The Role of Psychedelics in the Treatment of Substance Use Disorders: An Overview of Systematic Reviews
Abstract
1. Introduction
2. Materials and Methods
2.1. Psychedelic Classification
2.2. Search Strategy and Methods
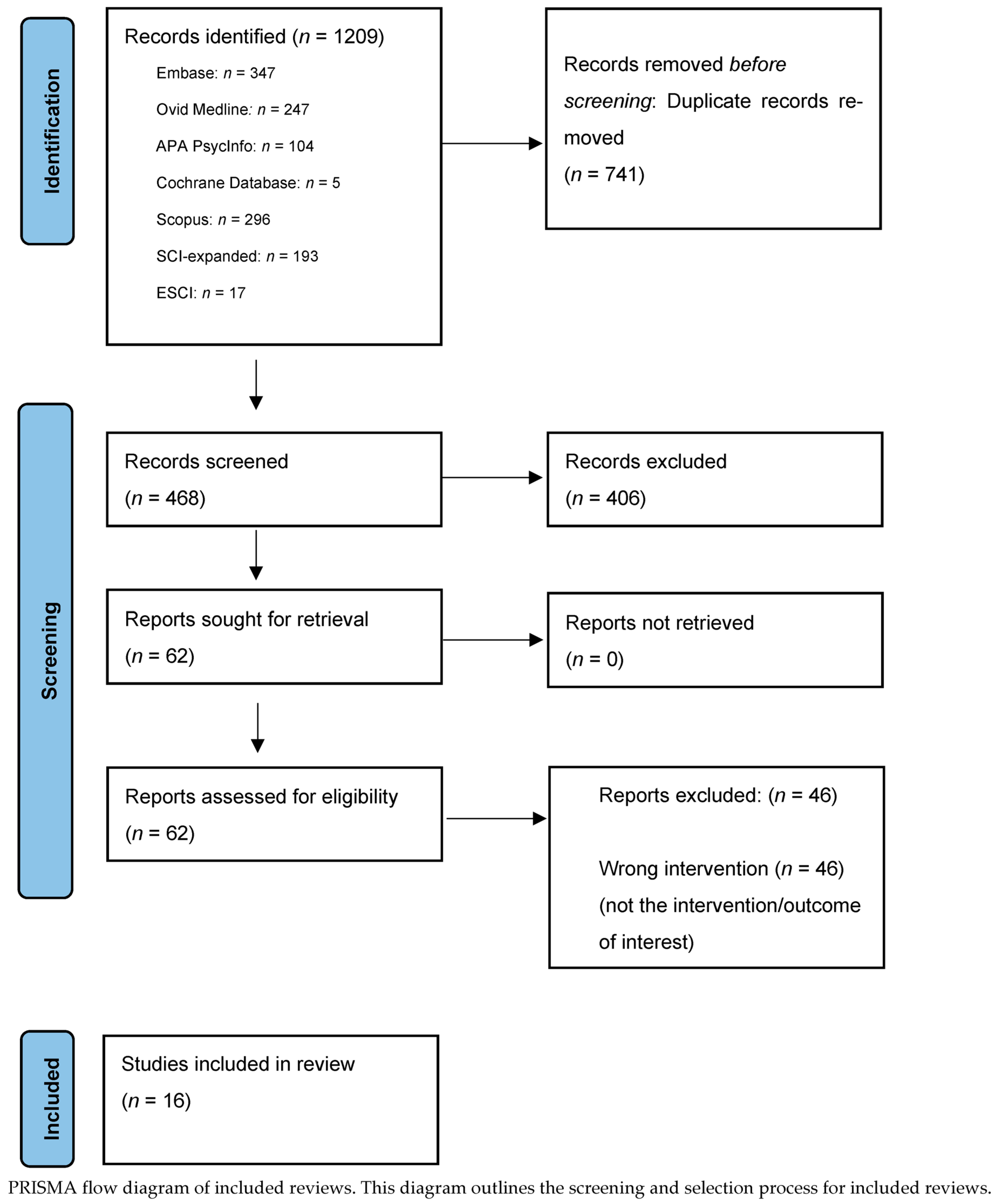
Overview of Systematic Reviews
3. Results
3.1. Psychedelic Drugs—Psilocybin, LSD, Ayahuasca, Ibogaine, MDMA, Mescaline
3.2. Ketamine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Whiteford, H.A.; Degenhardt, L.; Rehm, J.; Baxter, A.J.; Ferrari, A.J.; Erskine, H.E.; Charlson, F.J.; Norman, R.E.; Flaxman, A.D.; Johns, N.; et al. Global burden of disease attributable to mental and substance use disorders: Findings from the Global Burden of Disease Study 2010. Lancet 2013, 382, 1575–1586. [Google Scholar] [CrossRef]
- SAMHSA. Substance Abuse and Mental Health Services Administration: Results from the 2020 National Survey on Drug Use and Health: Detailed Tables; SAMHSA: Rockville, MD, USA, 2021. [Google Scholar]
- Mokdad, A.H.; Marks, J.S.; Stroup, D.F.; Gerberding, J.L. Actual causes of death in the United States, 2000. J. Am. Med. Assoc. 2004, 291, 1238–1245. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Mokdad, A.H.; Ballestros, K.; Echko, M.; Glenn, S.; Olsen, H.E.; Mullany, E.; Lee, A.; Khan, A.R.; Ahmadi, A.; et al. The state of US health, 1990-2016: Burden of diseases, injuries, and risk factors among US states. J. Am. Med. Assoc. 2018, 319, 1444–1472. [Google Scholar] [CrossRef]
- Adrian, M.; Barry, S.J. Physical and Mental Health Problems Associated with the Use of Alcohol and Drugs. Subst. Use Misuse 2003, 38, 1575–1614. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Blanco, C. The changing opioid crisis: Development, challenges and opportunities. Mol. Psychiatry 2021, 26, 218–233. [Google Scholar] [CrossRef] [PubMed]
- Substance Abuse and Mental Health Services Administration. Key Substance Use and Mental Health Indicators in the United States: Results from the 2023 National Survey on Drug Use and Health (HHS Publication No. PEP24-07-021, NSDUH Series H-59); Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration: Rockville, MD, USA, 2024.
