Electroacupuncture Reduced Fibromyalgia-Pain-like Behavior through Inactivating Transient Receptor Potential V1 and Interleukin-17 in Intermittent Cold Stress Mice Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Ethical Considerations
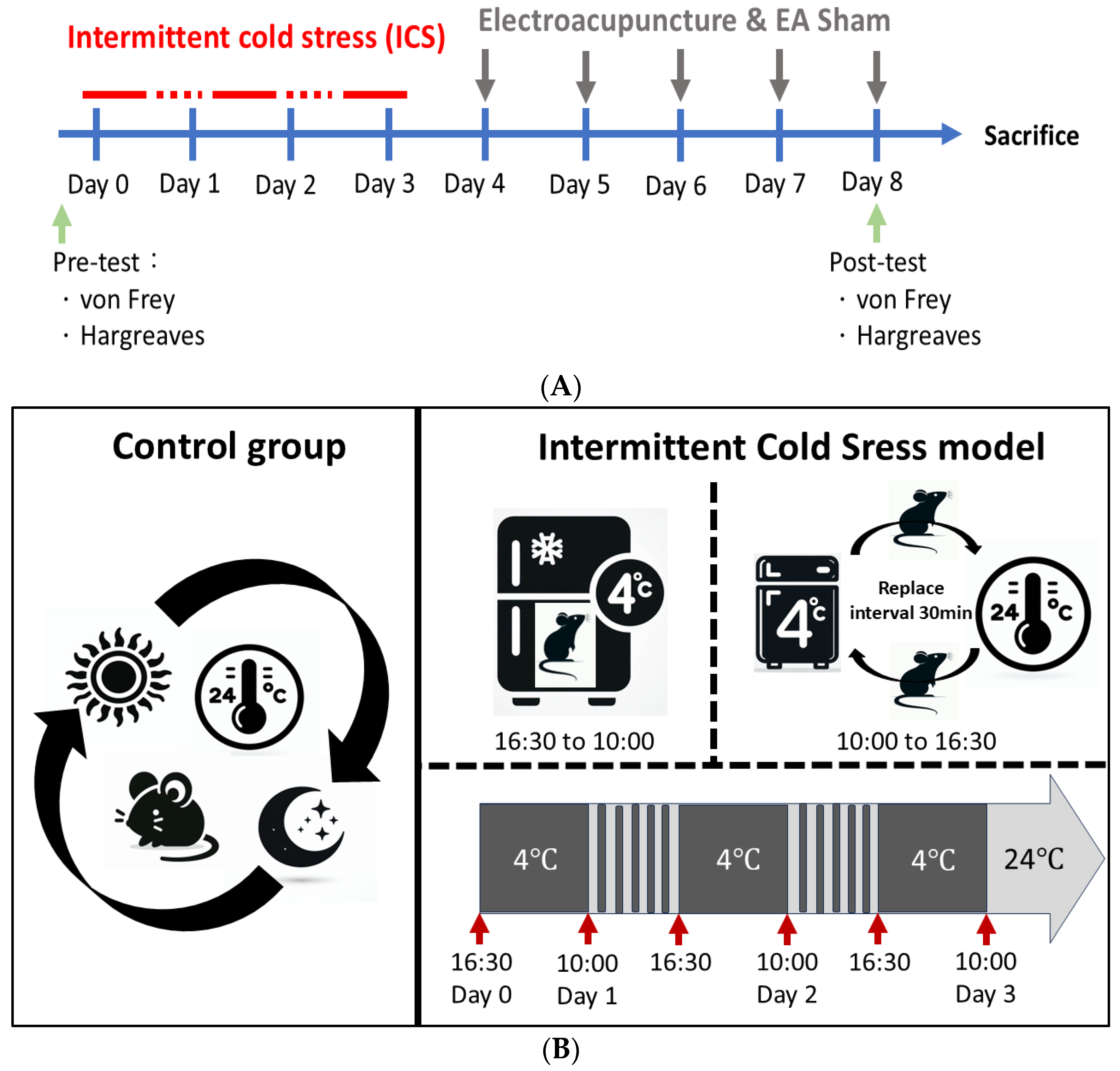
2.2. The Intermittent Cold Stress (ICS)-Induced Fibromyalgia (FM)-like Mice Model
2.3. Electroacupuncture (EA) and Sham EA Treatments
2.4. Behavior Test
2.5. Enzyme-Linked Immunosorbent Assay (ELISA) and Western Blot Analysis
2.6. Immunofluorescence
2.7. Statistical Analysis
3. Results
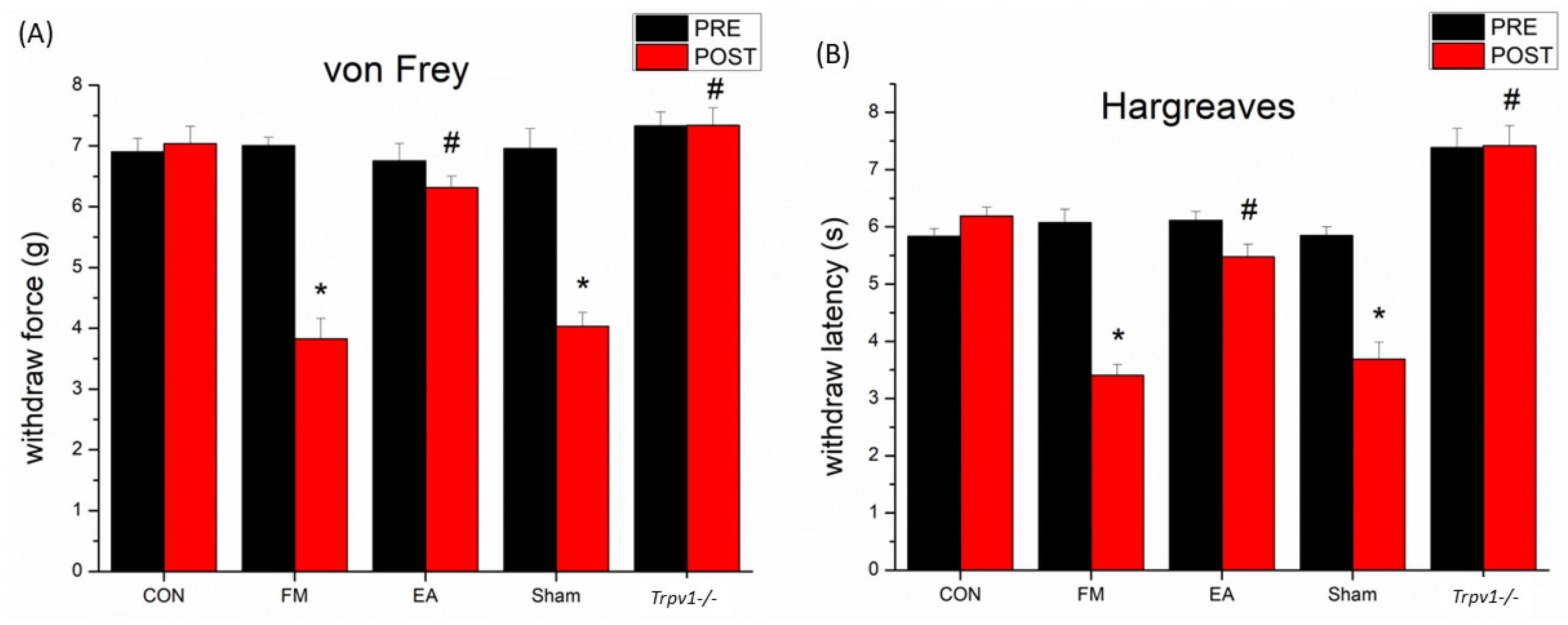
3.1. Effect of Electroacupuncture Treatment and TRPV1 Deletion on Pain-like Behavior and the Levels of Inflammatory Mediators in Fibromyalgia Model Mice
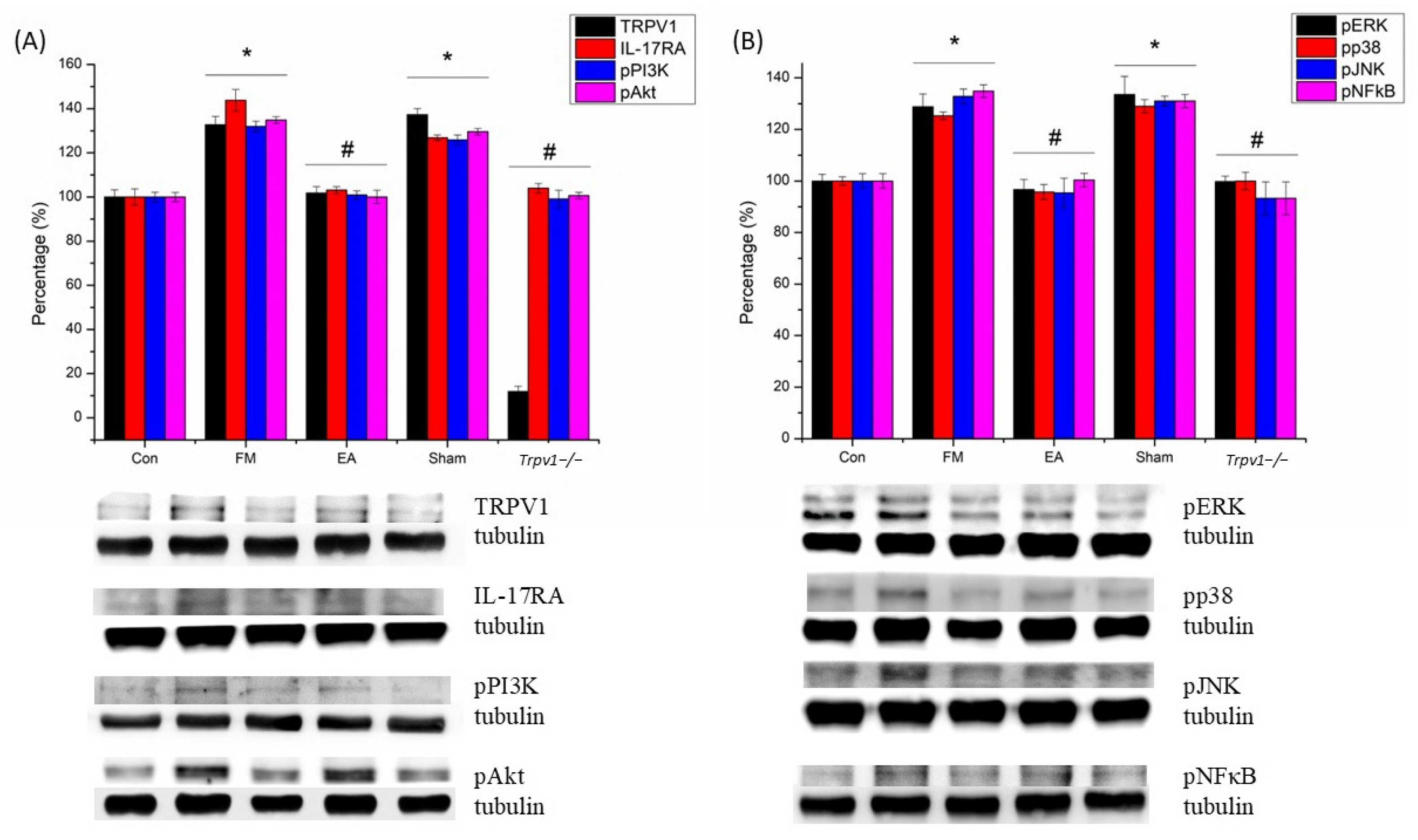
3.2. Electroacupuncture (EA) and TRPV1 Deletion (Trpv1−/−) but Not Sham EA Reduced IL-17-Related Signaling Pathways in the Somatosensory Cortex (SSC) of Mice
3.3. Electroacupuncture (EA) and TRPV1 Deletion (Trpv1−/−) but Not Sham EA Reduced IL-17-Related Signaling Pathways in the Cerebellum Lobe V (CB5) of Mice
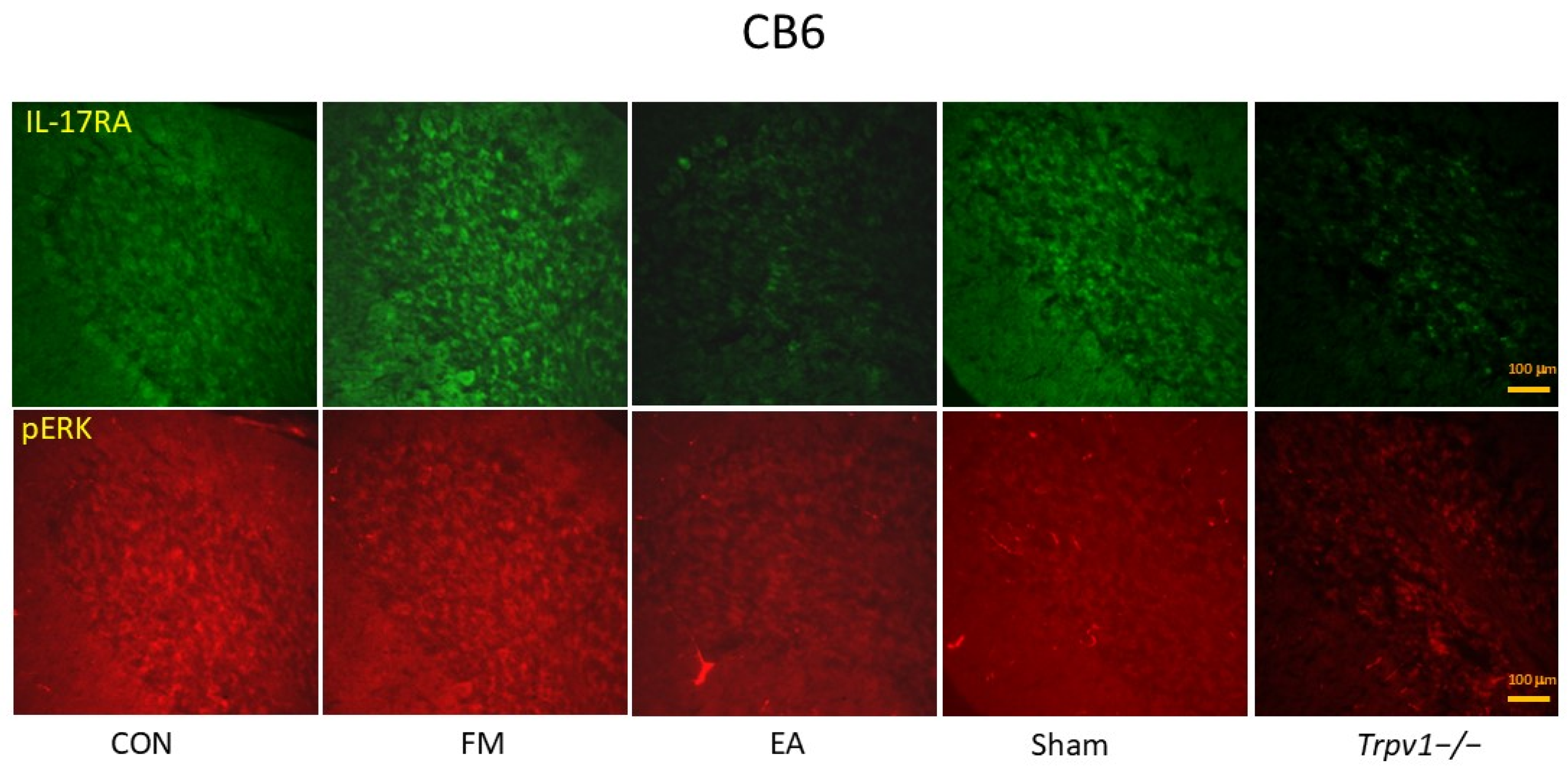
3.4. Electroacupuncture (EA) and TRPV1 Deletion (Trpv1−/−) but Not Sham EA Reduced IL-17-Related Signaling Pathways in the Cerebellum Lobe VI (CB6) of Mice
3.5. Electroacupuncture (EA) and TRPV1 Deletion (Trpv1−/−) but Not Sham EA Reduced IL-17-Related Signaling Pathways in the Cerebellum Lobe VII (CB7) of Mice
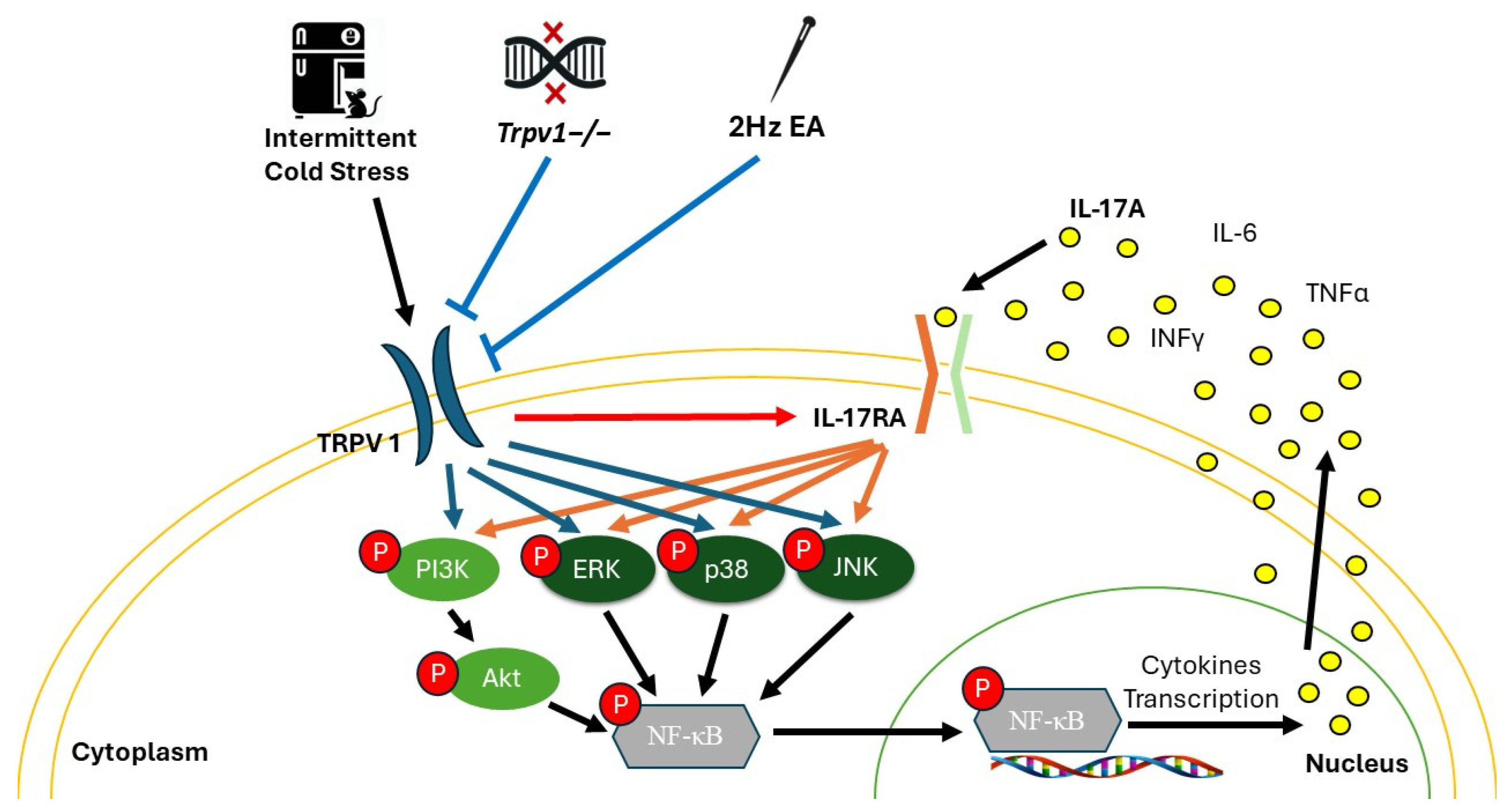
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giorgi, V.; Sirotti, S.; Romano, M.E.; Marotto, D.; Ablin, J.N.; Salaffi, F.; Sarzi-Puttini, P. Fibromyalgia: One year in review 2022. Clin. Exp. Rheumatol. 2022, 40, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010, 62, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Clauw, D.J. Fibromyalgia: A clinical review. Jama 2014, 311, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Ross, K.; Anderson, J.; Russell, I.J. Aspects of fibromyalgia in the general population: Sex, pain threshold, and fibromyalgia symptoms. J. Rheumatol. 1995, 22, 151–156. [Google Scholar]
- Queiroz, L.P. Worldwide epidemiology of fibromyalgia. Curr. Pain Headache Rep. 2013, 17, 356. [Google Scholar] [CrossRef]
- Bawazir, Y. Prevalence of fibromyalgia syndrome in Saudi Arabia: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2023, 24, 692. [Google Scholar] [CrossRef]
- Galvez-Sánchez, C.M.; Del Reyes Paso, G.A. Diagnostic Criteria for Fibromyalgia: Critical Review and Future Perspectives. J. Clin. Med. 2020, 9, 1219. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Schweinhardt, P. Dysfunctional neurotransmitter systems in fibromyalgia, their role in central stress circuitry and pharmacological actions on these systems. Pain Res. Treat. 2012, 2012, 741–746. [Google Scholar] [CrossRef]
- Ansari, A.H.; Pal, A.; Ramamurthy, A.; Kabat, M.; Jain, S.; Kumar, S. Fibromyalgia Pain and Depression: An Update on the Role of Repetitive Transcranial Magnetic Stimulation. ACS. Chem. Neurosci. 2021, 12, 256–270. [Google Scholar] [CrossRef]
- Sarzi-Puttini, P.; Giorgi, V.; Marotto, D.; Atzeni, F. Fibromyalgia: An update on clinical characteristics, aetiopathogenesis and treatment. Nat. Rev. Rheumatol. 2020, 16, 645–660. [Google Scholar] [CrossRef]
- Peck, M.M.; Maram, R.; Mohamed, A.; Ochoa Crespo, D.; Kaur, G.; Ashraf, I.; Malik, B.H. The Influence of Pro-inflammatory Cytokines and Genetic Variants in the Development of Fibromyalgia: A Traditional Review. Cureus 2020, 12, e10276. [Google Scholar] [CrossRef]
