AAV2retro Enters Axons of Passage and Extensively Transduces Corticospinal Neurons After Injection into Spinal White Matter
Abstract
1. Introduction
2. Materials and Methods
2.1. AAV Vector
2.2. Mouse Surgery and Sacrifice
2.3. Histology
2.4. Analysis and Statistics: Brain
2.5. Analysis and Statistics: Lumbar Spinal Cord
3. Results
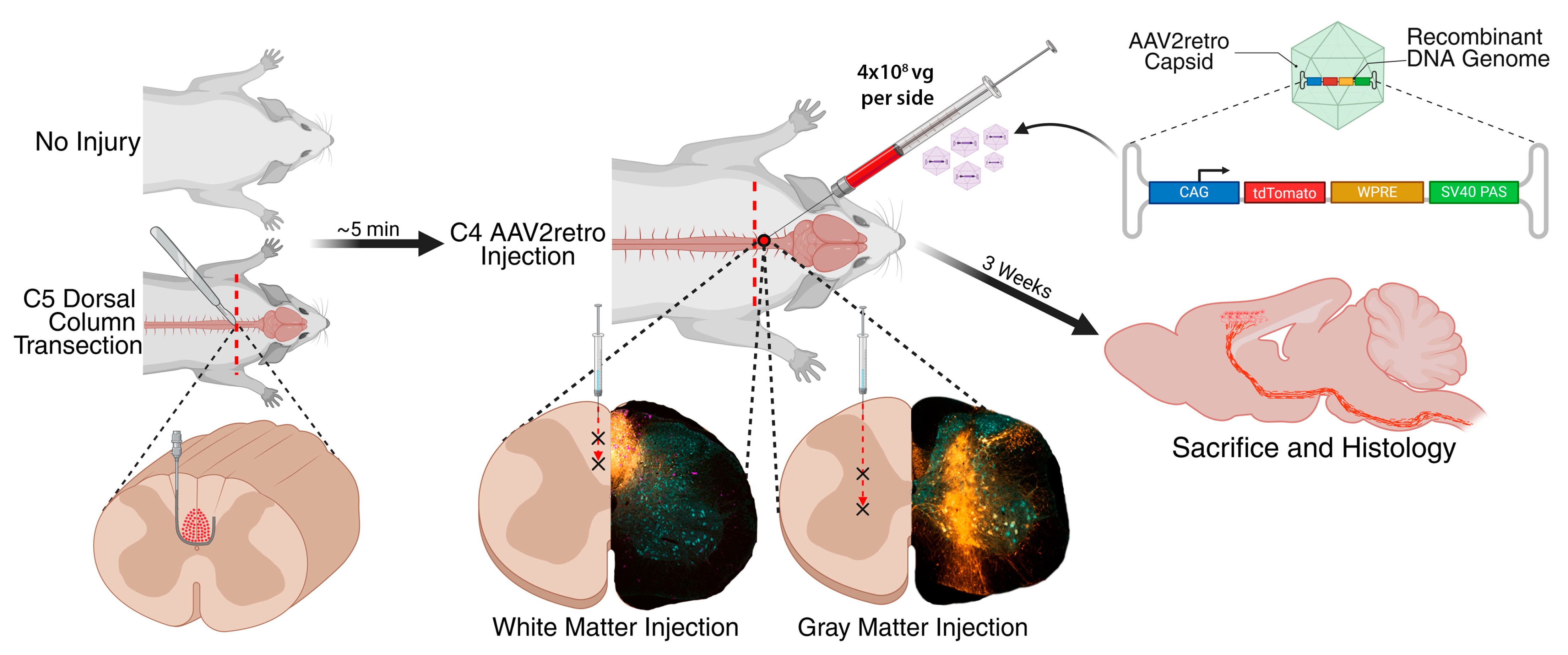
3.1. Experimental Design
- 1.
- Injection of AAV2retro into intact C4 gray matter;
- 2.
- Injection of AAV2retro into intact C4 dorsal column white matter;
- 3.
- Injection of AAV2retro into C4 gray matter bordering a C5 dorsal column lesion;
- 4.
- Injection of AAV2retro into C4 dorsal column white matter bordering a C5 dorsal column lesion.