- Esser, M.B.; Leung, G.; Sherk, A.; Bohm, M.K.; Liu, Y.; Lu, H.; Naimi, T.S. Estimated Deaths Attributable to Excessive Alcohol Use Among US Adults Aged 20 to 64 Years, 2015 to 2019. JAMA Netw. Open 2022, 5, e2239485. [Google Scholar] [CrossRef]
- Esser, M.B.; Sherk, A.; Liu, Y.; Naimi, T.S. Deaths from Excessive Alcohol Use—United States, 2016–2021. Morb. Mortal. Wkly. Rep. 2024, 73, 154–161. [Google Scholar] [CrossRef]
- Rehm, J.R.; Mathers, C.; Popova, S.; Thavorncharoensap, M.; Teerawattananon, Y.; Patra, J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 2009, 373, 2223–2233. [Google Scholar] [CrossRef]
- Sacks, J.J.; Gonzales, K.R.; Bouchery, E.E.; Tomedi, L.E.; Brewer, R.D. 2010 National and State Costs of Excessive Alcohol Consumption. Am. J. Prev. Med. 2015, 49, e73–e79. [Google Scholar] [CrossRef]
- Volkow, N.D.; Blanco, C. Substance use disorders: A comprehensive update of classification, epidemiology, neurobiology, clinical aspects, treatment and prevention. World Psychiatry 2023, 22, 203–229. [Google Scholar] [CrossRef]
- Bormann, N.L.; Burson, J.R.; Burson, E.M.; McGinnis, M.; Karpyak, V.; Coombes, B.J.; Gold, M.; Oesterle, T.S. How Treatment-Refractory Addiction Is Defined: A Scoping Review. J. Addict. Med. 2025, 10–1097. [Google Scholar] [CrossRef]
- Case, A.; Deaton, A. Rising morbidity and mortality in midlife among white non-Hispanic Americans in the 21st century. Proc. Natl. Acad. Sci. USA 2015, 112, 15078–15083. [Google Scholar] [CrossRef] [PubMed]
- Aksu, S.; Soyata, A.Z.; Şeker, S.; Akkaya, G.; Yılmaz, Y.; Kafalı, T.; Evren, C.; Umut, G. Transcranial direct current stimulation combined with cognitive training improves decision making and executive functions in opioid use disorder: A triple-blind sham-controlled pilot study. J. Addict. Dis. 2024, 42, 154–165. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Y.; Cao, X.; Shan, C.; Pan, J.; He, H.; Ma, Y.; Yuan, T.F. Transcranial direct current stimulation of the frontal-parietal-temporal area attenuates cue-induced craving for heroin. J. Psychiatr. Res. 2016, 79, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Bormann, N.L.; Oesterle, T.S.; Arndt, S.; Karpyak, V.M.; Croarkin, P.E. Systematic review and meta-analysis: Combining transcranial magnetic stimulation or direct current stimulation with pharmacotherapy for treatment of substance use disorders. Am. J. Addict. 2024, 33, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Tang, V.M.; Goud, R.; Zawertailo, L.; Selby, P.; Coroiu, A.; Sloan, M.E.; Chenoweth, M.J.; Buchman, D.; Ibrahim, C.; Blumberger, D.M.; et al. Repetitive transcranial magnetic stimulation for smoking cessation: Next steps for translation and implementation into clinical practice. Psychiatry Res. 2023, 326, 115340. [Google Scholar] [CrossRef]
- Li, S.; Jiang, A.; Ma, X.; Yang, B.; Ni, H.; Zheng, Y.; Wang, Z.; Dong, G.H. Repetitive transcranial magnetic stimulation reduces smoking cravings by decreasing cerebral blood flow in the dorsolateral prefrontal cortex. Brain Commun. 2025, 7, fcaf101. [Google Scholar] [CrossRef]
- Garcia-Romeu, A.; Davis, A.K.; Erowid, E.; Erowid, F.; Griffiths, R.R.; Johnson, M.W. Persisting Reductions in Cannabis, Opioid, and Stimulant Misuse After Naturalistic Psychedelic Use: An Online Survey. Front. Psychiatry 2020, 10, 995. [Google Scholar] [CrossRef]
- da Costa, S.C.; Oesterle, T.; Rummans, T.A.; Richelson, E.; Gold, M. Psychedelic drugs for psychiatric disorders. J. Neurol. Sci. 2022, 440, 120332. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L. How do psychedelics work? Curr. Opin. Psychiatry 2019, 32, 16–21. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Erritzoe, D.; Williams, T.; Stone, J.M.; Reed, L.J.; Colasanti, A.; Tyacke, R.J.; Leech, R.; Malizia, A.L.; Murphy, K.; et al. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc. Natl. Acad. Sci. USA 2012, 109, 2138–2143. [Google Scholar] [CrossRef]
- Nichols, D.E. Psychedelics. Pharmacol. Rev. 2016, 68, 264–355. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Goodwin, G.M. The Therapeutic Potential of Psychedelic Drugs: Past, Present, and Future. Neuropsychopharmacology 2017, 42, 2105–2113. [Google Scholar] [CrossRef] [PubMed]
- Schultes, R.E.; Hofmann, A.; Rätsch, C. Plants of the Gods: Their Sacred, Healing, and Hallucinogenic Powes; Healing Arts Press: Rochester, VT, USA, 2001. [Google Scholar]
- Metzner, R. Entheogenic rituals, shamanism and green psychology. Eur. J. Ecopsychol. 2013, 4, 64–77. [Google Scholar]
- Zarate, C.A. Do the dissociative side effects of ketamine mediate its antidepressant effects? J. Affect. Disord. 2014, 159, 56–61. [Google Scholar] [CrossRef]
- Newport, D.J.; Carpenter, L.L.; McDonald, W.M.; Potash, J.B.; Tohen, M.; Nemeroff, C.B. Ketamine and other NMDA antagonists: Early clinical trials and possible mechanisms in depression. Am. J. Psychiatry 2015, 172, 950–966. [Google Scholar] [CrossRef]
- Zunszain, P.A.; Horowitz, M.A.; Cattaneo, A.; Lupi, M.M.; Pariante, C.M. Ketamine: Synaptogenesis, immunomodulation and glycogen synthase kinase-3 as underlying mechanisms of its antidepressant properties. Mol. Psychiatry 2013, 18, 1236–1241. [Google Scholar] [CrossRef]
- Zanos, P.; Moaddel, R.; Morris, P.J.; Georgiou, P.; Fischell, J.; Elmer, G.I.; Alkondon, M.; Yuan, P.; Pribut, H.J.; Singh, N.S.; et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 2016, 533, 481–486. [Google Scholar] [CrossRef]