- Kumbhare, D.; Hassan, S.; Diep, D.; Duarte, F.C.K.; Hung, J.; Damodara, S.; West, D.W.D.; Selvaganapathy, P.R. Potential role of blood biomarkers in patients with fibromyalgia: A systematic review with meta-analysis. Pain 2022, 163, 1232–1253. [Google Scholar] [CrossRef]
- Pernambuco, A.P.; Schetino, L.P.; Alvim, C.C.; Murad, C.M.; Viana, R.S.; Carvalho, L.S.; Reis, D.Á. Increased levels of IL-17A in patients with fibromyalgia. Clin. Exp. Rheumatol. 2013, 31 (Suppl. S79), S60–S63. [Google Scholar] [PubMed]
- Dolcino, M.; Tinazzi, E.; Puccetti, A.; Lunardi, C. Gene Expression Profiling in Fibromyalgia Indicates an Autoimmune Origin of the Disease and Opens New Avenues for Targeted Therapy. J. Clin. Med. 2020, 9, 1814. [Google Scholar] [CrossRef] [PubMed]
- Andrés-Rodríguez, L.; Borràs, X.; Feliu-Soler, A.; Pérez-Aranda, A.; Angarita-Osorio, N.; Moreno-Peral, P.; Montero-Marin, J.; García-Campayo, J.; Carvalho, A.F.; Maes, M.; et al. Peripheral immune aberrations in fibromyalgia: A systematic review, meta-analysis and meta-regression. Brain Behav. Immun. 2020, 87, 881–889. [Google Scholar] [CrossRef]
- Liao, H.Y.; Lin, Y.W. Electroacupuncture reduces cold stress-induced pain through microglial inactivation and transient receptor potential V1 in mice. Chin. Med. 2021, 16, 43. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Gurney, A.L. IL-17: Prototype member of an emerging cytokine family. J. Leukoc. Biol. 2002, 71, 1–8. [Google Scholar] [CrossRef]
- Wynn, T.A. T(H)-17: A giant step from T(H)1 and T(H)2. Nat. Immunol. 2005, 6, 1069–1070. [Google Scholar] [CrossRef]
- Korn, T.; Bettelli, E.; Oukka, M.; Kuchroo, V.K. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009, 27, 485–517. [Google Scholar] [CrossRef]
- Amatya, N.; Garg, A.V.; Gaffen, S.L. IL-17 Signaling: The Yin and the Yang. Trends Immunol. 2017, 38, 310–322. [Google Scholar] [CrossRef]
- Bechara, R.; McGeachy, M.J.; Gaffen, S.L. The metabolism-modulating activity of IL-17 signaling in health and disease. J. Exp. Med. 2021, 218, e20202191. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, L.; Li, R.; Huang, Y.; Wang, S. The IL-17 family in diseases: From bench to bedside. Signal Transduct. Target. Ther. 2023, 8, 402. [Google Scholar] [CrossRef]
- McGeachy, M.J.; Cua, D.J.; Gaffen, S.L. The IL-17 Family of Cytokines in Health and Disease. Immunity 2019, 50, 892–906. [Google Scholar] [CrossRef]
- Kuwabara, T.; Ishikawa, F.; Kondo, M.; Kakiuchi, T. The Role of IL-17 and Related Cytokines in Inflammatory Autoimmune Diseases. Mediat. Inflamm. 2017, 2017, 3908061. [Google Scholar]
- Cao, L.; DeLeo, J.A. CNS-infiltrating CD4 + T lymphocytes contribute to murine spinal nerve transection-induced neuropathic pain. Eur. J. Immunol. 2008, 38, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, Y.; Lao, L.; Saito, R.; Li, A.; Bäckman, C.M.; Berman, B.M.; Ren, K.; Wei, P.K.; Zhang, R.X. Spinal interleukin-17 promotes thermal hyperalgesia and NMDA NR1 phosphorylation in an inflammatory pain rat model. Pain 2013, 154, 294–305. [Google Scholar] [CrossRef]
- Huang, F.; Kao, C.Y.; Wachi, S.; Thai, P.; Ryu, J.; Wu, R. Requirement for both JAK-mediated PI3K signaling and ACT1/TRAF6/TAK1-dependent NF-kappaB activation by IL-17A in enhancing cytokine expression in human airway epithelial cells. J. Immunol. 2007, 179, 6504–6513. [Google Scholar] [CrossRef]
- Qian, Y.; Kang, Z.; Liu, C.; Li, X. IL-17 signaling in host defense and inflammatory diseases. Cell. Mol. Immunol. 2010, 7, 328–333. [Google Scholar] [CrossRef]
