1. Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental condition characterized by persistent deficits in social communication and interaction, as well as restricted, repetitive patterns of behavior, interests, or activities. These symptoms typically emerge in early childhood and persist across the lifespan, though their manifestations may vary significantly over time and across individuals [
1,
2,
3]. Adolescence, in particular, represents a period of rapid and often volatile neurobiological and psychosocial transformation, during which the developmental trajectory of individuals with ASD may diverge markedly from that of typically developing peers [
4,
5].
This divergence is compounded by the profound heterogeneity observed in both the neurobiological underpinnings and behavioral expressions of ASD. Neuroimaging studies in adolescents have revealed wide-ranging alterations in cortical thickness, white matter connectivity, and functional network dynamics that are not uniform across individuals with ASD [
6,
7,
8]. Concurrently, behavioral profiles can vary from significant social withdrawal and sensory sensitivities to impulsivity, rigid thinking, and co-occurring conditions such as anxiety, ADHD, or mood disorders [
9,
10,
11]. These complexities create substantial challenges for clinicians, educators, and families attempting to support adolescents with ASD during this critical developmental window.
While the literature has extensively addressed the core features of ASD and its early developmental presentation, there is comparatively less integration of findings specific to adolescence, especially with regard to the interplay between neurobiological changes and behavioral heterogeneity. The adolescent brain is undergoing synaptic pruning, myelination, and hormonal shifts that influence cognitive control, emotion regulation, and social motivation—domains that are often atypical in individuals with ASD [
12,
13,
14]. As a result, adolescence may act as a tipping point where some individuals experience exacerbation of symptoms while others show compensatory adaptations or emerging strengths [
15].
Given this background, the aim of the present paper is to provide a structured, integrative overview of the neurobiological and behavioral heterogeneity observed in adolescents with ASD, with particular emphasis on how developmental changes during adolescence intersect with the core and associated features of autism. Rather than offering an exhaustive review, we focus on a critical synthesis of current findings from neuroimaging, behavioral, and psychosocial domains to highlight key patterns, inconsistencies, and implications for intervention.
Our guiding question is as follows: How do neurodevelopmental changes during adolescence contribute to the variability in behavioral and cognitive outcomes observed in individuals with ASD? By addressing this question, we aim to clarify how adolescence both magnifies and reframes heterogeneity in ASD, and why a one-size-fits-all approach to diagnosis, support, or therapy is especially problematic during this life stage.
We further argue that understanding adolescent-specific trajectories is crucial not only for improving clinical care but also for informing models of neurodevelopmental plasticity and resilience. In the sections that follow, we examine evidence for structural and functional brain variability (
Section 2), behavioral heterogeneity (
Section 3), contextual influences including environmental and educational factors (
Section 4), and implications for intervention and support (
Section 5). We conclude by proposing future research directions that can better capture the dynamic and multifaceted nature of adolescence in ASD.
2. Neurobiological Heterogeneity in Adolescents with ASD
The neurobiological heterogeneity observed in adolescents with ASD reflects a complex interplay between genuine individual variability and methodological inconsistency across studies. Adolescence is a critical period of brain development marked by rapid changes in cortical thickness, white matter organization, and functional network architecture. Consequently, studies attempting to characterize neural differences in adolescents with ASD are especially vulnerable to confounding effects introduced by sample composition, imaging technique, preprocessing choices, and analytic design. The following section critically evaluates the main categories of neuroimaging evidence—structural, white matter, and functional—highlighting where findings converge, where they diverge, and how methodological heterogeneity contributes to inconsistent outcomes.
2.1. Structural Brain Findings
Neuroanatomical studies examining gray matter volume and cortical thickness in adolescents with ASD have yielded mixed and often contradictory findings. Some report increased cortical thickness in medial prefrontal regions and decreased volume in temporal poles compared to typically developing peers [
12], while others observe no significant volumetric differences [
13]. A major source of variability stems from the reliance of many early studies on region-of-interest (ROI) analyses using small, homogenous samples—typically high-functioning, male adolescents. These ROI-based studies are susceptible to confirmation bias, as they focus narrowly on regions hypothesized a priori to be implicated in ASD. In contrast, whole-brain voxel-based morphometry (VBM) approaches offer broader spatial coverage but introduce their own variability due to differences in segmentation algorithms, smoothing kernels, and thresholding strategies [
14].
Moreover, the overwhelming majority of these studies are cross-sectional in design, limiting their ability to distinguish delayed maturation from deviant developmental trajectories. Longitudinal imaging, which would be more informative for tracking the evolving neuroanatomical phenotype of ASD, remains scarce in this age group. Compounding these limitations is the frequent omission or inconsistent control of key covariates such as IQ, co-occurring psychiatric conditions, and medication use. Together, these methodological and sample-related inconsistencies severely limit the comparability of structural findings across studies and may partially account for their divergent results.
2.2. White Matter Microstructure
Diffusion tensor imaging (DTI) studies investigating white matter integrity in adolescents with ASD have also reported highly variable findings, including both decreased and increased fractional anisotropy (FA) in major association tracts such as the corpus callosum, inferior longitudinal fasciculus, and uncinate fasciculus [
15,
16,
17]. These discrepancies are not easily explained by underlying biological differences alone. Rather, they are likely influenced by differences in tractography methods—some studies employ tract-based spatial statistics (TBSS), while others use deterministic or probabilistic tractography. These techniques differ substantially in their sensitivity to crossing fibers, partial volume effects, and noise, affecting both the location and magnitude of reported group differences.
Another major confound in DTI studies is head motion, which is more prevalent in individuals with ASD and can systematically bias diffusion metrics if not rigorously controlled. While some studies report motion parameters and exclude participants with excessive movement, many do not, and fewer still apply advanced correction techniques such as outlier replacement or motion-scrubbing. This omission is particularly problematic given the known susceptibility of FA to motion-induced artifacts, especially in frontal and temporal tracts.
Sample size is also a critical concern. Many DTI studies in this population include fewer than 30 participants per group, resulting in low statistical power and inflated risk of false positives. Furthermore, the ASD population itself is extremely heterogeneous—encompassing a wide range of verbal ability, cognitive function, and adaptive skills—yet most studies do not stratify participants or account for this internal variability. As a result, the literature on white matter integrity in adolescent ASD remains fragmented and difficult to synthesize into a coherent narrative.
