Effectiveness of Two Models of Telerehabilitation in Improving Recovery from Subacute Upper Limb Disability after Stroke: Robotic vs. Non-Robotic
Abstract
1. Introduction
2. Materials and Methods
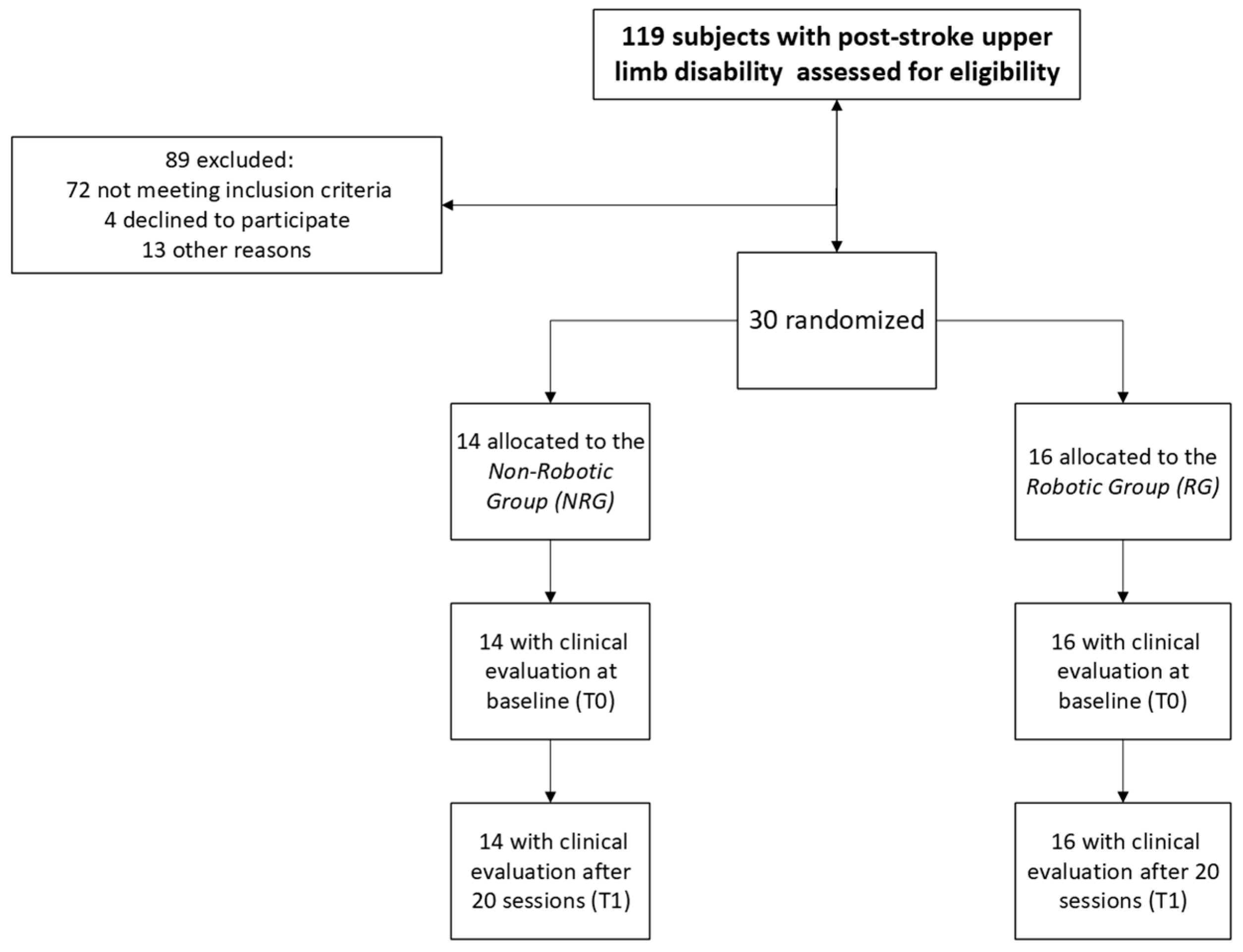
2.1. Study Design and Participants
2.2. Intervention
2.3. Clinical Evaluation
2.4. Statistical Analysis
3. Results
- a statistically significant interaction factor timeXgroup on the FMA-UE motor function (p = 0.006, η2 = 0.006), the BBT (p = 0.036, η2 = 0.148), and the TMT-A (p = 0.018, η2 = 0.011);
- a statistically significant main effect time on the FMA-UE motor function (p < 0.001, η2 = 0.033), the FMA-UE sensation (p < 0.001, η2 = 0.112), the ARAT (p < 0.001, η2 = 0.014), the BBT (p = 0.004, η2 = 0.263), the MoCA (p = 0.005, η2 = 0.027), the TMT-A (p < 0.001, η2 = 0.024), the TMT-B (p = 0.001, η2 = 0.032), and the BDI (p = 0.018, η2 = 0.039);
- no statistical relevance on NRS, SDMT, and STAY-Y1.