- Varshney, P.; Saini, N. PI3K/AKT/mTOR activation and autophagy inhibition plays a key role in increased cholesterol during IL-17A mediated inflammatory response in psoriasis. Biochim. Biophys. Acta Mol. Basis. Dis. 2018, 1864 5 Pt A, 1795–1803. [Google Scholar] [CrossRef]
- Rai, S.N.; Dilnashin, H.; Birla, H.; Singh, S.S.; Zahra, W.; Rathore, A.S.; Singh, B.K.; Singh, S.P. The Role of PI3K/Akt and ERK in Neurodegenerative Disorders. Neurotox. Res. 2019, 35, 775–795. [Google Scholar] [CrossRef]
- Long, H.Z.; Cheng, Y.; Zhou, Z.W.; Luo, H.Y.; Wen, D.D.; Gao, L.C. PI3K/AKT Signal Pathway: A Target of Natural Products in the Prevention and Treatment of Alzheimer’s Disease and Parkinson’s Disease. Front. Pharmacol. 2021, 12, 648636. [Google Scholar] [CrossRef]
- Wang, Q.; Shen, Z.N.; Zhang, S.J.; Sun, Y.; Zheng, F.J.; Li, Y.H. Protective effects and mechanism of puerarin targeting PI3K/Akt signal pathway on neurological diseases. Front. Pharmacol. 2022, 13, 1022053. [Google Scholar] [CrossRef]
- He, X.; Li, Y.; Deng, B.; Lin, A.; Zhang, G.; Ma, M.; Wang, Y.; Yang, Y.; Kang, X. The PI3K/AKT signalling pathway in inflammation, cell death and glial scar formation after traumatic spinal cord injury: Mechanisms and therapeutic opportunities. Cell Prolif. 2022, 55, e13275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Suo, M.; Yu, G.; Zhang, M. Antinociceptive and anti-inflammatory effects of cryptotanshinone through PI3K/Akt signaling pathway in a rat model of neuropathic pain. Chem. Biol. Interact. 2019, 305, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Wu, Z.; Zhu, D.; Gu, J.; Xu, M.; Zhang, M.; Duan, H.; Li, Y.; Chen, T. Proanthocyanidins Inhibit the Transmission of Spinal Pain Information Through a Presynaptic Mechanism in a Mouse Inflammatory Pain Model. Front. Neurosci. 2021, 15, 804722. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Gereau, R.W., 4th; Malcangio, M.; Strichartz, G.R. MAP kinase and pain. Brain Res. Rev. 2009, 60, 135–148. [Google Scholar] [CrossRef]
- Cheng, S.J.; Chen, C.C.; Yang, H.W.; Chang, Y.T.; Bai, S.W.; Chen, C.C.; Yen, C.T.; Min, M.Y. Role of extracellular signal-regulated kinase in synaptic transmission and plasticity of a nociceptive input on capsular central amygdaloid neurons in normal and acid-induced muscle pain mice. J. Neurosci. 2011, 31, 2258–2270. [Google Scholar] [CrossRef]
- Chen, W.N.; Lee, C.H.; Lin, S.H.; Wong, C.W.; Sun, W.H.; Wood, J.N.; Chen, C.C. Roles of ASIC3, TRPV1, and NaV1.8 in the transition from acute to chronic pain in a mouse model of fibromyalgia. Mol. Pain 2014, 10, 40. [Google Scholar] [CrossRef]
- Nugraha, B.; Scheibe, R.; Korallus, C.; Gaestel, M.; Gutenbrunner, C. The p38/MK2 Axis in Monocytes of Fibromyalgia Syndrome Patients: An Explorative Study. Medicina 2021, 57, 396. [Google Scholar] [CrossRef]
- Xie, S.; Li, J.; Wang, J.H.; Wu, Q.; Yang, P.; Hsu, H.C.; Smythies, L.E.; Mountz, J.D. IL-17 activates the canonical NF-kappaB signaling pathway in autoimmune B cells of BXD2 mice to upregulate the expression of regulators of G-protein signaling 16. J. Immunol. 2010, 184, 2289–2296. [Google Scholar] [CrossRef]
- Ghowsi, M.; Qalekhani, F.; Farzaei, M.H.; Mahmudii, F.; Yousofvand, N.; Joshi, T. Inflammation, oxidative stress, insulin resistance, and hypertension as mediators for adverse effects of obesity on the brain: A review. Biomedicine 2021, 11, 13–22. [Google Scholar] [CrossRef]