- Cornwell, B.R.; Salvadore, G.; Furey, M.; Marquardt, C.A.; Brutsche, N.E.; Grillon, C.; Zarate, C.A. Synaptic potentiation is critical for rapid antidepressant response to ketamine in treatment-resistant major depression. Biol. Psychiatry 2012, 72, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Vollenweider, F.X.; Kometer, M. The neurobiology of psychedelic drugs: Implications for the treatment of mood disorders. Nat. Rev. Neurosci. 2010, 11, 642–651. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. DSM 5; American Psychiatric Publishing, Inc.: Washington, DC, USA, 2013; p. 991. [Google Scholar]
- Williams, N.R.; Heifets, B.D.; Bentzley, B.S.; Blasey, C.; Sudheimer, K.D.; Hawkins, J.; Lyons, D.M.; Schatzberg, A.F. Attenuation of antidepressant and antisuicidal effects of ketamine by opioid receptor antagonism. Mol. Psychiatry 2019, 24, 1779–1786. [Google Scholar] [CrossRef]
- Williams, N.R.; Heifets, B.D.; Blasey, C.; Sudheimer, K.; Pannu, J.; Pankow, H.; Hawkins, J.; Birnbaum, J.; Lyons, D.M.; Rodriguez, C.I.; et al. Attenuation of Antidepressant Effects of Ketamine by Opioid Receptor Antagonism. Am. J. Psychiatry 2018, 175, 1205–1215. [Google Scholar] [CrossRef]
- Famula, A.; Radoszewski, J.; Czerwiec, T.; Sobis, J.; Wieckiewicz, G. Ketamine in Substance Use Disorder Treatment: A Narrative Review. Alpha Psychiatry 2024, 25, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Drug Enforcement Administration. Title 21 United States Code (USC) Controlled Substances Act—Section 801; Office of Diversion Control. United States Department of Justice: Springfield, VA, USA, 1970.
- Johnson, M.W.; Griffiths, R.R.; Hendricks, P.S.; Henningfield, J.E. The abuse potential of medical psilocybin according to the 8 factors of the Controlled Substances Act. Neuropharmacology 2018, 142, 143–166. [Google Scholar] [CrossRef]
- Janssen-Aguilar, R.; Meshkat, S.; Demchenko, I.; Zhang, Y.; Greenshaw, A.; Dunn, W.; Tanguay, R.; Mayo, L.M.; Swainson, J.; Jetly, R.; et al. Role of ketamine in the treatment of substance use disorders: A systematic review. J. Subst. Use Addict. Treat. 2025, 175, 209705. [Google Scholar] [CrossRef]
- Jones, J.L.; Mateus, C.F.; Malcolm, R.J.; Brady, K.T.; Back, S.E. Efficacy of Ketamine in the Treatment of Substance Use Disorders: A Systematic Review. Front. Psychiatry 2018, 9, 277. [Google Scholar] [CrossRef]
- Martinotti, G.; Chiappini, S.; Pettorruso, M.; Mosca, A.; Miuli, A.; Di Carlo, F.; D’Andrea, G.; Collevecchio, R.; Di Muzio, I.; Sensi, S.L.; et al. Therapeutic Potentials of Ketamine and Esketamine in Obsessive-Compulsive Disorder (OCD), Substance Use Disorders (SUD) and Eating Disorders (ED): A Review of the Current Literature. Brain Sci. 2021, 11, 856. [Google Scholar] [CrossRef]
- Walsh, Z.; Mollaahmetoglu, O.M.; Rootman, J.; Golsof, S.; Keeler, J.; Marsh, B.; Nutt, D.J.; Morgan, C.J.A. Ketamine for the treatment of mental health and substance use disorders: Comprehensive systematic review. BJPsych Open 2021, 8, e19. [Google Scholar] [CrossRef]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B.; Group, P.-S. PRISMA-S: An extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef]
- Calleja-Conde, J.; Morales-Garcia, J.A.; Echeverry-Alzate, V.; Buhler, K.M.; Gine, E.; Lopez-Moreno, J.A. Classic psychedelics and alcohol use disorders: A systematic review of human and animal studies. Addict. Biol. 2022, 27, e13229. [Google Scholar] [CrossRef] [PubMed]
- Sicignano, D.; Hernandez, A.V.; Schiff, B.; Elmahy, N.; White, C.M. The impact of psychedelics on patients with alcohol use disorder: A systematic review with meta-analysis. Curr. Med. Res. Opin. 2024, 40, 293–302. [Google Scholar] [CrossRef]
- Mosca, A.; Chiappini, S.; Miuli, A.; Mancusi, G.; Santovito, M.C.; Di Carlo, F.; Pettorruso, M.; Corkery, J.M.; Canessa, C.; Martinotti, G.; et al. Ibogaine/Noribogaine in the Treatment of Substance Use Disorders: A Systematic Review of the Current Literature. Curr. Neuropharmacol. 2023, 21, 2178–2194. [Google Scholar] [CrossRef]
- Spoelstra, S.K.; Schoevers, R.A.; Venema, S.D.; Knegtering, H. Psychedelics as a potential treatment for tobacco use disorder: A systematic review. Discov. Ment. Health 2024, 4, 37. [Google Scholar] [CrossRef]
- Rodrigues, L.S.; Rossi, G.N.; Rocha, J.M.; LOsório, F.; Bouso, J.C.; Hallak, J.E.C.; Dos Santos, R.G. Effects of ayahuasca and its alkaloids on substance use disorders: An updated (2016–2020) systematic review of preclinical and human studies. Eur. Arch. Psychiatry Clin. Neurosci. 2022, 272, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, J.J.; Fonseca, F.; Elices, M.; Farre, M.; Torrens, M. Therapeutic Use of LSD in Psychiatry: A Systematic Review of Randomized-Controlled Clinical Trials. Front. Psychiatry 2019, 10, 943. [Google Scholar] [CrossRef] [PubMed]
- Phan, A.N.; Terry, G.E. Systematic review and rationale of using psychedelics in the treatment of cannabis use disorder. Front. Psychiatry 2023, 14, 1144276. [Google Scholar] [CrossRef] [PubMed]
- van der Meer, P.B.; Fuentes, J.J.; Kaptein, A.A.; Schoones, J.W.; de Waal, M.M.; Goudriaan, A.E.; Kramers, K.; Schellekens, A.; Somers, M.; Bossong, M.G.; et al. Therapeutic effect of psilocybin in addiction: A systematic review. Front. Psychiatry 2023, 14, 1134454. [Google Scholar] [CrossRef]
- Sharma, R.; Batchelor, R.; Sin, J. Psychedelic Treatments for Substance Use Disorder and Substance Misuse: A Mixed Methods Systematic Review. J. Psychoact. Drugs 2023, 55, 612–630. [Google Scholar] [CrossRef]