2.3. Functional Connectivity and Network Dynamics
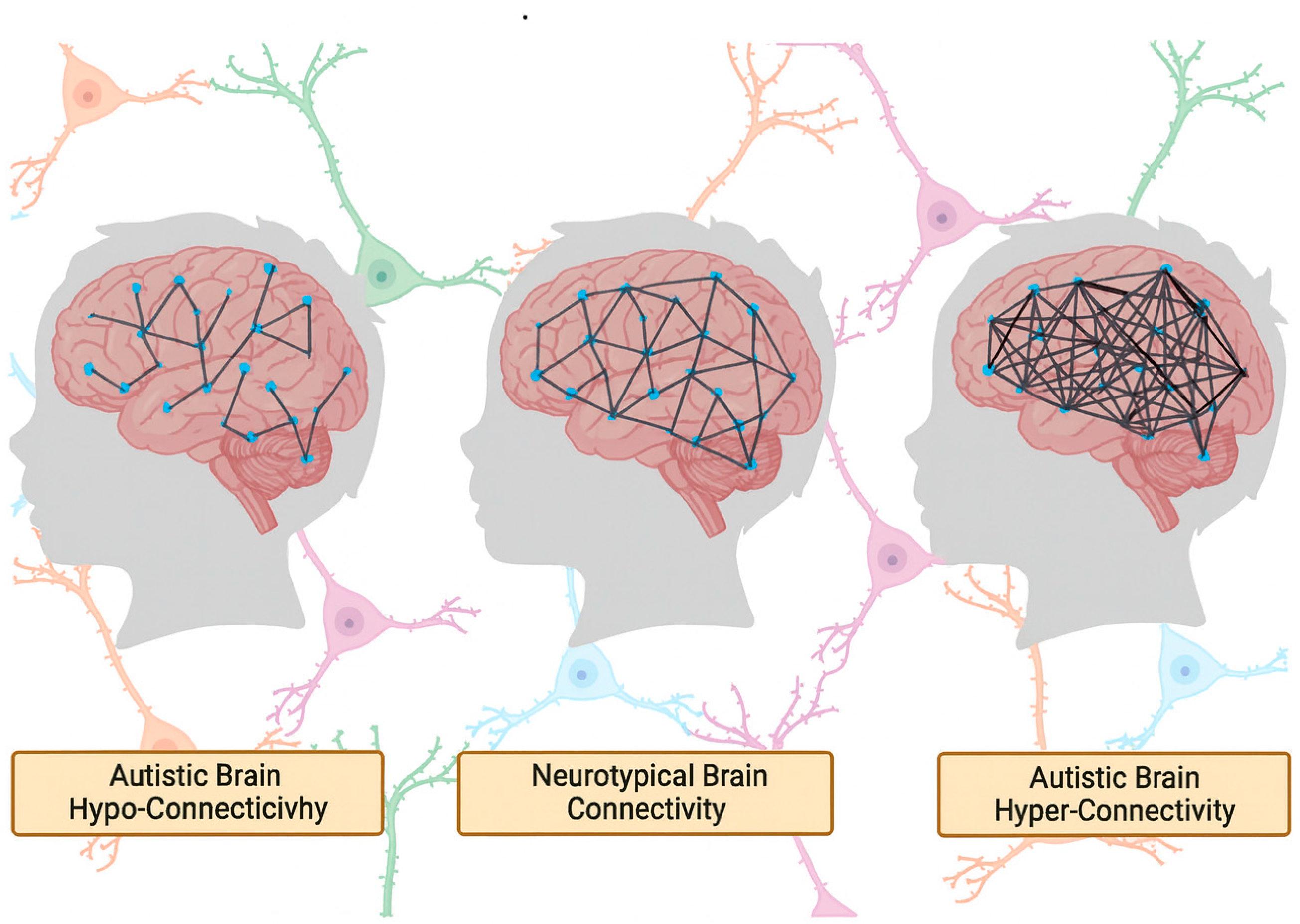
Resting-state functional MRI (rs-fMRI) has been widely used to investigate large-scale brain network organization in ASD, yet findings remain inconsistent. Some studies report long-range hypoconnectivity, especially between nodes of the default mode network (DMN) such as the medial prefrontal cortex and posterior cingulate cortex [
18], while others describe hyperconnectivity within salience or sensorimotor networks [
19,
20]. A key source of this inconsistency is the variability in preprocessing pipelines, particularly with regard to global signal regression (GSR). While GSR is intended to reduce physiological noise, its application can introduce spurious anti-correlations or invert the direction of observed group differences, leading to contradictory interpretations of the same underlying data [
21].
Additional variability arises from differences in scan duration (ranging from 5 to 10 min), participant instructions (eyes open vs. closed), motion scrubbing thresholds, and spatial normalization templates. These choices, often left underreported, directly affect the reliability and replicability of functional connectivity measures. Moreover, many studies do not adequately account for individual differences in alertness, compliance, or cognitive engagement during scanning—factors that are especially variable in adolescents with ASD.
More recently, studies have employed dynamic functional connectivity (dFC) approaches to assess time-varying fluctuations in connectivity patterns. While this approach offers greater ecological validity, its implementation is highly inconsistent across studies. Sliding window lengths, clustering algorithms, and the number of identified connectivity states vary widely, making it difficult to determine whether reported abnormalities reflect true neural dynamics or methodological artifacts [
22].
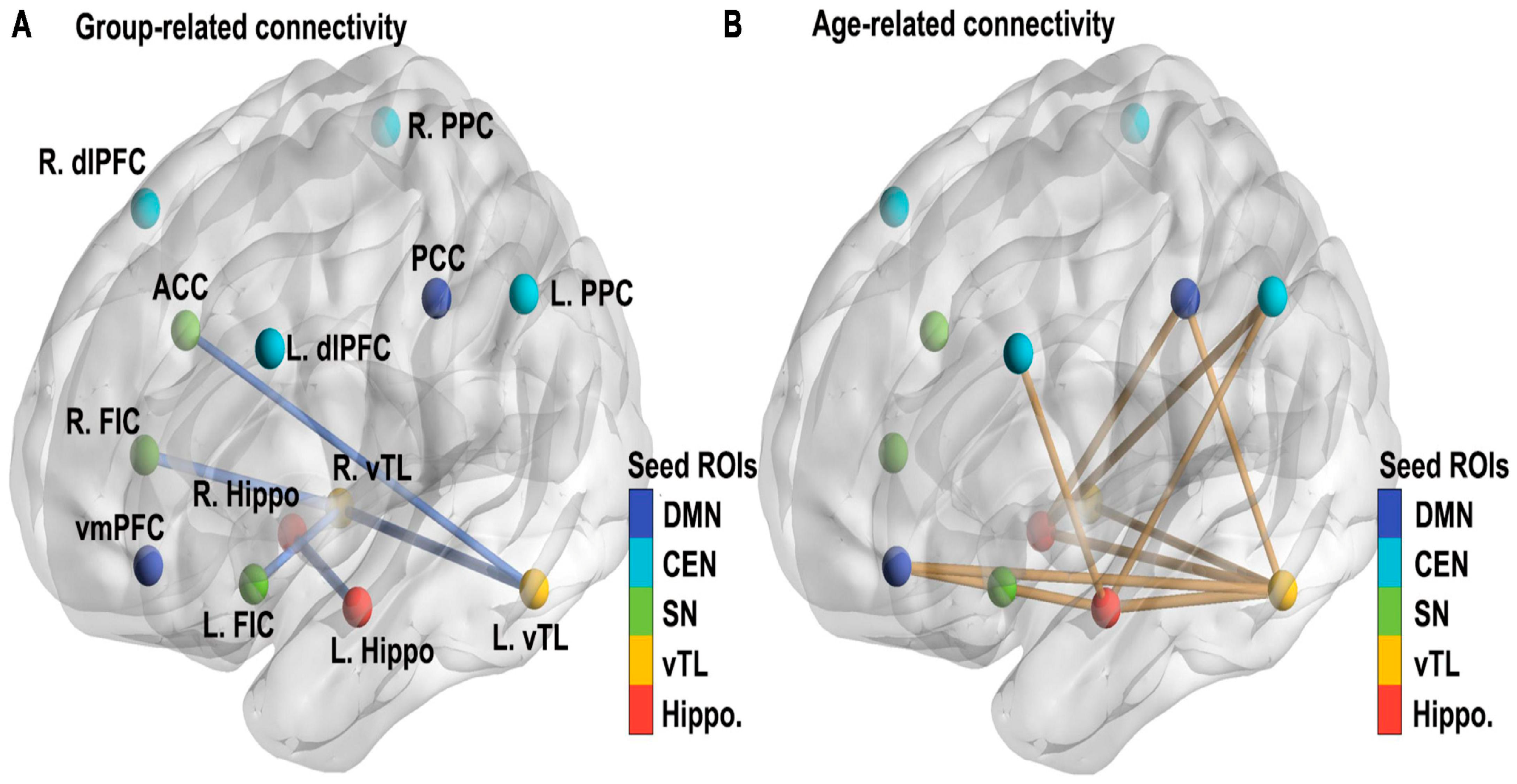
Furthermore, functional connectivity studies rarely stratify or statistically control for common ASD comorbidities such as ADHD or anxiety, despite clear evidence that these conditions independently alter functional network organization. Medication use—particularly of stimulants, SSRIs, or antipsychotics—is also frequently unreported or inconsistently excluded. These omissions further confound group comparisons and raise questions about the specificity of observed connectivity differences to ASD itself. “These connectivity patterns—characterized by relative underactivation in frontal control regions and overactivation in sensory-perceptual areas—are illustrated in
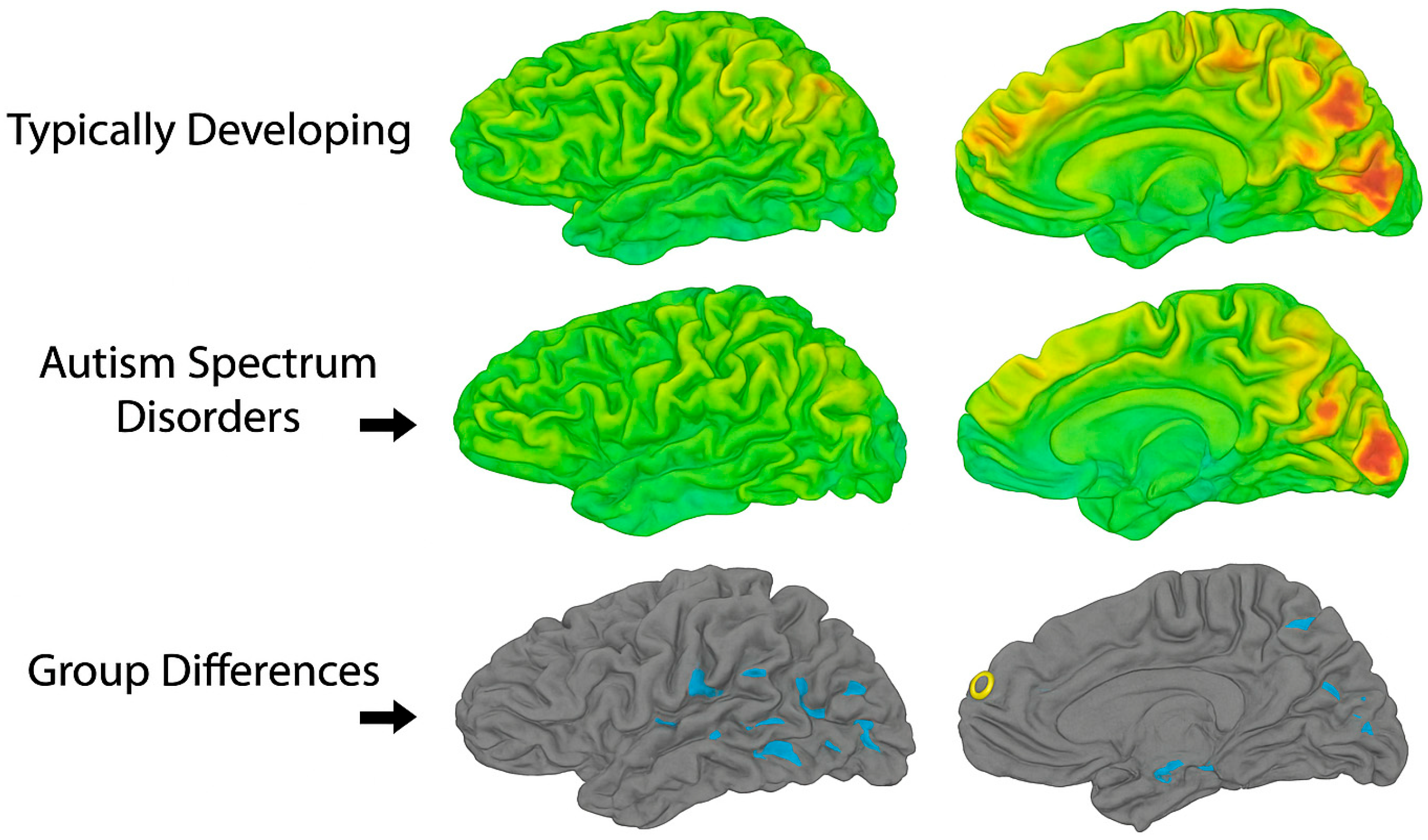
