26, e12971. [Google Scholar] [CrossRef]
- Mahler, S.V.; Smith, R.J.; Moorman, D.E.; Sartor, G.C.; Aston-Jones, G. Multiple Roles for Orexin/Hypocretin in Addiction. Prog. Brain Res. 2012, 198, 79–121. [Google Scholar]
- Bearn, J.; Buntwal, N.; Papadopoulos, A.; Checkley, S. Salivary Cortisol during Opiate Dependence and Withdrawal. Addict. Biol. 2001, 6, 157–162. [Google Scholar] [CrossRef]
- Di Sebastiano, A.R.; Coolen, L.M. Orexin and Natural Reward. In Feeding, Maternal, and Male Sexual Behavior, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2012; Volume 198, ISBN 9780444594891. [Google Scholar]
- Tsujino, N.; Sakurai, T. Role of Orexin in Modulating Arousal, Feeding and Motivation. Front. Behav. Neurosci. 2013, 7, 28. [Google Scholar] [CrossRef]
- Choi, D.L.; Davis, J.F.; Fitzgerald, M.E.; Benoit, S.C. The Role of Orexin-A in Food Motivation, Reward-Based Feeding Behavior and Food-Induced Neuronal Activation in Rats. Neuroscience 2010, 167, 11–20. [Google Scholar] [CrossRef]
- Muschamp, J.W.; Dominguez, J.M.; Sato, S.M.; Shen, R.Y.; Hull, E.M. A Role for Hypocretin (Orexin) in Male Sexual Behavior. J. Neurosci. 2007, 27, 2837–2845. [Google Scholar] [CrossRef]
- Peyron, C.; Tighe, D.K.; Van Den Pol, A.N.; De Lecea, L.; Heller, H.C.; Sutcliffe, J.G.; Kilduff, T.S. Neurons Containing Hypocretin (Orexin) Project to Multiple Neuronal Systems. J. Neurosci. 1998, 18, 9996–10015. [Google Scholar] [CrossRef]
- Smith, S.M.; Vale, W.W. The Role of the Hypothalamic-Pituitary-Adrenal Axis in Neuroendocrine Responses to Stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar] [CrossRef]
- Winsky-Sommerer, R.; Yamanaka, A.; Diano, S.; Borok, E.; Roberts, A.J.; Sakurai, T.; Kilduff, T.S.; Horvath, T.L.; De Lecea, L. Interaction between the Corticotropin-Releasing Factor System and Hypocretins (Orexins): A Novel Circuit Mediating Stress Response. J. Neurosci. 2004, 24, 11439–11448. [Google Scholar] [CrossRef]
- Martin-Fardon, R.; Zorrilla, E.P.; Ciccocioppo, R.; Weiss, F. Role of Innate and Drug-Induced Dysregulation of Brain Stress and Arousal Systems in Addiction: Focus on Corticotropin-Releasing Factor, Nociceptin/Orphanin FQ, and Orexin/Hypocretin. Brain Res. 2010, 1314, 145–161. [Google Scholar] [CrossRef]
- Plaza-Zabala, A.; Maldonado, R.; Berrendero, F. The Hypocretin/Orexin System: Implications for Drug Reward and Relapse. Mol. Neurobiol. 2012, 45, 424–439. [Google Scholar] [CrossRef]
- Matzeu, A.; Martin-Fardon, R. Drug Seeking and Relapse: New Evidence of a Role for Orexin and Dynorphin Co-Transmission in the Paraventricular Nucleus of the Thalamus. Front. Neurol. 2018, 9, 720. [Google Scholar] [CrossRef]
- Thompson, J.L.; Borgland, S.L. A Role for Hypocretin/Orexin in Motivation. Behav. Brain Res. 2011, 217, 446–453. [Google Scholar] [CrossRef]
- Matzeu, A.; Martin-Fardon, R. Understanding the Role of Orexin Neuropeptides in Drug Addiction: Preclinical Studies and Translational Value. Front. Behav. Neurosci. 2022, 15, 787595. [Google Scholar] [CrossRef]
- Zhou, L.; Sun, W.L.; See, R.E. Orexin Receptor Targets for Anti-Relapse Medication Development in Drug Addiction. Pharmaceuticals 2011, 4, 804–821. [Google Scholar] [CrossRef]
- Date, Y.; Ueta, Y.; Yamashita, H.; Yamaguchi, H.; Matsukura, S.; Kangawa, K.; Sakurai, T.; Yanagisawa, M.; Nakazato, M. Orexins, Orexigenic Hypothalamic Peptides, Interact with Autonomic, Neuroendocrine and Neuroregulatory Systems. Proc. Natl. Acad. Sci. USA 1999, 96, 748–753. [Google Scholar] [CrossRef]