- Trope, A.; Anderson, B.T.; Hooker, A.R.; Glick, G.; Stauffer, C.; Woolley, J.D. Psychedelic-Assisted Group Therapy: A Systematic Review. J. Psychoact. Drugs 2019, 51, 174–188. [Google Scholar] [CrossRef]
- Kelson, M.; Burnett, J.M.; Matthews, A.; Juneja, T. Ketamine Treatment for Alcohol Use Disorder: A Systematic Review. Cureus 2023, 15, e38498. [Google Scholar] [CrossRef]
- Drozdz, S.J.; Goel, A.; McGarr, M.W.; Katz, J.; Ritvo, P.; Mattina, G.F.; Bhat, V.; Diep, C.; Ladha, K.S. Ketamine Assisted Psychotherapy: A Systematic Narrative Review of the Literature. J. Pain. Res. 2022, 15, 1691–1706. [Google Scholar] [CrossRef]
- Garel, N.; McAnulty, C.; Greenway, K.T.; Lesperance, P.; Miron, J.P.; Rej, S.; Richard-Devantoy, S.; Jutras-Aswad, D. Efficacy of ketamine intervention to decrease alcohol use, cravings, and withdrawal symptoms in adults with problematic alcohol use or alcohol use disorder: A systematic review and comprehensive analysis of mechanism of actions. Drug Alcohol. Depend. 2022, 239, 109606. [Google Scholar] [CrossRef] [PubMed]
- Kew, B.M.; Porter, R.J.; Douglas, K.M.; Glue, P.; Mentzel, C.L.; Beaglehole, B. Ketamine and psychotherapy for the treatment of psychiatric disorders: Systematic review. BJPsych Open 2023, 9, e79. [Google Scholar] [CrossRef] [PubMed]
- Walsh, Z.; Mollaahmetoglu, O.M.; Rootman, J.; Golsof, S.; Keeler, J.; Marsh, B.; Nutt, D.J.; Morgan, C.J.A. Ketamine for the treatment of mental health and substance use disorders: Comprehensive systematic review—CORRIGENDUM. BJPsych Open 2022, 8, e29. [Google Scholar] [CrossRef]
- Osmond, H. A review of the clinical effects of psychotomimetic agents. Ann. N. Y. Acad. Sci. 1957, 66, 418–434. [Google Scholar] [CrossRef]
- Kelly, J.F.; Magill, M.; Stout, R.L. How do people recover from alcohol dependence? A systematic review of the research on mechanisms of behavior change in Alcoholics Anonymous. Addict. Res. 2009, 17, 236–259. [Google Scholar] [CrossRef]
- William, W. The Society of Alcoholics Anonymous. Am. J. Psychiatry 1949, 106, 370–375. [Google Scholar] [CrossRef]
- Kinnell, H.G.; Glatt, M.M. Alcoholics Anonymous. Lancet 1978, 311, 714–715. [Google Scholar] [CrossRef]
- Hunt, H.; Pollock, A.; Campbell, P.; Estcourt, L.; Brunton, G. An introduction to overviews of reviews: Planning a relevant research question and objective for an overview. Syst. Rev. 2018, 7, 39. [Google Scholar] [CrossRef]
- Nicholas, C.R.; Wang, J.B.; Coker, A.; Mitchell, J.M.; Klaire, S.S.; Yazar-Klosinski, B.; Emerson, A.; Brown, R.T.; Doblin, R. The effects of MDMA-assisted therapy on alcohol and substance use in a phase 3 trial for treatment of severe PTSD. Drug Alcohol. Depend. 2022, 233, 109356. [Google Scholar] [CrossRef]
- de Lima, J.; Beggs, S.; Howard, R. Neural toxicity of ketamine and othe NMDA antagonists. Pain 2000, 88, 311–312. [Google Scholar] [CrossRef]
- Kalsi, S.S.; Wood, D.M.; Dargan, P.I. The epidemiology and patterns of acute and chronic toxicity associated with recreational ketamine use. Emerg. Health Threat. J. 2011, 4, 7107. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.; Lui, L.M.W.; Rosenblat, J.D.; Teopiz, K.M.; Lipsitz, O.; Cha, D.S.; Xiong, J.; Nasri, F.; Lee, Y.; Kratiuk, K.; et al. Ketamine-induced urological toxicity: Potential mechanisms and translation for adults with mood disorders receiving ketamine treatment. Psychopharmacology 2021, 238, 917–926. [Google Scholar] [CrossRef]
- Orhurhu, V.J.; Vashisht, R.; Claus, L.E.; Cohen, S.P. Ketamine Toxicity; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Slikker, W., Jr.; Liu, F.; Rainosek, S.W.; Patterson, T.A.; Sadovova, N.; Hanig, J.P.; Paule, M.G.; Wang, C. Ketamine-Induced Toxicity in Neurons Differentiated from Neural Stem Cells. Mol. Neurobiol. 2015, 52, 959–969. [Google Scholar] [CrossRef]
- Yiu-Cheung, C. Acute and chronic toxicity pattern in ketamine abusers in Hong Kong. J. Med. Toxicol. 2012, 8, 267–270. [Google Scholar] [CrossRef]
- Ahmed, S.N.; Petchkovsky, L. Abuse of ketamine. Br. J. Psychiatry 1980, 137, 303. [Google Scholar] [CrossRef] [PubMed]
- Bobo, W.V.; Miller, S.C. Ketamine as a preferred substance of abuse. Am. J. Addict. 2002, 11, 332–334. [Google Scholar] [CrossRef]
- Bokor, G.; Anderson, P.D. Ketamine: An update on its abuse. J. Pharm. Pract. 2014, 27, 582–586. [Google Scholar] [CrossRef]
- Corkery, J.M.; Hung, W.C.; Claridge, H.; Goodair, C.; Copeland, C.S.; Schifano, F. Recreational ketamine-related deaths notified to the National Programme on Substance Abuse Deaths, England, 1997–2019. J. Psychopharmacol. 2021, 35, 1324–1348. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Lin, C.H.; Lane, H.Y. Mania following ketamine abuse. Neuropsychiatr. Dis. Treat. 2016, 12, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Nomiya, A.; Nishimatsu, H.; Homma, Y. Interstitial cystitis symptoms associated with ketamine abuse: The first Japanese case. Int. J. Urol. 2011, 18, 735. [Google Scholar] [CrossRef] [PubMed]
- Pappachan, J.M.; Raj, B.; Thomas, S.; Hanna, F.W. Multiorgan dysfunction related to chronic ketamine abuse. In Baylor University Medical Center Proceedings; Taylor & Francis: Oxfordshire, UK, 2014; Volume 27, pp. 223–225. [Google Scholar] [CrossRef]
- Sassano-Higgins, S.; Baron, D.; Juarez, G.; Esmaili, N.; Gold, M. A Review of Ketamine Abuse and Diversion. Depress. Anxiety 2016, 33, 718–727. [Google Scholar] [CrossRef] [PubMed]
| Class | Molecular Structure | Pharmacology and Background |
|---|---|---|