Figure 1, which visually contrasts functional brain activity in adolescents with ASD versus neurotypical controls.”
The yellow areas demonstrate underactivity in prefrontal cortical regions, related to planning, complex cognition, decision-making, and adaptive behavior. The blue occipital and temporal regions are typically overactive in ASD, and at the top center, marked in blue, is a region that is likewise overactive and responsible for sensory processing.
2.4. Developmental Considerations and Methodological Standardization
A persistent limitation across neuroimaging studies in adolescent ASD is the tendency to pool participants into broad age categories, often spanning from late childhood (e.g., 10 years) through young adulthood (e.g., 19 years). This approach masks critical neurodevelopmental inflection points that occur during mid-adolescence, including synaptic pruning, myelination, and hormonal changes that influence social and cognitive processing. Given the neurobiological dynamism of this period, age-stratified analyses are essential to disentangle developmental effects from ASD-specific alterations.
To address the methodological challenges identified throughout this section, future research should prioritize the use of large, multisite datasets such as the Autism Brain Imaging Data Exchange (ABIDE) and ENIGMA-ASD, which facilitate replication and allow for the application of harmonized preprocessing pipelines. These collaborative efforts also support the use of machine learning and data-driven clustering techniques to identify neurobiological subtypes within ASD. However, such approaches will only be effective if grounded in robust data acquisition practices, transparent reporting standards, and careful phenotypic characterization. Standardization of scan protocols, motion correction strategies, and inclusion criteria—combined with stratification by age, IQ, comorbidities, and medication status—will be critical for advancing the field toward more interpretable and clinically relevant findings. These challenges are conceptually summarized in
Figure 1, which illustrates how neuroimaging findings in adolescent ASD are shaped by both biological and methodological sources of heterogeneity.
4. Default Mode Network and Cognitive Function in Adolescents with ASD
4.1. Atypical DMN Connectivity in ASD
The DMN, a prominent large-scale brain network encompassing the medial prefrontal cortex, posterior cingulate cortex/precuneus, and angular gyrus, is implicated in self-referential thought, social cognition, and internally directed processes. Aberrations in DMN connectivity have been repeatedly reported in ASD populations, with findings pointing to both hypo- and hyper-connectivity patterns across different developmental stages and clinical subgroups.
