Decline in Sensory Integration in Old Age and Its Related Functional Brain Connectivity Correlates Observed during a Virtual Reality Task
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
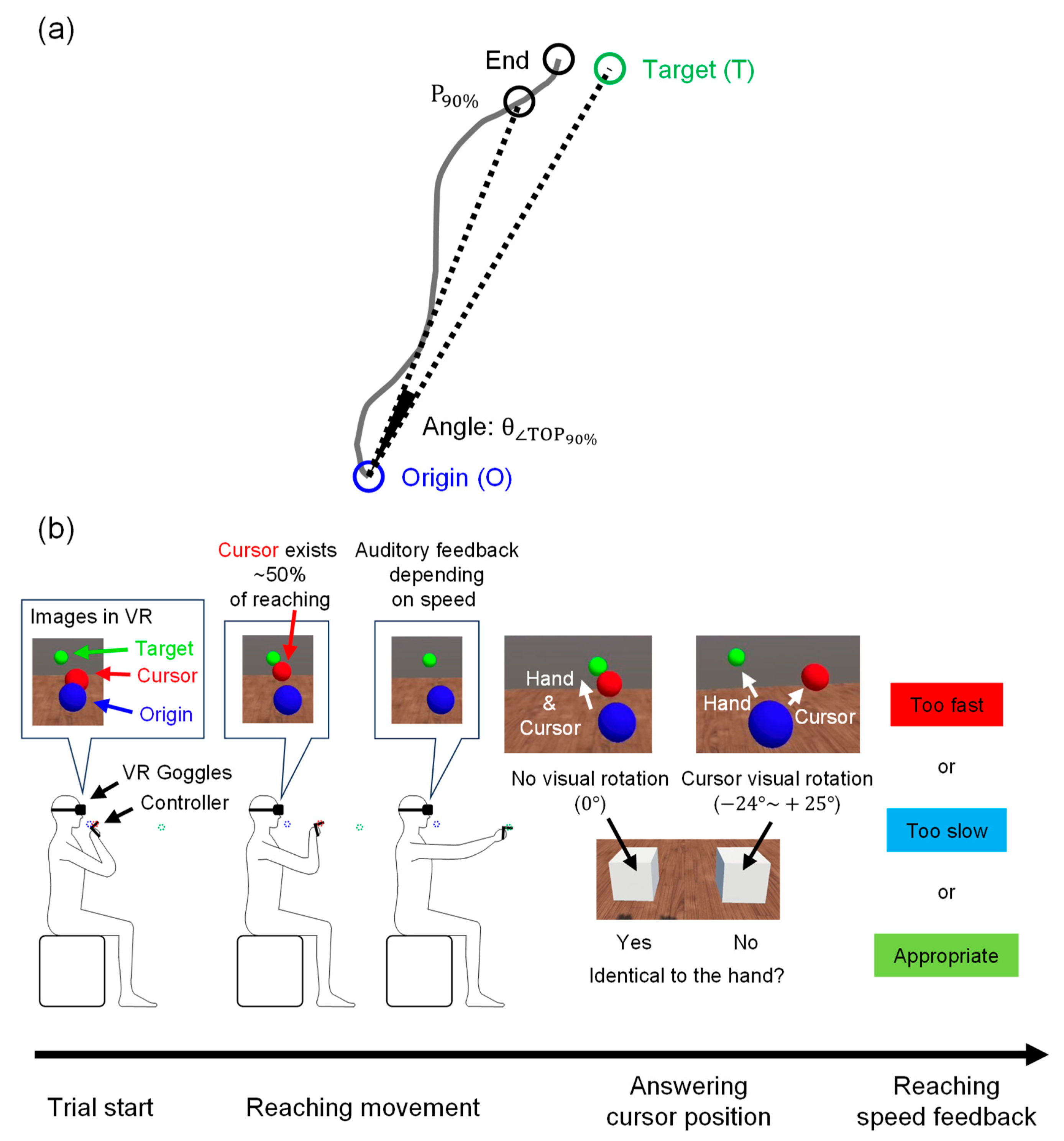
2.2. VR-HMD Arm-Reaching Task
2.3. Behavioral Data Analyses
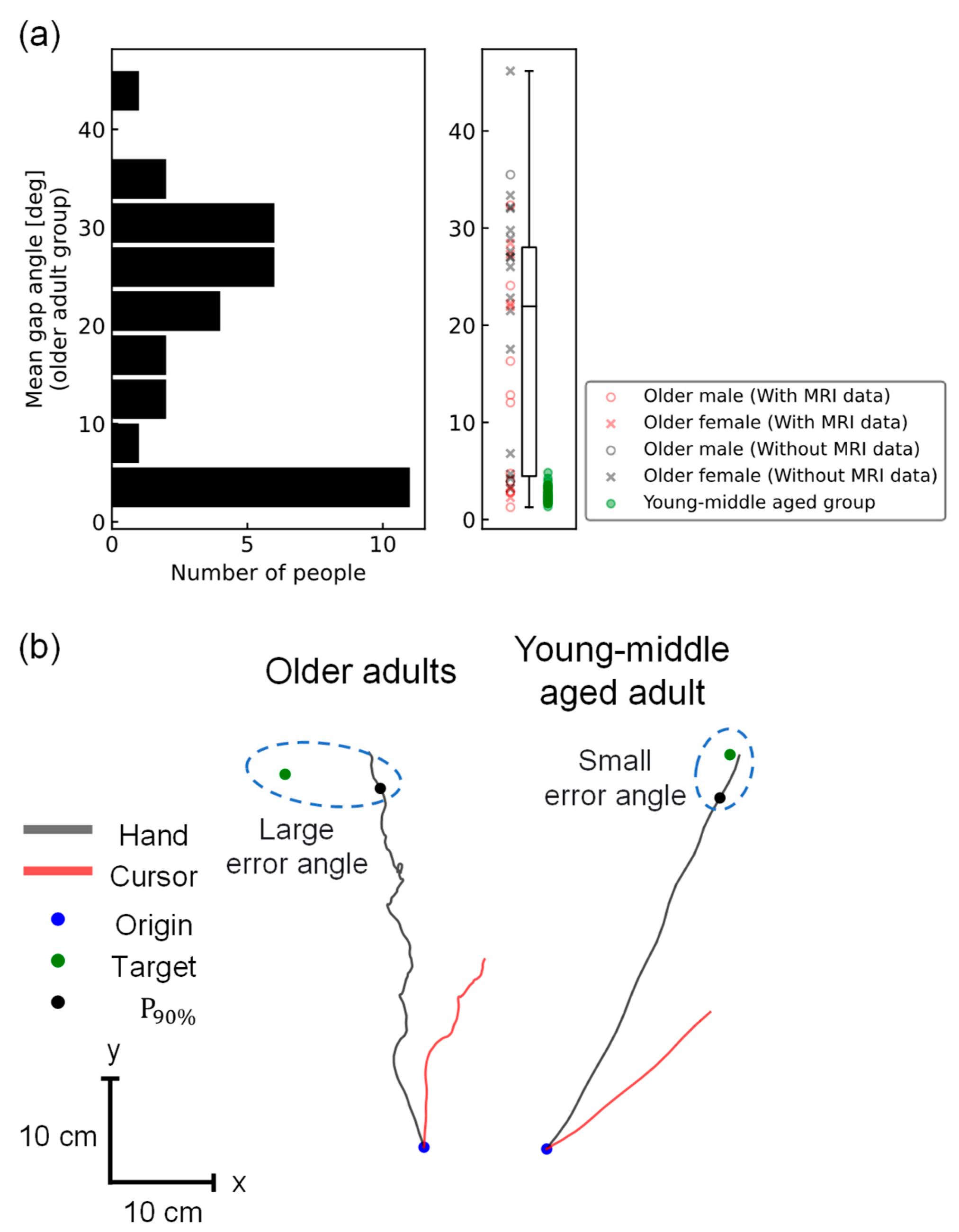
2.3.1. Mean Gap Angle Calculation
2.3.2. Statistical Analyses
2.4. MRI Acquisition
2.5. MRI Data Analyses
2.5.1. Preprocessing
2.5.2. Denoising
- Regression of white matter timeseries (utilizing 5 CompCor noise components)
- Regression of CSF timeseries (also using 5 CompCor noise components)
- Accounting for motion parameters and their first-order derivatives (12 factors) [34]
- Removal of outlier scans (up to 41 factors) [29]
- Adjustment for session and task effects, including their first-order derivatives (two factors)
- Correction for linear trends (two factors) within each functional run
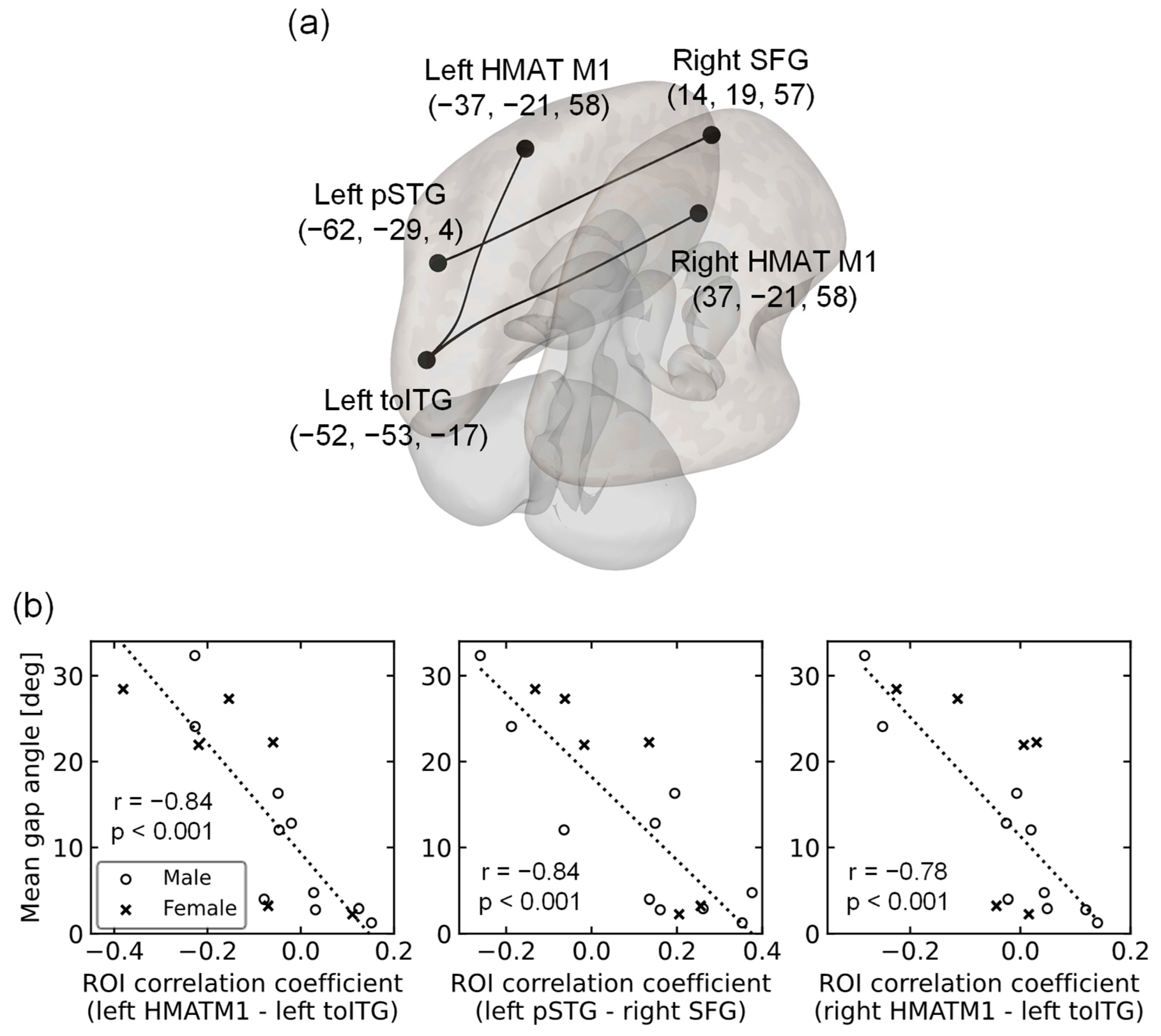
2.5.3. Regions of Interest (ROIs)
2.5.4. First-Level Analysis
2.5.5. Group-Level Analyses
3. Results
3.1. Behavioral Difference between Older Adults and Young/Middle-Aged Adults
3.2. rsFC of Older Adults Reflecting the Mean Gap Angle
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peterka, R.J. Sensorimotor integration in human postural control. J. Neurophysiol. 2002, 88, 1097–1118. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, W.; Zhu, Y.; Tian, E.; Kong, W. Impaired multisensory integration predisposes the elderly people to fall: A systematic review. Front. Neurosci. 2020, 14, 411. [Google Scholar] [CrossRef]
- Sturnieks, D.L.; St George, R.; Lord, S.R. Balance disorders in the elderly. Neurophysiol. Clin. 2008, 38, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Rand, M.K.; Wang, L.; Müsseler, J.; Heuer, H. Vision and proprioception in action monitoring by young and older adults. Neurobiol. Aging 2013, 34, 1864–1872. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, N.; Tsuda, H.; Aquino, D.; Takagi, A.; Ogata, Y.; Koike, Y.; Minati, L. Age-related decline of sensorimotor integration influences resting-state functional brain connectivity. Brain Sci. 2020, 10, 966. [Google Scholar] [CrossRef]