- Hartung, J.E.; Eskew, O.; Wong, T.; Tchivileva, I.E.; Oladosu, F.A.; O’Buckley, S.C.; Nackley, A.G. Nuclear factor-kappa B regulates pain and COMT expression in a rodent model of inflammation. Brain Behav. Immun. 2015, 50, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Singh, L.; Singh, N.; Bhatti, M.S.; Bhatti, R. Ameliorative effect of imperatorin in chemically induced fibromyalgia: Role of NMDA/NFkB mediated downstream signaling. Biochem. Pharmacol. 2019, 166, 56–69. [Google Scholar] [CrossRef]
- Cohen, J.A.; Edwards, T.N.; Liu, A.W.; Hirai, T.; Jones, M.R.; Wu, J.; Li, Y.; Zhang, S.; Ho, J.; Davis, B.M.; et al. Cutaneous TRPV1(+) Neurons Trigger Protective Innate Type 17 Anticipatory Immunity. Cell 2019, 178, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.P.M.; Brusco, I.; Brum, E.S.; Fialho, M.F.P.; Camponogara, C.; Scussel, R.; Machado-de-Ávila, R.A.; Trevisan, G.; Oliveira, S.M. Involvement of TRPV1 and the efficacy of α-spinasterol on experimental fibromyalgia symptoms in mice. Neurochem. Int. 2020, 134, 104673. [Google Scholar] [CrossRef] [PubMed]
- Patapoutian, A.; Tate, S.; Woolf, C.J. Transient receptor potential channels: Targeting pain at the source. Nat. Rev. Drug Discov. 2009, 8, 55–68. [Google Scholar] [CrossRef]
- Steenland, H.W.; Ko, S.W.; Wu, L.J.; Zhuo, M. Hot receptors in the brain. Mol. Pain. 2006, 2, 34. [Google Scholar] [CrossRef]
- Mallet, C.; Barrière, D.A.; Ermund, A.; Jönsson, B.A.; Eschalier, A.; Zygmunt, P.M.; Högestätt, E.D. TRPV1 in brain is involved in acetaminophen-induced antinociception. PLoS ONE 2010, 5, e12748. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, E.; Luongo, L.; de Novellis, V.; Berrino, L.; Rossi, F.; Maione, S. Moving towards supraspinal TRPV1 receptors for chronic pain relief. Mol. Pain 2010, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Giordano, C.; Cristino, L.; Luongo, L.; Siniscalco, D.; Petrosino, S.; Piscitelli, F.; Marabese, I.; Gatta, L.; Rossi, F.; Imperatore, R.; et al. TRPV1-dependent and -independent alterations in the limbic cortex of neuropathic mice: Impact on glial caspases and pain perception. Cereb. Cortex 2012, 22, 2495–2518. [Google Scholar] [CrossRef]
- Jurik, A.; Ressle, A.; Schmid, R.M.; Wotjak, C.T.; Thoeringer, C.K. Supraspinal TRPV1 modulates the emotional expression of abdominal pain. Pain 2014, 155, 2153–2160. [Google Scholar] [CrossRef]
- Kerckhove, N.; Mallet, C.; François, A.; Boudes, M.; Chemin, J.; Voets, T.; Bourinet, E.; Alloui, A.; Eschalier, A. Ca(v)3.2 calcium channels: The key protagonist in the supraspinal effect of paracetamol. Pain 2014, 155, 764–772. [Google Scholar] [CrossRef]
- Silva, M.; Martins, D.; Charrua, A.; Piscitelli, F.; Tavares, I.; Morgado, C.; Di Marzo, V. Endovanilloid control of pain modulation by the rostroventromedial medulla in an animal model of diabetic neuropathy. Neuropharmacology 2016, 107, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Martins, D.; Tavares, I.; Morgado, C. “Hotheaded”: The role OF TRPV1 in brain functions. Neuropharmacology 2014, 85, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Barrantes, R.; Cordova, C.; Poblete, H.; Muñoz, P.; Marchant, I.; Wianny, F.; Olivero, P. Perspectives of TRPV1 Function on the Neurogenesis and Neural Plasticity. Neural. Plast. 2016, 2016, 1568145. [Google Scholar] [CrossRef]
- Antunes, M.D.; Marques, A.P. The role of physiotherapy in fibromyalgia: Current and future perspectives. Front. Physiol. 2022, 13, 968292. [Google Scholar] [CrossRef]