Padmanabhan et al. [
24] employed resting-state fMRI to assess DMN function in children, adolescents, and adults with ASD. Their results indicated reduced long-range DMN connectivity in adolescents with ASD compared to age-matched controls. However, this study utilized a relatively small sample (N = 19 ASD adolescents), limiting generalizability. Additionally, the participants were not stratified by cognitive ability or symptom profile, obscuring whether the observed DMN disruptions were linked to specific subtypes or broader ASD characteristics. While their longitudinal design is commendable, more robust sample stratification and inclusion of behavioral correlates would improve interpretability. Furthermore, the use of seed-based correlation methods may have constrained the capacity to detect broader network-level disruptions or compensatory adaptations in adolescents with more intact cognitive functioning.
Lawrence et al. [
23], using a larger and developmentally focused cohort, found that adolescents with ASD exhibit atypical developmental trajectories in DMN functional connectivity, particularly within the medial prefrontal and posterior cingulate cortices. The divergence from typical age-related connectivity maturation underscores the importance of studying ASD within a neurodevelopmental framework. Yet, this study’s reliance on cross-sectional rather than longitudinal data weakens the strength of inferences about within-individual change. Importantly, the authors did not control for the impact of psychiatric comorbidities (e.g., anxiety, ADHD), which are known to affect DMN function and are prevalent in adolescents with ASD, raising questions about the specificity of their findings to ASD per se.
In contrast, Nair et al. [
17] provided a comparative review of DMN connectivity in adolescents with ASD and early-onset psychosis, revealing that both disorders exhibit disruptions in DMN integrity but with distinct topographical patterns. Their review critically synthesizes findings across multiple studies, though many of the included works employed varied preprocessing pipelines, ROI definitions, and head motion correction standards. These methodological inconsistencies make it difficult to determine whether reported DMN abnormalities reflect genuine disorder-specific alterations or analytic artifacts. Moreover, their review does not sufficiently address how developmental stage (early vs. mid-to-late adolescence) might moderate the nature or extent of DMN dysfunction.
These studies collectively highlight DMN abnormalities in adolescents with ASD, but methodological variability—including differences in preprocessing standards, parcellation schemes, and connectivity metrics—substantially limits cross-study comparability. Future investigations should adopt harmonized acquisition and analysis protocols, larger and phenotypically stratified samples, and developmental designs to discern which DMN alterations are truly diagnostic of ASD versus those reflecting developmental delay, comorbidity, or task-specific factors.
4.2. DMN and Social-Cognitive Impairments
Given the role of the DMN in social cognition, it is not surprising that aberrations in this network are frequently linked to social deficits in adolescents with ASD. Functional connectivity disruptions in the medial prefrontal cortex (mPFC) and posterior cingulate cortex (PCC) have been associated with reduced perspective-taking, impaired theory of mind, and diminished self-other differentiation.
Burrows et al. [
29] reported that adolescents with ASD show decreased functional connectivity between the mPFC and temporoparietal junction (TPJ), two regions crucial for mentalizing. However, their findings were based on a limited cohort (N = 13 adolescents with ASD), and the study lacked direct behavioral correlations, rendering the clinical relevance of the connectivity alterations ambiguous. Additionally, the exclusive use of resting-state paradigms fails to capture how DMN nodes engage during active social tasks. A combined resting-state and task-based fMRI approach would provide a more nuanced understanding of how DMN dysfunction impacts social cognitive processes in this population.
Padmanabhan et al. [
24], in the same cohort discussed earlier, also observed reduced coherence within the mPFC subnetwork during adolescence. Interestingly, in adults with ASD, some degree of normalization was seen, suggesting that DMN function may shift across developmental stages. However, their developmental interpretation is limited by the cross-sectional nature of the data, making it impossible to track within-subject neural trajectories. This introduces potential cohort effects and raises questions about whether observed “normalization” in adulthood reflects true recovery, compensatory reorganization, or sample bias (e.g., exclusion of more severely affected individuals due to compliance issues in scanning).
Moreover, studies like those by Dajani and Uddin [
56] emphasize the need to conceptualize DMN function within a broader framework of network flexibility and integration. They argue that DMN abnormalities in ASD may not be isolated but part of a broader pattern of impaired switching between task-positive and task-negative networks, implicating systems such as the salience network and executive control network. Yet, the empirical grounding of this hypothesis remains limited, particularly in adolescents. Future work should integrate multi-network analyses and examine how atypical DMN dynamics relate to real-world social functioning over time.
4.3. Restricted and Repetitive Behaviors (RRBs)
RRBs are among the core diagnostic features of ASD and are highly heterogeneous in their manifestation during adolescence. These behaviors range from simple motor stereotypies to complex cognitive rituals and routines. While some individuals may exhibit persistent hand-flapping or rocking, others may demonstrate intense, narrow interests or rigid adherence to routines. The severity and type of RRBs can change over time, particularly during adolescence, influenced by both neurodevelopmental changes and social contexts [
1,
7,
16].
Neuroimaging studies have suggested that RRBs may be linked to aberrant connectivity within cortico-striatal-thalamo-cortical circuits, including the basal ganglia and orbitofrontal cortex [
4,
9,
10]. However, critical examination reveals methodological inconsistencies across studies. For example, differences in how RRBs are defined, measured, and categorized pose significant challenges. Some studies rely on parent-reported instruments (e.g., the Repetitive Behavior Scale-Revised), while others use clinician-administered tools or direct behavioral observations. These discrepancies contribute to variations in reported prevalence and neurobiological correlates of RRBs.
Age is another crucial moderating factor. The work of Uddin et al. [
10] highlights that during adolescence, dynamic changes in brain plasticity may exacerbate or attenuate RRBs depending on contextual stimuli and environmental demands. However, findings remain mixed, with some longitudinal data indicating a reduction in lower-order repetitive behaviors (e.g., motor stereotypies) during adolescence, while higher-order behaviors (e.g., insistence on sameness, intense interests) may persist or even intensify [
7,
57].
Sample characteristics further complicate the landscape. Many studies examining RRBs in adolescents with ASD focus on high-functioning individuals, potentially neglecting more severe presentations. This bias may distort the generalizability of findings and reinforce stereotypes about RRBs being uniformly rigid or maladaptive. For example, some restricted interests may serve positive adaptive or coping functions in certain adolescents and thus warrant nuanced interpretation rather than pathologization.
In summary, while the neural correlates of RRBs are increasingly studied, the field suffers from inconsistent definitions, sampling bias, and lack of developmental specificity. A more rigorous stratification of subtypes, longitudinal designs, and harmonization of measurement tools are needed to disentangle the underlying neurobiology and developmental trajectory of RRBs in adolescence.
4.4. Sensory Processing Differences
Sensory processing atypicalities are frequently reported in adolescents with ASD and span hyperreactivity, hyporeactivity, and sensory-seeking behaviors across visual, auditory, tactile, olfactory, and proprioceptive modalities [
48]. These differences are not merely peripheral phenomena but are central to the lived experience of many adolescents with ASD and often influence social, academic, and behavioral functioning [
48,
57].
Neuroimaging studies implicate atypical activity and connectivity in primary sensory cortices, the insula, and thalamic regions in mediating sensory processing differences [
40,
58]. For instance, thalamocortical dysconnectivity has been proposed as a neural signature underlying sensory filtering difficulties, with evidence of reduced integration between the thalamus and sensory association cortices [
58]. Functional MRI studies also reveal abnormal activation in the insula and anterior cingulate cortex, suggesting disruptions in the salience network, which modulates attention to internal and external sensory stimuli [
24].
