Detection of EEG Activity in Response to the Surrounding Environment: A Neuro-Architecture Study
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Experimental Procedure
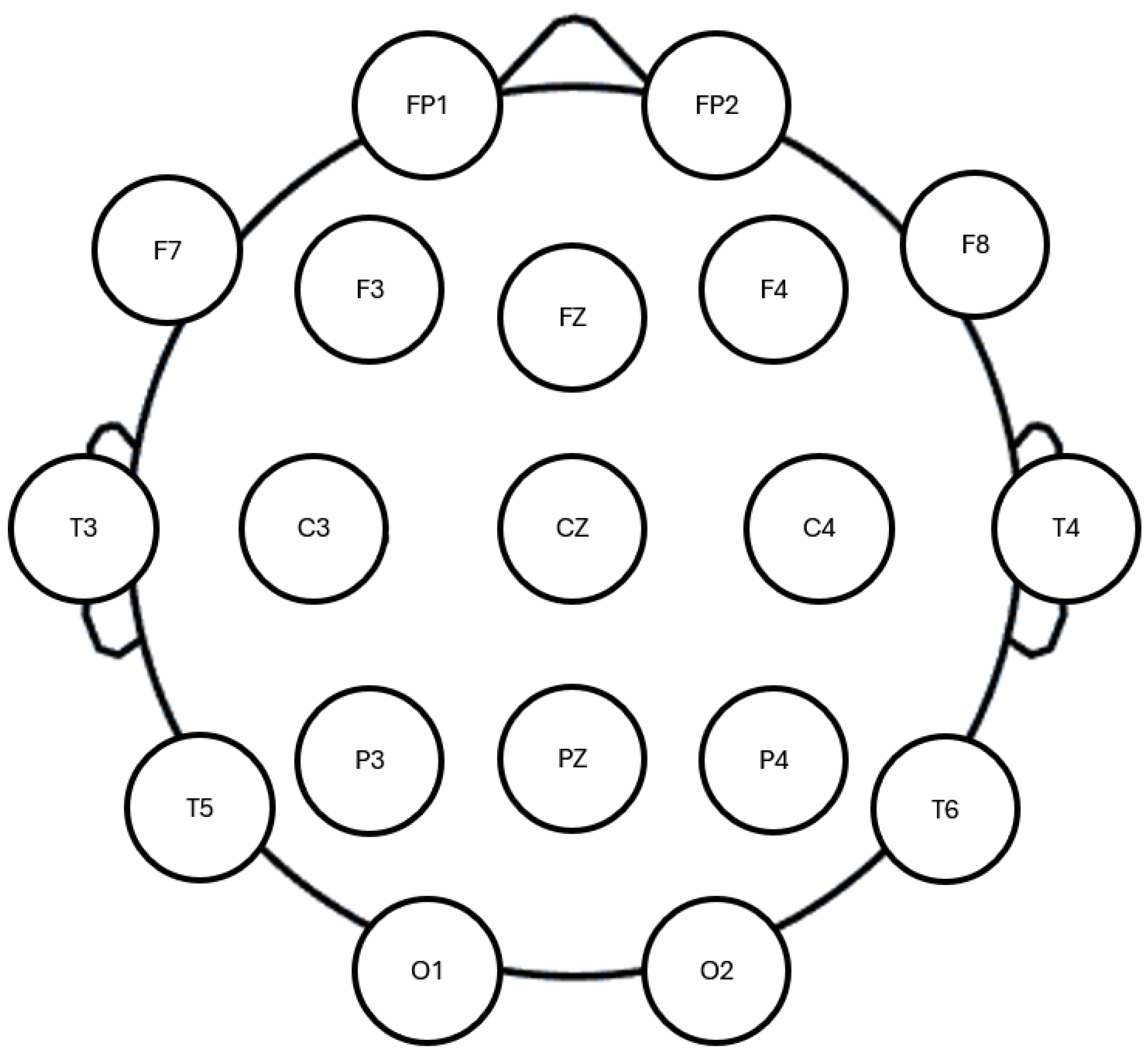
2.3. Equipment and Software
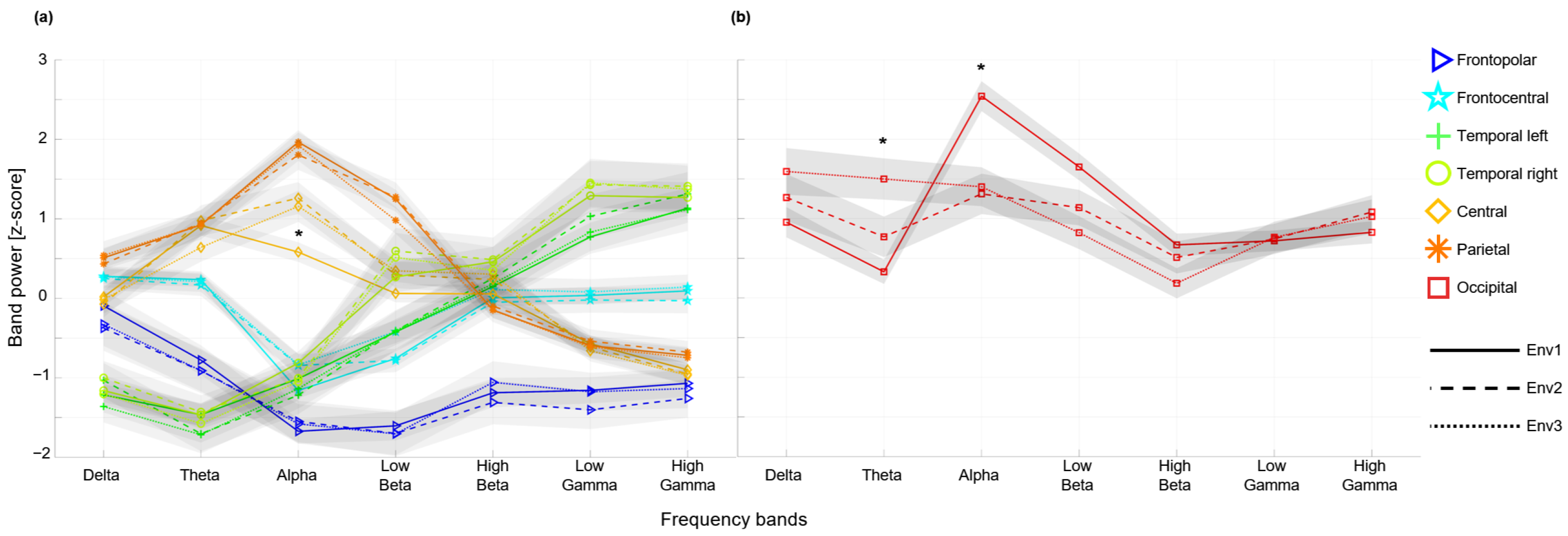
2.4. Statistical Analysis
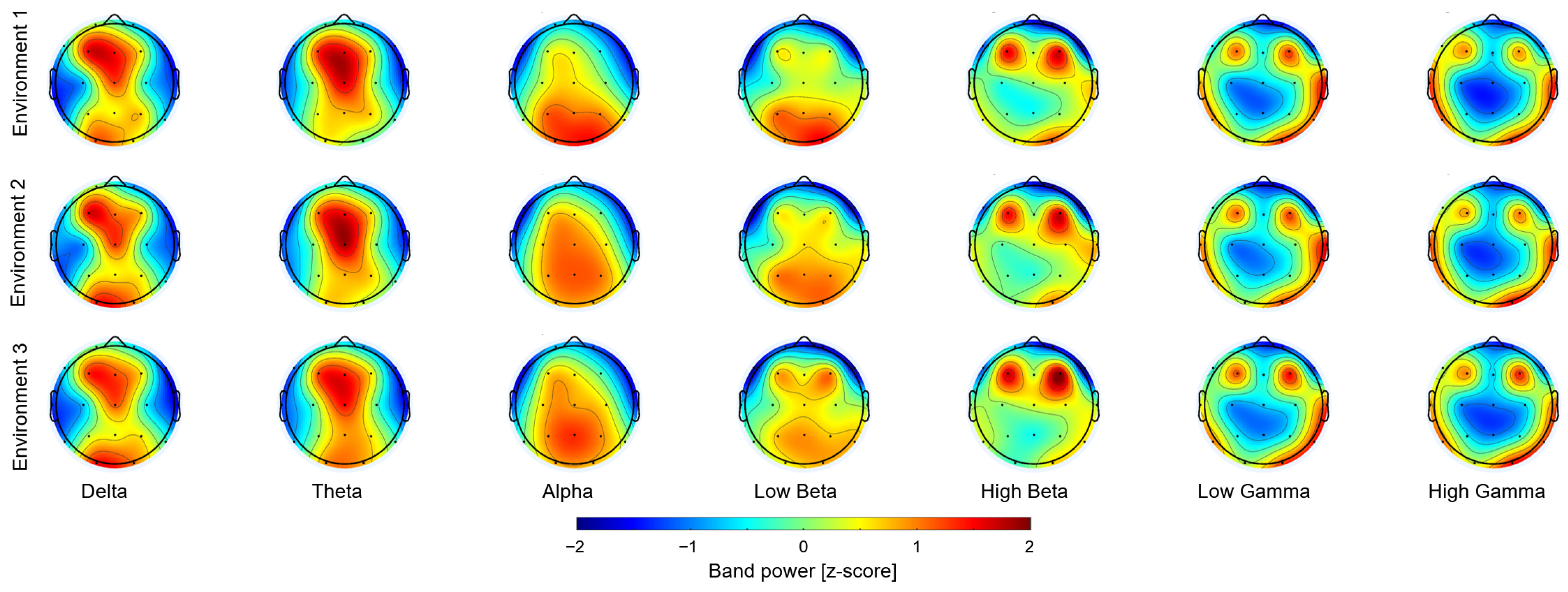
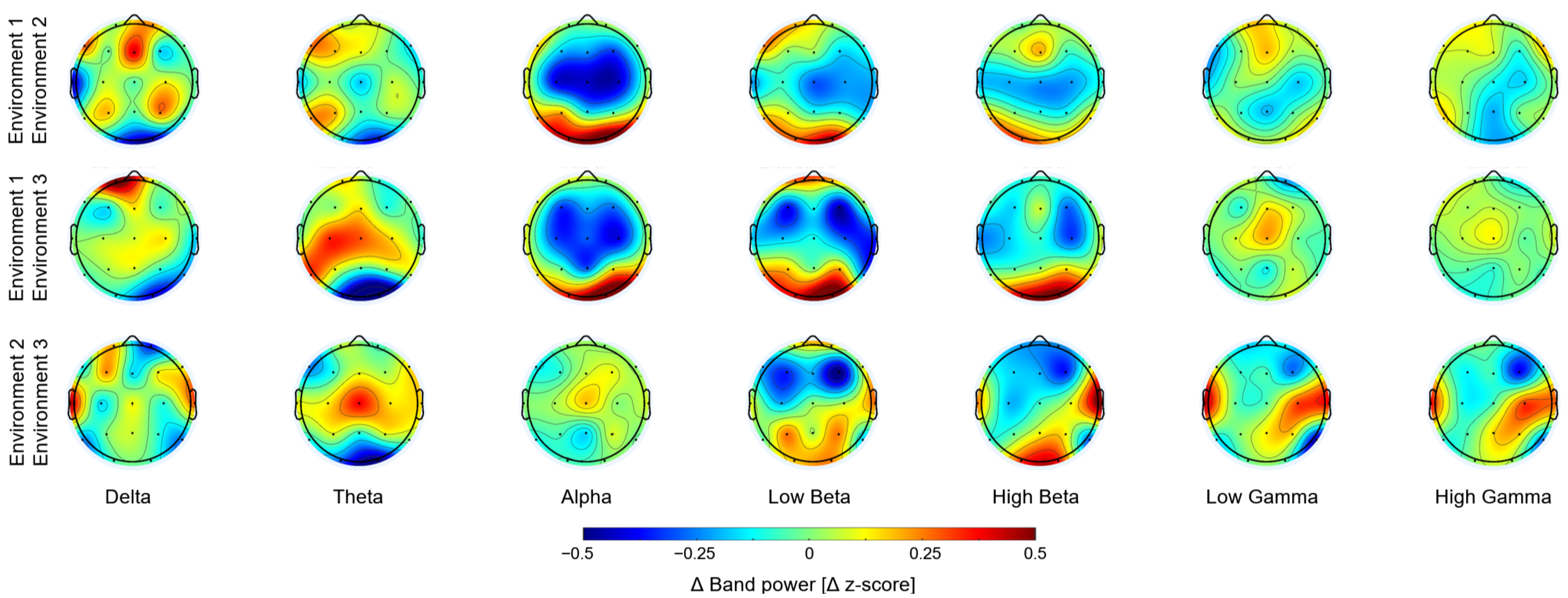
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
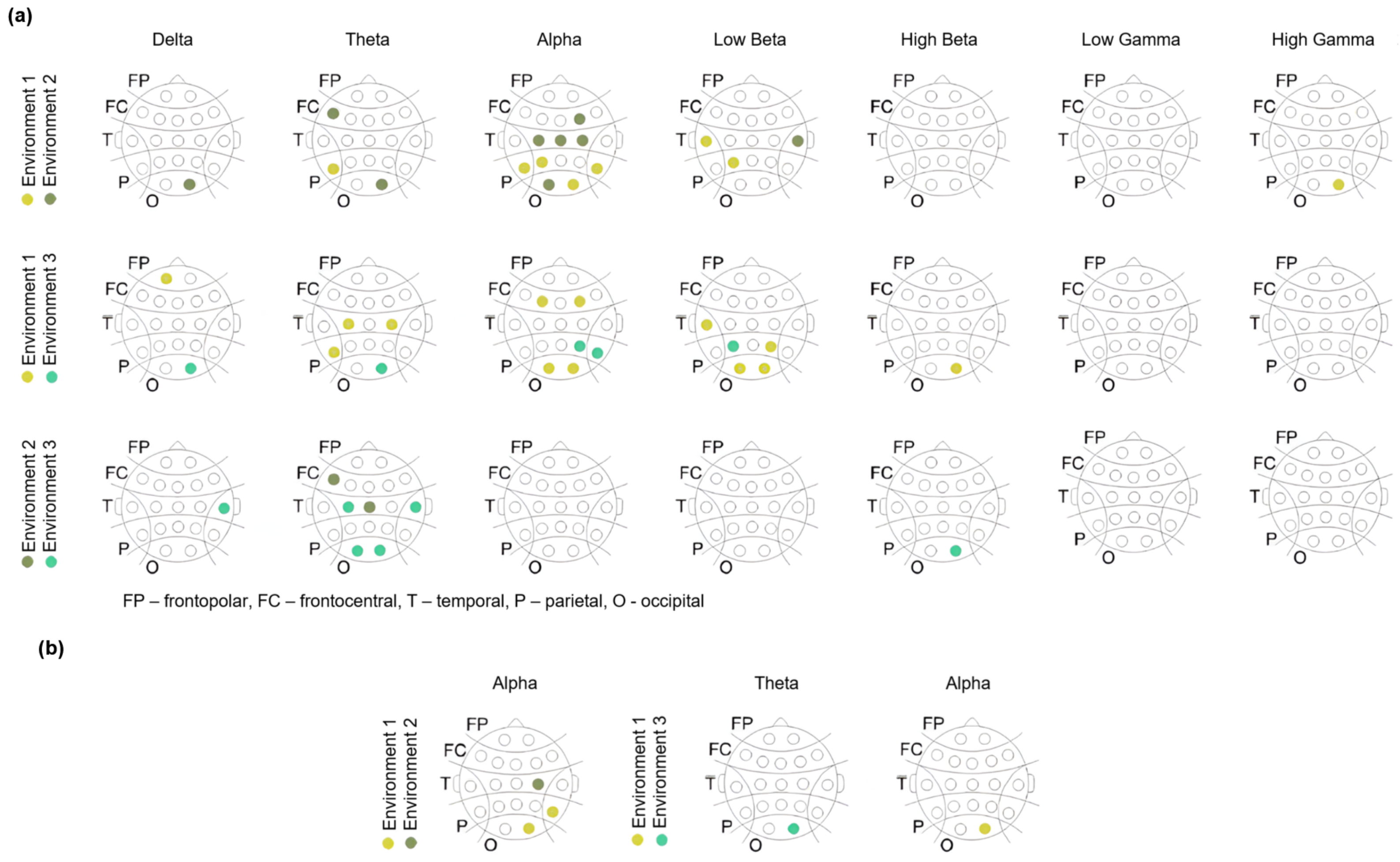
Appendix A. Supplementary Tables
| Freq. Bands | Channels | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Delta | O2 0.013 | |||||||||
| Theta | O2 0.028 | F7 0.033 | T5 0.024 | |||||||
| Alpha | F4 0.005 | C3 0.007 | C4 0.001 | P3 0.026 | O1 0.008 | O2 0.001 | F8 0.036 | T5 0.018 | T6 0.002 | CZ 0.003 |
| Low Beta | P3 0.013 | T3 0.036 | T4 0.039 | |||||||
| High Gamma | O2 0.046 | |||||||||
| Freq. Bands | Channels | |||||
|---|---|---|---|---|---|---|
| Delta | FP1 0.011 | O2 0.006 | ||||
