Task-Dependent Neural Activity in the Posterior Parietal Cortex Is Associated with Better Balance in Adults with Acquired Brain Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Outcome Measures
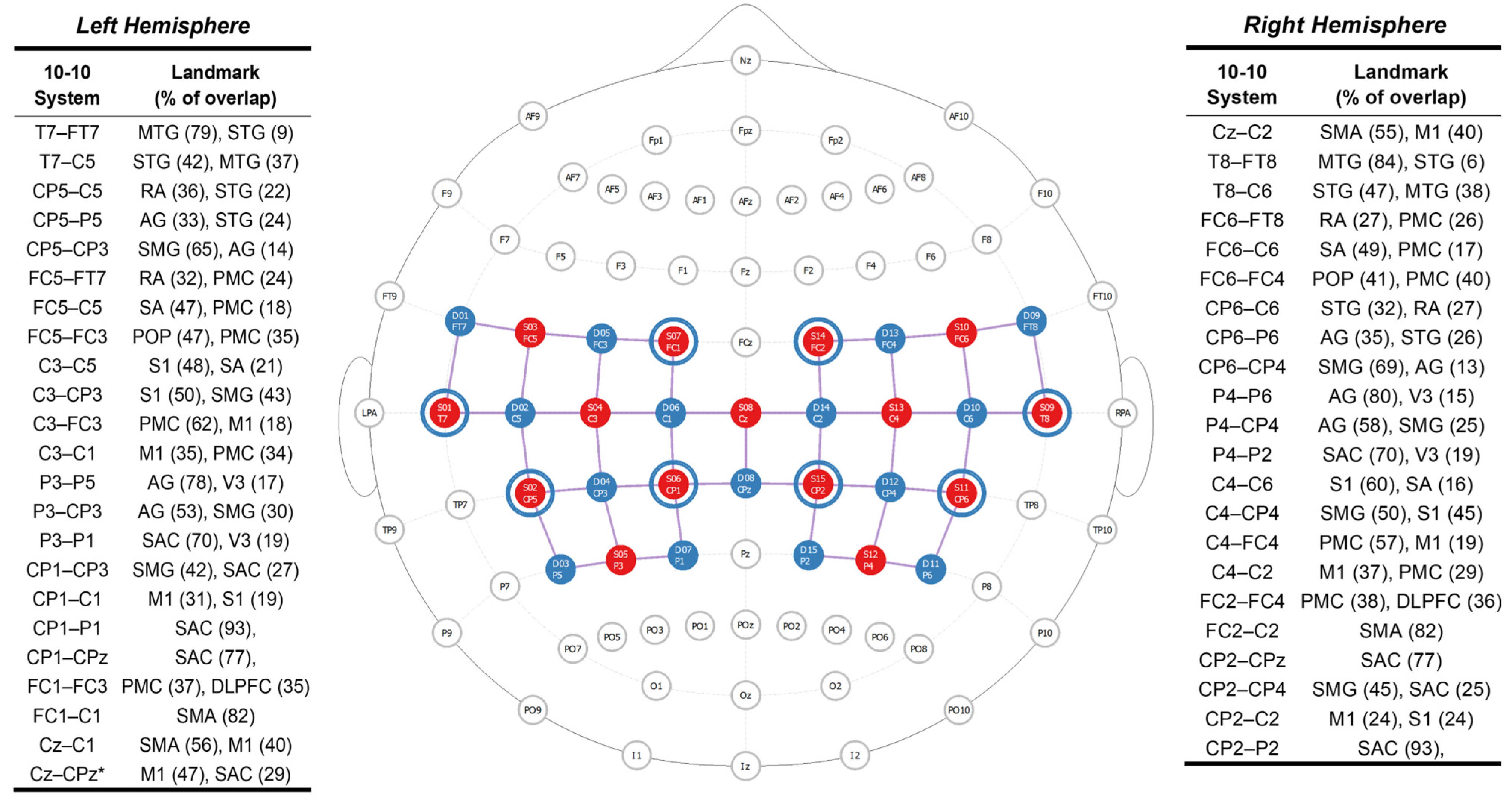
2.3.1. Task-Dependent Neural Activity
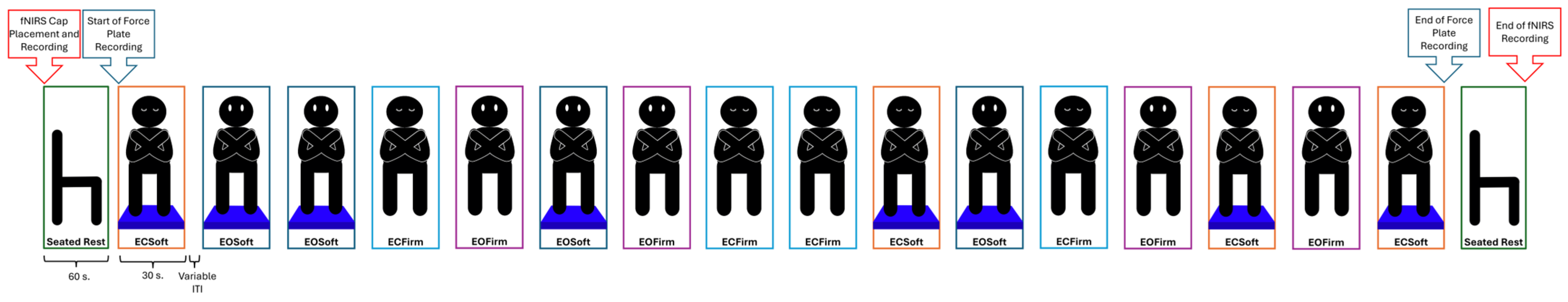
2.3.2. Balance Performance Data
2.4. Data Acquisition Procedures
2.5. Task-Dependent Neural Activity Data Processing
2.6. Balance Performance Data Reduction & Analysis
2.7. Statistical Analysis
3. Results
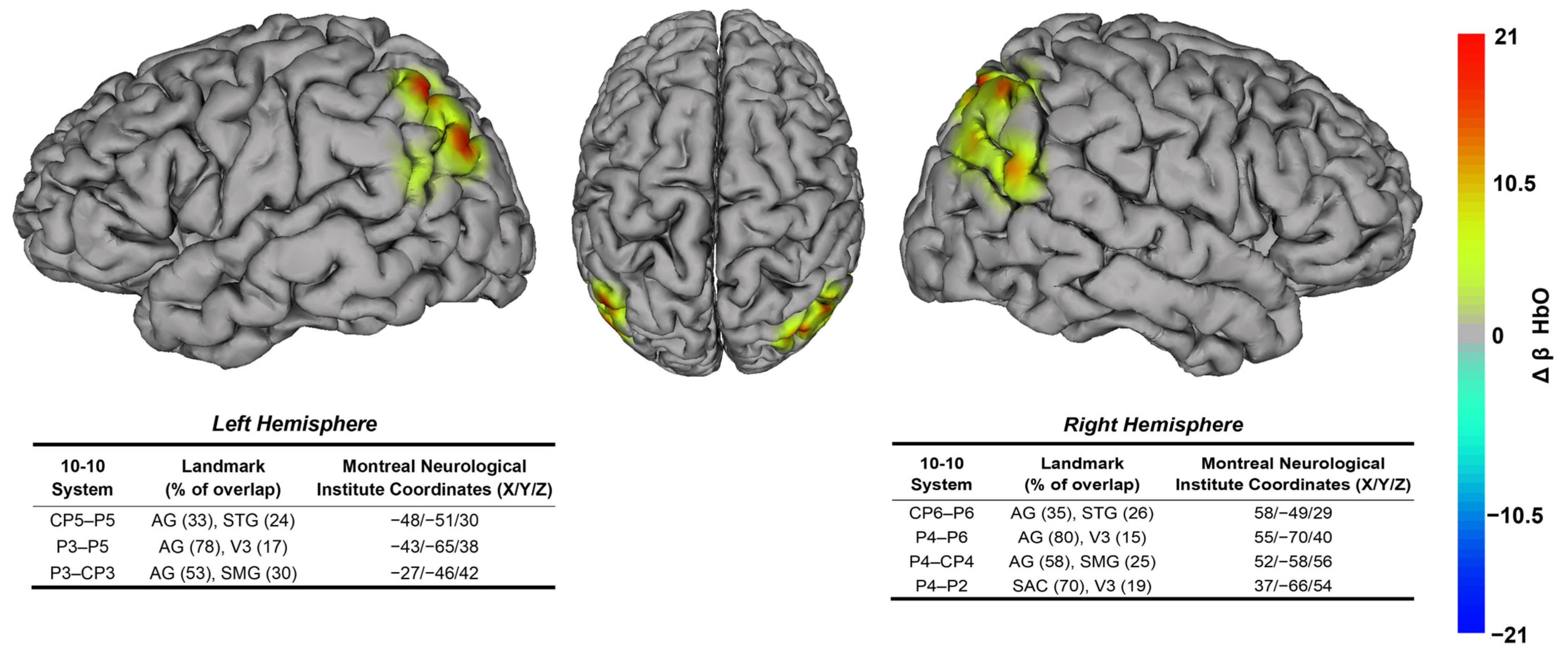
3.1. Task-Dependent Neural Activity Findings
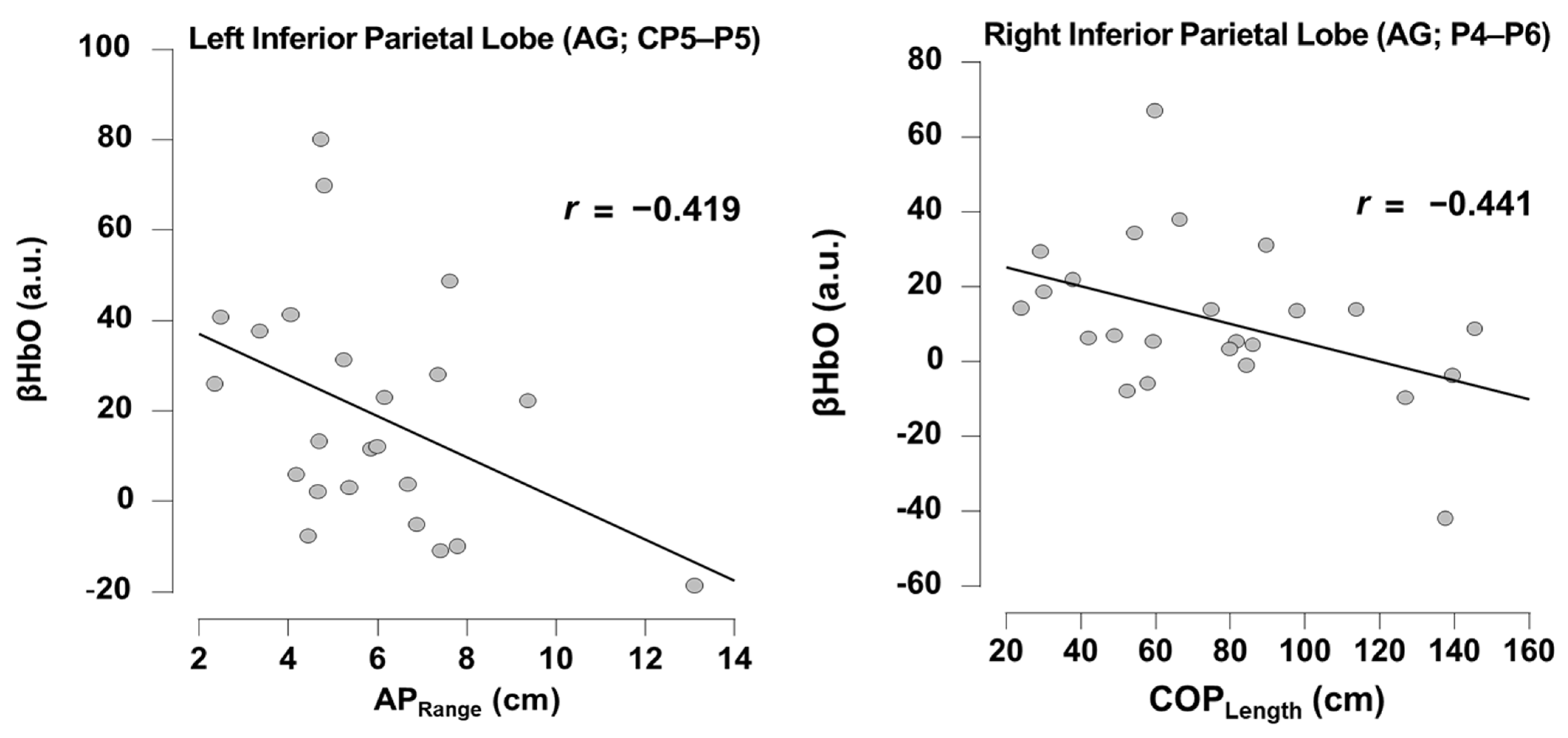
3.2. Relationship Between Task-Dependent Neural Activity and Balance
4. Discussion
4.1. Balance Performance Relates to Posterior Parietal Cortex Neural Substrates
4.2. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABI | Acquired Brain Injury |
| AP | Anterior–Posterior |
| APRange | Anterior–Posterior Range |
| CoP | Center of Pressure |
| COPLength | Center of Pressure Length |
| ECFirm | Eyes Closed Firm Surface |
| ECSoft | Eyes Closed Soft Surface |
| EOFirm | Eyes Open Firm Surface |
| EOSoft | Eyes Open Soft Surface |
| FDR | False Discovery Rate |
| fMRI | Functional Magnetic Resonance Imaging |
| fNIRS | Functional Near-infrared Spectroscopy |
| fOLD | FNIRS Optodes Location Decider |
| GLM | Generalized Linear Model |
| HbO | Oxygenated Hemoglobin |
| HbR | Deoxygenated Hemoglobin |
| IPL | Inferior Parietal Lobe |
| ITI | Inter-trial Interval |
| LED | Light-emitting Diode |
| ML | Medial–Lateral |
| MLRange | Medio-Lateral Range |