- Veilleux, J.C.; Colvin, P.J.; Anderson, J.; York, C.; Heinz, A.J. A Review of Opioid Dependence Treatment: Pharmacological and Psychosocial Interventions to Treat Opioid Addiction. Clin. Psychol. Rev. 2010, 30, 155–166. [Google Scholar] [CrossRef]
- Baimel, C.; Bartlett, S.E.; Chiou, L.C.; Lawrence, A.J.; Muschamp, J.W.; Patkar, O.; Tung, L.W.; Borgland, S.L. Orexin/Hypocretin Role in Reward: Implications for Opioid and Other Addictions. Br. J. Pharmacol. 2015, 172, 334–348. [Google Scholar] [CrossRef]
- Mohammadkhani, A.; James, M.H.; Pantazis, C.B.; Aston-Jones, G. Persistent Effects of the Orexin-1 Receptor Antagonist SB-334867 on Motivation for the Fast Acting Opioid Remifentanil. Brain Res. 2020, 1731, 146461. [Google Scholar] [CrossRef]
- Nogueira, D.D.S.; Corwin, C.; Rakholia, Y.; Punnuru, V.; Nampally, M.; Kohtz, A.S.; Aston-Jones, G. Fentanyl Demand and Seeking in Female Rats: Role of the Orexin System and Estrous Cycle. Addict. Neurosci. 2024, 13, 100178. [Google Scholar] [CrossRef]
- Suzuki, M.; Shiraishi, E.; Cronican, J.; Kimura, H. Effects of the Orexin Receptor 2 Agonist Danavorexton on Emergence from General Anaesthesia and Opioid-Induced Sedation, Respiratory Depression, and Analgesia in Rats and Monkeys. Br. J. Anaesth. 2024, 132, 541–552. [Google Scholar] [CrossRef]
- Smith, R.J.; Aston-Jones, G. Orexin/Hypocretin 1 Receptor Antagonist Reduces Heroin Self-Administration and Cue-Induced Heroin Seeking. Eur. J. Neurosci. 2012, 35, 798. [Google Scholar] [CrossRef]
- Sharf, R.; Sarhan, M.; DiLeone, R.J. Role of Orexin/Hypocretin in Dependence and Addiction. Brain Res. 2010, 1314, 130–138. [Google Scholar] [CrossRef]
- Calipari, E.S.; España, R.A. Hypocretin/Orexin Regulation of Dopamine Signaling: Implications for Reward and Reinforcement Mechanisms. Front. Behav. Neurosci. 2012, 6, 54. [Google Scholar] [CrossRef]
- Sakurai, T. The Role of Orexin in Motivated Behaviours. Nat. Rev. Neurosci. 2014, 15, 719–731. [Google Scholar] [CrossRef]
- Thomas, C.S.; Mohammadkhani, A.; Rana, M.; Qiao, M.; Baimel, C.; Borgland, S.L. Optogenetic Stimulation of Lateral Hypothalamic Orexin/Dynorphin Inputs in the Ventral Tegmental Area Potentiates Mesolimbic Dopamine Neurotransmission and Promotes Reward-Seeking Behaviours. Neuropsychopharmacology 2022, 47, 728–740. [Google Scholar] [CrossRef]
- Baimel, C.; Borgland, S.L. Hypocretin Modulation of Drug-Induced Synaptic Plasticity, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2012; Volume 198, ISBN 9780444594891. [Google Scholar]
- Spanagel, R. Cannabinoids and the Endocannabinoid System in Reward Processing and Addiction: From Mechanisms to Interventions. Dialogues Clin. Neurosci. 2020, 22, 241–250. [Google Scholar] [CrossRef]
- Rebassa, J.B.; Capó, T.; Lillo, J.; Raïch, I.; Reyes-Resina, I.; Navarro, G. Cannabinoid and Orexigenic Systems Interplay as a New Focus of Research in Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 5378. [Google Scholar] [CrossRef]
- Raïch, I.; Rebassa, J.B.; Lillo, J.; Cordomi, A.; Rivas-Santisteban, R.; Lillo, A.; Reyes-Resina, I.; Franco, R.; Navarro, G. Antagonization of OX1 Receptor Potentiates CB2 Receptor Function in Microglia from APPSw/Ind Mice Model. Int. J. Mol. Sci. 2022, 23, 12801. [Google Scholar] [CrossRef]
- Jäntti, M.H.; Mandrika, I.; Kukkonen, J.P. Human Orexin/Hypocretin Receptors Form Constitutive Homo- and Heteromeric Complexes with Each Other and with Human CB1 Cannabinoid Receptors. Biochem. Biophys. Res. Commun. 2014, 445, 486–490. [Google Scholar] [CrossRef]