- Zhang, Y.; Smeets, J.B.J.; Brenner, E.; Verschueren, S.; Duysens, J. Effects of ageing on responses to stepping-target displacements during walking. Eur. J. Appl. Physiol. 2021, 121, 127–140. [Google Scholar] [CrossRef]
- De Rond, V.; Orcioli-Silva, D.; Dijkstra, B.W.; Orban de Xivry, J.J.; Pantall, A.; Nieuwboer, A. Compromised brain activity with age during a game-like dynamic balance task: Single-vs. dual-task performance. Front. Aging Neurosci. 2021, 13, 657308. [Google Scholar] [CrossRef]
- Seidler, R.D.; Bernard, J.A.; Burutolu, T.B.; Fling, B.W.; Gordon, M.T.; Gwin, J.T.; Kwak, Y.; Lipps, D.B. Motor control and aging: Links to age-related brain structural, functional, and biochemical effects. Neurosci. Biobehav. Rev. 2010, 34, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Van Ruitenbeek, P.; Monteiro, T.S.; Chalavi, S.; King, B.R.; Cuypers, K.; Sunaert, S.; Peeters, R.; Swinnen, S.P. Interactions between the aging brain and motor task complexity across the lifespan: Balancing brain activity resource demand and supply. Cereb. Cortex. 2023, 33, 6420–6434. [Google Scholar] [CrossRef]
- Voelcker-Rehage, C. Motor-skill learning in older adults—A review of studies on age-related differences. Eur. Rev. Aging Phys. Act. 2008, 5, 5–16. [Google Scholar] [CrossRef]
- King, B.R.; Van Ruitenbeek, P.; Leunissen, I.; Cuypers, K.; Heise, K.-F.; Monteiro, T.S.; Hermans, L.; Levin, O.; Albouy, G.; Mantini, D.; et al. Age-related declines in motor performance are associated with decreased segregation of large-scale resting state brain networks. Cereb. Cortex. 2018, 28, 4390–4402. [Google Scholar] [CrossRef]
- Meijer, A.; Königs, M.; Pouwels, P.J.; Smith, J.; Visscher, C.; Bosker, R.J.; Hartman, E.; Oosterlaan, J. Resting state networks mediate the association between both cardiovascular fitness and gross motor skills with neurocognitive functioning. Child. Dev. 2022, 93, e412–e426. [Google Scholar] [CrossRef]