However, a critical examination of the literature reveals notable methodological limitations. Many studies fail to differentiate between types of sensory symptoms (e.g., hyper- vs. hypo-responsiveness), instead aggregating them into composite scores that may obscure distinct neural correlates. Furthermore, there is wide variation in the instruments used to assess sensory reactivity, from parent- or self-report questionnaires like the Sensory Profile to clinician-based observational tools, leading to significant measurement heterogeneity. This inconsistency hampers cross-study comparisons and contributes to variable findings.
Another challenge relates to sample heterogeneity. Much of the literature focuses on either younger children or mixed-age samples, with fewer studies isolating adolescents as a distinct developmental group. Given that adolescence is marked by rapid neurobiological changes, including myelination and synaptic pruning in sensory and integrative brain areas, the developmental trajectory of sensory processing differences may be particularly volatile during this period [
19,
44]. Yet, only a handful of longitudinal studies track sensory symptoms from childhood through adolescence, leaving questions about their developmental stability unresolved.
Sensory sensitivities in adolescence may compound difficulties in social interaction, school participation, and emotion regulation. For example, auditory hypersensitivity may lead to social withdrawal or avoidance of crowded environments, thereby exacerbating social skill deficits [
57]. However, relatively few studies explore these downstream functional consequences in depth. Moreover, the relationship between sensory symptoms and co-occurring anxiety or executive function difficulties remains underexplored, despite clinical evidence suggesting strong bidirectional influences.
To advance the field, future studies must adopt stratified methodologies that distinguish subtypes of sensory reactivity and link them to specific neural networks using well-powered, multimodal neuroimaging. In parallel, longitudinal designs are needed to assess the evolution of sensory symptoms and their predictive value for broader adaptive functioning.
4.5. Sleep Disturbances
Sleep disturbances are highly prevalent among adolescents with ASD, with estimates suggesting that between 40% and 80% of individuals experience some form of sleep dysregulation [
57]. These disturbances include difficulty initiating and maintaining sleep, reduced total sleep time, night awakenings, and abnormal circadian rhythms. While sleep issues are often framed as a secondary concern, mounting evidence suggests they may exacerbate core ASD symptoms and significantly impact daytime functioning.
Functional and structural neuroimaging studies implicate several brain regions in sleep regulation abnormalities in ASD. These include the brainstem, hypothalamus, and pineal gland, with additional modulation by the thalamus and frontal cortex [
40,
57]. Melatonin dysregulation, often due to abnormalities in serotonin pathways, has been consistently observed, suggesting a neurochemical basis for delayed sleep onset and poor sleep maintenance.
Despite these findings, few studies have specifically focused on adolescents as a distinct developmental group. Most research either collapses across wide age ranges or includes only children, limiting the ability to disentangle age-specific neurobiological contributors. This omission is particularly problematic given that adolescence is a period of normative shifts in sleep architecture and circadian phase delay, which may interact with ASD-related neural atypicalities to compound difficulties [
19].
Methodologically, studies investigating sleep in ASD vary considerably in terms of assessment tools. Objective measures like polysomnography and actigraphy are used inconsistently, and many studies rely solely on parent or self-report questionnaires, which are prone to bias and may not accurately capture sleep architecture. Sample sizes are often small and heterogeneous, and the inclusion of adolescents with comorbid psychiatric conditions (e.g., anxiety, ADHD) further muddies interpretation of findings. These factors together hinder the replicability and generalizability of results.
Furthermore, while several studies establish correlations between sleep quality and ASD symptom severity, few explore causality or directionality. For example, while disrupted sleep may exacerbate social-communication difficulties, it is also possible that anxiety related to social performance contributes to sleep onset insomnia—a bidirectional relationship that remains poorly understood.
Sleep disturbances in adolescents with ASD have been associated with impairments in attention, executive functioning, mood regulation, and adaptive behavior. Poor sleep may also amplify sensory sensitivities and contribute to irritability and aggression, underscoring its broad impact [
57]. However, there is a dearth of intervention studies targeting sleep in adolescents specifically. Most behavioral sleep interventions have been developed for younger children, and pharmacological treatments such as melatonin are prescribed off-label with limited long-term efficacy data.
There is a pressing need for longitudinal and intervention-based studies that can clarify the mechanisms linking sleep and ASD features across adolescence. Given the heterogeneity of sleep profiles in ASD, stratified approaches—possibly guided by circadian biomarkers or neuroimaging—could help identify subgroups who may benefit from tailored interventions.
4.6. Emotion Regulation and Comorbidities
Emotion regulation (ER), the capacity to modulate emotional responses in accordance with contextual demands, is a key domain in which many adolescents with ASD experience profound challenges. Deficits in ER are not only intrinsic to ASD but also central to the development and maintenance of comorbid psychiatric conditions, most notably anxiety and depression. These challenges often manifest behaviorally as affective lability, heightened irritability, meltdowns, or social withdrawal, with significant implications for adaptive functioning and quality of life [
55].
Neuroimaging studies have implicated altered connectivity within limbic circuits, particularly between the amygdala and prefrontal cortex, in emotion dysregulation among individuals with ASD [
50,
51,
55]. The amygdala, responsible for emotion detection and salience processing, often shows hypo- or hyper-reactivity depending on the context, while the medial mPFC demonstrates inconsistent patterns of engagement across studies. This disrupted amygdala–PFC circuitry is thought to underlie deficits in emotional insight, empathy, and regulation.
However, studies vary considerably in sample size, age ranges, and methodology. For instance, Guo et al. [
50] reported decreased amygdala functional connectivity in adolescents using resting-state fMRI, but their relatively small sample (n = 23 ASD; n = 21 controls) and the lack of task-based assessments limit the interpretability of findings. Other investigations, such as Braden et al. [
55] employed both structural and functional imaging in middle-aged adults, raising concerns about age-related generalizability to adolescence. These discrepancies underscore the need for adolescent-specific, multimodal research that integrates structure, function, and behavior.
Anxiety disorders are among the most common comorbid conditions in adolescents with ASD, with prevalence estimates as high as 40% to 50% [
54]. Anxiety often exacerbates social avoidance, leads to compulsive behaviors, and is strongly linked to intolerance of uncertainty—a trait observed across multiple ASD subgroups. Depression, although less frequently diagnosed in younger individuals with ASD, becomes increasingly prominent during adolescence and is associated with higher rates of suicidal ideation.
Studies examining these comorbidities often suffer from selection biases. Participants are typically high-functioning and verbal, potentially skewing estimates of prevalence and neural correlates. Additionally, many diagnostic instruments are not validated for ASD populations, especially when assessing internalizing symptoms, which may manifest atypically (e.g., as somatic complaints or increased repetitive behaviors).
Across studies on ER and comorbidity, heterogeneity in task paradigms and analysis pipelines complicates comparison. Emotion-processing tasks range from facial affect recognition to implicit regulation paradigms, with varying degrees of ecological validity. Furthermore, comorbid psychiatric conditions are often treated as exclusion criteria, eliminating individuals who may best represent the “real-world” complexity of ASD presentations. Few studies account for the potential confounding effects of medication use, pubertal status, or sleep disturbance, factors known to affect emotional processing. As such, claims regarding the universality or specificity of neural abnormalities in ER among adolescents with ASD must be viewed cautiously.