- Chandrasekera, P.C.; Wan, T.C.; Gizewski, E.T.; Auchampach, J.A.; Lasley, R.D. Adenosine A1 Receptors Heterodimerize with Β1- and Β2-Adrenergic Receptors Creating Novel Receptor Complexes with Altered G Protein Coupling and Signaling. Cell. Signal. 2013, 25, 736–742. [Google Scholar] [CrossRef]
- Navarro, G.; Quiroz, C.; Moreno-Delgado, D.; Sierakowiak, A.; McDowell, K.; Moreno, E.; Rea, W.; Cai, N.-S.; Aguinaga, D.; Howell, L.A.; et al. Orexin–Corticotropin-Releasing Factor Receptor Heteromers in the Ventral Tegmental Area as Targets for Cocaine. J. Neurosci. 2015, 35, 6639–6653. [Google Scholar] [CrossRef]
- Ellis, J.; Pediani, J.D.; Canals, M.; Milasta, S.; Milligan, G. Orexin-1 Receptor-Cannabinoid CB1 Receptor Heterodimerization Results in Both Ligand-Dependent and -Independent Coordinated Alterations of Receptor Localization and Function. J. Biol. Chem. 2006, 281, 38812–38824. [Google Scholar] [CrossRef]
- Flores, Á.; Maldonado, R.; Berrendero, F. Hypocretins/Orexins and Addiction: Role in Cannabis Dependence. In Handbook of Cannabis and Related Pathologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 533–542. [Google Scholar]
- Schwarzer, C. 30 Years of Dynorphins—New Insights on Their Functions in Neuropsychiatric Diseases. Pharmacol. Ther. 2009, 123, 353–370. [Google Scholar] [CrossRef]
- Bruchas, M.R.; Chavkin, C. Kinase Cascades and Ligand-Directed Signaling at the Kappa Opioid Receptor. Psychopharmacology 2010, 210, 137–147. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, R.; Chen, X.; Wang, C.; Cai, X.; Liu, H.; Jiang, Y.; Liu, C.; Bai, B. Heterodimerization of Human Orexin Receptor 1 and Kappa Opioid Receptor Promotes Protein Kinase A/CAMP-Response Element Binding Protein Signaling via a Gαs-Mediated Mechanism. Cell. Signal. 2015, 27, 1426–1438. [Google Scholar] [CrossRef]
- Zhang, R.; Li, D.; Mao, H.; Wei, X.; Xu, M.; Zhang, S.; Jiang, Y.; Wang, C.; Xin, Q.; Chen, X.; et al. Disruption of 5-Hydroxytryptamine 1A Receptor and Orexin Receptor 1 Heterodimer Formation Affects Novel G Protein-Dependent Signaling Pathways and Has Antidepressant Effects in Vivo. Transl. Psychiatry 2022, 12, 122. [Google Scholar] [CrossRef]
- Fujita, W.; Gomes, I.; Dove, L.S.; Prohaska, D.; McIntyre, G.; Devi, L.A. Molecular Characterization of Eluxadoline as a Potential Ligand Targeting Mu-Delta Opioid Receptor Heteromers. Biochem. Pharmacol. 2014, 92, 448–456. [Google Scholar] [CrossRef]
- Jörg, M.; May, L.T.; Mak, F.S.; Lee, K.C.K.; Miller, N.D.; Scammells, P.J.; Capuano, B. Synthesis and Pharmacological Evaluation of Dual Acting Ligands Targeting the Adenosine A 2A and Dopamine D 2 Receptors for the Potential Treatment of Parkinson’s Disease. J. Med. Chem. 2015, 58, 718–738. [Google Scholar] [CrossRef]
- Hasbi, A.; Perreault, M.L.; Shen, M.Y.F.; Fan, T.; Nguyen, T.; Alijaniaram, M.; Banasikowski, T.J.; Grace, A.A.; O’Dowd, B.F.; Fletcher, P.J.; et al. Activation of Dopamine D1-D2 Receptor Complex Attenuates Cocaine Reward and Reinstatement of Cocaine-Seeking through Inhibition of DARPP-32, ERK, and ΔFosB. Front. Pharmacol. 2018, 8, 924. [Google Scholar] [CrossRef]
- Gomes, I.; Fujita, W.; Gupta, A.; Saldanha, S.A.; Negri, A.; Pinello, C.E.; Eberhart, C.; Roberts, E.; Filizola, M.; Hodder, P.; et al. Identification of a μ-δ Opioid Receptor Heteromer-Biased Agonist with Antinociceptive Activity. Proc. Natl. Acad. Sci. USA 2013, 110, 12072–12077. [Google Scholar] [CrossRef] [PubMed]
| Substance of Abuse | Intervention | Subjects | Main Findings | Ref |
|---|---|---|---|---|
| Opioids | Orexin peptide KO | R (rat) | Attenuation of morphine dependence | [155] |
| Pre-pro orexin KO | M (mouse) | Abolishment of subcutaneous morphine-induced place preference and hyperlocomotion | [156] | |
