Restoration of Enzymatic Activity of Energy-Related Proteins in Rats with Traumatic Brain Injury Following Administration of Gamma-Glutamylcysteine Ethyl Ester
Abstract
1. Introduction
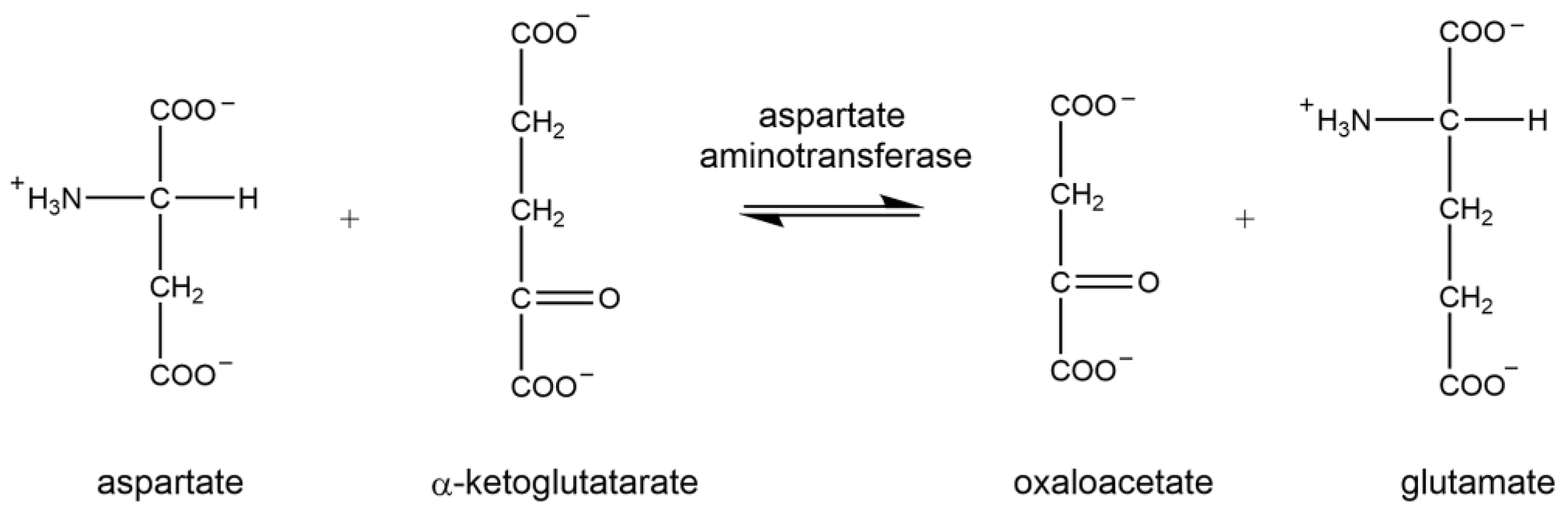
1.1. Aspartate Aminotransferase
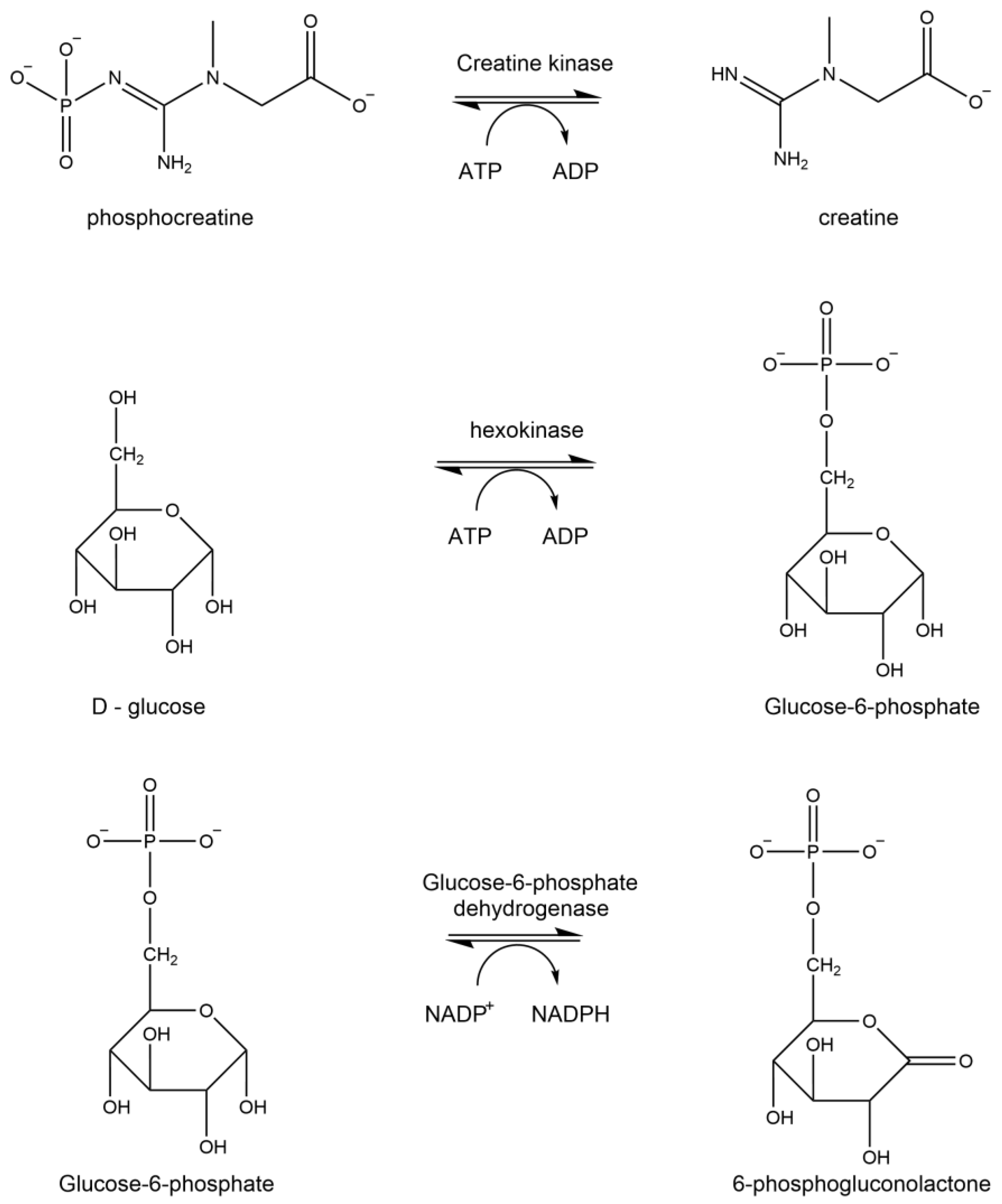
1.2. ATP Synthase Assay
1.3. Cytochrome C Oxidase
1.4. Creatine Kinase
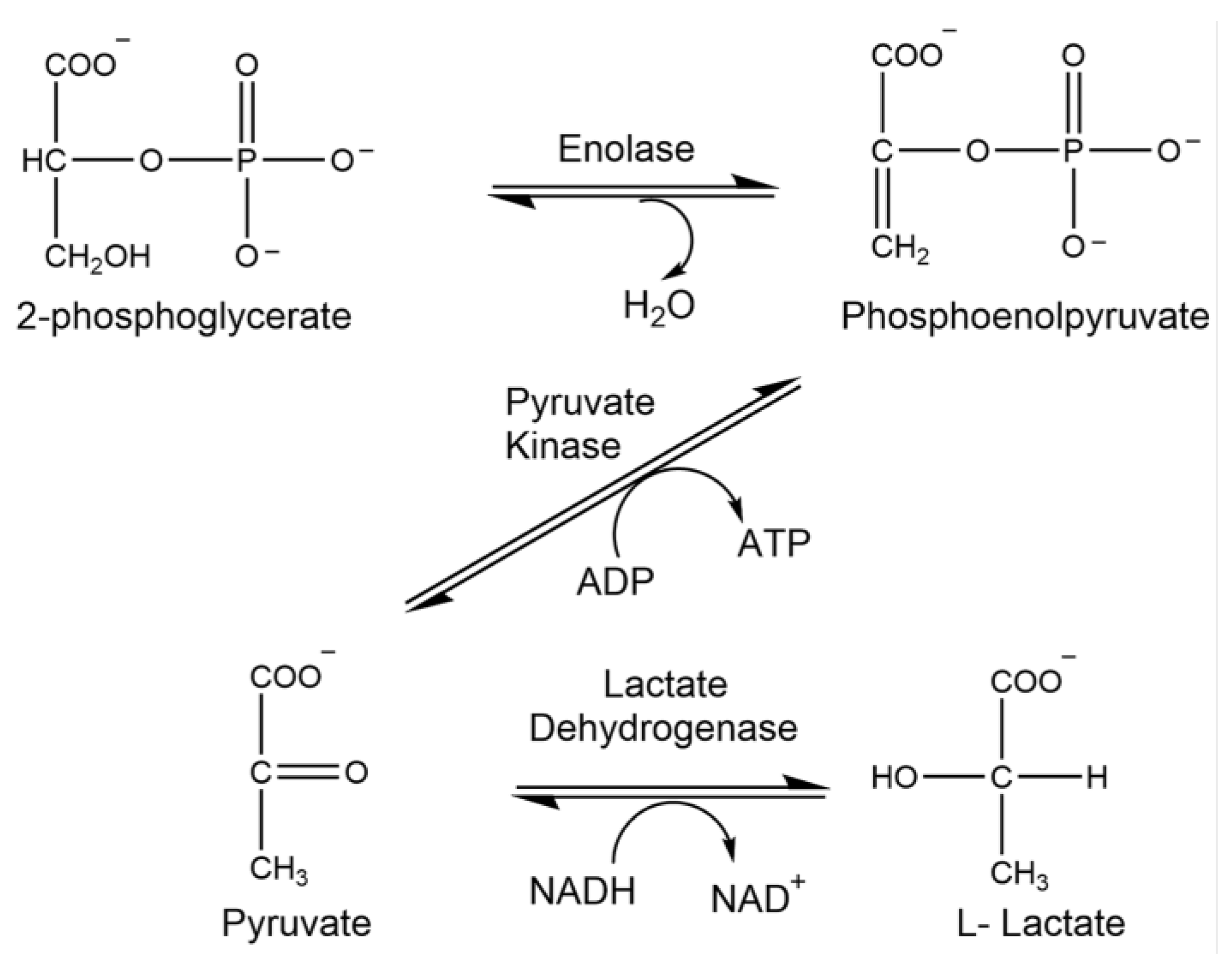
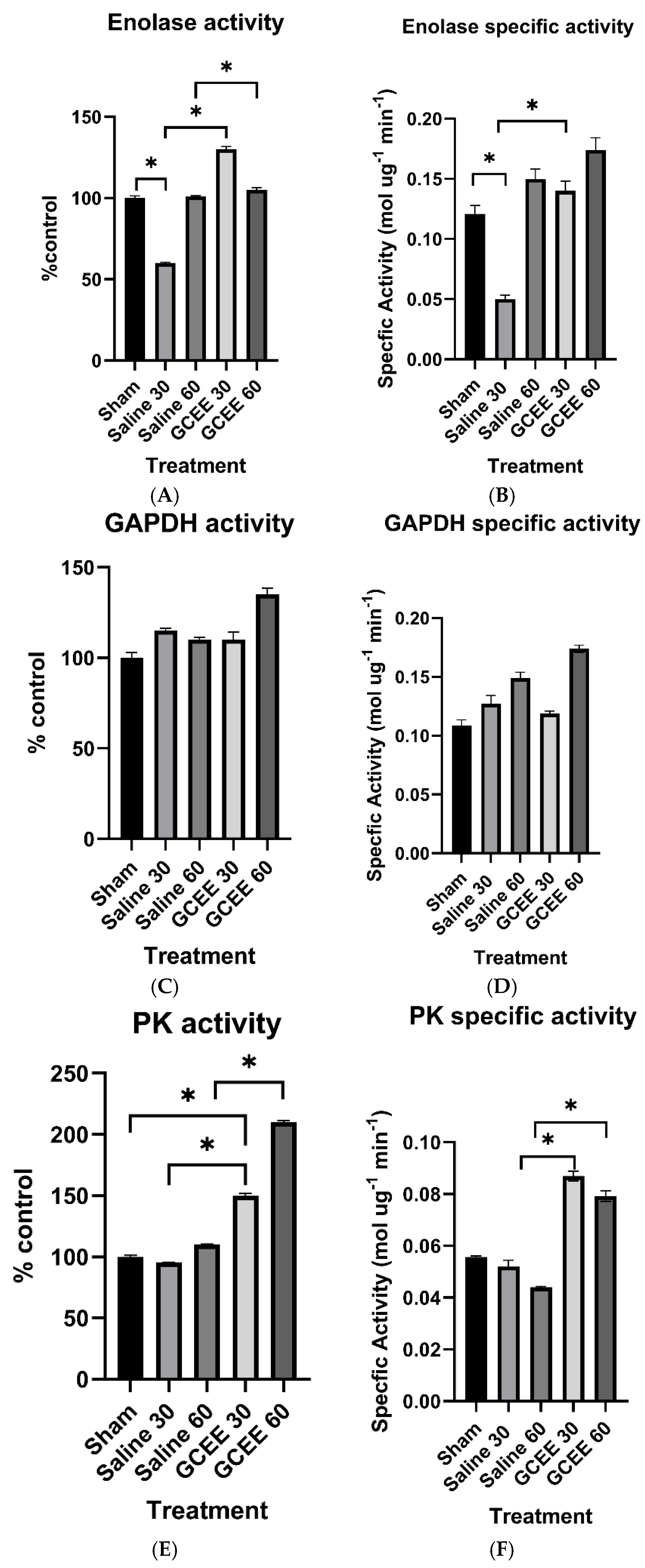
1.5. Enolase
1.6. Glyceraldehyde-3-Phosphate Dehydrogenase
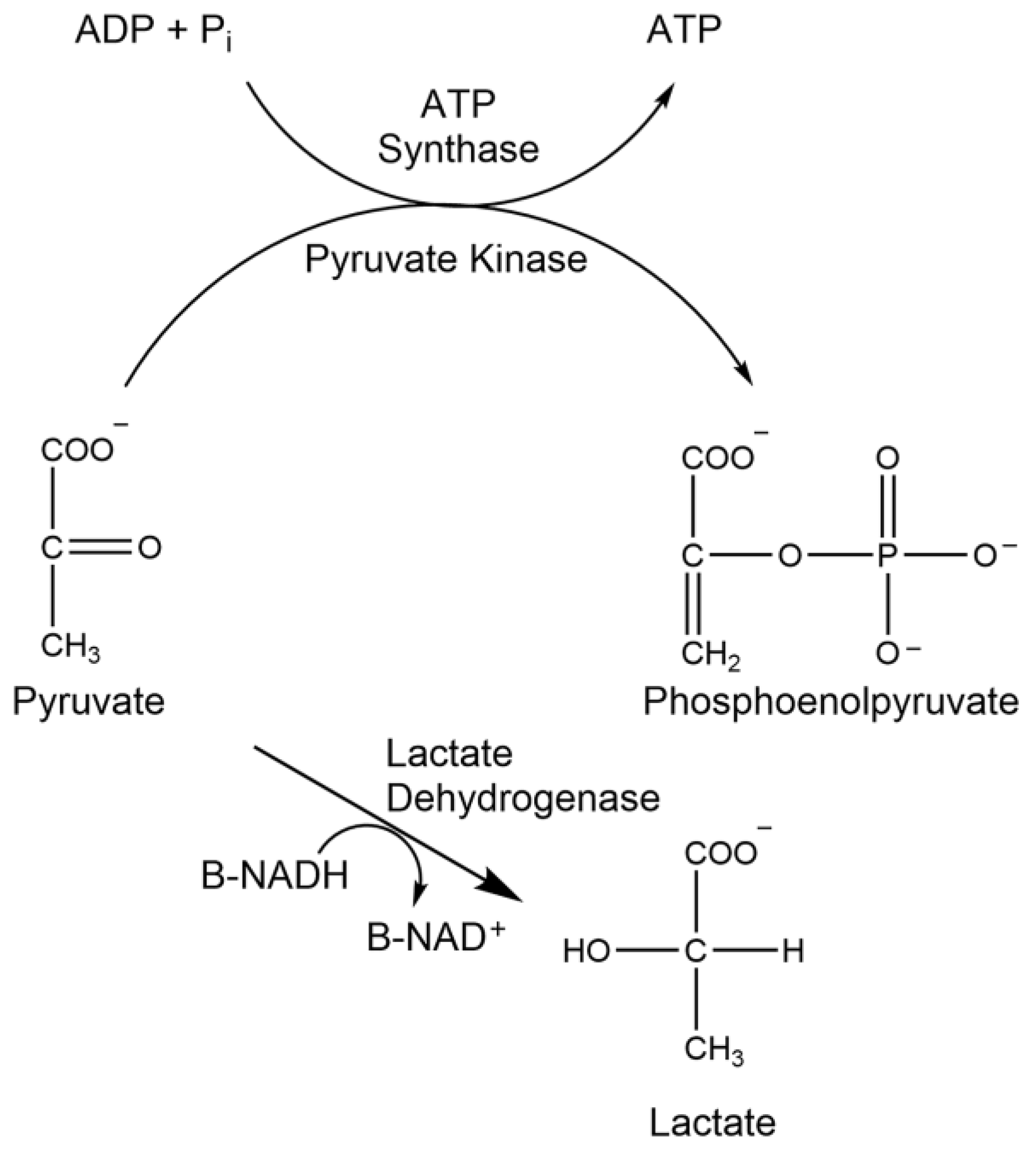
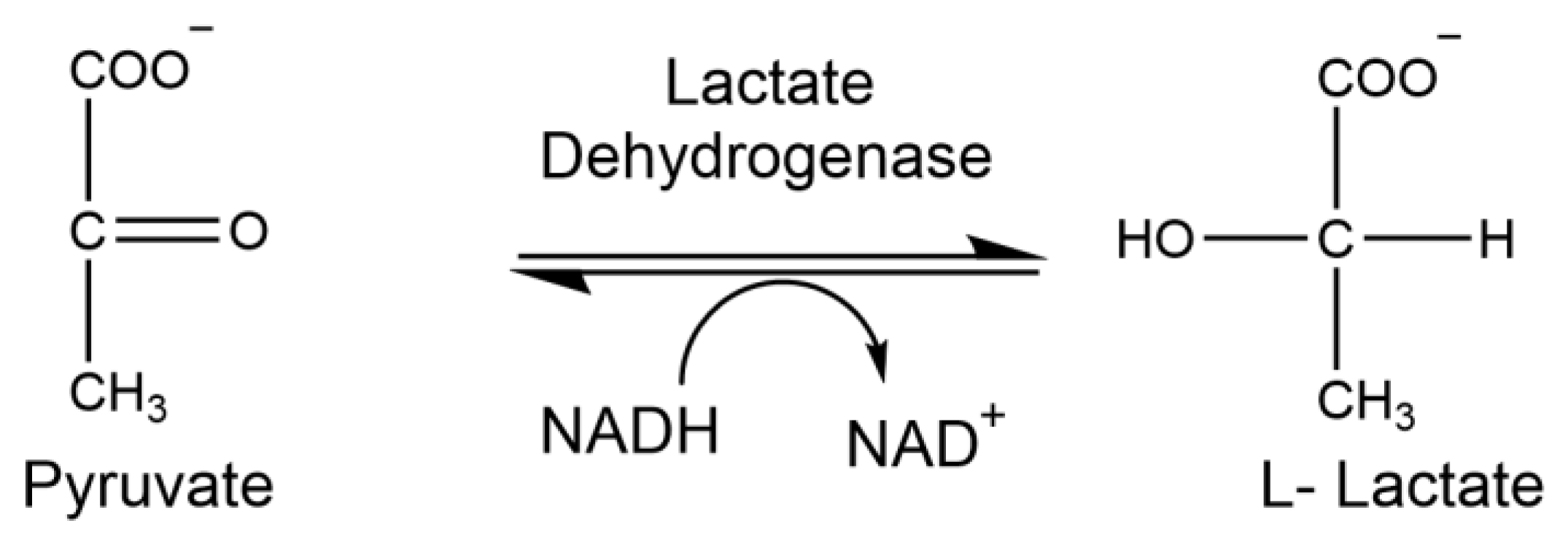
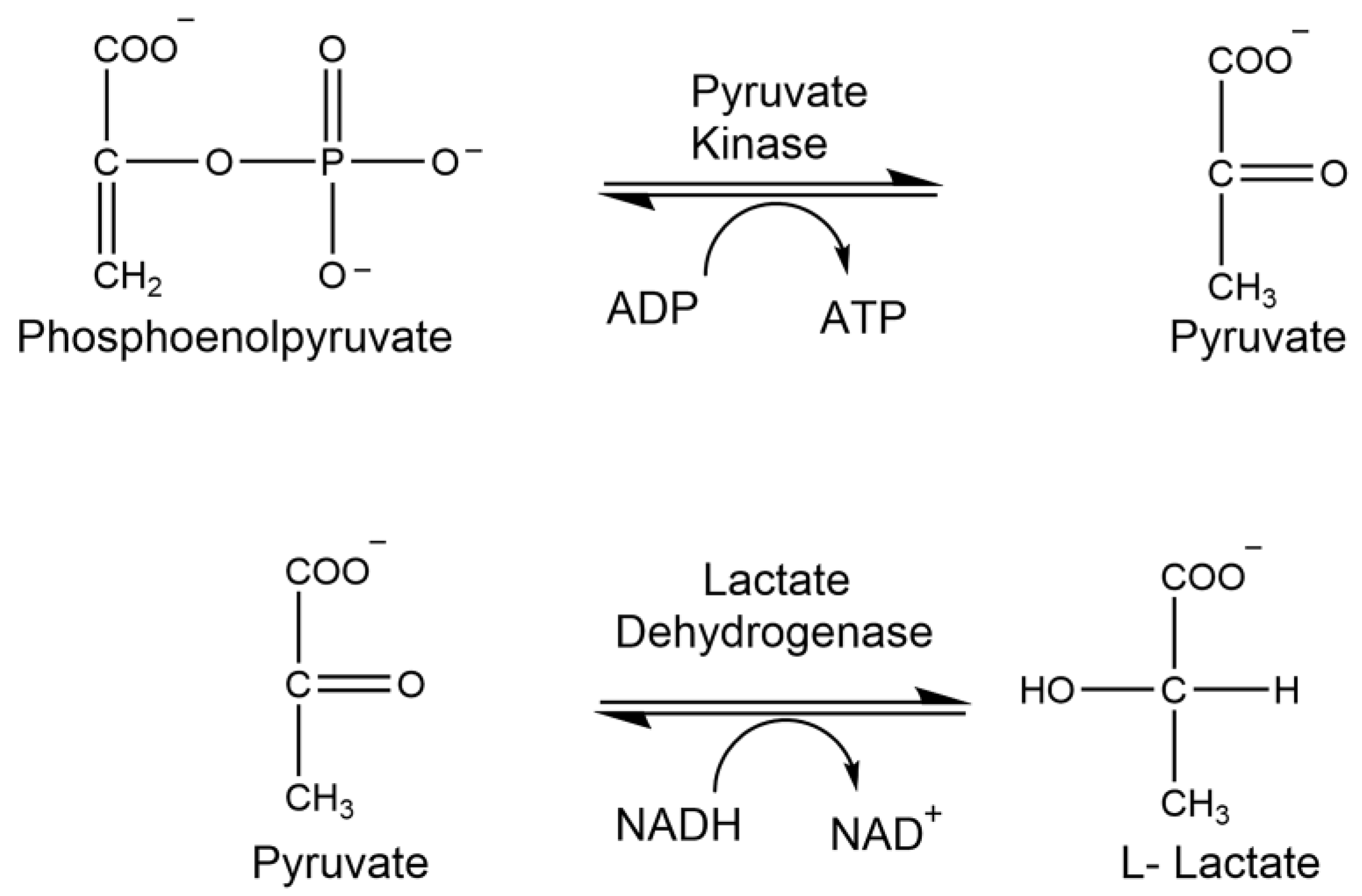
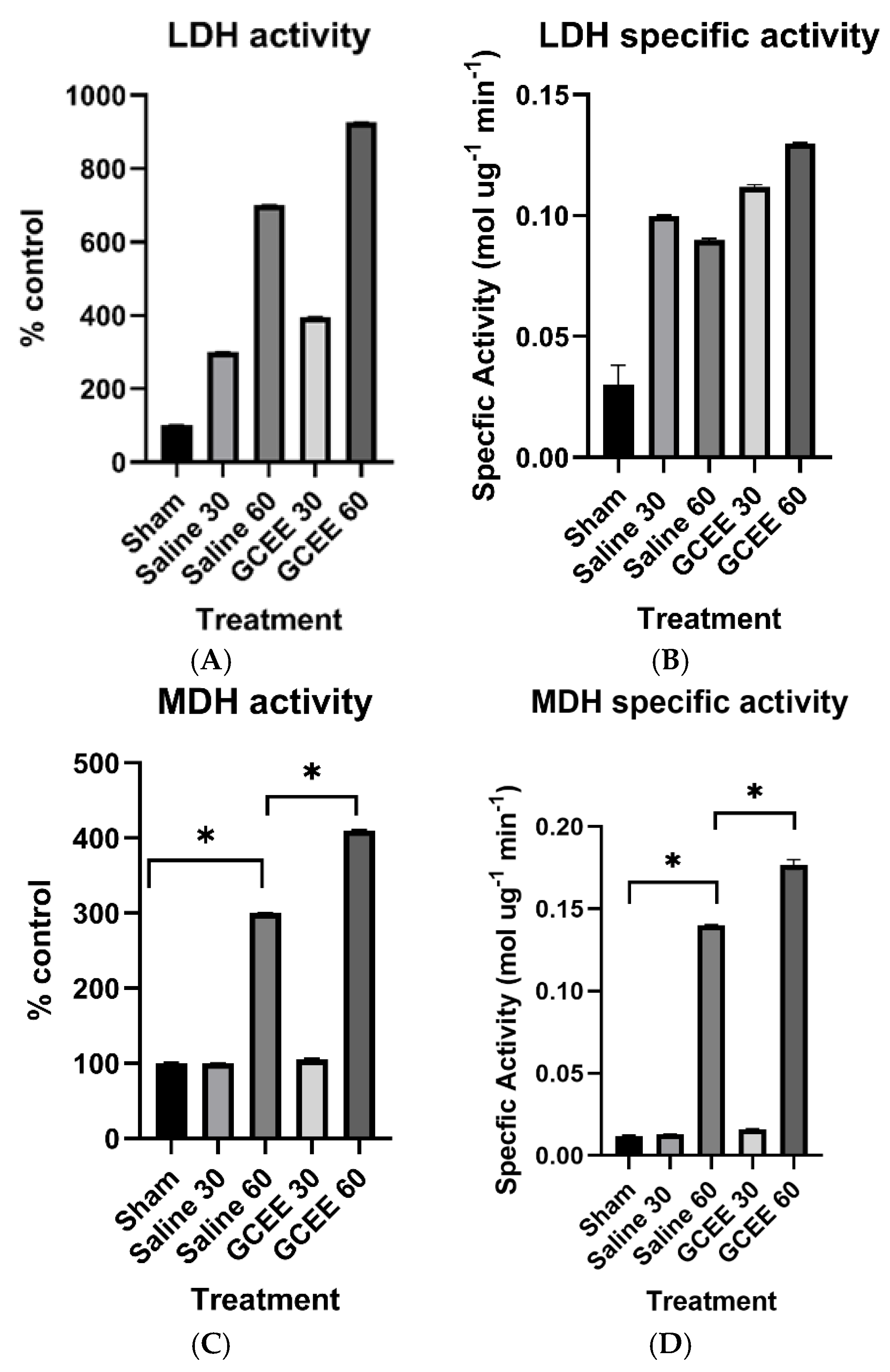
1.7. Lactate Dehydrogenase
1.8. Malate Dehydrogenase
1.9. Pyruvate Kinase
2. Materials and Methods
2.1. Chemicals
2.2. Surgical Methods
2.3. Analysis of Aspartate Aminotransferase Activity
2.4. Analysis of ATP Synthase Activity
2.5. Analysis of Cytochrome C Oxidase Activity
2.6. Analysis of Creatine Kinase Activity
2.7. Analysis of Enolase Activity
2.8. Analysis of Glyceraldehyde-3-Phosphate Dehydrogenase Activity
2.9. Analysis of Lactate Dehydrogenase Activity
2.10. Analysis of Malate Dehydrogenase Activity
2.11. Analysis of Pyruvate Kinase Activity
2.12. Specific Activity
2.13. Statistical Analysis
3. Results
3.1. Enzymatic Analysis
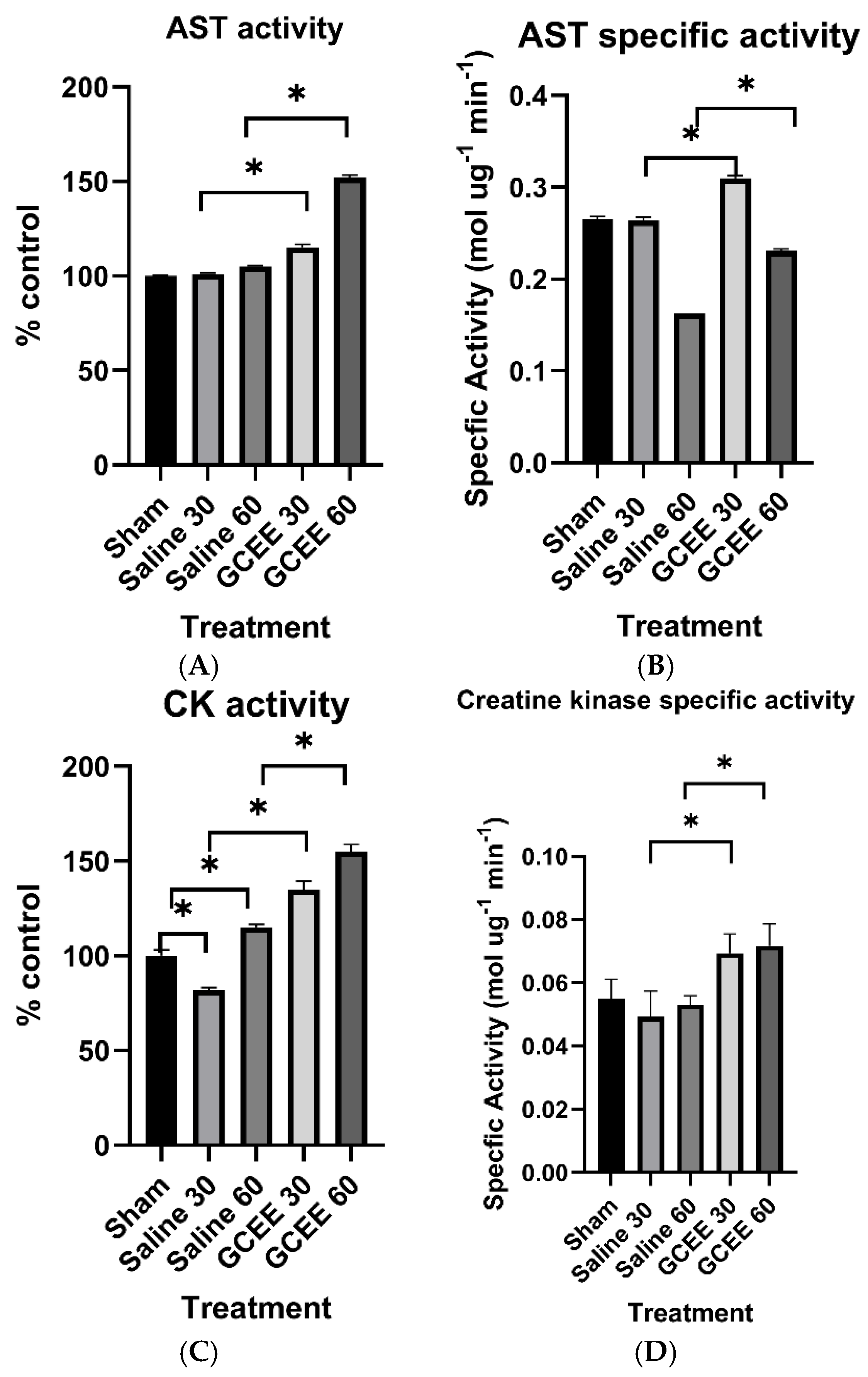
3.1.1. Transferase Enzymes (AST and CK Activities)
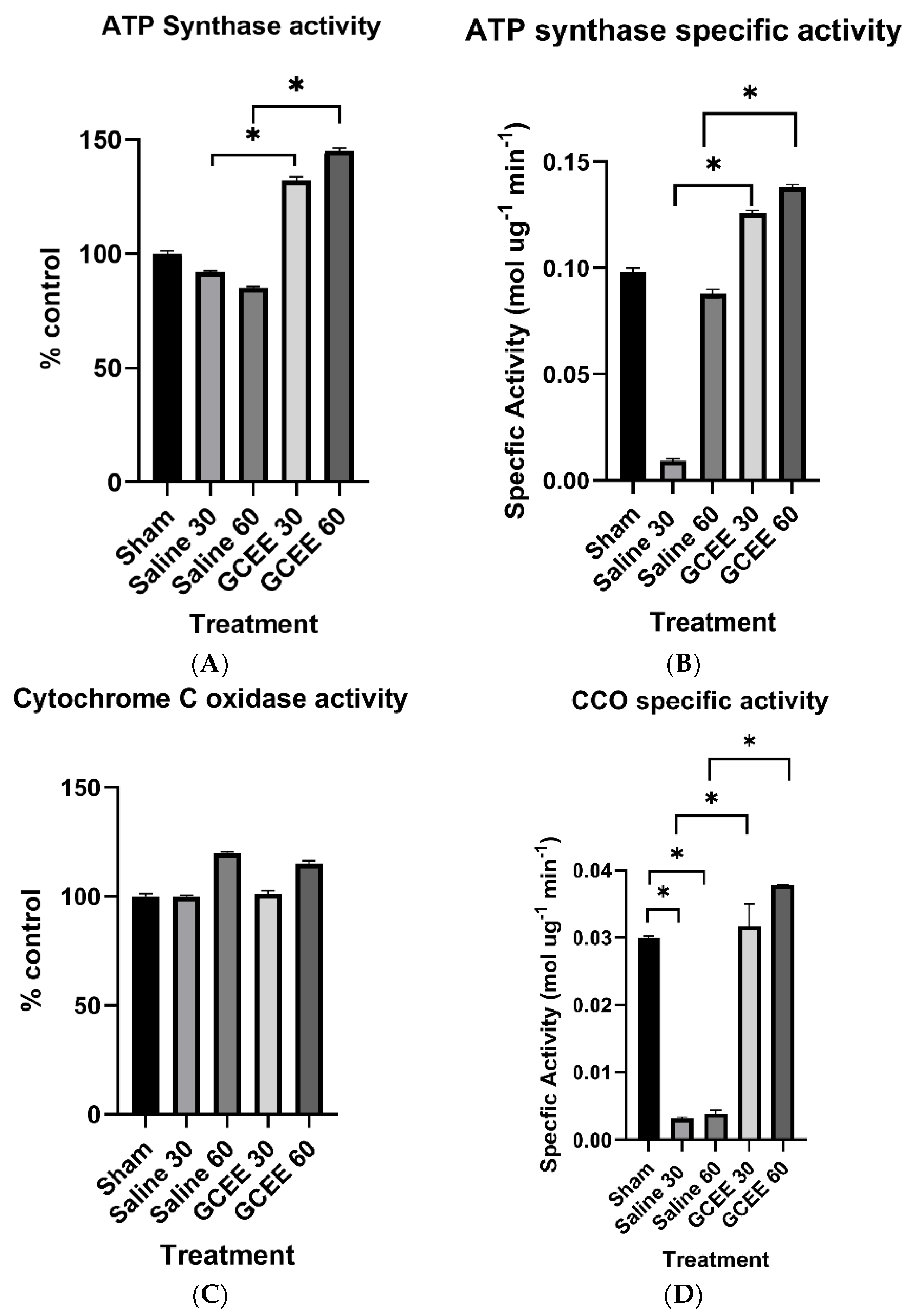
3.1.2. Oxidative Phosphorylation Proteins (ATP Synthase and CCO Activities)
3.1.3. Glycolytic Enzymes (ENO, GAPDH, and PK Activities)
3.1.4. Dehydrogenases (LDH and MDH Activities)
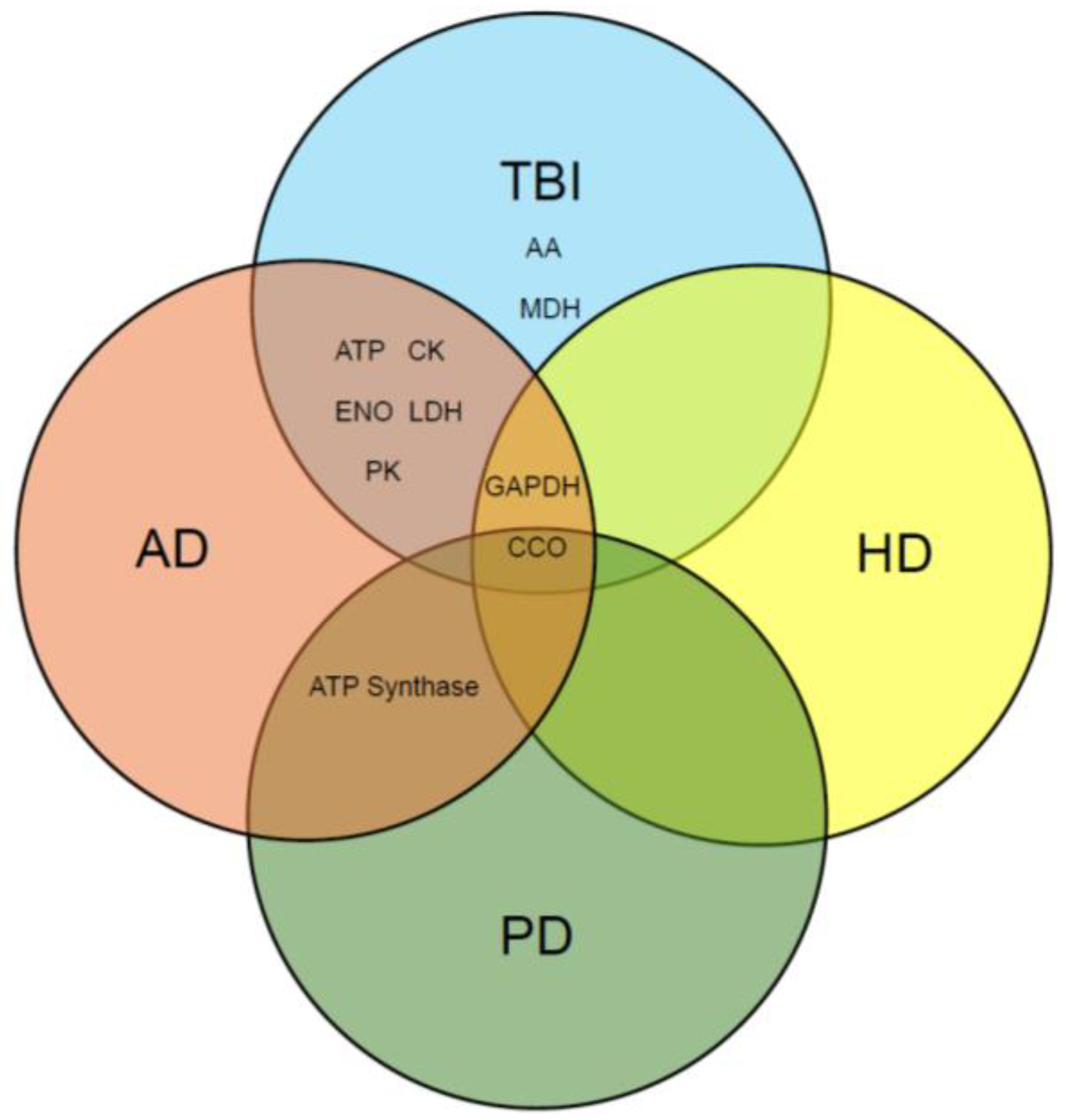
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dismuke-Greer, C.E.; Almeida, E.; Ryan, J.L.; Nakase-Richardson, R. Department of Defense Military Treatment Facility and Community Care Costs After Traumatic Brain Injury in Service Members and Veterans Treated in Veterans Affairs Polytrauma Rehabilitation Centers: A VA TBI Model Systems Study. J. Head Trauma Rehabil. 2025, 40, E300–E307. [Google Scholar] [CrossRef] [PubMed]