- Wu, J.N. A short history of acupuncture. J. Altern. Complement Med. 1996, 2, 19–21. [Google Scholar] [CrossRef]
- Zhang, X.C.; Chen, H.; Xu, W.T.; Song, Y.Y.; Gu, Y.H.; Ni, G.X. Acupuncture therapy for fibromyalgia: A systematic review and meta-analysis of randomized controlled trials. J. Pain Res. 2019, 12, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, R.O.; Souza, M.B.; Oliveira, M.X.; Lacerda, A.C.; Mendonça, V.A.; Henschke, N.; Oliveira, V.C. Association of Therapies with Reduced Pain and Improved Quality of Life in Patients With Fibromyalgia: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2021, 181, 104–112. [Google Scholar] [CrossRef]
- Deluze, C.; Bosia, L.; Zirbs, A.; Chantraine, A.; Vischer, T.L. Electroacupuncture in fibromyalgia: Results of a controlled trial. Bmj 1992, 305, 1249–1252. [Google Scholar] [CrossRef]
- Uğurlu, F.G.; Sezer, N.; Aktekin, L.; Fidan, F.; Tok, F.; Akkuş, S. The effects of acupuncture versus sham acupuncture in the treatment of fibromyalgia: A randomized controlled clinical trial. Acta Reum. Port. 2017, 42, 32–37. [Google Scholar]
- Mist, S.D.; Jones, D. Randomized Controlled Trial of Acupuncture for Women with Fibromyalgia: Group Acupuncture with Traditional Chinese Medicine Diagnosis-Based Point Selection. Pain Med. 2018, 19, 1862–1871. [Google Scholar] [CrossRef]
- Mawla, I.; Ichesco, E.; Zöllner, H.J.; Edden, R.A.; Chenevert, T.; Buchtel, H.; Bretz, M.D.; Sloan, H.; Kaplan, C.M.; Harte, S.E.; et al. Greater Somatosensory Afference with Acupuncture Increases Primary Somatosensory Connectivity and Alleviates Fibromyalgia Pain via Insular γ-Aminobutyric Acid: A Randomized Neuroimaging Trial. Arthritis Rheumatol. 2021, 73, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.M.; Hsieh, C.L.; Lin, Y.W. Electroacupuncture reduces chronic fibromyalgia pain through attenuation of transient receptor potential vanilloid 1 signaling pathway in mouse brains. Iran. J. Basic Med. Sci. 2020, 23, 894–900. [Google Scholar]
- Hsu, H.C.; Hsieh, C.L.; Lee, K.T.; Lin, Y.W. Electroacupuncture reduces fibromyalgia pain by downregulating the TRPV1-pERK signalling pathway in the mouse brain. Acupunct. Med. 2020, 38, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Lottering, B.; Lin, Y.W. Functional characterization of nociceptive mechanisms involved in fibromyalgia and electroacupuncture. Brain Res. 2021, 1755, 147260. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, I.H.; Lin, Y.W. Electroacupuncture Reduces Fibromyalgia Pain by Attenuating the HMGB1, S100B, and TRPV1 Signalling Pathways in the Mouse Brain. Evid. Based Complement Altern. Med. 2022, 2022, 2242074. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.G.; Hsieh, C.L.; Lin, Y.W. Analgesic Effect of Electroacupuncture in a Mouse Fibromyalgia Model: Roles of TRPV1, TRPV4, and pERK. PLoS ONE 2015, 10, e0128037. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, R.; Feng, Y.; Zhao, Z.; He, Y.Y.; Wang, D.W.; Wang, Q.; Pang, X.T.; Yao, Y.; Li, J.W.; Sun, Z.L. Effect of electroacupuncture on serum inflammatory cytokines in animal models with rheumatoid arthritis: A systematic review and meta-analysis. Eur. J. Integr. Med. 2022, 55, 102187. [Google Scholar] [CrossRef]
- Zeng, C.; Bai, X.; Qin, H.; Wang, H.; Rong, X.; Yan, J. Effect of adjuvant therapy with electroacupuncture on bone turnover markers and interleukin 17 in patients with rheumatoid arthritis. J. Tradit. Chin. Med. 2019, 39, 582–586. [Google Scholar]