| Theta | C3 0.026 | C4 0.049 | O2 0.0002 | T5 0.0424 | ||
| Alpha | F3 0.031 | F4 0.039 | P4 0.028 | O1 0.028 | O2 0.0002 | T6 0.024 |
| Low Beta | P3 0.006 | P4 0.008 | O1 0.022 | O2 0.003 | T3 0.0495 | |
| High Beta | O2 0.039 | |||||
| Freq. Bands | Channels | |||||
|---|---|---|---|---|---|---|
| Delta | T4 0.039 | |||||
| Theta | C3 0.031 | O1 0.031 | O2 0.018 | F7 0.045 | T4 0.033 | CZ 0.049 |
| High Beta | O2 0.039 | |||||
Appendix B. Supplementary Figures
References
- Di Pompeo, I.; D’Aurizio, G.; Burattini, C.; Bisegna, F.; Curcio, G. Positive mood induction to promote well-being and health: A systematic review from real settings to virtual reality. J. Environ. Psychol. 2023, 91, 102095. [Google Scholar] [CrossRef]
- Klepeis, N.; Nelson, W.; Ott, W.; Robinson, J.; Tsang, A.; Switzer, P.; Behar, J.; Hern, S.; Engelmann, W. The National Human Activity Pattern Survey (NHAPS): A resource for assessing exposure to environmental pollutants. J. Expo. Sci. Environ. Epidemiol. 2001, 11, 231–252. Available online: http://www.nature.com/articles/7500165 (accessed on 3 October 2025). [CrossRef]