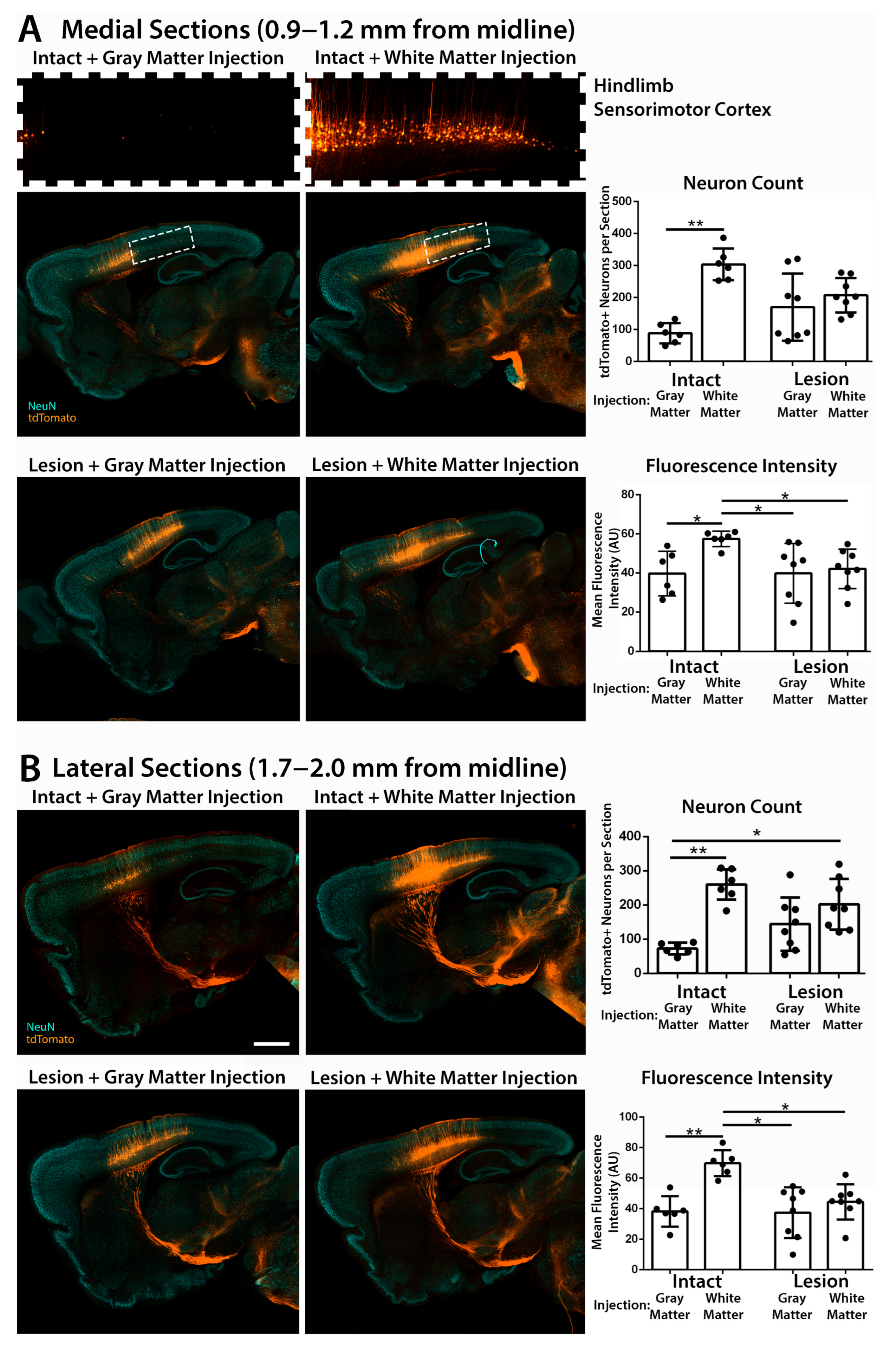
3.2. Retrograde Transduction of Corticospinal Neurons in the Sensorimotor Cortex
3.3. Quantification of Transduced Corticospinal Axons in the Lumbar Spinal Cord
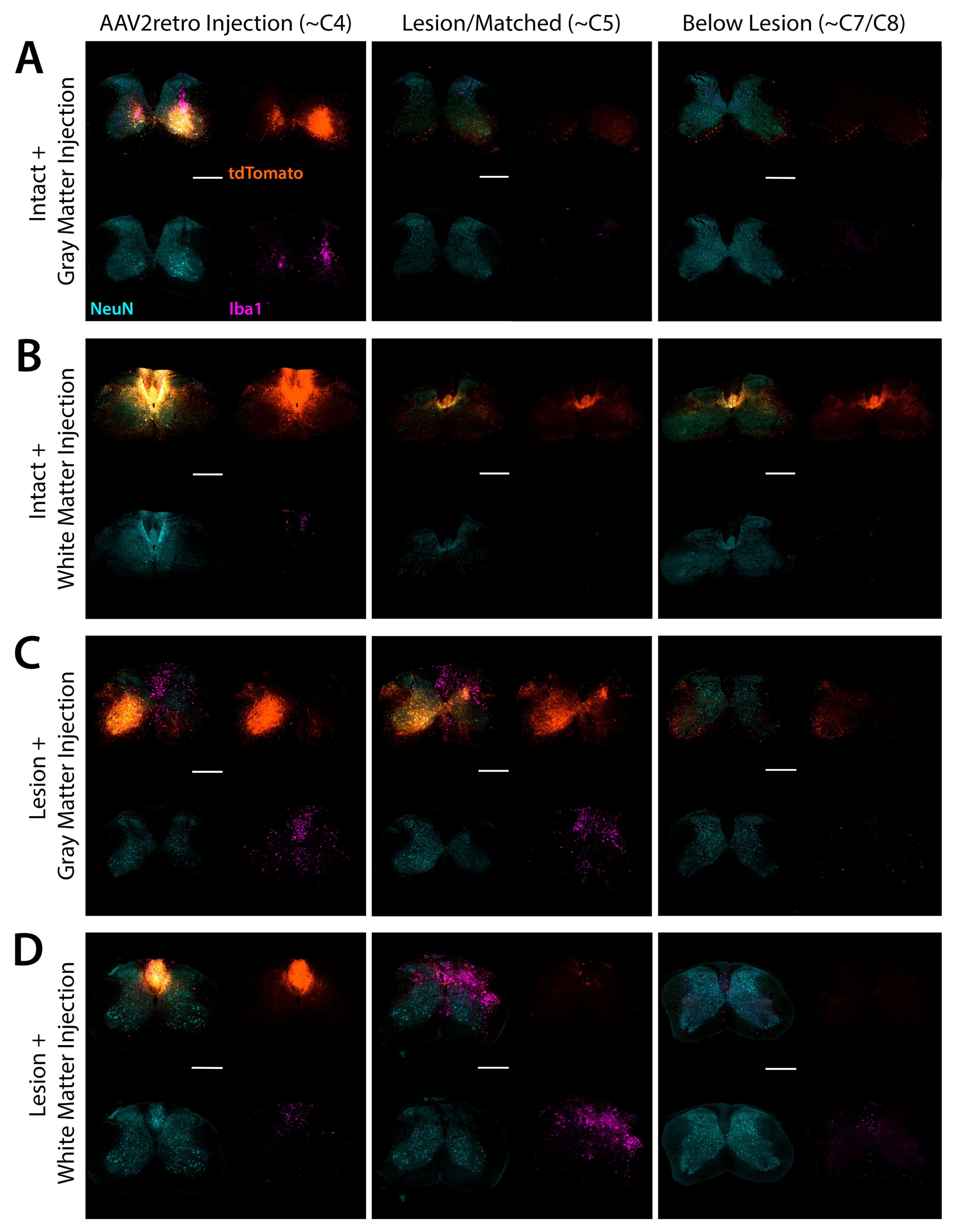
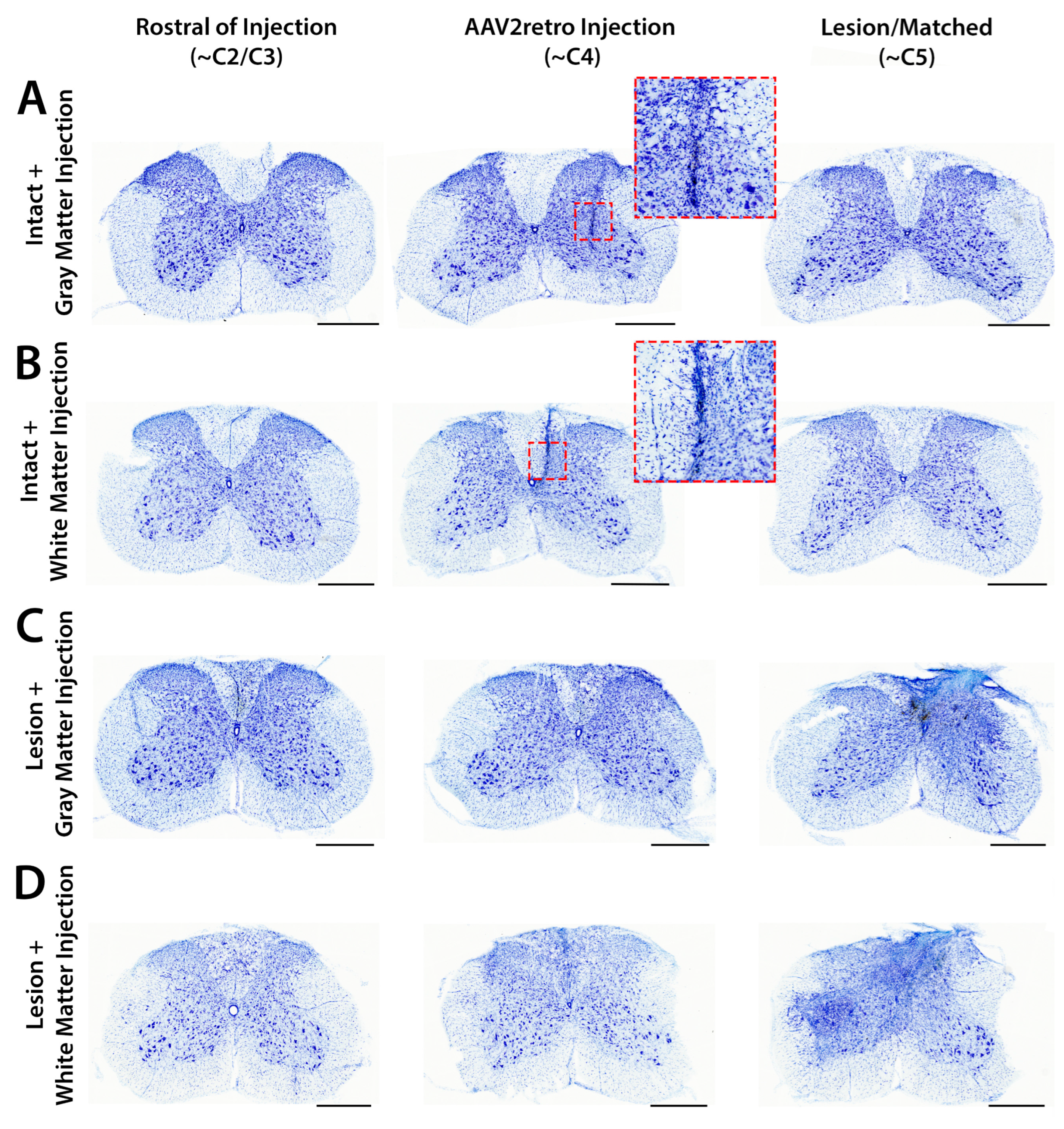
3.4. Accuracy and Safety of Spinal Cord Injections by Immunofluorescent and Nissl Staining
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAV | Adeno-associated virus |
| AF | Alexa Fluor |
| AU | Arbitrary units |
| CAG | Chicken beta-actin gene |
| CI | Confidence interval |
| CNS | Central nervous system |
| DRG | Dorsal root ganglion |
| PBS | Phosphate-buffered saline |
| RT | Room temperature |
| SCI | Spinal cord injury |
| SV40 PAS | Simian virus 40 polyadenylation signal |
| TBS | Tris-buffered saline |
| WPRE | Woodchuck hepatitis virus post-transcriptional regulatory element |
References
- Sinopoulou, E.; Rosenzweig, E.S.; Conner, J.M.; Gibbs, D.; Weinholtz, C.A.; Weber, J.L.; Brock, J.H.; Nout-Lomas, Y.S.; Ovruchesky, E.; Takashima, Y.; et al. Rhesus Macaque versus Rat Divergence in the Corticospinal Projectome. Neuron 2022, 110, 2970–2983.e4. [Google Scholar] [CrossRef]
- Golan, N.; Ehrlich, D.; Bonanno, J.; O’Brien, R.F.; Murillo, M.; Kauer, S.D.; Ravindra, N.; Van Dijk, D.; Cafferty, W.B. Anatomical Diversity of the Adult Corticospinal Tract Revealed by Single-Cell Transcriptional Profiling. J. Neurosci. 2023, 43, 7929–7945. [Google Scholar] [CrossRef]
- Xie, F.; Zheng, B. White Matter Inhibitors in CNS Axon Regeneration Failure. Exp. Neurol. 2008, 209, 302–312. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Jin, Y. Intrinsic Control of Axon Regeneration. Neuron 2016, 90, 437–451. [Google Scholar] [CrossRef]
- Poplawski, G.H.D.; Kawaguchi, R.; Van Niekerk, E.; Lu, P.; Mehta, N.; Canete, P.; Lie, R.; Dragatsis, I.; Meves, J.M.; Zheng, B.; et al. Injured Adult Neurons Regress to an Embryonic Transcriptional Growth State. Nature 2020, 581, 77–82. [Google Scholar] [CrossRef]
- Wang, Z.; Reynolds, A.; Kirry, A.; Nienhaus, C.; Blackmore, M.G. Overexpression of Sox11 Promotes Corticospinal Tract Regeneration after Spinal Injury While Interfering with Functional Recovery. J. Neurosci. 2015, 35, 3139–3145. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.A.; Olson, G.M.; Chakraborty, A.; Blackmore, M.G. Promotion of Corticospinal Tract Growth by KLF6 Requires an Injury Stimulus and Occurs within Four Weeks of Treatment. Exp. Neurol. 2021, 339, 113644. [Google Scholar] [CrossRef] [PubMed]