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atella, V.; Piano Mortari, A.; Kopinska, J.; Belotti, F.; Lapi, F.; Cricelli, C.; Fontana, L. Trends in age-related disease burden and healthcare utilization. Aging Cell 2019, 18, e12861. [Google Scholar] [CrossRef] [PubMed]
- Camussi, E.; Meneghetti, D.; Sbarra, M.L.; Rella, R.; Barillà, F.; Sassi, C.; Montali, L.; Annovazzi, C. COVID-19, people with disabilities, and the Italian government recovery: Investigating the impact and promoting psychological resources to prevent future emergencies. Front. Psychol. 2023, 14, 1260853. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G.; et al. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Thayabaranathan, T.; Donnan, G.A.; Howard, G.; Howard, V.J.; Rothwell, P.M.; Feigin, V.; Norrving, B.; Owolabi, M.; Pandian, J.; et al. Global Stroke Statistics 2019. Int. J. Stroke Off. J. Int. Stroke Soc. 2020, 15, 819–838. [Google Scholar] [CrossRef] [PubMed]
- Cieza, A.; Causey, K.; Kamenov, K.; Hanson, S.W.; Chatterji, S.; Vos, T. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 2006–2017. [Google Scholar] [CrossRef]
- Smith, L. Management of Patients with Stroke: Rehabilitation, Prevention and Management of Complications, and Discharge Planning: A National Clinical Guideline. June 2010. Available online: https://collections.nlm.nih.gov/catalog/nlm:nlmuid-101609293-pdf (accessed on 12 September 2024).
- Aprile, I.; Germanotta, M.; Cruciani, A.; Loreti, S.; Pecchioli, C.; Cecchi, F.; Montesano, A.; Galeri, S.; Diverio, M.; Falsini, C.; et al. Upper Limb Robotic Rehabilitation After Stroke: A Multicenter, Randomized Clinical Trial. J. Neurol. Phys. Ther. 2020, 44, 3–14. [Google Scholar] [CrossRef]
- Brunner, I.; Skouen, J.S.; Hofstad, H.; Aßmus, J.; Becker, F.; Sanders, A.-M.; Pallesen, H.; Qvist Kristensen, L.; Michielsen, M.; Thijs, L.; et al. Virtual Reality Training for Upper Extremity in Subacute Stroke (VIRTUES): A multicenter RCT. Neurology 2017, 89, 2413–2421. [Google Scholar] [CrossRef]
- Lee, H.Y.; Kim, Y.L.; Lee, S.M. Effects of virtual reality-based training and task-oriented training on balance performance in stroke patients. J. Phys. Ther. Sci. 2015, 27, 1883–1888. [Google Scholar] [CrossRef]
- Kwakkel, G.; Kollen, B.J.; Krebs, H.I. Effects of robot-assisted therapy on upper limb recovery after stroke: A systematic review. Neurorehabilit. Neural Repair. 2008, 22, 111–121. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Z.; Gu, J.; Tan, J.; Hu, T. Effectiveness of soft robotic glove versus repetitive transcranial magnetic stimulation in post-stroke patients with severe upper limb dysfunction: A randomised controlled trial. Front. Neurol. 2022, 13, 887205. [Google Scholar] [CrossRef]
- Chen, J.; Or, C.K.; Chen, T. Effectiveness of Using Virtual Reality–Supported Exercise Therapy for Upper Extremity Motor Rehabilitation in Patients With Stroke: Systematic Review and Meta-analysis of Randomized Controlled Trials. J. Med. Internet Res. 2022, 24, e24111. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, H.; Bosomworth, H.; Krebs, H.I.; van Wijck, F.; Howel, D.; Wilson, N.; Aird, L.; Alvarado, N.; Andole, S.; Cohen, D.L.; et al. Robot assisted training for the upper limb after stroke (RATULS): A multicentre randomised controlled trial. Lancet 2019, 394, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Morone, G.; Palomba, A.; Martino Cinnera, A.; Agostini, M.; Aprile, I.; Arienti, C.; Paci, M.; Casanova, E.; Marino, D.; LA Rosa, G.; et al. Systematic review of guidelines to identify recommendations for upper limb robotic rehabilitation after stroke. Eur. J. Phys. Rehabil. Med. 2021, 57, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, R.S.; Sorrentino, G.; Cassio, A.; Mazzoli, D.; Andrenelli, E.; Bizzarini, E.; Campanini, I.; Carmignano, S.M.; Cerulli, S.; Chisari, C.; et al. Robotic-assisted gait rehabilitation following stroke: A systematic review of current guidelines and practical clinical recommendations. Eur. J. Phys. Rehabil. Med. 2021, 57, 460–471. [Google Scholar] [CrossRef]
- Pavan, A.; Fasano, A.; Cortellini, L.; Lattanzi, S.; Papadopoulou, D.; Insalaco, S.; Germanotta, M.; Aprile, I. Implementation of a robot-mediated upper limb rehabilitation protocol for a customized treatment after stroke: A retrospective analysis. NeuroRehabilitation 2024, preprint, 1–10. [Google Scholar] [CrossRef]
- Campagnini, S.; Arienti, C.; Patrini, M.; Liuzzi, P.; Mannini, A.; Carrozza, M.C. Machine learning methods for functional recovery prediction and prognosis in post-stroke rehabilitation: A systematic review. J. Neuroeng. Rehabil. 2022, 19, 54. [Google Scholar] [CrossRef]
- Germanotta, M.; Cruciani, A.; Pecchioli, C.; Loreti, S.; Spedicato, A.; Meotti, M.; Mosca, R.; Speranza, G.; Cecchi, F.; Giannarelli, G.; et al. Reliability, validity and discriminant ability of the instrumental indices provided by a novel planar robotic device for upper limb rehabilitation. J. Neuroeng. Rehabil. 2018, 15, 39. [Google Scholar] [CrossRef]
- Germanotta, M.; Gower, V.; Papadopoulou, D.; Cruciani, A.; Pecchioli, C.; Mosca, R.; Speranza, G.; Falsini, C.; Cecchi, F.; Vannetti, F.; et al. Reliability, validity and discriminant ability of a robotic device for finger training in patients with subacute stroke. J. Neuroeng. Rehabil. 2020, 17, 1. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Topol, E.J. Telemedicine 2020 and the next decade. Lancet 2020, 395, 859. [Google Scholar] [CrossRef]
- Rossetto, F.; Mestanza Mattos, F.G.; Gervasoni, E.; Germanotta, M.; Pavan, A.; Cattaneo, D.; Aprile, I.; Baglio, F. Efficacy of telerehabilitation with digital and robotic tools for the continuity of care of people with chronic neurological disorders: The TELENEURO@REHAB protocol for a randomized controlled trial. Digit. Health 2024, 10, 20552076241228930. [Google Scholar] [CrossRef]
- Bouabida, K.; Lebouche, B.; Pomey, M.-P. Telehealth and COVID-19 Pandemic: An Overview of the Telehealth Use, Advantages, Challenges, and Opportunities during COVID-19 Pandemic. Healthcare 2022, 10, 2293. [Google Scholar] [CrossRef] [PubMed]
- Wosik, J.; Fudim, M.; Cameron, B.; Gellad, Z.F.; Cho, A.; Phinney, D.; Curtis, S.; Roman, M.; Poon, E.G.; Ferranti, J.; et al. Telehealth transformation: COVID-19 and the rise of virtual care. J. Am. Med. Inform. Assoc. 2020, 27, 957–962. [Google Scholar] [CrossRef]