- Salingaros, N.; Masden, K. Chapter 5: Neuroscience, the Natural Environment, and Building Design. In Biophilic Design: The Theory, Science and Practice of Bringing Buildings to Life; John Wiley and Sons: Hoboken, NJ, USA, 2008; pp. 59–83. [Google Scholar]
- Chang, C.-C.; Cox, D.T.C.; Fan, Q.; Nghiem, T.; Tan, C.; Oh, R.; Lin, B.; Shanahan, D.; Fuller, R.; Gaston, K.; et al. People’s desire to be in nature and how they experience it are partially heritable. PLoS Biol. 2022, 20, e3001500. [Google Scholar] [CrossRef]
- Barbiero, G.; Berto, R. Biophilia as Evolutionary Adaptation: An Onto- and Phylogenetic Framework for Biophilic Design. Front. Psychol. 2021, 12, 700709. Available online: https://www.frontiersin.org/articles/10.3389/fpsyg.2021.700709/full (accessed on 3 October 2025). [CrossRef] [PubMed]
- Albrecht, G. Solastalgia. Altern. J. (AJ)-Canada’s Environ. Voice 2006, 32, 34–36. [Google Scholar]
- Sanders, S.; Deming, A.; Ray, S.; Brorby, T.; Passarello, E.; Atkinson, J.; Ray, J.; Bogard, P.; Wilkinson, M.; Shukla, P.; et al. Solastalgia: An Anthology of Emotion in a Disappearing World; University of Virginia Press: Charlottesville, VA, USA, 2023; Available online: http://muse.jhu.edu/pub/191/edited%5C_volume/book/109723 (accessed on 3 October 2025).
- Ulrich, R. Natural Versus Urban Scenes. Environ. Behav. 1981, 13, 523–556. [Google Scholar] [CrossRef]
- Mayer, F.; Frantz, C.; Bruehlman-Senecal, E.; Dolliver, K. Why Is Nature Beneficial? Environ. Behav. 2009, 41, 607–643. [Google Scholar] [CrossRef]
- Ancora, L.; Blanco-Mora, D.; Alves, I.; Bonifácio, A.; Morgado, P.; Miranda, B. Cities and neuroscience research: A systematic literature review. Front. Psychiatry 2022, 13, 983352. Available online: http://www.frontiersin.org/articles/10.3389/fpsyt.2022.983352/full (accessed on 3 October 2025). [CrossRef]
- Ulrich, R. View Through a Window May Influence Recovery from Surgery. Science 1984, 224, 420–421. [Google Scholar] [CrossRef]
- Ulrich, R.; Zimring, C.; Zhu, X.; DuBose, J.; Seo, H.; Choi, Y.; Quan, X.; Joseph, A. A Review of the Research Literature on Evidence-Based Healthcare Design. HERD Health Environ. Res. Des. J. 2008, 1, 61–125. [Google Scholar] [CrossRef]
- Ulrich, R.; Simons, R.; Losito, B.; Fiorito, E.; Miles, M.; Zelson, M. Stress recovery during exposure to natural and urban environments. J. Environ. Psychol. 1991, 11, 201–230. Available online: http://linkinghub.elsevier.com/retrieve/pii/S0272494405801847 (accessed on 3 October 2025). [CrossRef]
- Kaplan, R.; Kaplan, S. The Experience of Nature: A Psychological Perspective; Cambridge University Press: Cambridge, UK, 1989; Volume 7, Available online: https://books.google.com/books/about/The%5C_Experience%5C_of%5C_Nature.html?hl=&id=7l80AAAAIAAJ (accessed on 3 October 2025).
