Pork Liver Decomposition Product May Improve Frontal Lobe Function in Humans—Open Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. PLDP
2.3. Study Design
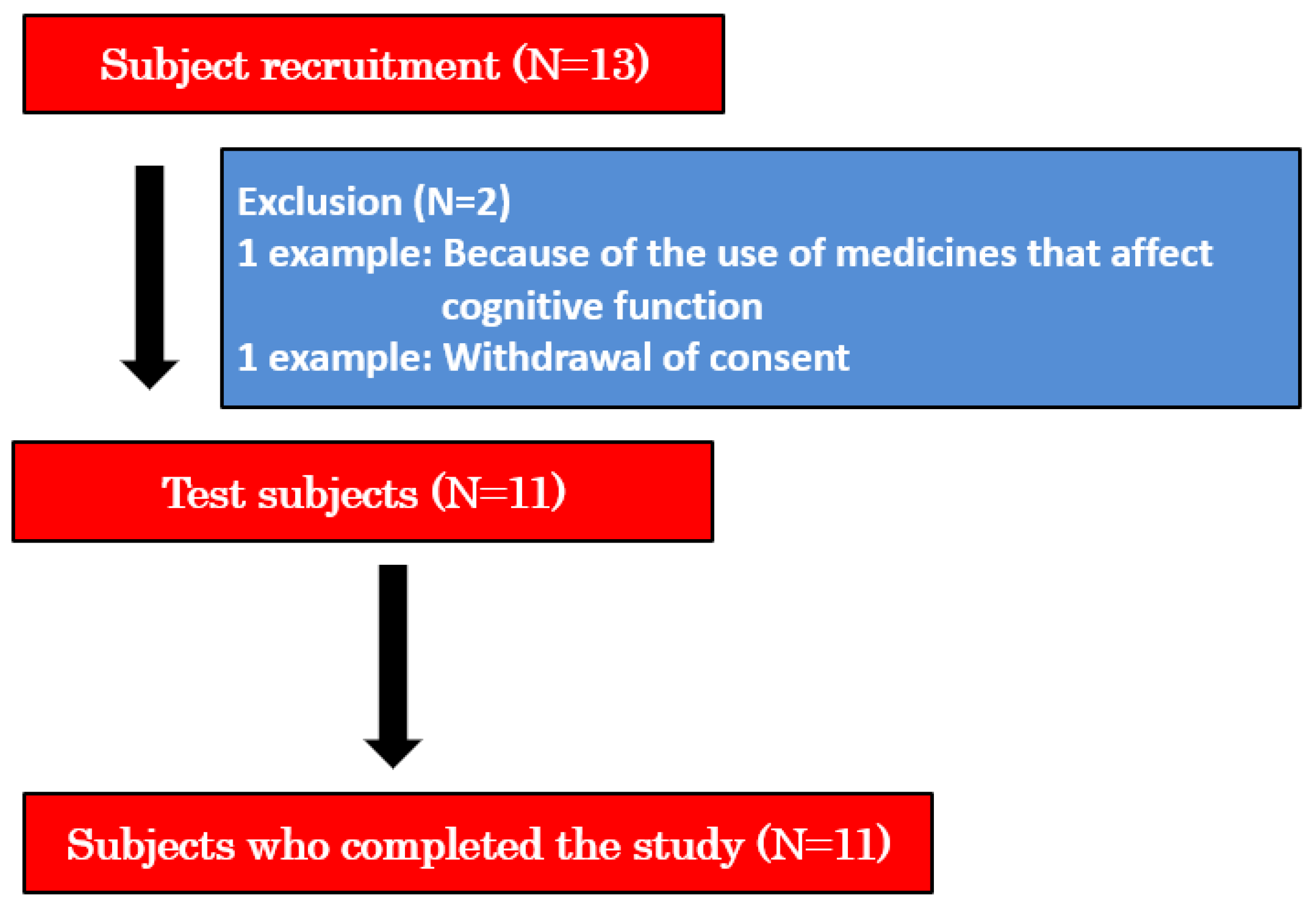
2.4. Recruitment of Participants
- Those who are 20 years of age or older on the date of obtaining consent for the test.
- Those with a score of 15 or more and 23 or less on the HDS-R.
- A person who can explain the purpose and content of the test before the test and obtain written consent from the participant.
- Those who must continue to take anti-dementia drugs or those who will start taking anti-dementia drugs (this study will not be successful).
- Those taking medicines that are expected to affect the HDS-R (this study will fail).
- Individuals with a history of brain disease or complications that affect the HDS-R (this study will not be successful).