- Omboni, S.; Padwal, R.S.; Alessa, T.; Benczúr, B.; Green, B.B.; Hubbard, I.; Kario, K.; Khan, N.A.; Konradi, A.; Logan, A.G.; et al. The worldwide impact of telemedicine during COVID-19: Current evidence and recommendations for the future. Connect. Health 2022, 1, 7–35. [Google Scholar] [CrossRef] [PubMed]
- Arshad Ali, S.; Bin Arif, T.; Maab, H.; Baloch, M.; Manazir, S.; Jawed, F.; Ochani, R.K. Global Interest in Telehealth During COVID-19 Pandemic: An Analysis of Google TrendsTM. Cureus 2020, 12, e10487. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, A.; Howes, S.; Murphy, P.J.; Deutsch, J.E.; Stokes, M.; Pedlow, K.; McDonough, S.M. Factors influencing the delivery of telerehabilitation for stroke: A systematic review. PLoS ONE 2022, 17, e0265828. [Google Scholar] [CrossRef]
- Lebioda, L.A.; Pedroso, B.; Dos Santos, M.E.C.; Pinto, G.M.C.; Welling, L.C. Neurological telerehabilitation in the COVID-19 era—Current perspectives through a bibliometric analysis. Front. Neurol. 2023, 14, 1227846. [Google Scholar] [CrossRef]
- Su, Z.; Guo, Z.; Wang, W.; Liu, Y.; Liu, Y.; Chen, W.; Zheng, M.; Michael, N.; Lu, S.; Wang, W.; et al. The effect of telerehabilitation on balance in stroke patients: Is it more effective than the traditional rehabilitation model? A meta-analysis of randomized controlled trials published during the COVID-19 pandemic. Front. Neurol. 2023, 14, 1156473. [Google Scholar] [CrossRef] [PubMed]
- Térémetz, M.; Garcia Alvarez, A.; Hanneton, S.; Roby-Brami, A.; Roche, N.; Bensmail, D.; Lindberg, P.; Robertson, J.V.G. Improving upper-limb and trunk kinematics by interactive gaming in individuals with chronic stroke: A single-blinded RCT. Ann. Phys. Rehabil. Med. 2022, 65, 101622. [Google Scholar] [CrossRef]
- Chien, W.-T.; Chong, Y.-Y.; Tse, M.-K.; Chien, C.-W.; Cheng, H.-Y. Robot-assisted therapy for upper-limb rehabilitation in subacute stroke patients: A systematic review and meta-analysis. Brain Behav. 2020, 10, e01742. [Google Scholar] [CrossRef]
- Jakob, I.; Kollreider, A.; Germanotta, M.; Benetti, F.; Cruciani, A.; Padua, L.; Aprile, I. Robotic and Sensor Technology for Upper Limb Rehabilitation. PM&R 2018, 10, S189–S197. [Google Scholar] [CrossRef]
- Conti, S.; Bonazzi, S.; Laiacona, M.; Masina, M.; Coralli, M.V. Montreal Cognitive Assessment (MoCA)-Italian version: Regression based norms and equivalent scores. Neurol. Sci. 2015, 36, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.L.Y.; Mcnamara, R.J.; Dennis, S.M.; Moddel, C.; Alison, J.A.; Mckenzie, D.K.; Mckeough, Z.J. Satisfaction and Experience With a Supervised Home-Based Real-Time Videoconferencing Telerehabilitation Exercise Program in People with Chronic Obstructive Pulmonary Disease (COPD). Int. J. Telerehabilitation 2016, 8, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Federici, S.; Bracalenti, M.; Meloni, F.; Luciano, J.V. World Health Organization disability assessment schedule 2.0: An international systematic review. Disabil. Rehabil. 2017, 39, 2347–2380. [Google Scholar] [CrossRef]
- World Health Organization. International Classification of Functioning, Disability and Health: Children and Youth Version: ICF-CY; World Health Organization: Geneva, Switzerland, 2007; ISBN 978-961-6659-38-3. Available online: https://iris.who.int/handle/10665/43737 (accessed on 9 October 2023).