- Campion, T.J.; Sheikh, I.S.; Smit, R.D.; Iffland, P.H.; Chen, J.; Junker, I.P.; Krynska, B.; Crino, P.B.; Smith, G.M. Viral Expression of Constitutively Active AKT3 Induces CST Axonal Sprouting and Regeneration, but Also Promotes Seizures. Exp. Neurol. 2022, 349, 113961. [Google Scholar] [CrossRef]
- Liu, K.; Lu, Y.; Lee, J.K.; Samara, R.; Willenberg, R.; Sears-Kraxberger, I.; Tedeschi, A.; Park, K.K.; Jin, D.; Cai, B.; et al. PTEN Deletion Enhances the Regenerative Ability of Adult Corticospinal Neurons. Nat. Neurosci. 2010, 13, 1075–1081. [Google Scholar] [CrossRef]
- Geoffroy, C.G.; Lorenzana, A.O.; Kwan, J.P.; Lin, K.; Ghassemi, O.; Ma, A.; Xu, N.; Creger, D.; Liu, K.; He, Z.; et al. Effects of PTEN and Nogo Codeletion on Corticospinal Axon Sprouting and Regeneration in Mice. J. Neurosci. 2015, 35, 6413–6428. [Google Scholar] [CrossRef]
- Jin, D.; Liu, Y.; Sun, F.; Wang, X.; Liu, X.; He, Z. Restoration of Skilled Locomotion by Sprouting Corticospinal Axons Induced by Co-Deletion of PTEN and SOCS3. Nat. Commun. 2015, 6, 8074. [Google Scholar] [CrossRef]
- Castle, M.J.; Turunen, H.T.; Vandenberghe, L.H.; Wolfe, J.H. Controlling AAV Tropism in the Nervous System with Natural and Engineered Capsids. Methods Mol. Biol. 2016, 1382, 133–149. [Google Scholar] [CrossRef]
- Ling, Q.; Herstine, J.A.; Bradbury, A.; Gray, S.J. AAV-Based in Vivo Gene Therapy for Neurological Disorders. Nat. Rev. Drug Discov. 2023, 22, 789–806. [Google Scholar] [CrossRef] [PubMed]
- Rafii, M.S.; Baumann, T.L.; Bakay, R.A.E.; Ostrove, J.M.; Siffert, J.; Fleisher, A.S.; Herzog, C.D.; Barba, D.; Pay, M.; Salmon, D.P.; et al. A Phase1 Study of Stereotactic Gene Delivery of AAV2-NGF for Alzheimer’s Disease. Alzheimers Dement. 2014, 10, 571–581. [Google Scholar] [CrossRef]
- Van Laar, A.D.; Christine, C.W.; Phielipp, N.; Larson, P.S.; Elder, J.B.; Merola, A.; San Sebastian, W.; Fiandaca, M.S.; Kells, A.P.; Wisniewski, M.E.; et al. Intraputaminal Delivery of Adeno-Associated Virus Serotype 2-Glial Cell Line-Derived Neurotrophic Factor in Mild or Moderate Parkinson’s Disease. Mov. Disord. 2025, 40, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Chan, K.Y.; Wu, J.; Botticello-Romero, N.R.; Zheng, Q.; Lou, S.; Keyes, C.; Svanbergsson, A.; Johnston, J.; Mills, A.; et al. An AAV Capsid Reprogrammed to Bind Human Transferrin Receptor Mediates Brain-Wide Gene Delivery. Science 2024, 384, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Moyer, T.C.; Hoffman, B.A.; Chen, W.; Shah, I.; Ren, X.