| RCT | Randomized Control Trial |
| ROI | Region of Interest |
| SPL | Superior Parietal Lobe |
| SSC | Short-separation Channel |
| TBI | Traumatic Brain Injury |
| TDDR | Temporal Derivative Distribution Repair |
References
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.-C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the Global Incidence of Traumatic Brain Injury. J. Neurosurg. 2019, 130, 1080–1097. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, Regional, and National Burden of Stroke and Its Risk Factors, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Hoofien, D.; Gilboa, A.; Vakil, E.; Donovick, P.J. Traumatic Brain Injury (TBI) 10–20 Years Later: A Comprehensive Outcome Study of Psychiatric Symptomatology, Cognitive Abilities and Psychosocial Functioning. Brain Inj. 2001, 15, 189–209. [Google Scholar] [CrossRef] [PubMed]
- Grauwmeijer, E.; Heijenbrok-Kal, M.H.; Peppel, L.D.; Hartjes, C.J.; Haitsma, I.K.; De Koning, I.; Ribbers, G.M. Cognition, Health-Related Quality of Life, and Depression Ten Years after Moderate to Severe Traumatic Brain Injury: A Prospective Cohort Study. J. Neurotrauma 2018, 35, 1543–1551. [Google Scholar] [CrossRef]
- Tasseel-Ponche, S.; Yelnik, A.P.; Bonan, I.V. Motor Strategies of Postural Control after Hemispheric Stroke. Neurophysiol. Clin. Neurophysiol. 2015, 45, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.A.; Van Puymbroeck, M.; Altenburger, P.A.; Miller, K.K.; Combs, S.A.; Page, S.J. Balance Is Associated with Quality of Life in Chronic Stroke. Top. Stroke Rehabil. 2013, 20, 340–346. [Google Scholar] [CrossRef]