- Monteiro, T.S.; King, B.R.; Seer, C.; Mantini, D.; Swinnen, S.P. Network-specific differences in transient brain activity at rest are associated with age-related reductions in motor performance. NeuroImage 2022, 252, 119025. [Google Scholar] [CrossRef]
- Chen, J.; Or, C.K.; Chen, T. Effectiveness of using virtual reality–supported exercise therapy for upper extremity motor rehabilitation in patients with stroke: Systematic review and meta-analysis of randomized controlled trials. J. Med. Internet Res. 2022, 24, e24111. [Google Scholar] [CrossRef] [PubMed]
- De Miguel-Rubio, A.; Rubio, M.D.; Alba-Rueda, A.; Salazar, A.; Moral-Munoz, J.A.; Lucena-Anton, D. Virtual reality systems for upper limb motor function recovery in patients with spinal cord injury: Systematic review and meta-analysis. JMIR Mhealth Uhealth 2020, 8, e22537. [Google Scholar] [CrossRef]
- Ketcham, C.J.; Stelmach, G.E. Movement control in the older adult. In Technology for Adaptive Aging; National Academies Press: Washington, DC, USA, 2004. [Google Scholar]
- Jones, J.A.; Swan, J.E.; Singh, G.; Kolstad, E.; Ellis, S.R. The effects of virtual reality, augmented reality, and motion parallax on egocentric depth perception. In Proceedings of the 5th Symposium on Applied Perception in Graphics and Visualization, Los Angeles, CA, USA, 9–10 August 2008; pp. 9–14. [Google Scholar] [CrossRef]
- Whitfield-Gabrieli, S.; Nieto-Castanon, A. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012, 2, 125–141. [Google Scholar] [CrossRef]
- Nieto-Castanon, A.; Whitfield-Gabrieli, S. CONN Functional Connectivity Toolbox: RRID SCR_009550, Release 22; Hilbert Press: Boston, MA, USA, 2022. [Google Scholar] [CrossRef]
- Penny, W.D.; Friston, K.J.; Ashburner, J.T.; Kiebel, S.J.; Nichols, T.E. (Eds.) Statistical Parametric Mapping: The Analysis of Functional Brain Images; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Nieto-Castanon, A. FMRI minimal preprocessing pipeline. In Handbook of Functional Connectivity Magnetic Resonance Imaging Methods in CONN; Hilbert Press: Boston, MA, USA, 2020; pp. 3–16. [Google Scholar]