-Q.; Knox, T.; Liu, J.; Wang, W.; Li, J.; Khalid, H.; et al. Highly Conserved Brain Vascular Receptor ALPL Mediates Transport of Engineered AAV Vectors across the Blood-Brain Barrier. Mol. Ther. 2025, 33, 3902–3916. [Google Scholar] [CrossRef]
- Castle, M.J.; Cheng, Y.; Asokan, A.; Tuszynski, M.H. Physical Positioning Markedly Enhances Brain Transduction after Intrathecal AAV9 Infusion. Sci. Adv. 2018, 4, eaau9859. [Google Scholar] [CrossRef]
- Hordeaux, J.; Buza, E.L.; Dyer, C.; Goode, T.; Mitchell, T.W.; Richman, L.; Denton, N.; Hinderer, C.; Katz, N.; Schmid, R.; et al. Adeno-Associated Virus-Induced Dorsal Root Ganglion Pathology. Hum. Gene Ther. 2020, 31, 808–818. [Google Scholar] [CrossRef]
- Kussick, E.; Johansen, N.; Taskin, N.; Chowdhury, A.; Quinlan, M.A.; Fraser, A.; Clark, A.G.; Wynalda, B.; Martinez, R.; Groce, E.L.; et al. Enhancer AAVs for Targeting Spinal Motor Neurons and Descending Motor Pathways in Rodents and Macaque. Cell Rep. 2025, 26, 115730. [Google Scholar] [CrossRef]
- Ben-Simon, Y.; Hooper, M.; Narayan, S.; Daigle, T.L.; Dwivedi, D.; Way, S.W.; Oster, A.; Stafford, D.A.; Mich, J.K.; Taormina, M.J.; et al. A Suite of Enhancer AAVs and Transgenic Mouse Lines for Genetic Access to Cortical Cell Types. Cell 2025, 188, 3045–3064.e23. [Google Scholar] [CrossRef]
- Mortberg, M.A.; Gentile, J.E.; Nadaf, N.M.; Vanderburg, C.; Simmons, S.; Dubinsky, D.; Slamin, A.; Maldonado, S.; Petersen, C.L.; Jones, N.; et al. A Single-Cell Map of Antisense Oligonucleotide Activity in the Brain. Nucleic Acids Res. 2023, 51, 7109–7124. [Google Scholar] [CrossRef] [PubMed]
- Khare, P.; Edgecomb, S.X.; Hamadani, C.M.; Tanner, E.E.L.; Manickam, D.S. Lipid Nanoparticle-Mediated Drug Delivery to the Brain. Adv. Drug Deliv. Rev. 2023, 197, 114861. [Google Scholar] [CrossRef] [PubMed]
- Klaw, M.C.; Xu, C.; Tom, V.J. Intraspinal AAV Injections Immediately Rostral to a Thoracic Spinal Cord Injury Site Efficiently Transduces Neurons in Spinal Cord and Brain. Mol. Ther. Nucleic Acids 2013, 2, e108. [Google Scholar] [CrossRef]
- Castle, M.J.; Perlson, E.; Holzbaur, E.L.; Wolfe, J.H. Long-Distance Axonal Transport of AAV9 Is Driven by Dynein and Kinesin-2 and Is Trafficked in a Highly Motile Rab7-Positive Compartment. Mol. Ther. 2014, 22, 554–566. [Google Scholar] [CrossRef] [PubMed]