- Slobounov, S.; Wu, T.; Hallett, M. Neural Basis Subserving the Detection of Postural Instability: An fMRI Study. Mot. Control 2006, 10, 69–89. [Google Scholar] [CrossRef]
- Surgent, O.J.; Dadalko, O.I.; Pickett, K.A.; Travers, B.G. Balance and the Brain: A Review of Structural Brain Correlates of Postural Balance and Balance Training in Humans. Gait Posture 2019, 71, 245–252. [Google Scholar] [CrossRef]
- Taube, W.; Mouthon, M.; Leukel, C.; Hoogewoud, H.-M.; Annoni, J.-M.; Keller, M. Brain Activity during Observation and Motor Imagery of Different Balance Tasks: An fMRI Study. Cortex 2015, 64, 102–114. [Google Scholar] [CrossRef]
- Scarapicchia, V.; Brown, C.; Mayo, C.; Gawryluk, J.R. Functional Magnetic Resonance Imaging and Functional Near-Infrared Spectroscopy: Insights from Combined Recording Studies. Front. Hum. Neurosci. 2017, 11, 419. [Google Scholar] [CrossRef]
- Pinti, P.; Aichelburg, C.; Gilbert, S.; Hamilton, A.; Hirsch, J.; Burgess, P.; Tachtsidis, I. A Review on the Use of Wearable Functional Near-Infrared Spectroscopy in Naturalistic Environments. Jpn. Psychol. Res. 2018, 60, 347–373. [Google Scholar] [CrossRef] [PubMed]
- Pinti, P.; Tachtsidis, I.; Hamilton, A.; Hirsch, J.; Aichelburg, C.; Gilbert, S.; Burgess, P.W. The Present and Future Use of Functional Near-infrared Spectroscopy (fNIRS) for Cognitive Neuroscience. Ann. N. Y. Acad. Sci. 2020, 1464, 5–29. [Google Scholar] [CrossRef]
- Scholkmann, F.; Kleiser, S.; Metz, A.J.; Zimmermann, R.; Mata Pavia, J.; Wolf, U.; Wolf, M. A Review on Continuous Wave Functional Near-Infrared Spectroscopy and Imaging Instrumentation and Methodology. NeuroImage 2014, 85, 6–27. [Google Scholar] [CrossRef]