- Fugl-Meyer, A.R.; Jääskö, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar] [CrossRef]
- Lyle, R.C. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int. J. Rehabil. Res. 1981, 4, 483–492. [Google Scholar] [CrossRef]
- Desrosiers, J.; Bravo, G.; Hébert, R.; Dutil, E.; Mercier, L. Validation of the Box and Block Test as a measure of dexterity of elderly people: Reliability, validity, and norms studies. Arch. Phys. Med. Rehabil. 1994, 75, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Downie, W.W.; Leatham, P.A.; Rhind, V.M.; Wright, V.; Branco, J.A.; Anderson, J.A. Studies with pain rating scales. Ann. Rheum. Dis. 1978, 37, 378–381. [Google Scholar] [CrossRef]
- Santangelo, G.; Siciliano, M.; Pedone, R.; Vitale, C.; Falco, F.; Bisogno, R.; Siano, P.; Barone, P.; Grossi, D.; Santangelo, F.; et al. Normative data for the Montreal Cognitive Assessment in an Italian population sample. Neurol. Sci. 2015, 36, 585–591. [Google Scholar] [CrossRef]
- Giovagnoli, A.R.; Del Pesce, M.; Mascheroni, S.; Simoncelli, M.; Laiacona, M.; Capitani, E. Trail making test: Normative values from 287 normal adult controls. Ital. J. Neurol. Sci. 1996, 17, 305–309. [Google Scholar] [CrossRef]
- Nocentini, U.; Giordano, A.; Di Vincenzo, S.; Panella, M.; Pasqualetti, P. The Symbol Digit Modalities Test—Oral version: Italian normative data. Funct. Neurol. 2006, 21, 93–96. [Google Scholar]
- Steer, R.A.; Cavalieri, T.A.; Leonard, D.M.; Beck, A.T. Use of the Beck depression inventory for primary care to screen for major depression disorders. Gen. Hosp. Psychiatry 1999, 21, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Spielberger, C.D.; Gorsuch, R.; Lushene, R.E.; Vagg, P.R.; Jacobs, G.A. Manual for the State-Trait Anxiety Inventory (Form Y1–Y2). In The Use of Psychological Testing for Treatment Planning and Outcomes Assessment, 2nd ed.; Consulting Psychologists Press: Palo Alto, CA, USA, 1983. [Google Scholar]
- Piron, L.; Tonin, P.; Trivello, E.; Battistin, L.; Dam, M. Motor tele-rehabilitation in post-stroke patients. Med. Inform. Internet Med. 2004, 29, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Piron, L.; Turolla, A.; Agostini, M.; Zucconi, C.; Cortese, F.; Zampolini, M.; Zannini, M.; Dam, M.; Ventura, L.; Battauz, M.; et al. Exercises for paretic upper limb after stroke: A combined virtual-reality and telemedicine approach. J. Rehabil. Med. 2009, 41, 1016–1102. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jin, W.; Dong, W.S.; Jin, Y.; Qiao, F.L.; Zhou, Y.F.; Ren, C.C. Effects of Home-based Telesupervising Rehabilitation on Physical Function for Stroke Survivors with Hemiplegia: A Randomized Controlled Trial. Am. J. Phys. Med. Rehabil. 2017, 96, 152–160. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, M.; Crotty, M.; Liu, E.; Killington, M.; Kwakkel, G.; van Wegen, E. Early Supported Discharge by Caregiver-Mediated Exercises and e-Health Support After Stroke: A Proof-of-Concept Trial. Stroke 2016, 47, 1885–1892. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Tomás, M.T.; Burillo-Lafuente, M.; Vicente-Parra, A.; Sanz-Rubio, M.C.; Suarez-Serrano, C.; Marcén-Román, Y.; Franco-Sierra, M.Á. Telerehabilitation as a Therapeutic Exercise Tool versus Face-to-Face Physiotherapy: A Systematic Review. Int. J. Environ. Res. Public Health 2023, 20, 4358. [Google Scholar] [CrossRef]