Future research should prioritize stratification based on ER profiles using dimensional approaches rather than categorical diagnoses. Neurobiologically informed subtyping, possibly through machine learning applied to neuroimaging data, could improve the predictive utility of findings and guide intervention. Furthermore, research should explicitly investigate how ER difficulties interact with other ASD features, such as sensory processing or executive dysfunction, to produce distinct behavioral phenotypes.
Interventions targeting ER, such as mindfulness-based programs, cognitive behavioral therapy, or biofeedback, have shown promise but remain understudied in adolescents with ASD. Incorporating insights from neurobiological studies into these interventions could enhance their specificity and efficacy. For example, neurofeedback protocols targeting amygdala–PFC connectivity patterns could provide novel avenues for remediation.
4.7. Social Cognition and Theory of Mind
Deficits in social cognition—including the ability to infer others’ beliefs, emotions, and intentions—are among the most salient and impairing features of ASD. These deficits, often conceptualized under the framework of Theory of Mind (ToM), become particularly pronounced during adolescence, when social demands intensify. While impairments in ToM are not universal in ASD, they are sufficiently common to warrant in-depth neurobiological and behavioral scrutiny [
16,
49,
54].
Functional neuroimaging consistently implicates a network of brain regions associated with ToM, including the mPFC, TPJ, superior temporal sulcus (STS), and PCC [
16]. In adolescents with ASD, hypoactivation in these regions during ToM tasks has been documented in several studies. For instance, Pelphrey et al. [
16] found reduced activation in the STS and TPJ when individuals with ASD attempted to infer the intentions of others. Sato et al. [
49] similarly reported diminished gray matter volume in the so-called “social brain” network, suggesting potential structural underpinnings of social cognition deficits.
While such findings are compelling, several methodological caveats warrant attention. Sample sizes in many studies are modest (often <30 per group), limiting statistical power and inflating the risk of both false positives and false negatives. Furthermore, most tasks used to assess ToM rely on artificial or static stimuli, such as cartoon stories or still images, which may fail to capture the dynamic and context-dependent nature of real-world social interactions.
Age-related variability is another critical concern. Some studies pool data from children, adolescents, and adults, obscuring developmental nuances. For example, findings in younger children may reflect delayed maturation of social brain networks, whereas findings in adolescents may represent developmental divergence. Additionally, sex differences in ToM processing—which may be particularly relevant in light of the proposed “female autism phenotype”—are rarely considered in analyses, even though females with ASD may show more subtle ToM deficits or use compensatory strategies.
ToM tasks are often cognitively demanding, requiring not only social inference but also executive functioning, working memory, and language comprehension. Consequently, it can be difficult to disentangle core ToM deficits from more general cognitive limitations. This issue is especially relevant in samples that include individuals with intellectual disability or language impairments. Failure to control for these variables leads to heterogeneity in findings and reduces the specificity of conclusions.
Additionally, fMRI studies assessing ToM are often task-based and cross-sectional, which limits inferences about causality or developmental trajectories. Few studies examine how changes in social cognition relate to evolving brain connectivity or structural development over time.
It is also important to note that some adolescents with ASD exhibit near-typical performance on ToM tasks despite atypical neural activation patterns. This has led to the hypothesis that compensatory mechanisms—such as increased reliance on executive control or rote memorization of social rules—may support behaviorally normative performance. However, these strategies may be brittle and less effective in real-life, unstructured contexts.
Studies like that of Braden et al. [
55] support this notion by showing preserved performance despite atypical activation in medial prefrontal and temporal regions during social-cognitive tasks. However, these compensatory routes have not been systematically mapped, and their neural basis remains poorly understood.
To address these limitations, future research should include larger and more diverse samples, use ecologically valid paradigms (e.g., video-based or interactive tasks), and apply longitudinal designs to track developmental trajectories. Stratification by sex, IQ, and co-occurring conditions will be essential to tease apart subgroup-specific patterns. Moreover, integrating neuroimaging with behavioral interventions may allow identification of biomarkers predictive of treatment response.
Understanding the neural and cognitive underpinnings of ToM deficits is critical for developing targeted interventions. Social cognition training programs, such as those based on the UCLA PEERS
® model, have demonstrated modest improvements in social functioning [
54]. However, individual differences in neurobiological substrates may moderate treatment outcomes—a hypothesis that remains to be systematically tested.
4.8. Executive Function and Adaptive Behavior
Executive function (EF) encompasses a set of higher-order cognitive processes that support goal-directed behavior, including planning, working memory, cognitive flexibility, inhibition, and attentional control. These abilities are crucial for everyday adaptive functioning, especially during adolescence—a developmental window marked by growing demands for autonomy, organization, and emotional regulation. In adolescents with ASD, deficits in EF are among the most consistently observed cognitive impairments [
55], and they strongly predict challenges in academic achievement, social competence, and independent living skills [
59].
EF is supported by a distributed network of brain regions centered on the PFC, including dorsolateral and ventromedial regions, as well as the anterior cingulate cortex (ACC) and parietal lobes. In individuals with ASD, neuroimaging studies have reported hypoactivation in these regions during EF tasks, along with atypical structural connectivity between the PFC and other cortical and subcortical regions [
55]. For instance, Braden and colleagues [
55] documented both structural and functional abnormalities in frontal systems in middle-aged adults with ASD, which may reflect persistent developmental divergence originating in adolescence [
55].
However, many studies of EF in ASD do not isolate adolescents as a discrete developmental group. This is a significant limitation, as adolescence is associated with a normative increase in PFC myelination, synaptic pruning, and connectivity reorganization [
19,
60]—processes that may be altered in ASD. Thus, findings from adult or pediatric populations may not generalize to adolescents, and studies that conflate these age groups risk obscuring critical developmental trajectories. The methodological heterogeneity in EF research is substantial. Studies use a wide range of cognitive tasks (e.g., Wisconsin Card Sorting Test, Tower of London, Stroop tasks), each tapping different EF subdomains. Performance on these tasks is also influenced by motivational factors, anxiety, and familiarity with test formats. Additionally, test–retest reliability for many EF tasks is modest, and ecological validity is often low, raising questions about the generalizability of lab-based findings to real-world behaviors.
Sample sizes in neuroimaging studies of EF in ASD are frequently small, and the inclusion criteria vary widely. Some studies include only high-functioning individuals, while others fail to control for comorbid ADHD, which itself is associated with EF deficits. As a result, effect sizes are inconsistent, and replication is limited.
While cognitive EF tasks assess abstract problem-solving abilities, adaptive behavior refers to the real-world execution of everyday tasks such as managing time, hygiene, communication, and social interaction. Adolescents with ASD often exhibit a striking dissociation between intellectual ability and adaptive behavior, a phenomenon known as the “IQ–adaptive behavior gap” [
59]. This gap may reflect impairments in EF, as well as difficulties in generalizing learned skills to novel or unstructured environments.
Yet, few studies integrate direct EF assessments with standardized measures of adaptive behavior (e.g., Vineland Adaptive Behavior Scales or the ABAS-II). Moreover, the neurobiological mechanisms linking EF deficits to adaptive outcomes remain underexplored. While Braden et al. [
55] and others have noted that EF deficits may be associated with reduced integrity of frontoparietal networks [
55], these associations have rarely been tested longitudinally.
The trajectory of EF development in ASD is heterogeneous. Some adolescents show gradual improvement in EF skills, while others exhibit persistent or worsening difficulties. Longitudinal studies such as Andrews et al. [
42] have begun to chart white matter development in relation to ASD symptomatology, but few have specifically linked these findings to EF or adaptive skill growth.