- Castle, M.J.; Gershenson, Z.T.; Giles, A.R.; Holzbaur, E.L.F.; Wolfe, J.H. Adeno-Associated Virus Serotypes 1, 8, and 9 Share Conserved Mechanisms for Anterograde and Retrograde Axonal Transport. Hum. Gene Ther. 2014, 25, 705–720. [Google Scholar] [CrossRef]
- Tervo, D.G.R.; Hwang, B.-Y.; Viswanathan, S.; Gaj, T.; Lavzin, M.; Ritola, K.D.; Lindo, S.; Michael, S.; Kuleshova, E.; Ojala, D.; et al. A Designer AAV Variant Permits Efficient Retrograde Access to Projection Neurons. Neuron 2016, 92, 372–382. [Google Scholar] [CrossRef]
- Wang, Z.; Maunze, B.; Wang, Y.; Tsoulfas, P.; Blackmore, M.G. Global Connectivity and Function of Descending Spinal Input Revealed by 3D Microscopy and Retrograde Transduction. J. Neurosci. 2018, 38, 10566–10581. [Google Scholar] [CrossRef]
- Skorput, A.G.J.; Gore, R.; Schorn, R.; Riedl, M.S.; Marron Fernandez de Velasco, E.; Hadlich, B.; Kitto, K.F.; Fairbanks, C.A.; Vulchanova, L. Targeting the Somatosensory System with AAV9 and AAV2retro Viral Vectors. PLoS ONE 2022, 17, e0264938. [Google Scholar] [CrossRef]
- Metcalfe, M.; Steward, O. PTEN Deletion in Spinal Pathways via Retrograde Transduction with AAV-RG Enhances Forelimb Motor Recovery after Cervical Spinal Cord Injury; Sex Differences and Late-Onset Pathophysiologies. Exp. Neurol. 2023, 370, 114551. [Google Scholar] [CrossRef]
- Harrison, M.; O’Brien, A.; Adams, L.; Cowin, G.; Ruitenberg, M.J.; Sengul, G.; Watson, C. Vertebral Landmarks for the Identification of Spinal Cord Segments in the Mouse. Neuroimage 2013, 68, 22–29. [Google Scholar] [CrossRef]
- Kumamaru, H.; Lu, P.; Rosenzweig, E.S.; Kadoya, K.; Tuszynski, M.H. Regenerating Corticospinal Axons Innervate Phenotypically Appropriate Neurons within Neural Stem Cell Grafts. Cell Rep. 2019, 26, 2329–2339.e4. [Google Scholar] [CrossRef]
- Lu, P.; Gomes-Leal, W.; Anil, S.; Dobkins, G.; Huie, J.R.; Ferguson, A.R.; Graham, L.; Tuszynski, M. Origins of Neural Progenitor Cell-Derived Axons Projecting Caudally after Spinal Cord Injury. Stem Cell Rep. 2019, 13, 105–114. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Friedli, L.; Rosenzweig, E.S.; Barraud, Q.; Schubert, M.; Dominici, N.; Awai, L.; Nielson, J.L.; Musienko, P.; Nout-Lomas, Y.; Zhong, H.; et al. Pronounced Species Divergence in Corticospinal Tract Reorganization and Functional Recovery after Lateralized Spinal Cord Injury Favors Primates. Sci. Transl. Med. 2015, 7, 302ra134. [Google Scholar] [CrossRef] [PubMed]