- Morone, G.; Pichiorri, F. Post-Stroke Rehabilitation: Challenges and New Perspectives. J. Clin. Med. 2023, 12, 550. [Google Scholar] [CrossRef]
- Özden, F.; Sarı, Z.; Karaman, Ö.N.; Aydoğmuş, H. The effect of video exercise-based telerehabilitation on clinical outcomes, expectation, satisfaction, and motivation in patients with chronic low back pain. Ir. J. Med. Sci. 2022, 191, 1229–1239. [Google Scholar] [CrossRef]
- Aprile, I.; Briani, C.; Pazzaglia, C.; Cecchi, F.; Negrini, S.; Padua, L.; Don Carlo Gnocchi Pain-Rehab Group. Pain in stroke patients: Characteristics and impact on the rehabilitation treatment. A multicenter cross-sectional study. Eur. J. Phys. Rehabil. Med. 2015, 51, 725–736. [Google Scholar]
- Aprile, I.; Germanotta, M.; Cruciani, A.; Pecchioli, C.; Loreti, S.; Papadopoulou, D.; Montesano, A.; Galeri, S.; Diverio, M.; Falsini, C.; et al. Poststroke shoulder pain in subacute patients and its correlation with upper limb recovery after robotic or conventional treatment: A secondary analysis of a multicenter randomized controlled trial. Int. J. Stroke 2021, 16, 396–405. [Google Scholar] [CrossRef]
- Tchero, H.; Tabue Teguo, M.; Lannuzel, A.; Rusch, E. Telerehabilitation for Stroke Survivors: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2018, 20, e10867. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sun, D.; Zhang, S.; Shi, Y.; Qiao, F.; Zhou, Y.; Liu, J.; Ren, C. Effects of home-based telerehabilitation in patients with stroke: A randomized controlled trial. Neurology 2020, 95, e2318–e2330. [Google Scholar] [CrossRef] [PubMed]
- Piron, L.; Turolla, A.; Tonin, P.; Piccione, F.; Lain, L.; Dam, M. Satisfaction with care in post-stroke patients undergoing a telerehabilitation programme at home. J. Telemed. Telecare 2008, 14, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Jung, K.B. Effect of Physiotherapy to Correct Rounded Shoulder Posture in 30 Patients During the COVID-19 Pandemic in South Korea Using a Telerehabilitation Exercise Program to Improve Posture, Physical Function, and Reduced Pain, with Evaluation of Patient Satisfaction. Med. Sci. Monit. 2022, 28, e938926. [Google Scholar] [CrossRef]
- Hyun, A.-H.; Cho, J.-Y. Effect of 8 Weeks Un-tact Pilates Home Training on Body Composition, Abdominal Obesity, Pelvic Tilt and Strength, Back Pain in Overweight Women after Childbirth. Exerc. Sci. 2021, 30, 61–69. [Google Scholar] [CrossRef]
- Durfee, W.; Carey, J.; Nuckley, D.; Deng, J. Design and implementation of a home stroke telerehabilitation system. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; Volume 2009, pp. 2422–2425. [Google Scholar] [CrossRef]


| Deficit/Pain | Parameters | Exergame | Exergame’s Repetitions | Repetitions’ Series |
|---|---|---|---|---|
| Severe impairment | Exergames with an assistance level between 6 and 8 | All from the library | 16 | 2 |
| Moderate impairment | Exergames with an assistance level between 3 and 5 | All from the library | 32 | 2 |