- Tsai, S.T.; Yang, C.C.; Liao, H.Y.; Lin, Y.W. Electroacupuncture Reduces Fibromyalgia Pain via Neuronal/Microglial Inactivation and Toll-like Receptor 4 in the Mouse Brain: Precise Interpretation of Chemogenetics. Biomedicines 2024, 12, 387. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Neyama, H. Fibromyalgia Animal Models Using Intermittent Cold and Psychological Stress. Biomedicines 2023, 12, 56. [Google Scholar] [CrossRef]
- Nishiyori, M.; Ueda, H. Prolonged gabapentin analgesia in an experimental mouse model of fibromyalgia. Mol. Pain 2008, 4, 52. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Chen, W.H.; Hsieh, C.L.; Lin, Y.W. Abundant expression and functional participation of TRPV1 at Zusanli acupoint (ST36) in mice: Mechanosensitive TRPV1 as an “acupuncture-responding channel. BMC Complement Altern. Med. 2014, 14, 96. [Google Scholar] [CrossRef]
- Nishiyori, M.; Nagai, J.; Nakazawa, T.; Ueda, H. Absence of morphine analgesia and its underlying descending serotonergic activation in an experimental mouse model of fibromyalgia. Neurosci. Lett. 2010, 472, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Yunus, M.B. The role of gender in fibromyalgia syndrome. Curr. Rheumatol. Rep. 2001, 3, 128–134. [Google Scholar] [CrossRef]
- Mäkinen, T.M.; Palinkas, L.A.; Reeves, D.L.; Pääkkönen, T.; Rintamäki, H.; Leppäluoto, J.; Hassi, J. Effect of repeated exposures to cold on cognitive performance in humans. Physiol. Behav. 2006, 87, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Jin, H.; Cong, P.; Zhang, Y.; Tong, C.; Shi, X.; Liu, X.; Tong, Z.; Shi, L.; et al. Cold stress-induced brain injury regulates TRPV1 channels and the PI3K/AKT signaling pathway. Brain Res. 2017, 1670, 201–207. [Google Scholar] [CrossRef]
- Ji, R.R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef]
- Muir, W.W., 3rd; Woolf, C.J. Mechanisms of pain and their therapeutic implications. J. Am. Vet. Med. Assoc. 2001, 219, 1346–1356. [Google Scholar] [CrossRef]
- Vierck, C.J., Jr. Mechanisms underlying development of spatially distributed chronic pain (fibromyalgia). Pain 2006, 124, 242–263. [Google Scholar] [CrossRef] [PubMed]
- Littlejohn, G.; Guymer, E. Neurogenic inflammation in fibromyalgia. Semin. Immunopathol. 2018, 40, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Singh Gautam, A.; Kumar Singh, R. Therapeutic potential of targeting IL-17 and its receptor signaling in neuroinflammation. Drug Discov. Today 2023, 28, 103517. [Google Scholar] [CrossRef]
- Jiang, X.; Zhou, R.; Zhang, Y.; Zhu, T.; Li, Q.; Zhang, W. Interleukin-17 as a potential therapeutic target for chronic pain. Front. Immunol. 2022, 13, 999407. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.W.; Chou, A.I.W.; Su, H.; Su, K.P. Transient receptor potential V1 (TRPV1) modulates the therapeutic effects for comorbidity of pain and depression: The common molecular implication for electroacupuncture and omega-3 polyunsaturated fatty acids. Brain Behav. Immun. 2020, 89, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.C.; Yen, C.M.; Lin, M.C.; Chen, Y.H.; Liao, H.Y.; Huang, Y.W.; Lin, Y.W. Electroacupuncture Attenuates Fibromyalgia Pain via Toll-like Receptor 4 in the Mouse Brain. Life 2023, 13, 1160. [Google Scholar] [CrossRef] [PubMed]
- Marrone, M.C.; Morabito, A.; Giustizieri, M.; Chiurchiù, V.; Leuti, A.; Mattioli, M.; Marinelli, S.; Riganti, L.; Lombardi, M.; Murana, E.; et al. TRPV1 channels are critical brain inflammation detectors and neuropathic pain biomarkers in mice. Nature Commun. 2017, 8, 15292. [Google Scholar] [CrossRef]