- Kaplan, S. The restorative benefits of nature: Toward an integrative framework. J. Environ. Psychol. 1995, 15, 169–182. Available online: http://linkinghub.elsevier.com/retrieve/pii/0272494495900012 (accessed on 3 October 2025). [CrossRef]
- Hartig, T.; Mitchell, R.; Vries, S.; Frumkin, H. Nature and Health. Annu. Rev. Public Health 2014, 35, 207–228. [Google Scholar] [CrossRef]
- Hartig, T.; Mang, M.; Evans, G. Restorative Effects of Natural Environment Experiences. Environ. Behav. 1991, 23, 3–26. [Google Scholar] [CrossRef]
- Wells, N.; Evans, G. Nearby Nature. Environ. Behav. 2003, 35, 311–330. [Google Scholar] [CrossRef]
- Grahn, P.; Stigsdotter, U. Landscape planning and stress. Urban For. Urban Green. 2003, 2, 1–18. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1618866704700199 (accessed on 3 October 2025). [CrossRef]
- Herzog, T.; Black, A.; Fountaine, K.; Knotts, D. Reflection and attentional recovery as distinctive benefits of restorative environments. J. Environ. Psychol. 1997, 17, 165–170. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0272494497900517 (accessed on 3 October 2025). [CrossRef]
- Berman, M.; Jonides, J.; Kaplan, S. The Cognitive Benefits of Interacting With Nature. Psychol. Sci. 2008, 19, 1207–1212. [Google Scholar] [CrossRef]
- Hartig, T.; Evans, G.; Jamner, L.; Davis, D.; Gärling, T. Tracking restoration in natural and urban field settings. J. Environ. Psychol. 2003, 23, 109–123. Available online: http://linkinghub.elsevier.com/retrieve/pii/S0272494402001093 (accessed on 3 October 2025). [CrossRef]
- Berto, R. Exposure to restorative environments helps restore attentional capacity. J. Environ. Psychol. 2005, 25, 249–259. Available online: http://linkinghub.elsevier.com/retrieve/pii/S0272494405000381 (accessed on 3 October 2025). [CrossRef]
- Presti, P.; Ruzzon, D.; Avanzini, P.; Caruana, F.; Rizzolatti, G.; Vecchiato, G. Measuring arousal and valence generated by the dynamic experience of architectural forms in virtual environments. Sci. Rep. 2022, 12, 13376. Available online: http://www.nature.com/articles/s41598-022-17689-9 (accessed on 3 October 2025). [CrossRef] [PubMed]
- Martínez-Soto, J.; Gonzales-Santos, L.; Pasaye, E.; Barrios, F. Exploration of neural correlates of restorative environment exposure through functional magnetic resonance. Intell. Build. Int. 2013, 5, 10–28. [Google Scholar] [CrossRef]
- Henderson, J.; Larson, C.; Zhu, D. Cortical activation to indoor versus outdoor scenes: An fMRI study. Exp. Brain Res. 2007, 179, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Jeong, G.; Baek, H.; Kim, G.; Sundaram, T.; Kang, H.; Lee, S.; Kim, H.; Song, J. Human brain activation in response to visual stimulation with rural and urban scenery pictures: A functional magnetic resonance imaging study. Sci. Total Environ. 2010, 408, 2600–2607. Available online: http://linkinghub.elsevier.com/retrieve/pii/S0048969710001695 (accessed on 3 October 2025). [CrossRef]
- Jiang, X.; Hu, Y.; Larsen, L.; Chang, C.; Sullivan, W. Impacts of urban green infrastructure on attentional functioning: Insights from an fMRI study. Front Psychol 2023, 14, 1047993. [Google Scholar] [CrossRef]
- Burattini, C.; Bisegna, F.; D’Aurizio, G.; Curcio, G. The effect of an audio-video stimulation on emotions: A virtual reality study. In Proceedings of the 2023 Immersive and 3D Audio: From Architecture to Automotive (I3DA), Bologna, Italy, 5–7 September 2023; pp. 1–4. [Google Scholar] [CrossRef]
- Burattini, C.; D’Aurizio, G.; Broszio, K. Environmental psychology and health: Research in VR and real settings. J. Environ. Psychol. 2024, 100, 102478. [Google Scholar] [CrossRef]
- Yeom, S.; Kim, H.; Hong, T. Psychological and physiological effects of a green wall on occupants: A cross-over study in virtual reality. Build. Environ. 2021, 204, 108134. Available online: http://linkinghub.elsevier.com/retrieve/pii/S0360132321005357 (accessed on 3 October 2025). [CrossRef]