| Mild impairment | Exergames with an assistance level between 0 and 2 or exercises in adaptive mode | All from the library | 32 | 2 |
| Pain ≤ 4 | Exergames, regardless of UL impairment, with assistance greater than 6 | All from the library | 16 | 2 |
| Non-Robotic Group (n = 14) | Robotic Group (n = 16) | p-Value | ||
|---|---|---|---|---|
| Age (years) | 65.7 (±13.3) | 65.7 (±11.3) | 0.995 | |
| Sex | Men | 6 (42.9%) | 11 (68.8%) | 0.153 |
| Women | 8 (57.1%) | 5 (31.3%) | ||
| Latency (days) | 122.7 (±73.9) | 126.8 (85.3) | 0.890 | |
| Schooling (years) | 11.1 (±3.2) | 9.5 (±3.2) | 0.164 | |
| Occupation | Employed | 1 (7.1%) | 1 (6.3%) | 0.922 |
| Unemployed/retired | 13 (92.2%) | 15 (93.8%) | ||
| Primary outcome | World Health Organization Disability Assessment Schedule 2.0 | 31.1 (16.3) | 34.9 (12.1) | 0.678 |
| Secondary outcomes | Fugl-Meyer Assessment for Upper Extremity-motor function | 30.8 (19.5) | 28.9 (22.3) | 0.677 |
| Fugl-Meyer Assessment for Upper Extremity-sensation | 9.4 (3.5) | 8.0 (4.1) | 0.495 | |
| Action Research Arm Test | 21.7 (22.2) | 21.4 (24.5) | 1.000 | |
| Box and Block Test-Right | 31.2 (21.8) | 38.9 (21.0) | 0.560 | |
| Box and Block Test-Left | 23.4 (22.9) | 17.8 (22.7) | 0.430 | |
| Numeric Rating Scale | 3.5 (3.3) | 3.2 (3.0) | 0.780 | |
| Montreal Cognitive Assessment | 22.7 (4.2) | 22.6 (4.8) | 0.950 | |
| Trail Making Test-A | 70.9 (68.8) | 89.9 (55.4) | 0.129 | |
| Trail Making Test-B | 191.1 (92.7) | 214.6 (85.8) | 0.349 | |
| Symbol Digit Modalities Test | 27.6 (11.9) | 28.5 (11.8) | 1.000 | |
| Beck Depression Inventory | 10.4 (7.3) | 15.6 (12.4) | 0.211 | |
| State Trait Anxiety Inventory—Y1 | 44.6 (5.2) | 45.0 (3.6) | 0.723 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavan, A.; Fasano, A.; Lattanzi, S.; Cortellini, L.; Cipollini, V.; Insalaco, S.; Mauro, M.C.; Germanotta, M.; Aprile, I.G. Effectiveness of Two Models of Telerehabilitation in Improving Recovery from Subacute Upper Limb Disability after Stroke: Robotic vs. Non-Robotic. Brain Sci. 2024, 14, 941. https://doi.org/10.3390/brainsci14090941
Pavan A, Fasano A, Lattanzi S, Cortellini L, Cipollini V, Insalaco S, Mauro MC, Germanotta M, Aprile IG. Effectiveness of Two Models of Telerehabilitation in Improving Recovery from Subacute Upper Limb Disability after Stroke: Robotic vs. Non-Robotic. Brain Sciences. 2024; 14(9):941. https://doi.org/10.3390/brainsci14090941
Chicago/Turabian StylePavan, Arianna, Alessio Fasano, Stefania Lattanzi, Laura Cortellini, Valeria Cipollini, Sabina Insalaco, Maria Cristina Mauro, Marco Germanotta, and Irene Giovanna Aprile. 2024. "Effectiveness of Two Models of Telerehabilitation in Improving Recovery from Subacute Upper Limb Disability after Stroke: Robotic vs. Non-Robotic" Brain Sciences 14, no. 9: 941. https://doi.org/10.3390/brainsci14090941
APA StylePavan, A., Fasano, A., Lattanzi, S., Cortellini, L., Cipollini, V., Insalaco, S., Mauro, M. C., Germanotta, M., & Aprile, I. G. (2024). Effectiveness of Two Models of Telerehabilitation in Improving Recovery from Subacute Upper Limb Disability after Stroke: Robotic vs. Non-Robotic. Brain Sciences, 14(9), 941. https://doi.org/10.3390/brainsci14090941