- Friston, K.J. Statistical parametric mapping. In Neuroscience Databases: A Practical Guide; Springer: Berlin/Heidelberg, Germany, 2003; pp. 237–250. [Google Scholar]
- Andersson, J.L.; Hutton, C.; Ashburner, J.; Turner, R.; Friston, K.J. Modeling geometric deformations in EPI time series. Neuroimage 2001, 13, 903–919. [Google Scholar] [CrossRef]
- Friston, K.J.; Ashburner, J.; Frith, C.D.; Poline, J.-B.; Heather, J.D.; Frackowiak RS, J. Spatial registration and normalization of images. Hum. Brain Mapp. 1995, 3, 165–189. [Google Scholar] [CrossRef]
- Henson RN, A.; Buechel, C.; Josephs, O.; Friston, K.J. The slice-timing problem in event-related fMRI. NeuroImage 1999, 9, 125. [Google Scholar]
- Sladky, R.; Friston, K.J.; Tröstl, J.; Cunnington, R.; Moser, E.; Windischberger, C. Slice-timing effects and their correction in functional MRI. Neuroimage 2011, 58, 588–594. [Google Scholar] [CrossRef]
- Whitfield-Gabrieli, S.; Nieto-Castanon, A.; Ghosh, S. Artifact Detection Tools (ART); Release version; NITRC: Cambridge, MA, USA, 2011. [Google Scholar]
- Nieto-Castanon, A. Preparing fMRI data for statistical analysis. In fMRI Techniques and Protocols; Filippi, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar] [CrossRef]
- Power, J.D.; Mitra, A.; Laumann, T.O.; Snyder, A.Z.; Schlaggar, B.L.; Petersen, S.E. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 2014, 84, 320–341. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, V.D.; Wager, T.D.; Krishnan, A.; Rosch, K.S.; Seymour, K.E.; Nebel, M.B.; Mostofsky, S.H.; Nyalakanai, P.; Kiehl, K. The impact of T1 versus EPI spatial normalization templates for fMRI data analyses. Hum. Brain Mapp. 2017, 38, 5331–5342. [Google Scholar] [CrossRef]
- Ashburner, J. A fast diffeomorphic image registration algorithm. Neuroimage 2007, 38, 95–113. [Google Scholar] [CrossRef]
- Ashburner, J.; Friston, K.J. Unified segmentation. Neuroimage 2005, 26, 839–851. [Google Scholar] [CrossRef]
- Nieto-Castanon, A. FMRI denoising pipeline. In Handbook of Functional Connectivity Magnetic Resonance Imaging Methods in CONN; Hilbert Press: Boston, MA, USA, 2020; pp. 17–25. [Google Scholar]