- Kamiyama, T.; Kameda, H.; Murabe, N.; Fukuda, S.; Yoshioka, N.; Mizukami, H.; Ozawa, K.; Sakurai, M. Corticospinal Tract Development and Spinal Cord Innervation Differ between Cervical and Lumbar Targets. J. Neurosci. 2015, 35, 1181–1191. [Google Scholar] [CrossRef]
- Gu, Z.; Matsuura, K.; Letelier, A.; Basista, M.; Craig, C.; Imai, F.; Yoshida, Y. Axon Fasciculation, Mediated by Transmembrane Semaphorins, Is Critical for the Establishment of Segmental Specificity of Corticospinal Circuits. J. Neurosci. 2023, 43, 5753–5768. [Google Scholar] [CrossRef]
- Tardieu, M.; Zérah, M.; Gougeon, M.-L.; Ausseil, J.; de Bournonville, S.; Husson, B.; Zafeiriou, D.; Parenti, G.; Bourget, P.; Poirier, B.; et al. Intracerebral Gene Therapy in Children with Mucopolysaccharidosis Type IIIB Syndrome: An Uncontrolled Phase 1/2 Clinical Trial. Lancet Neurol. 2017, 16, 712–720. [Google Scholar] [CrossRef]
- Bolon, B.; Buza, E.; Galbreath, E.; Wicks, J.; Cargnin, F.; Hordeaux, J. Neuropathological Findings in Nonclinical Species Following Administration of Adeno-Associated Virus (AAV)-Based Gene Therapy Vectors. Toxicol. Pathol. 2024, 52, 489–505. [Google Scholar] [CrossRef] [PubMed]
- Paterna, J.-C.; Feldon, J.; Büeler, H. Transduction Profiles of Recombinant Adeno-Associated Virus Vectors Derived from Serotypes 2 and 5 in the Nigrostriatal System of Rats. J. Virol. 2004, 78, 6808–6817. [Google Scholar] [CrossRef]
- Boulis, N.M.; Willmarth, N.E.; Song, D.K.; Feldman, E.L.; Imperiale, M.J. Intraneural Colchicine Inhibition of Adenoviral and Adeno-Associated Viral Vector Remote Spinal Cord Gene Delivery. Neurosurgery 2003, 52, 381–387; discussion 387. [Google Scholar] [CrossRef]
- Kudo, M.; Wupuer, S.; Fujiwara, M.; Saito, Y.; Kubota, S.; Inoue, K.-I.; Takada, M.; Seki, K. Specific Gene Expression in Unmyelinated Dorsal Root Ganglion Neurons in Nonhuman Primates by Intra-Nerve Injection of AAV 6 Vector. Mol. Ther. Methods Clin. Dev. 2021, 23, 11–22. [Google Scholar] [CrossRef]
- Hollis, E.R.; Jamshidi, P.; Lorenzana, A.O.; Lee, J.K.; Gray, S.J.; Samulski, R.J.; Zheng, B.; Tuszynski, M.H. Transient Demyelination Increases the Efficiency of Retrograde AAV Transduction. Mol. Ther. 2010, 18, 1496–1500. [Google Scholar] [CrossRef] [PubMed]