- Ferrari, M.; Quaresima, V. A Brief Review on the History of Human Functional Near-Infrared Spectroscopy (fNIRS) Development and Fields of Application. NeuroImage 2012, 63, 921–935. [Google Scholar] [CrossRef]
- Mihara, M.; Miyai, I.; Hatakenaka, M.; Kubota, K.; Sakoda, S. Role of the Prefrontal Cortex in Human Balance Control. NeuroImage 2008, 43, 329–336. [Google Scholar] [CrossRef]
- Takakura, H.; Nishijo, H.; Ishikawa, A.; Shojaku, H. Cerebral Hemodynamic Responses During Dynamic Posturography: Analysis with a Multichannel Near-Infrared Spectroscopy System. Front. Hum. Neurosci. 2015, 9, 620. [Google Scholar] [CrossRef] [PubMed]
- Mihara, M.; Miyai, I.; Hattori, N.; Hatakenaka, M.; Yagura, H.; Kawano, T.; Kubota, K. Cortical Control of Postural Balance in Patients with Hemiplegic Stroke. NeuroReport 2012, 23, 314–319. [Google Scholar] [CrossRef]
- Stephens, J.A.; Hernandez-Sarabia, J.A.; Sharp, J.L.; Leach, H.J.; Bell, C.; Thomas, M.L.; Buryznska, A.Z.; Weaver, J.A.; Schmid, A.A. Adaptive Yoga versus Low-Impact Exercise for Adults with Chronic Acquired Brain Injury: A Pilot Randomized Control Trial Protocol. Front. Hum. Neurosci. 2023, 17, 1291094. [Google Scholar] [CrossRef]
- King, P.R.; Donnelly, K.T.; Donnelly, J.P.; Dunnam, M.; Warner, G.; Kittleson, C.J.; Bradshaw, C.B.; Alt, M.; Meier, S.T. Psychometric Study of the Neurobehavioral Symptom Inventory. J. Rehabil. Res. Dev. 2012, 49, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Zimeo Morais, G.A.; Balardin, J.B.; Sato, J.R. fNIRS Optodes’ Location Decider (fOLD): A Toolbox for Probe Arrangement Guided by Brain Regions-of-Interest. Sci. Rep. 2018, 8, 3341. [Google Scholar] [CrossRef]