- Friston, K.J.; Williams, S.; Howard, R.; Frackowiak, R.S.; Turner, R. Movement-related effects in fMRI time-series. Magn. Reson. Med. 1996, 35, 346–355. [Google Scholar] [CrossRef]
- Hallquist, M.N.; Hwang, K.; Luna, B. The nuisance of nuisance regression: Spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. Neuroimage 2013, 82, 208–225. [Google Scholar] [CrossRef]
- Behzadi, Y.; Restom, K.; Liau, J.; Liu, T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 2007, 37, 90–101. [Google Scholar] [CrossRef]
- Chai, X.J.; Castañón, A.N.; Ongür, D.; Whitfield-Gabrieli, S. Anticorrelations in resting state networks without global signal regression. Neuroimage 2012, 59, 1420–1428. [Google Scholar] [CrossRef]
- Mayka, M.A.; Corcos, D.M.; Leurgans, S.E.; Vaillancourt, D.E. Three-dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: A meta-analysis. Neuroimage 2006, 31, 1453–1474. [Google Scholar] [CrossRef] [PubMed]
- Zapparoli, L.; Mariano, M.; Paulesu, E. How the motor system copes with aging: A quantitative meta-analysis of the effect of aging on motor function control. Commun. Biol. 2022, 5, 79. [Google Scholar] [CrossRef]
- Koen, J.D.; Rugg, M.D. Neural dedifferentiation in the aging brain. Trends Cogn. Sci. 2019, 23, 547–559. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morcom, A.M.; Johnson, W. Neural reorganization and compensation in aging. J. Cogn. Neurosci. 2015, 27, 1275–1285. [Google Scholar] [CrossRef]
- Nieto-Castanon, A. Functional connectivity measures. In Handbook of Functional Connectivity Magnetic Resonance Imaging Methods in CONN; Hilbert Press: Boston, MA, USA, 2020; pp. 26–62. [Google Scholar]
- Rohr, L.E. Gender-specific movement strategies using a computer-pointing task. J. Mot. Behav. 2006, 38, 431–437. [Google Scholar] [CrossRef]
- Smith, C.D.; Umberger, G.H.; Manning, E.L.; Slevin, J.T.; Wekstein, D.R.; Schmitt, F.A.; Markesbery, W.R.; Zhang, Z.; Gerhardt, G.A.; Kryscio, R.J.; et al. Critical decline in fine motor hand movements in human aging. Neurology 1999, 53, 1458. [Google Scholar] [CrossRef]