| Ergolines LSD * semisynthetic | 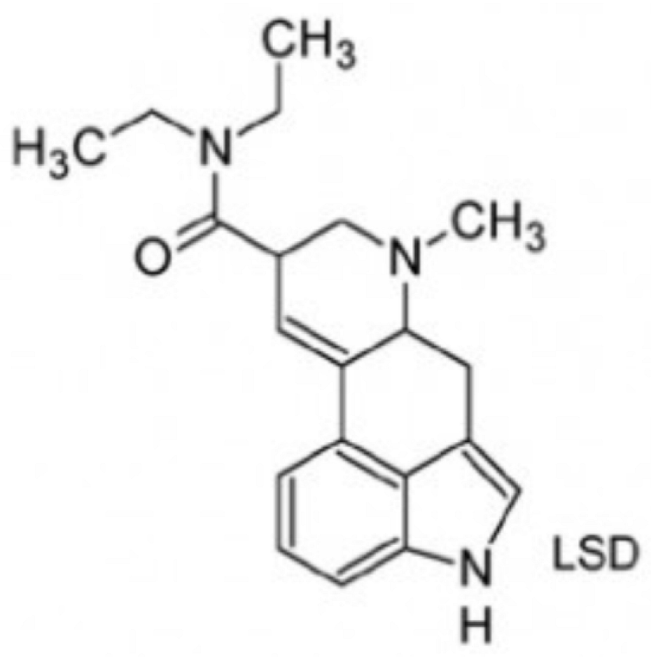 | Semisynthetic, derived from the class of ergolines (Claviceps purpurea). Mechanism of action—primarily mediated by the serotonergic agonism (5HT-1A and 5HT-2A) in the dorsal raphe and effects on dopaminergic, Trace Amine Associate receptor 1 (TAAR1) and 5HT-2A systems in the ventral tegmental area. LSD has high potency and high affinity for 5HT-2A receptors. First synthesized by the Swiss scientist Albert Hofmann in 1938, LSD was marketed under the brand name of Delysid® (LSD 25) until 1965, when Sandoz removed it from the market due to extensive non-medical use and experimentation with this drug. |
Indole ethylamines psilocybin (“magic mushrooms”) DMT (Psychotria viridis, Mimosa hostilis) natural—plant-based hallucinogens |  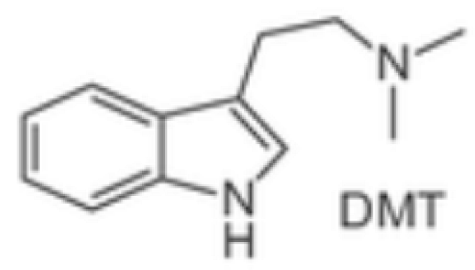  | Psilocybin—naturally occurring alkaloid from the class of indole ethylamine present in over 200 different species of fungus also known as “magic mushrooms.” Psilocybin has been used as an entheogenic ** substance in religious and sacramental ceremonies by South and Central American natives for centuries. Curiously, the Swiss scientist Albert Hofmann, who synthesized LSD in 1938, also isolated psilocybin. Similarly to LSD, psilocybin was also marketed by Sandoz in the 1950s under the brand name Indocybin®. Ayahuasca is a decoction prepared from Banisteriopsis caapi and a dimethyltryptamine (DMT), used by Indigenous cultures in South America (Brazil, Peru, Colombia and Ecuador) as part of traditional medicine and religion (Santo Daime, Uniao do Vegetal). Ayahuasca’s psychoactive effects derive mainly from DMT, which is only made possible through the combination of Banisteriopsis caapi and its effects as a selective and reversible MAO-A inhibitor, preventing DMT degradation by MAO-A. |
Phenylethylamines Mescaline, natural—plant-based MDMA, synthetic * |  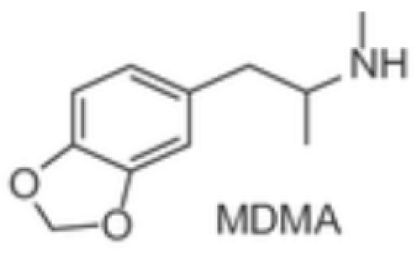 | Naturally occurring, mescaline is a psychedelic alkaloid found in cacti like peyote used for medical, recreational, and spiritual purposes in the US, Mexico, and South America. First scientific research with psychedelics is believed to date back to 1897, when Arthur Heffter initially isolated mescaline from the peyote cactus and further characterized its psychoactive properties. MDMA is a synthetic empathogen or entactogen drug with a complex pharmacology and both stimulant and hallucinogenic effects. First synthesized in 1912 by chemist Anton Kollisch, MDMA likely holds the highest potential for misuse, being scheduled as class I by the DEA in 1985. MDMA is also known as “ecstasy” or “molly” and considered a “club drug” commonly used in raves, festivals, and clubs. |
| Arylcyclohexylamine Ketamine synthetic |  | Synthetic noncompetitive NMDA-r antagonist; ketamine also modulates glutamatergic neurotransmission and influences dopaminergic and serotonergic circuits through effects on GABAergic interneurons. It seems to exert effects on mu, kappa, and delta opioid receptors. Ketamine is a dissociative anesthetic synthesized in the early 1960s by Parke-Davis as a replacement for phencyclidine. Ketamine has been extensively used in human and veterinarian medicine |
| Authors | Target Condition | Inclusion/Exclusion Criteria | Intervention | Outcomes |
|---|---|---|---|---|
| Javier Calleja-Conde et al. [45]. | Alcohol use/use disorder | Number of studies included: 27 20 human studies 7 preclinical studies Inclusion criteria: research papers, experimental and observational data discussing the association between classic psychedelic use and alcohol consumption conducted in both human and animal studies published from 2000. Exclusion criteria: review, commentary, conference and interview papers, qualitative data studies, and no full-text available articles. Studies focused on other psychedelic substances, such as MDMA or ketamine. | LSD clinical: self-reported 11 ug/kg LSD preclinical: 25–50 ug/lg Psilocybin clinical: 0.3–0.4 mg/kg Psilocybin preclinical: 1–2.5 mg/kg Ayahuasca (DMT) clinical: Ayahuasca (DMT) preclinical: 0.13–500 mg/kg No additional psychotherapies were listed for any of the interventions | LSD/Psilocybin clinical: 86.7% of participants reported a diminished effect of alcohol; 83% no longer met criteria for AUD. A total of 58% reduced or stopped alcohol consumption. LSD preclinical: alcohol consumption in mice reduced. Psilocybin clinical: mixed reports, some studies reported reduced alcohol consumption. Other studies reported no significant difference. Psilocybin preclinical: 40–50% reduction in alcohol consumption. Ayahuasca (DMT) clinical: 46.3% reduced alcohol consumption. Generally associated with improvement in AUD outcomes. One study reported 89.3% of participants consuming alcohol in past 30 days with 31.4% of participants reporting binge drinking behavior. Ayahuasca (DMT) preclinical: prevented alcohol-induced behavioral sensitization. Mescaline clinical: n individuals with previous AUD, 76% reported improvements in alcohol consumption. Another study reported increased alcohol consumption in youth. |