| SB-334867A (selective OX1R antagonist) | R | Suppression of morphine-induced place preference | [156] | |
| NBI-80713 (selective OX2R antagonist) | R | Reduction in morphine self-administration | [157] | |
| SB-334867 | R | Decreased motivation for fentanyl | [158] | |
| SB-334867 | R | Decreased motivation for remifentanyl | [159] | |
| SB-334867 | R | Reduction in oxycodone intake | [160] | |
| SB-334867 | R | Reversion of fentanyl-induced addiction state | [161] | |
| Elimination of orexin neurons | M | Reduction in the somatic and affective symptoms of withdrawal | [162] | |
| Suvorexant (dual OX1/2R antagonist) | Humans | Decreased diurnal salivary cortisol levels and self-reported stress in humans undergoing opioid withdrawal | [163] | |
| Suvorexant | M | Decreased morphine tolerance and dependence/decreased increased levels of CREB and p-ERK proteins | [164] | |
| SB-334867 | M | Prevented morphine-induced sensitivity to locomotor activity in mice | [165] | |
| SB-334867 | R | Significantly reduced naloxone-induced withdrawal syndrome physical symptoms in morphine-dependent rats | [166] | |
| SB-334867 | R | Microinjection into LC dramatically suppresses glutamate-induced morphine withdrawal | [167] | |
| SB-334867 | M | Attenuated the symptoms of naloxone-induced withdrawal | [168] | |
| SB-334867 | R | Attenuation of morphine-induced CPP (acquisition and expression/micro-injection into VTA) | [169] | |
| SB-334867 | R | Intra-DG (dentate gyrus) administration dose-dependently reduced morphine priming-induced reinstatement | [170] | |
| SB-334867 | R | Decreased motivation and the cue-induced reinstatement of remifentanil-seeking | [171] | |
| SB-334867 | R | Inhibition of increased activity of LC neurons following naloxone administration in morphine-dependent rats | [172] | |
| SB-334867 | R | Prevention of naloxone-induced neuronal activation within the LC in morphine-dependent rats/Decreased cAMP concentration in LC neurons | [173] | |
| SB-334867 | R | Significant reduction in physical symptoms of morphine withdrawal syndrome induced by naloxone95 | [174] | |
| TCS-OX2-29 (OX2R antagonist) | R | Intra-DG administration dose-dependently reduced morphine priming-induced reinstatement | [170] | |
| TCS-OX2-29 | R | Attenuation of morphine-induced CPP (acquisition and expression/micro-injection into VTA) | [169] | |
| Alcohol | SB-334867 | R | Reduction in ethanol self-administration and reinstatement | [113] |
| Suvorexant | R | Reduced the latency to REM sleep and sleep and slow-wave-sleep (SWS) onset in a dose-dependent manner/produced REM sleep and SWS fragmentation | [175] | |
| Almorexant (dual OX1/2R antagonist) | Healthy humans | Almorexant did not affect the pharmacokinetics of ethanol and did not synergize its effects | [176] | |
| Almorexant | R | Diminished alcohol self-administration (Systemic or VTA administration) | [134] | |
| Almorexant | R | It did not enhance the sedative effect of alcohol | [177] | |
| SB-334867 | R | Reduced alcohol intake and preference (Intra-NAc infusions) | [178] | |
| SB-334867 | R | Decreased alcohol relapse drinking | [179] | |
| GSK1059865 (OX1R antagonist) | M | Significantly reduced alcohol consumption in ethanol-dependent animals | [180] | |
| TCS-OX2-29 | R | Microinjections of TCS-OX2-29 (into the aPVT) reduced intermittent-access ethanol drinking | [181] | |
| Cocaine | SB-334867 | R | Blockade of footshock-induced reinstatement of previously extinguished cocaine-seeking behavior | [138] |