- Brigadoi, S.; Cooper, R.J. How Short Is Short? Optimum Source–Detector Distance for Short-Separation Channels in Functional near-Infrared Spectroscopy. Neurophotonics 2015, 2, 025005. [Google Scholar] [CrossRef]
- Tachtsidis, I.; Scholkmann, F. False Positives and False Negatives in Functional Near-Infrared Spectroscopy: Issues, Challenges, and the Way Forward. Neurophotonics 2016, 3, 031405. [Google Scholar] [CrossRef] [PubMed]
- Goble, D.J.; Khan, E.; Baweja, H.S.; O’Connor, S.M. A Point of Application Study to Determine the Accuracy, Precision and Reliability of a Low-Cost Balance Plate for Center of Pressure Measurement. J. Biomech. 2018, 71, 277–280. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, S.M.; Baweja, H.S.; Goble, D.J. Validating the BTrackS Balance Plate as a Low Cost Alternative for the Measurement of Sway-Induced Center of Pressure. J. Biomech. 2016, 49, 4142–4145. [Google Scholar] [CrossRef]
- Richmond, S.B.; Dames, K.D.; Goble, D.J.; Fling, B.W. Leveling the Playing Field: Evaluation of a Portable Instrument for Quantifying Balance Performance. J. Biomech. 2018, 75, 102–107. [Google Scholar] [CrossRef]
- Peirce, J.W. Generating Stimuli for Neuroscience Using PsychoPy. Front. Neuroinform. 2008, 2, 10. [Google Scholar] [CrossRef]
- Peirce, J.W. PsychoPy—Psychophysics Software in Python. J. Neurosci. Methods 2007, 162, 8–13. [Google Scholar] [CrossRef]
- Peirce, J.; Gray, J.R.; Simpson, S.; MacAskill, M.; Höchenberger, R.; Sogo, H.; Kastman, E.; Lindeløv, J.K. PsychoPy2: Experiments in Behavior Made Easy. Behav. Res. Methods 2019, 51, 195–203. [Google Scholar] [CrossRef]