- Picetti, E.; Galarza, L.; Arroyo Diez, M.; Badenes, R.; Ballesteros Sanz, M.A.; Barea-Mendoza, J.A.; Bortoli, R.G.; Bouzat, P.; Citerio, G.; Godoy, D.A.; et al. Staircase strategy, tier-three therapies, and effects on outcome in traumatic brain injured patients: The Triple-T TBI study. Intensive Care Med. 2025, 51, 681–691. [Google Scholar] [CrossRef]
- Zima, L.; Moore, A.N.; Smolen, P.; Kobori, N.; Noble, B.; Robinson, D.; Hood, K.N.; Homma, R.; Al Mamun, A.; Redell, J.B.; et al. The evolving pathophysiology of TBI and the advantages of temporally-guided combination therapies. Neurochem. Int. 2024, 180, 105874. [Google Scholar] [CrossRef] [PubMed]
- Bruns, J., Jr.; Hauser, W.A. The epidemiology of traumatic brain injury: A review. Epilepsia 2003, 44, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Bazarian, J.J.; Blyth, B.; Mookerjee, S.; He, H.; McDermott, M.P. Sex differences in outcome after mild traumatic brain injury. J. Neurotrauma 2010, 27, 527–539. [Google Scholar] [CrossRef]
- Ottochian, M.; Salim, A.; Berry, C.; Chan, L.S.; Wilson, M.T.; Margulies, D.R. Severe traumatic brain injury: Is there a gender difference in mortality? Am. J. Surg. 2009, 197, 155–158. [Google Scholar] [CrossRef]
- Dringen, R. Metabolism and functions of glutathione in brain. Prog. Neurobiol. 2000, 62, 649–671. [Google Scholar] [CrossRef]
- Lok, J.; Leung, W.; Zhao, S.; Pallast, S.; van Leyen, K.; Guo, S.; Wang, X.; Yalcin, A.; Lo, E.H. gamma-glutamylcysteine ethyl ester protects cerebral endothelial cells during injury and decreases blood-brain barrier permeability after experimental brain trauma. J. Neurochem. 2011, 118, 248–255. [Google Scholar] [CrossRef]