Factors contributing to this variability include sex, pubertal timing, environmental support, and intervention history. Additionally, cultural and socioeconomic factors may shape how EF and adaptive behavior are expressed and evaluated, yet these are often ignored in study designs.
Future studies should prioritize multi-method, longitudinal designs that integrate neuroimaging, cognitive testing, and adaptive behavior ratings. Stratified sampling based on EF profiles or trajectories could help identify subgroups with distinct support needs. Moreover, task-based fMRI studies should incorporate ecologically valid paradigms that simulate real-life executive demands.
Targeted EF interventions, such as those focusing on cognitive flexibility or time management, may yield substantial improvements in adolescents’ ability to function independently. However, few intervention studies to date have included neurobiological outcome measures, making it difficult to assess mechanistic change. Bridging this gap between brain and behavior will be critical for developing personalized, neuroscience-informed approaches to intervention.
4.9. Implications for Education and Social Integration
Adolescents with ASD face considerable challenges in educational and social contexts, particularly during the transition from structured primary school environments to the more demanding and less scaffolded settings of secondary education and beyond [
1,
21,
48]. These challenges are deeply intertwined with the neurobiological and cognitive heterogeneity documented in previous sections and are further modulated by contextual factors such as classroom environment, educator training, and the availability of peer supports.
Academic achievement in adolescents with ASD is highly variable and not reliably predicted by IQ alone [
21]. Some individuals perform at or above grade level, while others struggle with even basic literacy or numeracy. Cognitive heterogeneity, particularly in executive function, working memory, and processing speed, likely contributes to these disparate outcomes [
55,
56]. For instance, adolescents with intact language skills but poor cognitive flexibility may succeed in structured academic tasks yet falter in open-ended problem-solving or collaborative learning environments.
Unfortunately, educational assessments often fail to differentiate between core academic ability and the executive or attentional factors that may impede performance. Moreover, many studies that report on educational outcomes in ASD rely on caregiver or teacher reports rather than objective academic measures. This methodological limitation complicates efforts to identify specific neurocognitive predictors of academic success.
The adolescent period brings increasing emphasis on peer relationships, group belonging, and social identity formation. Adolescents with ASD are at elevated risk of social isolation, bullying, and mental health difficulties arising from poor peer integration [
1,
21,
54]. These difficulties are not solely a function of social cognition deficits (as discussed in
Section 4.6), but are also shaped by environmental factors such as classroom inclusion policies, peer attitudes, and educator expectations.
The literature evaluating social outcomes in adolescents with ASD is heterogeneous. Many studies use broad parent-report scales that conflate quantity of social interaction with quality or satisfaction. Others focus on observable behaviors without accounting for internal social motivation, which may be intact even in adolescents with marked communication difficulties. Consequently, the extent to which neurobiological differences translate into social exclusion remains difficult to quantify.
Intervention programs aimed at improving social functioning, such as the UCLA PEERS
® curriculum, have shown promise in enhancing social communication and peer interaction skills in adolescents and young adults with ASD [
54]. However, findings are inconsistent, and effect sizes vary widely across studies. One limitation is the short duration and limited generalizability of these interventions. Most rely on structured, clinician-led sessions and do not sufficiently address the transition of skills into unstructured, peer-driven environments such as lunchrooms, sports teams, or online platforms.
Furthermore, few intervention studies stratify participants based on neurobiological profiles, such as functional connectivity patterns or EF impairments. This limits the ability to determine which adolescents are most likely to benefit from specific programs. For instance, those with prominent amygdala-prefrontal dysconnectivity may struggle with emotion regulation in social situations, requiring different support than individuals with intact affective responses but poor planning or inhibition [
24,
50].
From a policy perspective, inclusion models that emphasize neurodiversity and individualized supports have gained traction, but implementation remains inconsistent. Educators often lack training in ASD-specific learning profiles, and school systems may not provide sufficient resources for one-on-one support, social skills coaching, or executive function accommodations.
A further complication is the relative scarcity of longitudinal studies assessing educational and social integration outcomes in adolescents with ASD. Without such data, it is difficult to determine the long-term impact of specific supports or identify critical transition points that require targeted intervention.
Research must move toward identifying neurocognitive and behavioral subtypes that predict differential responses to educational and social interventions. Multi-site, longitudinal studies with harmonized measures of academic performance, social functioning, and neurobiological change are urgently needed. Moreover, the inclusion of autistic voices in shaping research questions and educational practices will be essential to ensure ecological validity and ethical alignment.
Interdisciplinary collaboration between neuroscientists, educators, psychologists, and policy-makers is critical to translating complex findings into practical classroom strategies. This includes creating individualized education plans (IEPs) that are informed not only by test scores, but by profiles of cognitive flexibility, sensory sensitivity, and social motivation.
8. Discussion
Adolescents with ASD face multifaceted challenges during their transition to adulthood, encompassing neural, behavioral, social, and adaptive domains. These difficulties are closely linked to core ASD characteristics, including social communication deficits, restricted and repetitive behaviors, and impaired adaptive functioning [
1,
19]. While current research has made substantial progress in delineating neurobiological and behavioral heterogeneity, the developmental implications of these findings for adolescents remain inadequately addressed in the literature. This section synthesizes current evidence while critically evaluating methodological limitations, conflicting data, and unanswered questions specific to adolescents with ASD.
A central issue is the generalization of learned skills to novel environments such as postsecondary education and employment. Although this transition is expected in both ASD and NT adolescents, individuals with ASD often struggle with flexibility, social inference, and executive functioning, all of which impair skill transfer. However, much of the literature lacks clarity on whether these difficulties stem from persistent neurocognitive deficits, insufficient instructional support, or systemic educational shortcomings. Tailored transition planning is essential, but research has yet to determine which components of such programs are most effective for diverse ASD profiles.
Volkmar et al. [
1] emphasize the importance of coordinated efforts between families, educational institutions, and adult service providers. However, studies often neglect to evaluate the fidelity of such collaborative models or control for variability in socioeconomic status and access to services. Additionally, most outcome studies rely heavily on self-report or caregiver report, raising concerns about subjective bias and the lack of objective functional outcome measures.
Family support remains indispensable during this period, especially in contexts lacking public resources. Yet, the literature seldom accounts for cultural variations in family involvement or differences in caregiver burden across the ASD spectrum. There is a clear need for studies assessing how different family structures, caregiving styles, and support systems influence transition outcomes.
The plateau in daily living skills during adolescence and early adulthood, as described by O’Hearn et al. [
19], is well documented, yet the mechanisms remain equivocal. Is this stagnation primarily neurobiologically driven—due to halted white matter development—or is it a consequence of inadequate skills training or low expectations from caregivers and educators? Longitudinal studies with multimodal assessment (e.g., combining neuroimaging with ecological momentary assessment) could clarify this.
Social outcomes also show marked variability. While some individuals form romantic relationships and achieve independence, others remain socially isolated. Unfortunately, methodological heterogeneity—ranging from differing diagnostic criteria to nonstandardized outcome measures—hampers cross-study comparisons. Further complicating interpretation is the frequent underrepresentation of females and individuals from racially and ethnically diverse populations in these studies, limiting generalizability.