- Pollonini, L.; Olds, C.; Abaya, H.; Bortfeld, H.; Beauchamp, M.S.; Oghalai, J.S. Auditory Cortex Activation to Natural Speech and Simulated Cochlear Implant Speech Measured with Functional Near-Infrared Spectroscopy. Hear. Res. 2014, 309, 84–93. [Google Scholar] [CrossRef]
- Baker, W.B.; Parthasarathy, A.B.; Busch, D.R.; Mesquita, R.C.; Greenberg, J.H.; Yodh, A.G. Modified Beer-Lambert Law for Blood Flow. Biomed. Opt. Express 2014, 5, 4053–4075. [Google Scholar] [CrossRef]
- Fishburn, F.A.; Ludlum, R.S.; Vaidya, C.J.; Medvedev, A.V. Temporal Derivative Distribution Repair (TDDR): A Motion Correction Method for fNIRS. NeuroImage 2019, 184, 171–179. [Google Scholar] [CrossRef]
- Prieto, T.E.; Myklebust, J.B.; Hoffmann, R.G.; Lovett, E.G.; Myklebust, B.M. Measures of Postural Steadiness: Differences between Healthy Young and Elderly Adults. IEEE Trans. Biomed. Eng. 1996, 43, 956–966. [Google Scholar] [CrossRef]
- Goble, D.J.; Brown, E.C.; Marks, C.R.C.; Baweja, H.S. Expanded Normative Data for the Balance Tracking System Modified Clinical Test of Sensory Integration and Balance Protocol. Med. Devices Sens. 2020, 3, e10084. [Google Scholar] [CrossRef]
- Hernandez-Sarabia, J.A.; Schmid, A.A.; Sharp, J.L.; Stephens, J.A. Intervention-Induced Changes in Balance and Task-Dependent Neural Activity in Adults with Acquired Brain Injury: A Pilot Randomized Control Trial. Sensors 2024, 24, 4047. [Google Scholar] [CrossRef]
- JASP Team. JASP, version 0.19.0.0; JASP Team: Amsterdam, The Netherlands, 2024.