- Kawashima, R.; Roland, P.E.; O’Sullivan, B.T. Fields in human motor areas involved in preparation for reaching, actual reaching, and visuomotor learning: A positron emission tomography study. J. Neurosci. 1994, 14, 3462–3474. [Google Scholar] [CrossRef] [PubMed]
- Shulman, G.L.; Corbetta, M.; Buckner, R.L.; Fiez, J.A.; Miezin, F.M.; Raichle, M.E.; Petersen, S.E. Common blood flow changes across visual tasks: I. Increases in subcortical structures and cerebellum but not in nonvisual cortex. J. Cogn. Neurosci. 1997, 9, 624–647. [Google Scholar] [CrossRef]
- Buchsbaum, B.R.; Hickok, G.; Humphries, C. Role of left posterior superior temporal gyrus in phonological processing for speech perception and production. Cogn. Sci. 2001, 25, 663–678. [Google Scholar] [CrossRef]
- Graves, W.W.; Grabowski, T.J.; Mehta, S.; Gupta, P. The left posterior superior temporal gyrus participates specifically in accessing lexical phonology. J. Cogn. Neurosci. 2008, 20, 1698–1710. [Google Scholar] [CrossRef]
- Hu, S.; Ide, J.S.; Zhang, S.; Li, C.R. The right superior frontal gyrus and individual variation in proactive control of impulsive response. J. Neurosci. 2016, 36, 12688–12696. [Google Scholar] [CrossRef] [PubMed]
- Carp, J.; Park, J.; Hebrank, A.; Park, D.C.; Polk, T.A. Age-related neural dedifferentiation in the motor system. PLoS ONE 2011, 6, e29411. [Google Scholar] [CrossRef]
- Cassady, K.; Ruitenberg MF, L.; Reuter-Lorenz, P.A.; Tommerdahl, M.; Seidler, R.D. Neural dedifferentiation across the lifespan in the motor and somatosensory systems. Cereb. Cortex. 2020, 30, 3704–3716. [Google Scholar] [CrossRef] [PubMed]
- Naccarato, M.; Calautti, C.; Jones, P.S.; Day, D.J.; Carpenter, T.A.; Baron, J.C. Does healthy aging affect the hemispheric activation balance during paced index-to-thumb opposition task? An fMRI study. Neuroimage 2006, 32, 1250–1256. [Google Scholar] [CrossRef]
- Fujiyama, H.; Hinder, M.R.; Summers, J.J. Functional role of left PMd and left M1 during preparation and execution of left hand movements in older adults. J. Neurophysiol. 2013, 110, 1062–1069. [Google Scholar] [CrossRef]