- Bujak, J.K.; Kosmala, D.; Szopa, I.M.; Majchrzak, K.; Bednarczyk, P. Inflammation, Cancer and Immunity-Implication of TRPV1 Channel. Front. Oncol. 2019, 9, 1087. [Google Scholar] [CrossRef]
- Fernandes, E.S.; Liang, L.; Smillie, S.J.; Kaiser, F.; Purcell, R.; Rivett, D.W.; Alam, S.; Howat, S.; Collins, H.; Thompson, S. TRPV1 deletion enhances local inflammation and accelerates the onset of systemic inflammatory response syndrome. J. Immunol. 2012, 188, 5741–5751. [Google Scholar] [CrossRef]
- Kim, H.S.; Kwon, H.J.; Kim, G.E.; Cho, M.H.; Yoon, S.Y.; Davies, A.J.; Oh, S.B.; Lee, H.; Cho, Y.K.; Joo, C.H. Attenuation of natural killer cell functions by capsaicin through a direct and TRPV1-independent mechanism. Carcinogenesis 2014, 35, 1652–1660. [Google Scholar] [CrossRef]
- Majhi, R.K.; Sahoo, S.S.; Yadav, M.; Pratheek, B.M.; Chattopadhyay, S.; Goswami, C. Functional expression of TRPV channels in T cells and their implications in immune regulation. FEBS J. 2015, 282, 2661–2681. [Google Scholar] [CrossRef] [PubMed]
- Assas, M.B.; Wakid, M.H.; Zakai, H.A.; Miyan, J.A.; Pennock, J.L. Transient receptor potential vanilloid 1 expression and function in splenic dendritic cells: A potential role in immune homeostasis. Immunology 2016, 147, 292–304. [Google Scholar] [CrossRef]
- Luo, X.; Chen, O.; Wang, Z.; Bang, S.; Ji, J.; Lee, S.H.; Huh, Y.; Furutani, K.; He, Q.; Tao, X. IL-23/IL-17A/TRPV1 axis produces mechanical pain via macrophage-sensory neuron crosstalk in female mice. Neuron 2021, 109, 2691–2706. [Google Scholar] [CrossRef]
- Tan, Z.; Lin, Z.J.; Wu, L.J.; Zhou, L.J. The Macrophage IL-23/IL-17A Pathway: A New Neuro-Immune Mechanism in Female Mechanical Pain. Neurosci. Bull. 2022, 38, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.Y.; Yen, C.M.; Hsiao, I.-H.; Hsu, H.C.; Lin, Y.W. Eicosapentaenoic Acid Modulates Transient Receptor Potential V1 Expression in Specific Brain Areas in a Mouse Fibromyalgia Pain Model. Int. J. Mol. Sci. 2024, 25, 2901. [Google Scholar] [CrossRef]
- Guedj, E.; Cammilleri, S.; Niboyet, J.; Dupont, P.; Vidal, E.; Dropinski, J.P.; Mundler, O. Clinical Correlate of Brain SPECT Perfusion Abnormalities in Fibromyalgia. J. Nucl. Med. 2008, 49, 1798. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, J.; Loggia, M.L.; Cahalan, C.; Garcia, R.G.; Vangel, M.G.; Wasan, A.D.; Edwards, R.R.; Napadow, V. Fibromyalgia is characterized by altered frontal and cerebellar structural covariance brain networks. NeuroImage Clin. 2015, 7, 667–677. [Google Scholar] [CrossRef]
- Liu, W.; Lv, Y.; Ren, F. PI3K/Akt Pathway is Required for Spinal Central Sensitization in Neuropathic Pain. Cell. Mol. Neurobiol. 2018, 38, 747–755. [Google Scholar] [CrossRef]
- Chen, S.P.; Zhou, Y.Q.; Liu, D.Q.; Zhang, W.; Manyande, A.; Guan, X.H.; Tian, Y.K.; Ye, D.W.; Omar, D.M. PI3K/Akt Pathway: A Potential Therapeutic Target for Chronic Pain. Curr. Pharm. Des. 2017, 23, 1860–1868. [Google Scholar] [CrossRef]
- Xu, J.T.; Tu, H.Y.; Xin, W.J.; Liu, X.G.; Zhang, G.H.; Zhai, C.H. Activation of phosphatidylinositol 3-kinase and protein kinase B/Akt in dorsal root ganglia and spinal cord contributes to the neuropathic pain induced by spinal nerve ligation in rats. Exp. Neurol. 2007, 206, 269–279. [Google Scholar] [CrossRef]
- Pezet, S.; Marchand, F.; D’Mello, R.; Grist, J.; Clark, A.K.; Malcangio, M.; Dickenson, A.H.; Williams, R.J.; McMahon, S.B. Phosphatidylinositol 3-kinase is a key mediator of central sensitization in painful inflammatory conditions. J. Neurosci. 2008, 28, 4261–4270. [Google Scholar] [CrossRef]