| Juan José Fuentes et al. [50]. | Patients with a diagnosis of mental illness (neurotic symptoms, anxiety associated with life-threatening diseases) Alcohol/AUD and opioid (heroin) use disorder | Number of studies included: 11 Inclusion criteria: randomized controlled clinical trials involving patients with a diagnosis of mental illness. Exclusion criteria: experimental studies in healthy volunteers, trials with no control group or not randomized, animal studies, observational studies, review papers, qualitative studies, case reports, opinion pieces or comments, letters or editorials, conference abstract, posters and books chapters. | LSD clinical: 20–800 mcg/kg with group or individual therapy and without therapy | The vast majority of studies showed positive short-term changes in alcohol use patterns, abstinence, and reduced drinking behaviors following LSD intervention. Some studies, however, found no significant difference. |
| Alessio Mosca et al. [47]. | Alcohol, opioids/heroin, cocaine, cannabis, crack, psilocybin, LSD, benzodiazepine, amphetamine, ecstasy, morphine, mushrooms | Number of studies included: 31 Included: case report/series, open-label studies, observational study, survey, double-blind placebo-controlled study. Exclusion criteria: non-original studies (e.g., review, commentary, editorial, book chapter); non-full-text articles (e.g., conference abstract); language other than English; animal/in vitro studies: articles not dealing with ibogaine/noribogaine; articles not dealing with SUD treatment | Ibogaine clinical: 70 mg/kg no additional psychotherapy listed | The results show some efficacy of ibogaine in the treatment of SUDs, but its cardiotoxicity and mortality are concerning. Some studies included showed opioid use cessation and reduced opioid withdrawal symptoms, reduced alcohol cravings and related outcomes. Many studies reported severe adverse side effects of ibogaine and noribogaine, including cardiac toxicity (cardiac arrest, bradycardia, QT prolongation, Torsade de Pointes), multi-organ failure, and death. |
| Angela N. Phan and Garth E. Terry [51]. | Cannabis use disorder | Number of studies included: 4 Inclusion criteria: primary research and reports in individuals with or at risk for cannabis use disorder (e.g., individuals who use cannabis heavily or frequently, cannabis abuse, cannabis dependence), and in whom cannabis use and/or risks associated with CUD were reported before and after receipt of a psychedelic. Exclusion criteria: literature that included psychedelics and CUD that are without direct interaction (i.e., indirectly described in the clinical sample but not under study, and/or cannabis use, or risks associated with CUD were not reported after psychedelic use or administration), observational or epidemiological studies evaluating polysubstance abuse that included cannabis and repeated or chronic use of psychedelics (e.g., cohort description of cannabis use in a study of those who use MDMA regularly). Reviews and basic science (e.g., animal studies). | MDMA clinical: 40–180 mg/kg with psychotherapy LSD clinical: self-reported, no additional psychotherapy reported | MDMA clinical: No significant difference. LSD clinical: Cannabis use reduced. |
| Lucas Silva Rodrigues et al. [49]. | Amphetamine and Alcohol | Number of studies included: 9 Inclusion criteria: randomized and open-label clinical studies using ayahuasca or any of its alkaloids in the treatment of SUDs; observational studies describing the use of ayahuasca or any of its alkaloids in the treatment of SUDs; quantitative studies with a statistical analysis comparing two or more groups at the same time or at least two different time points in the same group; studies that used standardized instruments to assess the pattern of use of associated substances or symptoms, preclinical studies using ayahuasca or any of its alkaloids in experimental models of SUDs, studies published between 2016 and 2020; and studies published in English, Spanish or Portuguese. Exclusion criteria: studies used as results from the previous review | Ayahuasca clinical: dose not listed and no additional psychotherapy Ayahuasca preclinical: 0.26–2 mL/kg, 30–300 mg/kg | Ayahuasca clinical: reduced alcohol and tobacco consumption, reduced prevalence of AUD, and significant decrease in dependency. Ayahuasca preclinical: prevented preference for amphetamines, reduced psychomotor agitation caused by amphetamines, decreased alcohol-induced conditioned place preference. Ayahuasca seems to prevent an increase in Fos levels in the nucleus accumbens after re-exposure to methylphenidate. |
| Raman Sharma et al. [53]. | Alcohol, Opioid, Nicotine and Cocaine Problematic substance use | Number of studies included: 7 studies across 10 papers Inclusion criteria: empirical studies using any study design as long as SUD or substance misuse was the primary diagnosis of the participants in the studies; they were included regardless of the presence of other comorbid physical or mental health problems. Exclusion criteria: if there was insufficient or unclear information on treatment regimen using psychedelics (e.g., substance used, and dosage) or psychedelic-assisted psychotherapy (e.g., type of psychotherapy, duration, frequency), prohibiting reliable replication of treatment and rigorous assessment of study against eligibility criteria. Also excluded were non-primary studies, ineligible population, or intervention, no usable outcome data, ongoing studies and unable to access. | Psilocybin clinical: 0.3–70 mg/kg, with and without psychotherapy Ibogaine clinical: 3–12 mg/kg, with and without additional psychotherapy Noribogaine clinical: 60–240 mg, no additional psychotherapy Ayahuasca clinical: dose not listed but included psychotherapy | Psilocybin clinical: decreased number of alcohol drinking days and heavy drinking days. Reduced cravings and improved alcohol abstinence. Also reduced nicotine withdrawals and cravings and improved abstinence. Ibogaine clinical: improved opioid abstinence and opioid withdrawal. Noribogaine clinical: withdrawal increased prior to medications for opioid use disorder. Ayahuasca clinical: reduced alcohol, nicotine, and cocaine use. No difference in opioid use, and increased hallucinogen use. Useful for drug problems = 91.7% -Given insight into self-destructive behaviors = 86.7% -Mindful of need to become sober/abstinent now = 68.3% |