- Joshi, G.; Hardas, S.; Sultana, R.; St Clair, D.K.; Vore, M.; Butterfield, D.A. Glutathione elevation by gamma-glutamyl cysteine ethyl ester as a potential therapeutic strategy for preventing oxidative stress in brain mediated by in vivo administration of adriamycin: Implication for chemobrain. J. Neurosci. Res. 2007, 85, 497–503. [Google Scholar] [CrossRef]
- Song, J.; Kang, S.M.; Lee, W.T.; Park, K.A.; Lee, K.M.; Lee, J.E. Glutathione protects brain endothelial cells from hydrogen peroxide-induced oxidative stress by increasing nrf2 expression. Exp. Neurobiol. 2014, 23, 93–103. [Google Scholar] [CrossRef]
- Park, M.K.; Choi, B.Y.; Kho, A.R.; Lee, S.H.; Hong, D.K.; Kang, B.S.; Lee, C.J.; Yang, H.W.; Woo, S.Y.; Park, S.W.; et al. L-theanine ameliorates traumatic-brain-injury-induced hippocampal neuronal death in rats. Phytomedicine 2025, 139, 156457. [Google Scholar] [CrossRef]
- Tu, Y.; Han, D.; Liu, Y.; Hong, D.; Chen, R. Nicorandil attenuates cognitive impairment after traumatic brain injury via inhibiting oxidative stress and inflammation: Involvement of BDNF and NGF. Brain Behav. 2024, 14, e3356. [Google Scholar] [CrossRef]
- Ni, H.; Rui, Q.; Kan, X.; Gao, R.; Zhang, L.; Zhang, B. Catalpol Ameliorates Oxidative Stress and Neuroinflammation after Traumatic Brain Injury in Rats. Neurochem. Res. 2023, 48, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Karatoprak, D.E.; Engin, R.; Sahin, S.; Iclek, I.; Durak, M.A. Investigation of Neuroprotective Efficacy of Dexpanthenol in an Experimental Head Injury Model. J. Korean Neurosurg. Soc. 2024, 67, 521–530. [Google Scholar] [CrossRef]
- Nishida, K.; Ohta, Y.; Ito, M.; Nagamura, Y.; Kitahara, S.; Fujii, K.; Ishiguro, I. Conversion of gamma-glutamylcysteinylethyl ester to glutathione in rat hepatocytes. Biochim. Biophys. Acta 1996, 1313, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Drake, J.; Kanski, J.; Varadarajan, S.; Tsoras, M.; Butterfield, D.A. Elevation of brain glutathione