- McCaman, R.E.; McKenna, D.G.; Ono, J.K. A Pressure System for Intracellular and Extracellular Ejections of Picoliter Volumes. Brain Res. 1977, 136, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Bobo, R.H.; Laske, D.W.; Akbasak, A.; Morrison, P.F.; Dedrick, R.L.; Oldfield, E.H. Convection-Enhanced Delivery of Macromolecules in the Brain. Proc. Natl. Acad. Sci. USA 1994, 91, 2076–2080. [Google Scholar] [CrossRef]
- Cunningham, J.; Oiwa, Y.; Nagy, D.; Podsakoff, G.; Colosi, P.; Bankiewicz, K.S. Distribution of AAV-TK Following Intracranial Convection-Enhanced Delivery into Rats. Cell Transplant. 2000, 9, 585–594. [Google Scholar] [CrossRef]
- Sanftner, L.M.; Sommer, J.M.; Suzuki, B.M.; Smith, P.H.; Vijay, S.; Vargas, J.A.; Forsayeth, J.R.; Cunningham, J.; Bankiewicz, K.S.; Kao, H.; et al. AAV2-Mediated Gene Delivery to Monkey Putamen: Evaluation of an Infusion Device and Delivery Parameters. Exp. Neurol. 2005, 194, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Kügler, S.; Kilic, E.; Bähr, M. Human Synapsin 1 Gene Promoter Confers Highly Neuron-Specific Long-Term Transgene Expression from an Adenoviral Vector in the Adult Rat Brain Depending on the Transduced Area. Gene Ther. 2003, 10, 337–347. [Google Scholar] [CrossRef]
- Tedeschi, A.; Bradke, F. Spatial and Temporal Arrangement of Neuronal Intrinsic and Extrinsic Mechanisms Controlling Axon Regeneration. Curr. Opin. Neurobiol. 2017, 42, 118–127. [Google Scholar] [CrossRef]
- de Freria, C.M.; Van Niekerk, E.; Blesch, A.; Lu, P. Neural Stem Cells: Promoting Axonal Regeneration and Spinal Cord Connectivity. Cells 2021, 10, 3296. [Google Scholar] [CrossRef]
- Kadoya, K.; Lu, P.; Nguyen, K.; Lee-Kubli, C.; Kumamaru, H.; Yao, L.; Knackert, J.; Poplawski, G.; Dulin, J.; Strobl, H.; et al. Spinal Cord Reconstitution with Homologous Neural Grafts Enables Robust Corticospinal Regeneration. Nat. Med. 2016, 22, 479–487. [Google Scholar] [CrossRef]
- Koffler, J.; Zhu, W.; Qu, X.; Platoshyn, O.; Dulin, J.N.; Brock, J.; Graham, L.; Lu, P.; Sakamoto, J.; Marsala, M.; et al. Biomimetic 3D-Printed Scaffolds for Spinal Cord Injury Repair. Nat. Med. 2019, 25, 263–269. [Google Scholar] [CrossRef]
- Dum, R.P.; Strick, P.L. The Origin of Corticospinal Projections from the Premotor Areas in the Frontal Lobe. J. Neurosci. 1991, 11, 667–689. [Google Scholar] [CrossRef]