| Dakota Sicignano et al. [46]. | Alcohol use disorder | Number of studies included: 6 Inclusion criteria: randomized controlled trials (RCTs) where >90% of the participants had alcohol use disorder and where a psychedelic therapy (LSD, MDMA, psilocybin, mescaline, ayahuasca, dipropyltryptamine or dimethyltrypamine) was compared to any control. Exclusion criteria: observational studies, reviews, letters to editor, and conference abstracts, written in any language other than English and trials conducted entirely in patients with a history of schizophrenia or psychosis. | Psilocybin clinical: 25/70 mg/kg–25–40/70 mg/kg, no additional psychotherapy LSD clinical: 3–800 ug, no additional psychotherapy | Psilocybin clinical: associated with abstinence or substantial reductions in alcohol use. LSD clinical: abstinence or substantial reductions in alcohol use. |
| S. K. Spoelstra et al. [48]. | Nicotine and tobacco use disorder (smoking) | Number of studies included: 8 Inclusion criteria: research that focused on the relation between the use of classic or non-classic psychedelics and outcomes related to nicotine smoking (no other addictions), utilized qualitative or quantitative empirical data (both clinical and non-clinical studies were included, was published in English and peer reviewed Exclusion criteria: case reports, systematic reviews, and case studies, or studies with no focus on smoking | Ayahuasca clinical: no dose and no additional psychotherapy Psilocybin clinical: some with and some without psychotherapy, most doses were not listed and only one study used a precise dose of 20–30/70 mg/kg Psychedelics clinical: (“magic”) mushrooms, LSD, morning glory seeds, mescaline, peyote cactus, San Pedro cactus, dimethyltryptamine (DMT), and Ayahuasca | Ayahuasca clinical: 69.2% of participants self-reported quitting smoking, 18.3% reported reducing smoking, and 12.5% reported quitting for a period of time, then returning to tobacco smoking. Psilocybin clinical: 67% of participants were abstainers; another study reported 80% abstinence at 6-month follow-up. Psychedelics clinical: 38% of participants reported continuous smoking cessation. |
| Alexander Trope et al. [54]. | Alcohol use disorder | Number of studies included: 12 Inclusion criteria: article that contained some description of group methods, demographic and diagnostic information, and quantified outcome data. Exclusion criteria: inability to locate the abstract or full text in English or Spanish | LSD clinical: 25–1000 ug, with psychotherapy | LSD clinical: no significant differences in abstinence of alcohol between groups over 6–18-month follow-up. Cannot reliably determine the safety of interventions given that the assessment and reporting of adverse events were inconsistent. |
| Pim B. van der Meer et al. [52]. | Alcohol use disorder and tobacco use disorder | Number of studies included: 6 Inclusion criteria: intervention with ≥1 dose of psilocybin; clinical trial (open-label [pilot] studies, single-blind, or double-blind [placebo-controlled] trials); diagnosis of a SUD or non-substance-related disorder [i.e., diagnosed by the general practitioner or by a structured clinical interview based on Diagnostic and Statistical Manual of Mental Disorders (DSM) or International Classification of Diseases (ICD) criteria], adult patients (18 years); patients, and language of the manuscript was English, Spanish, Portuguese, Dutch, German, or French. Exclusion criteria: animal studies; experimental studies in healthy volunteers; observational studies; review papers; qualitative studies; opinion pieces or comments; letters or editorials; conference abstracts or posters; and case reports. | Psilocybin clinical: 6–70 mg with and without psychotherapy LSD clinical: 100–800 mcg | Psilocybin clinical: all four clinical trials indicated a beneficial effect of psilocybin-assisted therapy on SUD symptoms. Number of heavy alcohol drinking days reduced, and abstinence increased. Nicotine abstinence increased—7-day point prevalence abstinence 80% at week 26 and 67% at week 52 based on both biomarkers and self-report. Both studies that alternated LSD and psilocybin as well as included or did not include psychotherapy resulted in improvements in alcohol and nicotine use disorder. |
| Authors | Target Condition | Inclusion/Exclusion Criteria | Interventions | Outcomes |
|---|---|---|---|---|
| Kelson et al. (2023) [55]. | AUD | Number of studies included: 11 studies with 854 participants Inclusion: adults diagnosed with AUD; studies assessing ketamine efficacy for AUD. Exclusion: non-AUD populations: studies focused solely on ketamine metabolites. | Ketamine IV: 0.5–2.0 mg/kg. Psychotherapy, behavioral therapy, standard AUD medications (acamprosate, naltrexone, disulfiram). | The results were mixed with respect to relapse, craving, and withdrawal symptoms. Ketamine may improve heavy drinking and increase the number of post-infusion abstinent days. It may be an effective therapeutic modality for people with alcohol use disorders who fail to respond to FDA-approved first-line agents. |