by gamma-glutamylcysteine ethyl ester protects against peroxynitrite-induced oxidative stress. J. Neurosci. Res. 2002, 68, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Reed, T.T.; Owen, J.; Pierce, W.M.; Sebastian, A.; Sullivan, P.G.; Butterfield, D.A. Proteomic identification of nitrated brain proteins in traumatic brain-injured rats treated postinjury with gamma-glutamylcysteine ethyl ester: Insights into the role of elevation of glutathione as a potential therapeutic strategy for traumatic brain injury. J. Neurosci. Res. 2009, 87, 408–417. [Google Scholar] [CrossRef]
- Mattson, M.P.; Pedersen, W.A.; Duan, W.; Culmsee, C.; Camandola, S. Cellular and molecular mechanisms underlying perturbed energy metabolism and neuronal degeneration in Alzheimer’s and Parkinson’s diseases. Ann. N. Y. Acad. Sci. 1999, 893, 154–175. [Google Scholar] [CrossRef]
- Koroshetz, W.J.; Jenkins, B.G.; Rosen, B.R.; Beal, M.F. Energy metabolism defects in Huntington’s disease and effects of coenzyme Q10. Ann. Neurol. 1997, 41, 160–165. [Google Scholar] [CrossRef]
- Henderson, M.; Rice, B.; Sebastian, A.; Sullivan, P.G.; King, C.; Robinson, R.A.; Reed, T.T. Neuroproteomic study of nitrated proteins in moderate traumatic brain injured rats treated with gamma glutamyl cysteine ethyl ester administration post injury: Insight into the role of glutathione elevation in nitrosative stress. Proteom. Clin. Appl. 2016, 10, 1218–1224. [Google Scholar] [CrossRef]
- Ferrer, I.; Perez, E.; Dalfo, E.; Barrachina, M. Abnormal levels of prohibitin and ATP synthase in the substantia nigra and frontal cortex in Parkinson’s disease. Neurosci. Lett. 2007, 415, 205–209. [Google Scholar] [CrossRef]
- Tramutola, A.; Lanzillotta, C.; Perluigi, M.; Butterfield, D.A. Oxidative stress, protein modification and Alzheimer disease. Brain Res. Bull. 2017, 133, 88–96. [Google Scholar] [CrossRef]