- Galea, M.P.; Darian-Smith, I. Multiple Corticospinal Neuron Populations in the Macaque Monkey Are Specified by Their Unique Cortical Origins, Spinal Terminations, and Connections. Cereb. Cortex 1994, 4, 166–194. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, E.S.; Brock, J.H.; Culbertson, M.D.; Lu, P.; Moseanko, R.; Edgerton, V.R.; Havton, L.A.; Tuszynski, M.H. Extensive Spinal Decussation and Bilateral Termination of Cervical Corticospinal Projections in Rhesus Monkeys. J. Comp. Neurol. 2009, 513, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.N.; Kumari, R.; Bailey, W.M.; Glaser, E.P.; Bosse-Joseph, C.C.; Park, K.A.; Hammers, G.V.; Wireman, O.H.; Gensel, J.C. PTEN Knockout Using Retrogradely Transported AAVs Transiently Restores Locomotor Abilities in Both Acute and Chronic Spinal Cord Injury. Exp. Neurol. 2023, 368, 114502. [Google Scholar] [CrossRef] [PubMed]
- Monteys, A.M.; Hundley, A.A.; Ranum, P.T.; Tecedor, L.; Muehlmatt, A.; Lim, E.; Lukashev, D.; Sivasankaran, R.; Davidson, B.L. Regulated Control of Gene Therapies by Drug-Induced Splicing. Nature 2021, 596, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.E. A View from the Ending: Axonal Dieback and Regeneration Following SCI. Neurosci. Lett. 2017, 652, 11–24. [Google Scholar] [CrossRef]
- Du, K.; Zheng, S.; Zhang, Q.; Li, S.; Gao, X.; Wang, J.; Jiang, L.; Liu, K. Pten Deletion Promotes Regrowth of Corticospinal Tract Axons 1 Year after Spinal Cord Injury. J. Neurosci. 2015, 35, 9754–9763. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakashima, K.T.; Wang, S.; Castle, M.J. AAV2retro Enters Axons of Passage and Extensively Transduces Corticospinal Neurons After Injection into Spinal White Matter. Brain Sci. 2025, 15, 1058. https://doi.org/10.3390/brainsci15101058
Nakashima KT, Wang S, Castle MJ. AAV2retro Enters Axons of Passage and Extensively Transduces Corticospinal Neurons After Injection into Spinal White Matter. Brain Sciences. 2025; 15(10):1058. https://doi.org/10.3390/brainsci15101058
Chicago/Turabian StyleNakashima, Kazuki T., Shanshan Wang, and Michael J. Castle. 2025. "AAV2retro Enters Axons of Passage and Extensively Transduces Corticospinal Neurons After Injection into Spinal White Matter" Brain Sciences 15, no. 10: 1058. https://doi.org/10.3390/brainsci15101058
APA StyleNakashima, K. T., Wang, S., & Castle, M. J. (2025). AAV2retro Enters Axons of Passage and Extensively Transduces Corticospinal Neurons After Injection into Spinal White Matter. Brain Sciences, 15(10), 1058. https://doi.org/10.3390/brainsci15101058