- Kim, N.; Gero, J. Neurophysiological Responses to Biophilic Design: A Pilot Experiment Using VR and EEG. In Design Computing And Cognition’22; Springer: Berlin/Heidelberg, Germany, 2023; pp. 235–253. [Google Scholar] [CrossRef]
- Yin, J.; Arfaei, N.; MacNaughton, P.; Catalano, P.; Allen, J.; Spengler, J. Effects of biophilic interventions in office on stress reaction and cognitive function: A randomized crossover study in virtual reality. Indoor Air 2019, 29, 1028–1039. [Google Scholar] [CrossRef]
- Jung, D.; Kim, D.; Kim, N. Bringing nature into hospital architecture: Machine learning-based EEG analysis of the biophilia effect in virtual reality. J. Environ. Psychol. 2023, 89, 102033. Available online: http://linkinghub.elsevier.com/retrieve/pii/S0272494423000816 (accessed on 3 October 2025). [CrossRef]
- Higuera-Trujillo, J.; Millán, C.; Aviñó, A.; Rojas, J. Multisensory stress reduction: A neuro-architecture study of paediatric waiting rooms. Build. Res. Inf. 2020, 48, 269–285. [Google Scholar] [CrossRef]
- Song, R.; Chen, Q.; Zhang, Y.; Jia, Q.; He, H.; Gao, T.; Qiu, L. Psychophysiological restorative potential in cancer patients by virtual reality (VR)-based perception of natural environment. Front. Psychol. 2022, 13, 1003497. Available online: http://www.frontiersin.org/articles/10.3389/fpsyg.2022.1003497/full (accessed on 3 October 2025). [CrossRef] [PubMed]
- Lei, Q.; Yuan, C.; Lau, S. A quantitative study for indoor workplace biophilic design to improve health and productivity performance. J. Clean. Prod. 2021, 324, 129168. Available online: http://linkinghub.elsevier.com/retrieve/pii/S0959652621033540 (accessed on 3 October 2025). [CrossRef]
- Yeom, S.; Kim, H.; Hong, T.; Park, H.; Lee, D. An integrated psychological score for occupants based on their perception and emotional response according to the windows’ outdoor view size. Build. Environ. 2020, 180, 107019. Available online: http://linkinghub.elsevier.com/retrieve/pii/S0360132320303991 (accessed on 3 October 2025). [CrossRef]
- Jeong, J.; Park, S. Physiological and Psychological Effects of Visual Stimulation with Green Plant Types. Int. J. Environ. Res. Public Health 2021, 18, 12932. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. Available online: http://linkinghub.elsevier.com/retrieve/pii/S0165027003003479 (accessed on 3 October 2025). [CrossRef]
- Pion-Tonachini, L.; Kreutz-Delgado, K.; Makeig, S. ICLabel: An automated electroencephalographic independent component classifier, dataset, and website. NeuroImage 2019, 198, 181–197. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1053811919304185 (accessed on 3 October 2025). [CrossRef]
- Buzsáki, G. Rhythms of the Brain; Oxford University Press: Oxford, UK, 2006; Volume 10. [Google Scholar] [CrossRef]
- Klimesch, W.; Sauseng, P.; Hanslmayr, S. EEG alpha oscillations: The inhibition–timing hypothesis. Brain Res. Rev. 2007, 53, 63–88. Available online: http://linkinghub.elsevier.com/retrieve/pii/S016501730600083X (accessed on 3 October 2025). [CrossRef]
- Klimesch, W. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. 2012, 16, 606–617. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1364661312002434 (accessed on 3 October 2025). [CrossRef]
- Michelmann, S.; Griffiths, B.; Hanslmayr, S. The role of alpha and beta oscillations in the human EEG during perception and memory processes. In The Oxford Handbook of EEG Frequency; Oxford University Press: New York, NY, USA, 2022; pp. 202–219. [Google Scholar]
- Jensen, O.; Mazaheri, A. Shaping Functional Architecture by Oscillatory Alpha Activity: Gating by Inhibition. Front. Hum. Neurosci. 2010, 4, 186. Available online: http://www.frontiersin.org/journals/human-neuroscience/articles/10.3389/fnhum.2010.00186 (accessed on 3 October 2025).