- Abulimiti, A.; Bae, H.; Ali, A.; Balakrishnan, S.; Tsujishita, M.; Gveric, D.; Tierney, T.S.; Jonas, E.A.; Smith, P.J.S.; Gentleman, S.M.; et al. Reduced DJ-1-F1Fo ATP synthase association correlates with midbrain dopaminergic neuron vulnerability in idiopathic Parkinson’s disease. Sci. Adv. 2025, 11, eads3051. [Google Scholar] [CrossRef] [PubMed]
- Opii, W.O.; Nukala, V.N.; Sultana, R.; Pandya, J.D.; Day, K.M.; Merchant, M.L.; Klein, J.B.; Sullivan, P.G.; Butterfield, D.A. Proteomic identification of oxidized mitochondrial proteins following experimental traumatic brain injury. J. Neurotrauma 2007, 24, 772–789. [Google Scholar] [CrossRef]
- Ekici, B.; Aydinli, N. Subthalamic nuclei involvement in Leigh disease with cytochrome c oxidase deficiency. Acta Neurol. Belg. 2011, 111, 168–169. [Google Scholar] [PubMed]
- Gan, Z.S.; Stein, S.C.; Swanson, R.; Guan, S.; Garcia, L.; Mehta, D.; Smith, D.H. Blood Biomarkers for Traumatic Brain Injury: A Quantitative Assessment of Diagnostic and Prognostic Accuracy. Front. Neurol. 2019, 10, 446. [Google Scholar] [CrossRef]
- Rabow, L.; Hedman, G. CKBB-isoenzymes as a sign of cerebral injury. Acta Neurochir. Suppl. 1979, 28, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Karpman, R.R.; Weinstein, P.R.; Finley, P.R.; Karst-Sabin, B. Serum CPK isoenzyme BB as an indicator of brain tissue damage following head injury. J. Trauma 1981, 21, 148–151. [Google Scholar] [CrossRef]
- Kenney, K.; Landau, M.E.; Gonzalez, R.S.; Hundertmark, J.; O’Brien, K.; Campbell, W.W. Serum creatine kinase after exercise: Drawing the line between physiological response and exertional rhabdomyolysis. Muscle Nerve 2012, 45, 356–362. [Google Scholar] [CrossRef]
- Lynn, B.C.; Wang, J.; Markesbery, W.R.; Lovell, M.A. Quantitative changes in the mitochondrial proteome from subjects with mild cognitive impairment, early stage, and late stage Alzheimer’s disease. J. Alzheimers Dis. 2010, 19, 325–339. [Google Scholar] [CrossRef]