- Choi, J.I.; Svensson, C.I.; Koehrn, F.J.; Bhuskute, A.; Sorkin, L.S. Peripheral inflammation induces tumor necrosis factor dependent AMPA receptor trafficking and Akt phosphorylation in spinal cord in addition to pain behavior. Pain 2010, 149, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.-R.; Woolf, C.J. Neuronal Plasticity and Signal Transduction in Nociceptive Neurons: Implications for the Initiation and Maintenance of Pathological Pain. Neurobiol. Dis. 2001, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bhave, G.; Gereau, R.W.T. Posttranslational mechanisms of peripheral sensitization. J. Neurobiol. 2004, 61, 88–106. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, S.; Nishida, E. MAPK signalling: ERK5 versus ERK1/2. EMBO Rep. 2006, 7, 782–786. [Google Scholar] [CrossRef]
- Peyravian, N.; Deo, S.; Daunert, S.; Jimenez, J.J. Cannabidiol as a Novel Therapeutic for Immune Modulation. Immunotargets Ther. 2020, 9, 131–140. [Google Scholar] [CrossRef]
- Hot, A.; Zrioual, S.; Toh, M.L.; Lenief, V.; Miossec, P. IL-17A- versus IL-17F-induced intracellular signal transduction pathways and modulation by IL-17RA and IL-17RC RNA interference in rheumatoid synoviocytes. Ann. Rheum. Dis. 2011, 70, 341–348. [Google Scholar] [CrossRef]
- Yi, H.; Bai, Y.; Zhu, X.; Lin, L.; Zhao, L.; Wu, X.; Buch, S.; Wang, L.; Chao, J.; Yao, H. IL-17A induces MIP-1α expression in primary astrocytes via Src/MAPK/PI3K/NF-kB pathways: Implications for multiple sclerosis. J. Neuroimmune Pharmacol. 2014, 9, 629–641. [Google Scholar] [CrossRef]
- Suyama, K.; Sakai, D.; Watanabe, M. The Role of IL-17-Mediated Inflammatory Processes in the Pathogenesis of Intervertebral Disc Degeneration and Herniation: A Comprehensive Review. Front. Cell Dev. Biol. 2022, 10, 857164. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, M.; Huo, M.; Si, Y.; Zhang, Y.; Fang, Y.; Zhang, D. Mechanisms of acupuncture–electroacupuncture on inflammatory pain. Mol. Pain 2023, 19, 17448069231202882. [Google Scholar] [CrossRef]
- Zhang, R.; Lao, L.; Ren, K.; Berman, B.M. Mechanisms of acupuncture-electroacupuncture on persistent pain. Anesthesiology 2014, 120, 482–503. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, Y.-A.; Liao, H.-Y.; Hsiao, I.-H.; Hsu, H.-C.; Lin, Y.-W. Electroacupuncture Reduced Fibromyalgia-Pain-like Behavior through Inactivating Transient Receptor Potential V1 and Interleukin-17 in Intermittent Cold Stress Mice Model. Brain Sci. 2024, 14, 869. https://doi.org/10.3390/brainsci14090869
Yeh Y-A, Liao H-Y, Hsiao I-H, Hsu H-C, Lin Y-W. Electroacupuncture Reduced Fibromyalgia-Pain-like Behavior through Inactivating Transient Receptor Potential V1 and Interleukin-17 in Intermittent Cold Stress Mice Model. Brain Sciences. 2024; 14(9):869. https://doi.org/10.3390/brainsci14090869
Chicago/Turabian StyleYeh, Yu-An, Hsien-Yin Liao, I-Han Hsiao, Hsin-Cheng Hsu, and Yi-Wen Lin. 2024. "Electroacupuncture Reduced Fibromyalgia-Pain-like Behavior through Inactivating Transient Receptor Potential V1 and Interleukin-17 in Intermittent Cold Stress Mice Model" Brain Sciences 14, no. 9: 869. https://doi.org/10.3390/brainsci14090869
APA StyleYeh, Y.-A., Liao, H.-Y., Hsiao, I.-H., Hsu, H.-C., & Lin, Y.-W. (2024). Electroacupuncture Reduced Fibromyalgia-Pain-like Behavior through Inactivating Transient Receptor Potential V1 and Interleukin-17 in Intermittent Cold Stress Mice Model. Brain Sciences, 14(9), 869. https://doi.org/10.3390/brainsci14090869