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Fujimoto, H.; Mihara, M.; Hattori, N.; Hatakenaka, M.; Kawano, T.; Yagura, H.; Miyai, I.; Mochizuki, H. Cortical Changes Underlying Balance Recovery in Patients with Hemiplegic Stroke. NeuroImage 2014, 85, 547–554. [Google Scholar] [CrossRef]
- Goble, D.J.; Coxon, J.P.; Van Impe, A.; Geurts, M.; Doumas, M.; Wenderoth, N.; Swinnen, S.P. Brain Activity during Ankle Proprioceptive Stimulation Predicts Balance Performance in Young and Older Adults. J. Neurosci. 2011, 31, 16344–16352. [Google Scholar] [CrossRef] [PubMed]
- Ribot-Ciscar, E.; Rossi-Durand, C.; Roll, J.-P. Muscle Spindle Activity Following Muscle Tendon Vibration in Man. Neurosci. Lett. 1998, 258, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Pellijeff, A.; Bonilha, L.; Morgan, P.S.; McKenzie, K.; Jackson, S.R. Parietal Updating of Limb Posture: An Event-Related fMRI Study. Neuropsychologia 2006, 44, 2685–2690. [Google Scholar] [CrossRef]
- Naito, E.; Scheperjans, F.; Eickhoff, S.B.; Amunts, K.; Roland, P.E.; Zilles, K.; Ehrsson, H.H. Human Superior Parietal Lobule Is Involved in Somatic Perception of Bimanual Interaction With an External Object. J. Neurophysiol. 2008, 99, 695–703. [Google Scholar] [CrossRef]
- Hagura, N.; Takei, T.; Hirose, S.; Aramaki, Y.; Matsumura, M.; Sadato, N.; Naito, E. Activity in the Posterior Parietal Cortex Mediates Visual Dominance over Kinesthesia. J. Neurosci. 2007, 27, 7047–7053. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Mottola, L.; Quaresima, V. Principles, Techniques, and Limitations of Near Infrared Spectroscopy. Can. J. Appl. Physiol. 2004, 29, 463–487. [Google Scholar] [CrossRef] [PubMed]
| All (N = 27) | Female (n = 18) | Male (n = 9) | |
|---|---|---|---|
| Mean ± SD (Min–Max) or N (%) | Mean ± SD or n (%) | Mean ± SD or n (%) | |
| Age (years) | 51.30 ± 18.67 (19.00–82.00) | 50.22 ± 16.14 | 53.44 ± 23.91 |
| Time Since Most Recent Injury (years) | 13.30 ± 17.38 (0.50–62.00) | 15.51 ± 19.48 | 8.89 ± 11.93 |
| Current Time in Rehabilitation (years) | 0.88 ± 1.33 (0.00–5.00) * | 0.78 ± 1.18 * | 1.06 ± 1.63 |
| Ethnicity (not Hispanic) | 26 (96.30) | 17 (94.44) | 9 (100.00) |
| Race (white) | 27 (100.00) | 18 (100.00) | 9 (100.00) |
| Brain Injury Type | |||
| Non-TBI | 11 (40.74) | 6 (33.33) | 5 (55.56) |
| TBI | 14 (51.85) | 11 (61.11) | 3 (33.33) |
| Both | 2 (7.41) | 1 (5.56) | 1 (11.11) |
| Educational Level | |||
| High School | 3 (11.11) | 0 (0.00) | 3 (33.33) |
| Some College | 8 (29.63) | 5 (27.78) | 3 (33.33) |
| College Graduate | 8 (29.63) | 7 (38.89) | 1 (11.11) |
| Some Post-graduate | 1 (3.70) | 1 (5.56) | 0 (0.00) |
| Post-graduate Degree | 7 (25.93) | 5 (27.78) | 2 (22.22) |
| Loss of Balance | |||
| Moderate | 21 (77.78) | 16 (88.89) | 5 (55.56) |
| Moderate to Severe | 3 (11.11) | 2 (11.11) | 1 (11.11) |
| Severe | 3 (11.11) | 0 (0.00) | 3 (33.33) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez-Sarabia, J.A.; Schmid, A.A.; Stephens, J.A. Task-Dependent Neural Activity in the Posterior Parietal Cortex Is Associated with Better Balance in Adults with Acquired Brain Injury. Brain Sci. 2025, 15, 1049. https://doi.org/10.3390/brainsci15101049
Hernandez-Sarabia JA, Schmid AA, Stephens JA. Task-Dependent Neural Activity in the Posterior Parietal Cortex Is Associated with Better Balance in Adults with Acquired Brain Injury. Brain Sciences. 2025; 15(10):1049. https://doi.org/10.3390/brainsci15101049
Chicago/Turabian StyleHernandez-Sarabia, Jesus A., Arlene A. Schmid, and Jaclyn A. Stephens. 2025. "Task-Dependent Neural Activity in the Posterior Parietal Cortex Is Associated with Better Balance in Adults with Acquired Brain Injury" Brain Sciences 15, no. 10: 1049. https://doi.org/10.3390/brainsci15101049
APA StyleHernandez-Sarabia, J. A., Schmid, A. A., & Stephens, J. A. (2025). Task-Dependent Neural Activity in the Posterior Parietal Cortex Is Associated with Better Balance in Adults with Acquired Brain Injury. Brain Sciences, 15(10), 1049. https://doi.org/10.3390/brainsci15101049