- Lee, S.; Jang, K.I.; Lee, H.; Jo, Y.S.; Kwon, D.; Park, G.; Bae, S.; Kwon, Y.W.; Jang, J.H.; Oh, Y.S.; et al. Multi-proteomic analyses of 5xFAD mice reveal new molecular signatures of early-stage Alzheimer’s disease. Aging Cell 2024, 23, e14137. [Google Scholar] [CrossRef]
- Kilianski, J.; Peeters, S.; Debad, J.; Mohmed, J.; Wolf, S.E.; Minei, J.P.; Diaz-Arrastia, R.; Gatson, J.W. Plasma creatine kinase B correlates with injury severity and symptoms in professional boxers. J. Clin. Neurosci. 2017, 45, 100–104. [Google Scholar] [CrossRef]
- Meierhans, R.; Bechir, M.; Ludwig, S.; Sommerfeld, J.; Brandi, G.; Haberthur, C.; Stocker, R.; Stover, J.F. Brain metabolism is significantly impaired at blood glucose below 6 mM and brain glucose below 1 mM in patients with severe traumatic brain injury. Crit. Care 2010, 14, R13. [Google Scholar] [CrossRef] [PubMed]
- Chuang, D.M.; Hough, C.; Senatorov, V.V. Glyceraldehyde-3-phosphate dehydrogenase, apoptosis, and neurodegenerative diseases. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 269–290. [Google Scholar] [CrossRef] [PubMed]
- Tristan, C.; Shahani, N.; Sedlak, T.W.; Sawa, A. The diverse functions of GAPDH: Views from different subcellular compartments. Cell Signal 2011, 23, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Goto, S.; Takaoka, Y.; Tseng, H.P.; Fujimura, T.; Kawamoto, S.; Ono, K.; Chen, C.L. A novel moonlight function of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) for immunomodulation. Biofactors 2018, 44, 597–608. [Google Scholar] [CrossRef]
- Shi, Q.; Gibson, G.E. Up-regulation of the mitochondrial malate dehydrogenase by oxidative stress is mediated by miR-743a. J. Neurochem. 2011, 118, 440–448. [Google Scholar] [CrossRef]
- Maciak, K.; Adamowicz-Salach, A.; Poznanski, J.; Gora, M.; Fronk, J.; Burzynska, B. A Family Affected by a Life-Threatening Erythrocyte Defect Caused by Pyruvate Kinase Deficiency With Normal Iron Status: A Case Report. Front. Genet. 2020, 11, 560248. [Google Scholar] [CrossRef]