- Butler, J.S.; Burkhauser, R.V.; Mitchell, J.M.; Pincus, T.P. Measurement error in self-reported health variables. Rev. Econ. Stat. 1987, 69, 644–650. [Google Scholar] [CrossRef]
- Adams, S.A.; Matthews, C.E.; Ebbeling, C.B.; Moore, C.G.; Cunningham, J.E.; Fulton, J.; Hebert, J.R. The effect of social desirability and social approval on self-reports of physical activity. Am. J. Epidemiol. 2005, 161, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Fjell, A.M.; McEvoy, L.; Holland, D.; Dale, A.M.; Walhovd, K.B.; Alzheimer’s Disease Neuroimaging Initiative. Brain changes in older adults at very low risk for Alzheimer’s disease. J. Neurosci. 2013, 33, 8237–8242. [Google Scholar] [CrossRef]
- Zwergal, A.; Linn, J.; Xiong, G.; Brandt, T.; Strupp, M.; Jahn, K. Aging of human supraspinal locomotor and postural control in fMRI. Neurobiol. Aging 2012, 33, 1073–1084. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inagaki, S.; Matsuura, H.; Sakurai, K.; Minati, L.; Yoshimura, N. Decline in Sensory Integration in Old Age and Its Related Functional Brain Connectivity Correlates Observed during a Virtual Reality Task. Brain Sci. 2024, 14, 840. https://doi.org/10.3390/brainsci14080840
Inagaki S, Matsuura H, Sakurai K, Minati L, Yoshimura N. Decline in Sensory Integration in Old Age and Its Related Functional Brain Connectivity Correlates Observed during a Virtual Reality Task. Brain Sciences. 2024; 14(8):840. https://doi.org/10.3390/brainsci14080840
Chicago/Turabian StyleInagaki, Satoru, Hirokazu Matsuura, Kazuki Sakurai, Ludovico Minati, and Natsue Yoshimura. 2024. "Decline in Sensory Integration in Old Age and Its Related Functional Brain Connectivity Correlates Observed during a Virtual Reality Task" Brain Sciences 14, no. 8: 840. https://doi.org/10.3390/brainsci14080840
APA StyleInagaki, S., Matsuura, H., Sakurai, K., Minati, L., & Yoshimura, N. (2024). Decline in Sensory Integration in Old Age and Its Related Functional Brain Connectivity Correlates Observed during a Virtual Reality Task. Brain Sciences, 14(8), 840. https://doi.org/10.3390/brainsci14080840