| SB-334867 | R | Reduction in work to self-administer cocaine or high fat food pellets | [182] | |
| SB-334867 | R | Dose-dependent decrease in cue-induced reinstatement of cocaine-seeking | [115] | |
| SB-334867 | R | Blockade of cue-induced reinstatement of cocaine-seeking | [122] | |
| SB-334867 | M | Blockade of CPP induced by micro-injection of orexin in VTA | [183] | |
| SB-334867 | R | Reduced motivation for cocaine | [123] | |
| OX1R knock-down | M | Reduced dopaminergic response to cocaine and motivation to seek the drug | [121] | |
| Suvorexant | R | Attenuated cocaine-induced impulsive behaviors (systematic or direct injection in VTA) | [143] | |
| Suvorexant | R | Attenuation of the hedonic and motivational effect induced by cocaine | [184] | |
| SB-334867 | R | Counteracts the development of cocaine self-administration and attenuates the induction of amphetamine-induced CPP | [119] | |
| SB-334867 | R | Decreased cocaine intake (in a dose-dependent manner) | [185] | |
| SB-334867 | M | Attenuated impulsive-like behavior, LH self-stimulation, and cocaine self-administration | [186] | |
| SB-334867 | Female monkeys (rhesus) | Reduced cocaine self-administration | [187] | |
| SB-334867 | R | Blocking OX1R or OX1R and OX2R together reduces the effect of cocaine on dopamine signaling and cocaine motivation, but blocking OX2R alone showed no effect | [142] | |
| Almorexant | R | Decreased cocaine self-administration and weakened cocaine-induced dopamine uptake inhibition | [142] | |
| RTIOX-276 (OX1R antagonist) | R | Attenuation of cocaine-induced inhibition of dopamine uptake | [188] | |
| Amphetamine | SB-334867 | R | Reduced amphetamine-evoked DA outflow in the NAc and reduced amphetamine-induced sensitization | [118] |
| Almorexant | R | Decreased cocaine and amphetamine-induced CPP expression but did not affect morphine-induced CPP expression CPP expression/Interfered with morphine-induced locomotor sensitization but had no effect on cocaine and amphetamine-induced locomotor sensitization | [189] | |
| Cannabis | SB-334867 | M | Reduced the reinforcing and motivational properties of WIN55,212-2 (TCS-OX2-29 had no effect) | [190] |
| Nicotine | SB-334867 | M | Reduced somatic signs of nicotine-induced withdrawal (TCS-OX2-29 had no effect) | [117] |
| TCS 1102 (dual OX1/2R antagonist) | R | No effect on nicotine-seeking behavior | [191] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capó, T.; Lillo, J.; Rebassa, J.B.; Badia, P.; Raïch, I.; Cubeles-Juberias, E.; Reyes-Resina, I.; Navarro, G. The Orexin System in Addiction: Neuromodulatory Interactions and Therapeutic Potential. Brain Sci. 2025, 15, 1105. https://doi.org/10.3390/brainsci15101105
Capó T, Lillo J, Rebassa JB, Badia P, Raïch I, Cubeles-Juberias E, Reyes-Resina I, Navarro G. The Orexin System in Addiction: Neuromodulatory Interactions and Therapeutic Potential. Brain Sciences. 2025; 15(10):1105. https://doi.org/10.3390/brainsci15101105
Chicago/Turabian StyleCapó, Toni, Jaume Lillo, Joan Biel Rebassa, Pau Badia, Iu Raïch, Erik Cubeles-Juberias, Irene Reyes-Resina, and Gemma Navarro. 2025. "The Orexin System in Addiction: Neuromodulatory Interactions and Therapeutic Potential" Brain Sciences 15, no. 10: 1105. https://doi.org/10.3390/brainsci15101105
APA StyleCapó, T., Lillo, J., Rebassa, J. B., Badia, P., Raïch, I., Cubeles-Juberias, E., Reyes-Resina, I., & Navarro, G. (2025). The Orexin System in Addiction: Neuromodulatory Interactions and Therapeutic Potential. Brain Sciences, 15(10), 1105. https://doi.org/10.3390/brainsci15101105