- van Berkel, T.J.; Koster, J.F.; Staal, G.E. On the molecular basis of pyruvate kinase deficiency. I. Primary defect or conseqence of increased glutathione disulfide concentration. Biochim. Biophys. Acta 1973, 321, 496–502. [Google Scholar] [CrossRef]
- Mazzola, J.L.; Sirover, M.A. Reduction of glyceraldehyde-3-phosphate dehydrogenase activity in Alzheimer’s disease and in Huntington’s disease fibroblasts. J. Neurochem. 2001, 76, 442–449. [Google Scholar] [CrossRef]
- Perluigi, M.; Poon, H.F.; Maragos, W.; Pierce, W.M.; Klein, J.B.; Calabrese, V.; Cini, C.; De Marco, C.; Butterfield, D.A. Proteomic analysis of protein expression and oxidative modification in r6/2 transgenic mice: A model of Huntington disease. Mol. Cell Proteom. 2005, 4, 1849–1861. [Google Scholar] [CrossRef]
- Garcia-Fernandez, M.D.; Larrea, A.; Fernandez, R.; Rodriguez-Puertas, R.; Astigarraga, E.; Manuel, I.; Barreda-Gomez, G. Microarrays, Enzymatic Assays, and MALDI-MS for Determining Specific Alterations to Mitochondrial Electron Transport Chain Activity, ROS Formation, and Lipid Composition in a Monkey Model of Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 5470. [Google Scholar] [CrossRef]
- Zheng, T.; Kotol, D.; Sjoberg, R.; Mitsios, N.; Uhlen, M.; Zhong, W.; Edfors, F.; Mulder, J. Characterization of reduced astrocyte creatine kinase levels in Alzheimer’s disease. Glia 2024, 72, 1590–1603. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.G.; Keller, J.N.; Bussen, W.L.; Scheff, S.W. Cytochrome c release and caspase activation after traumatic brain injury. Brain Res. 2002, 949, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, S. Protein and Enzyme Explorer. Available online: https://www.sigmaaldrich.com/US/en/products/protein-biology/proteins-and-enzymes#explorer (accessed on 24 July 2024).