| Garel et al. (2022) [57] | AUD | Number of studies included: 8 studies, 634 participants Inclusion: studies examining ketamine use for AUD; short-term and long-term alcohol consumption reduction. Exclusion: non-peer-reviewed studies; non-human studies. | Ketamine IV: 0.5–2.0 mg/kg. Psychotherapy, psychedelics, alcohol-related memory retrieval exercises. | Ketamine interventions appear to be safe and possibly effective for alcohol use/abstinence and cravings. Equivocal evidence related to alcohol withdrawal management. All the studies were determined to be at critical risk of bias. |
| Jones et al. (2018) [41] | CUD, OUD, cannabis use disorder | Number of studies included: 7 studies—2 on AUD, 2 on cocaine use disorder, and 3 on OUD Inclusion: studies on ketamine for stimulant and OUD; impact on cravings and abstinence. Exclusion: studies lacking ketamine treatment; non-human trials. | Ketamine IV: 0.4–2.0 mg/kg. Ketamine IM: 2–3 mg/kg. Behavioral therapy, psychotherapy, pharmacotherapy for OUD, psychedelic-assisted therapy. | Both cocaine studies found improvements in cravings and decreased cocaine use rates. Studies of alcohol and opioid use disorders found improvement in abstinence rates in the ketamine group, with significant between-group effects noted for up to two years following a single infusion. Results suggest that ketamine may facilitate abstinence across multiple substances of abuse. |
| Kew et al. (2023) [58] | PTSD, anxiety, OCD | Number of studies included: 19 studies (n = 1008 participants)—7 studies examined ketamine for the treatment of SUD—n = 3 for AUD, n = 2 for OUD (“heroin dependence”), n = 1 for cocaine use disorder, n = 1 for cannabis use disorder Inclusion: studies combining ketamine with psychotherapy for psychiatric disorders. Exclusion: case reports, non-human studies. | Ketamine IV: 0.5 mg/kg (includes repeated infusions). CBT, supportive psychotherapy, group therapy. | For AUD, ketamine treatment performed better than saline control and treatment as usual. For OUD (heroin dependence), patients allocated to higher-dose ketamine and repeated ketamine/psychotherapy groups had better outcomes (abstinence). Abstinence from cocaine was observed in 48.2% of patients receiving ketamine and MBRP compared with 10.7% of patients receiving midazolam and MBRP. Twelve days post-infusion, there was a significantly greater proportion of cannabis-abstinent patients in the ketamine group compared with the midazolam group. |
| Walsh et al. (2022) [59] | Mental health, SUDs | Number of studies included: 83 published reports—33 systematic reviews, 29 RCT, two randomized trials without placebo, three non-randomized trials with controls, six open-label trials and 10 retrospective reviews. 14 studies examining ketamine as a treatment for SUD, including six studies focusing on AUD, five on cocaine use disorder, and three on OUD Inclusion: human studies on ketamine for mental health disorders, systematic reviews, meta-analyses. Exclusion: animal studies. | Ketamine IV, IM, and IN: 0.4–3 mg/kg. Psychotherapy, CBT, standard mental health pharmacotherapies. | Systematic reviews and meta-analyses provide support for robust, rapid and transient antidepressant and anti-suicidal effects of ketamine. Evidence for other indications is less robust, although it suggests similarly positive yet short-lived effects. |
| Drozdz et al. (2022) [56] | KAP for SUD and mental health disorders | Number of studies included: 17 (7 RCT, 5 case studies or case series; 4 were open-label trials; and 1 retrospective study.) Inclusion: studies reporting on ketamine-assisted psychotherapy for pain, mental health, and SUDs. Exclusion: animal studies; studies focused on recreational ketamine use. | ketamine IV, IM, IN, and SL: 0.5–2.0 mg/kg (doses varied). Psychotherapy, psychedelic-assisted therapy, opioid tapering programs. | Ketamine in combination with psychotherapy appears to promote abstinence, enhance motivation, and improve cravings and relapse prevention in AUD, cocaine use disorder, cannabis use disorder, and OUD. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correa da Costa, S.; Bormann, N.L.; Oesterle, T.; McGinnis, M.T.; Ho, M.-F.; Vettleson-Trutza, S.A.; Rummans, T.; Gold, M.S. The Role of Psychedelics in the Treatment of Substance Use Disorders: An Overview of Systematic Reviews. Brain Sci. 2025, 15, 1056. https://doi.org/10.3390/brainsci15101056
Correa da Costa S, Bormann NL, Oesterle T, McGinnis MT, Ho M-F, Vettleson-Trutza SA, Rummans T, Gold MS. The Role of Psychedelics in the Treatment of Substance Use Disorders: An Overview of Systematic Reviews. Brain Sciences. 2025; 15(10):1056. https://doi.org/10.3390/brainsci15101056
Chicago/Turabian StyleCorrea da Costa, Sabrina, Nicholas L. Bormann, Tyler Oesterle, Michele T. McGinnis, Ming-Fen Ho, Sara A. Vettleson-Trutza, Teresa Rummans, and Mark S. Gold. 2025. "The Role of Psychedelics in the Treatment of Substance Use Disorders: An Overview of Systematic Reviews" Brain Sciences 15, no. 10: 1056. https://doi.org/10.3390/brainsci15101056
APA StyleCorrea da Costa, S., Bormann, N. L., Oesterle, T., McGinnis, M. T., Ho, M.-F., Vettleson-Trutza, S. A., Rummans, T., & Gold, M. S. (2025). The Role of Psychedelics in the Treatment of Substance Use Disorders: An Overview of Systematic Reviews. Brain Sciences, 15(10), 1056. https://doi.org/10.3390/brainsci15101056