On the neural level, altered connectivity patterns, particularly involving the amygdala, are repeatedly implicated in ASD-related social and emotional regulation deficits [
23,
50]. However, developmental trajectories remain unclear due to the predominance of cross-sectional rather than longitudinal designs. Moreover, the relationship between neural patterns and functional behavior is often assumed rather than directly tested, leaving gaps in causal inference.
Partial symptom improvement across adolescence has been reported, particularly in social communication [
53]. Yet, repetitive behaviors remain relatively intractable. This raises questions about differential neural plasticity across behavioral domains and whether certain interventions selectively target more malleable functions. Research has yet to clarify the neurobiological correlates of intervention responsiveness during adolescence.
Psychiatric comorbidities such as anxiety and ADHD significantly impact transition readiness and long-term outcomes. However, much of the comorbidity research aggregates children, adolescents, and adults, obscuring the unique patterns of comorbidity emergence and persistence during adolescence specifically. More age-stratified research is urgently needed to guide stage-appropriate interventions.
Recent advances in ASD research have deepened our understanding of atypical information processing, such as prolonged intrinsic neural timescales and their correlation with symptom severity [
18]. However, these findings are often based on small samples or poorly matched control groups, limiting statistical power and generalizability. Moreover, replication studies are scarce. The reliability and clinical relevance of these biomarkers remain open questions.
Functional connectivity studies have revealed age-dependent differences in global and regional brain networks. For example, interhemispheric hypo-connectivity and regional hyper-connectivity continue to be identified, with evidence suggesting maturational differences between adolescents and adults [
22,
25]. Yet, inconsistencies in imaging methodology, preprocessing techniques, and region-of-interest definitions persist across studies. Task-based paradigms offer potential to resolve some of these inconsistencies, but they remain underutilized in developmental ASD research.
The role of the DMN in self-referential processing and social cognition has received increased attention. However, its developmental trajectory during adolescence—when social identity and autonomy emerge—is not well understood. Similarly, the early origins of DMN disruptions remain speculative, with few studies linking fetal or infant neural development to later adolescent outcomes [
24,
29].
Evidence-based intervention research continues to expand, but translation to practice remains limited, particularly in low-resource environments. While guidelines informed by multidisciplinary perspectives are being developed [
48], their uptake and effectiveness in real-world settings require better evaluation. Furthermore, despite meta-analytic data on maladaptive behaviors [
7], the field lacks consensus on which behaviors most impair transition outcomes and how they should be prioritized in interventions.
A particularly promising yet underexplored area is the late acquisition of daily living skills. Contrary to the longstanding belief that development plateaus in adolescence, newer studies suggest that appropriate environmental scaffolding can promote continued growth into adulthood [
56]. However, mechanisms underlying this “late bloom” phenomenon remain speculative.
Finally, while the integration of genetic, neurobiological, and behavioral data is widely endorsed [
1], few studies achieve this level of interdisciplinarity. Future research must prioritize larger, diverse samples and longitudinal, multimodal designs to advance precision intervention and personalized support planning [
59].
Integration of Outstanding Questions and Knowledge Gaps
Despite significant progress, several key questions remain unanswered in the study of adolescents with ASD. One pressing issue is the identification of neurobiological features that reliably predict adaptive versus maladaptive outcomes during the transition to adulthood. It is unclear which brain-based markers—such as specific connectivity patterns or developmental trajectories in white matter maturation—are most indicative of successful independence or persistent functional limitations.
Additionally, inconsistencies across studies highlight the need to critically assess how methodological variables—such as sample size, imaging modality, diagnostic thresholds, and task-based versus resting-state paradigms—contribute to the divergent findings regarding neural connectivity abnormalities in ASD. These methodological issues complicate the field’s ability to build a coherent understanding of developmental brain differences specific to adolescence.
Another important question pertains to the environmental and instructional factors that support continued acquisition of daily living skills beyond adolescence. Although emerging evidence suggests that skill growth does not universally plateau, the mechanisms by which certain adolescents continue to develop while others stagnate remain poorly understood. Identifying contextual and neurocognitive moderators of this growth is a priority [
59].
The role of sociocultural and demographic variables—including gender, ethnicity, and socioeconomic status—also warrants deeper investigation. Many existing studies lack representative samples, limiting the generalizability of findings and potentially obscuring meaningful subgroups within the adolescent ASD population.
Lastly, the field still lacks consensus regarding the impact of psychiatric comorbidities, particularly ADHD and anxiety, during adolescence. Clarifying how these co-occurring conditions influence developmental trajectories, intervention responsiveness, and adult outcomes remains a critical research need [
56].
Answering these questions will require longitudinal, multimodal research designs, greater methodological standardization, and stronger integration across behavioral, cognitive, neurobiological, and environmental domains.
9. Conclusions
ASD during adolescence presents a distinctive constellation of neurobiological, cognitive, and behavioral challenges that are both developmentally dynamic and highly heterogeneous. Throughout this review, we have identified converging evidence that highlights widespread atypicalities in brain connectivity, functional specialization, and adaptive behavior in adolescents with ASD. These include, but are not limited to, underconnectivity within and between frontoparietal and default mode networks, overconnectivity in sensorimotor and subcortical systems, delayed maturation in white matter tracts, and atypical trajectories in cognitive domains such as executive function, social processing, and emotion regulation.
What is known with reasonable confidence is that these neurobiological alterations contribute to a broad spectrum of cognitive and adaptive outcomes. Structural and functional neuroimaging consistently implicates disruptions in regions subserving higher-order cognitive control, theory of mind, and interoception. Developmental patterns, while variable, show divergence from NT norms particularly in the transition from adolescence into adulthood, where adaptive skill acquisition often plateaus and psychiatric comorbidities become more pronounced.
Yet several areas remain equivocal. Considerable variability exists in study outcomes due to methodological differences in sample characterization (e.g., IQ, sex distribution, comorbidity profiles), task design (e.g., passive viewing vs. active engagement paradigms), and neuroimaging techniques (e.g., resting-state vs. task-based fMRI). The significance of observed brain-behavior correlations is often constrained by cross-sectional designs, insufficient longitudinal data, and lack of replication. For instance, while hypo-connectivity in default mode regions is widely reported, its exact developmental onset, behavioral correlates, and plasticity remain debated.
Unknowns persist regarding the mechanisms that drive individual differences in adolescent ASD outcomes. Questions remain about which neurodevelopmental markers reliably predict positive adaptation, what role environmental modifiers play in shaping trajectories, and how interventions might be tailored to emerging adolescent needs. The extent to which neural compensation versus persistent deficits account for changes in behavior is poorly understood, and the influence of hormonal, sociocultural, and ecological transitions during adolescence on brain and behavior in ASD remains underexplored.
Given these challenges, it is clear that supporting adolescents with ASD requires both precision and breadth: precision in identifying neural and behavioral phenotypes that guide personalized interventions, and breadth in constructing systemic supports—educational, social, clinical—that can accommodate the full spectrum of needs. There is a pressing need for longitudinal, multimodal, and interdisciplinary approaches that bridge neurodevelopmental research with real-world implementation in clinical and educational settings.
Ultimately, ASD in adolescence must be understood as a lifespan issue, with adolescence representing a critical inflection point where neurobiological plasticity intersects with escalating environmental demands. Continued research that interrogates the intersection of biology, behavior, and context will be essential to refining diagnostic models, informing intervention timing and targets, and ultimately improving long-term outcomes for individuals with ASD and their families.