- Zheng, J.; Ramirez, V.D. Purification and identification of an estrogen binding protein from rat brain: Oligomycin sensitivity-conferring protein (OSCP), a subunit of mitochondrial F0F1-ATP synthase/ATPase. J. Steroid Biochem. Mol. Biol. 1999, 68, 65–75. [Google Scholar] [CrossRef]
- Hayashi, A.; Kidoguchi, K.; Suzuki, T.; Yamamura, Y.; Miwa, S.; Imai, K. Application of an automatic oxygenation technique to analysis of oxygen equilibrium curves for hemoglobinopathic red cells and functional screening of clinically important hemoglobinopathies. Hemoglobin 1979, 3, 429–450. [Google Scholar] [CrossRef] [PubMed]
- Arun, P.; Abu-Taleb, R.; Oguntayo, S.; Wang, Y.; Valiyaveettil, M.; Long, J.B.; Nambiar, M.P. Acute mitochondrial dysfunction after blast exposure: Potential role of mitochondrial glutamate oxaloacetate transaminase. J. Neurotrauma 2013, 30, 1645–1651. [Google Scholar] [CrossRef]
- Arun, P.; Oguntayo, S.; Alamneh, Y.; Honnold, C.; Wang, Y.; Valiyaveettil, M.; Long, J.B.; Nambiar, M.P. Rapid release of tissue enzymes into blood after blast exposure: Potential use as biological dosimeters. PLoS ONE 2012, 7, e33798. [Google Scholar] [CrossRef]
- Koza, L.; Linseman, D.A. Glutathione precursors shield the brain from trauma. Neural Regen. Res. 2019, 14, 1701–1702. [Google Scholar] [CrossRef]
- Srinivasan, S.; Avadhani, N.G. Cytochrome c oxidase dysfunction in oxidative stress. Free Radic Biol. Med. 2012, 53, 1252–1263. [Google Scholar] [CrossRef]
- Hovda, D.A.; Yoshino, A.; Kawamata, T.; Katayama, Y.; Becker, D.P. Diffuse prolonged depression of cerebral oxidative metabolism following concussive brain injury in the rat: A cytochrome oxidase histochemistry study. Brain Res. 1991, 567, 1–10. [Google Scholar] [CrossRef]
- Wallimann, T.; Tokarska-Schlattner, M.; Schlattner, U. The creatine kinase system and pleiotropic effects of creatine. Amino Acids 2011, 40, 1271–1296. [Google Scholar] [CrossRef]
- Pette, D.; Reichmann, H. The principle of determining relative enzyme activities by comparative kinetic microphotometry in situ. Histochem. J. 1989, 21, 531–534. [Google Scholar] [CrossRef]
- Guingab-Cagmat, J.D.; Cagmat, E.B.; Hayes, R.L.; Anagli, J. Integration of proteomics, bioinformatics, and systems biology in traumatic brain injury biomarker discovery. Front. Neurol. 2013, 4, 61. [Google Scholar] [CrossRef]
- Ingebrigtsen, T.; Romner, B. Biochemical serum markers for brain damage: A short review with emphasis on clinical utility in mild head injury. Restor. Neurol. Neurosci. 2003, 21, 171–176. [Google Scholar] [CrossRef]
- Selakovic, V.; Raicevic, R.; Radenovic, L. The increase of neuron-specific enolase in cerebrospinal fluid and plasma as a marker of neuronal damage in patients with acute brain infarction. J. Clin. Neurosci. 2005, 12, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Hardas, S.S.; Lange, M.L. Oxidatively modified glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and Alzheimer’s disease: Many pathways to neurodegeneration. J. Alzheimers Dis. 2010, 20, 369–393. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.R.; Cascio, M.B.; Sawa, A. GAPDH as a sensor of NO stress. Biochim. Biophys. Acta 2006, 1762, 502–509. [Google Scholar] [CrossRef]
- Nicholls, C.; Li, H.; Liu, J.P. GAPDH: A common enzyme with uncommon functions. Clin. Exp. Pharmacol. Physiol. 2012, 39, 674–679. [Google Scholar] [CrossRef]
- Murata-Kamiya, N.; Kamiya, H. Methylglyoxal, an endogenous aldehyde, crosslinks DNA polymerase and the substrate DNA. Nucleic Acids Res. 2001, 29, 3433–3438. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Perluigi, M.; Sultana, R. Oxidative stress in Alzheimer’s disease brain: New insights from redox proteomics. Eur. J. Pharmacol. 2006, 545, 39–50. [Google Scholar] [CrossRef]
- Hoyer, S. Causes and consequences of disturbances of cerebral glucose metabolism in sporadic Alzheimer disease: Therapeutic implications. Adv. Exp. Med. Biol. 2004, 541, 135–152. [Google Scholar] [CrossRef]
- Reed, T.; Perluigi, M.; Sultana, R.; Pierce, W.M.; Klein, J.B.; Turner, D.M.; Coccia, R.; Markesbery, W.R.; Butterfield, D.A. Redox proteomic identification of 4-hydroxy-2-nonenal-modified brain proteins in amnestic mild cognitive impairment: Insight into the role of lipid peroxidation in the progression and pathogenesis of Alzheimer’s disease. Neurobiol. Dis. 2008, 30, 107–120. [Google Scholar] [CrossRef]
- Banoei, M.M.; Lee, C.H.; Hutchison, J.; Panenka, W.; Wellington, C.; Wishart, D.S.; Winston, B.W.; The Canadian Biobank, Database for Traumatic Brain Injury (CanTBI) Investigators; The Canadian Critical Care Translational Biology Group (CCCTBG); The Canadian Traumatic Brain Injury Research, Clinical Network (CTRC). Using metabolomics to predict severe traumatic brain injury outcome (GOSE) at 3 and 12 months. Crit. Care 2023, 27, 295. [Google Scholar] [CrossRef] [PubMed]
- Guglielmetti, C.; Chou, A.; Krukowski, K.; Najac, C.; Feng, X.; Riparip, L.K.; Rosi, S.; Chaumeil, M.M. In vivo metabolic imaging of Traumatic Brain Injury. Sci. Rep. 2017, 7, 17525. [Google Scholar] [CrossRef] [PubMed]
- Bubber, P.; Haroutunian, V.; Fisch, G.; Blass, J.P.; Gibson, G.E. Mitochondrial abnormalities in Alzheimer brain: Mechanistic implications. Ann. Neurol. 2005, 57, 695–703. [Google Scholar] [CrossRef]
- Op den Velde, W.; Stam, F.C. Some cerebral proteins and enzyme systems in Alzheimer’s presenile and senile dementia. J. Am. Geriatr. Soc. 1976, 24, 12–16. [Google Scholar] [CrossRef]
- Zlotnik, A.; Gruenbaum, S.E.; Artru, A.A.; Rozet, I.; Dubilet, M.; Tkachov, S.; Brotfain, E.; Klin, Y.; Shapira, Y.; Teichberg, V.I. The neuroprotective effects of oxaloacetate in closed head injury in rats is mediated by its blood glutamate scavenging activity: Evidence from the use of maleate. J. Neurosurg. Anesthesiol. 2009, 21, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Ichai, C.; Armando, G.; Orban, J.C.; Berthier, F.; Rami, L.; Samat-Long, C.; Grimaud, D.; Leverve, X. Sodium lactate versus mannitol in the treatment of intracranial hypertensive episodes in severe traumatic brain-injured patients. Intensive Care Med. 2009, 35, 471–479. [Google Scholar] [CrossRef]




| Enzymatic Assays | Sham n = 6 | TBI + Vehicle at 30 min n = 6 | TBI + GCEE at 30 min n = 6 | TBI + Vehicle at 60 min n = 6 | TBI + GCEE at 60 min n = 6 |
|---|---|---|---|---|---|
| AST | Yes | Yes | Yes | Yes | Yes |
| ATP synthase | Yes | Yes | Yes | Yes | Yes |
| CCO | Yes | Yes | Yes | Yes | Yes |
| CK | Yes | Yes | Yes | Yes | Yes |
| ENO | Yes | Yes | Yes | Yes | Yes |
| GAPDH | Yes | Yes | Yes | Yes | Yes |
| LDH | Yes | Yes | Yes | Yes | Yes |
| MDH | Yes | Yes | Yes | Yes | Yes |
| PK | Yes | Yes | Yes | Yes | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rice, B.; Overbay, J.; Sebastian, A.; Sullivan, P.G.; Reed, T.T. Restoration of Enzymatic Activity of Energy-Related Proteins in Rats with Traumatic Brain Injury Following Administration of Gamma-Glutamylcysteine Ethyl Ester. Brain Sci. 2025, 15, 1067. https://doi.org/10.3390/brainsci15101067
Rice B, Overbay J, Sebastian A, Sullivan PG, Reed TT. Restoration of Enzymatic Activity of Energy-Related Proteins in Rats with Traumatic Brain Injury Following Administration of Gamma-Glutamylcysteine Ethyl Ester. Brain Sciences. 2025; 15(10):1067. https://doi.org/10.3390/brainsci15101067
Chicago/Turabian StyleRice, Brittany, Jonathan Overbay, Andrea Sebastian, Patrick G. Sullivan, and Tanea T. Reed. 2025. "Restoration of Enzymatic Activity of Energy-Related Proteins in Rats with Traumatic Brain Injury Following Administration of Gamma-Glutamylcysteine Ethyl Ester" Brain Sciences 15, no. 10: 1067. https://doi.org/10.3390/brainsci15101067
APA StyleRice, B., Overbay, J., Sebastian, A., Sullivan, P. G., & Reed, T. T. (2025). Restoration of Enzymatic Activity of Energy-Related Proteins in Rats with Traumatic Brain Injury Following Administration of Gamma-Glutamylcysteine Ethyl Ester. Brain Sciences, 15(10), 1067. https://doi.org/10.3390/brainsci15101067