- Magosso, E.; Ricci, G.; Ursino, M. Alpha and theta mechanisms operating in internal-external attention competition. J. Integr. Neurosci. 2021, 20, 1. [Google Scholar] [CrossRef]
- Ricci, G.; De Crescenzio, F.; Santhosh, S.; Magosso, E.; Ursino, M. Relationship between electroencephalographic data and comfort perception captured in a Virtual Reality design environment of an aircraft cabin. Sci. Rep. 2022, 12, 10938. [Google Scholar] [CrossRef]
- Chen, Z.; He, Y.; Yu, Y. Attention restoration during environmental exposure via alpha-theta oscillations and synchronization. J. Environ. Psychol. 2020, 68, 101406. [Google Scholar] [CrossRef]
- Grassini, S.; Segurini, G.; Koivisto, M. Watching Nature Videos Promotes Physiological Restoration: Evidence From the Modulation of Alpha Waves in Electroencephalography. Front. Psychol. 2022, 13, 871143. Available online: http://www.frontiersin.org/articles/10.3389/fpsyg.2022.871143/full (accessed on 3 October 2025). [CrossRef]
- Wang, F.; Ke, H.; Ma, H.; Tang, Y. Deep Wavelet Temporal-Frequency Attention for nonlinear fMRI factorization in ASD. Pattern Recognit. 2025, 165, 111543. [Google Scholar] [CrossRef]
- Morales, S.; Bowers, M.E. Time-frequency analysis methods and their application in developmental EEG data. Dev. Cogn. Neurosci. 2022, 54, 101067. [Google Scholar] [CrossRef]
- Murugappan, M.; Rizon, M.; Nagarajan, R.; Yaacob, S.; Hazry, D.; Zunaidi, I. Time-Frequency Analysis of EEG Signals for Human Emotion Detection. In 4th Kuala Lumpur International Conference on Biomedical Engineering 2008; Abu Osman, N.A., Ibrahim, F., Wan Abas, W.A.B., Abdul Rahman, H.S., Ting, H.-N., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 262–265. [Google Scholar] [CrossRef]
- Ignacio, P.; Shealy, T. Effects of biophilic restorative experiences on designers’ bodies, brains, and minds. Proc. Des. Soc. 2023, 3, 1565–1574. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Salinas, J.S.; Wróblewska, A.; Kucewicz, M.T. Detection of EEG Activity in Response to the Surrounding Environment: A Neuro-Architecture Study. Brain Sci. 2025, 15, 1103. https://doi.org/10.3390/brainsci15101103
García-Salinas JS, Wróblewska A, Kucewicz MT. Detection of EEG Activity in Response to the Surrounding Environment: A Neuro-Architecture Study. Brain Sciences. 2025; 15(10):1103. https://doi.org/10.3390/brainsci15101103
Chicago/Turabian StyleGarcía-Salinas, Jesús S., Anna Wróblewska, and Michal T. Kucewicz. 2025. "Detection of EEG Activity in Response to the Surrounding Environment: A Neuro-Architecture Study" Brain Sciences 15, no. 10: 1103. https://doi.org/10.3390/brainsci15101103
APA StyleGarcía-Salinas, J. S., Wróblewska, A., & Kucewicz, M. T. (2025). Detection of EEG Activity in Response to the Surrounding Environment: A Neuro-Architecture Study. Brain Sciences, 15(10), 1103. https://doi.org/10.3390/brainsci15101103