- People who have difficulty swallowing (as this product may cause aspiration).
- Not understanding the purpose of this study (because it may lead to adverse effects).
- Participants whose treatment may change during this study (because the study will be unsuccessful).
- Difficulty conducting intelligence tests (because the test will fail).
- Allergic to pork liver (to ensure the safety of the participants).
- Persons with malignant tumors or complications, such as severe heart, kidney, or liver disorders; respiratory diseases; or circulatory diseases (to ensure the safety of the participants).
- Other persons judged inappropriate for inclusion by the doctor in charge of the study.
2.5. Statistical Analysis
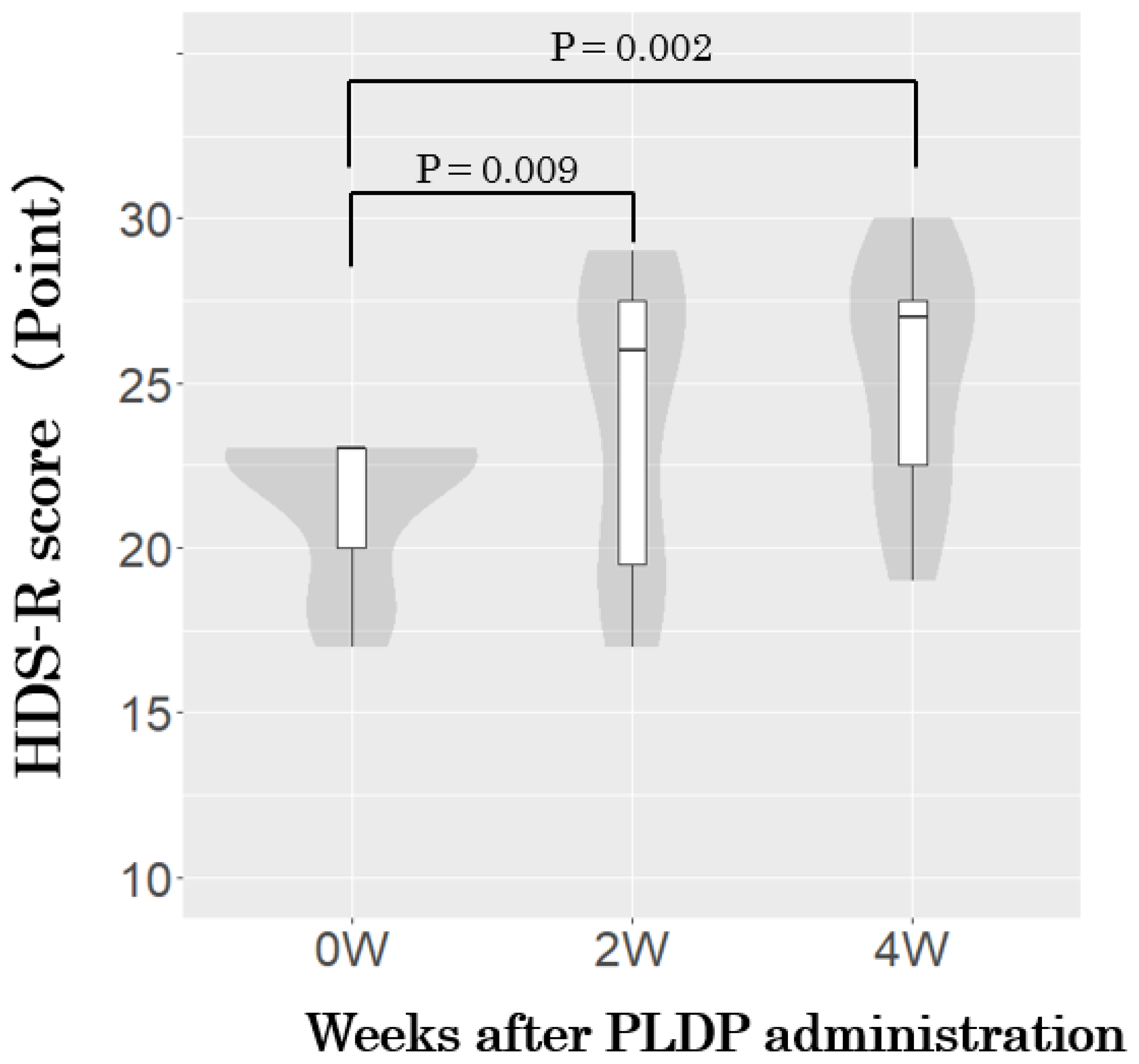
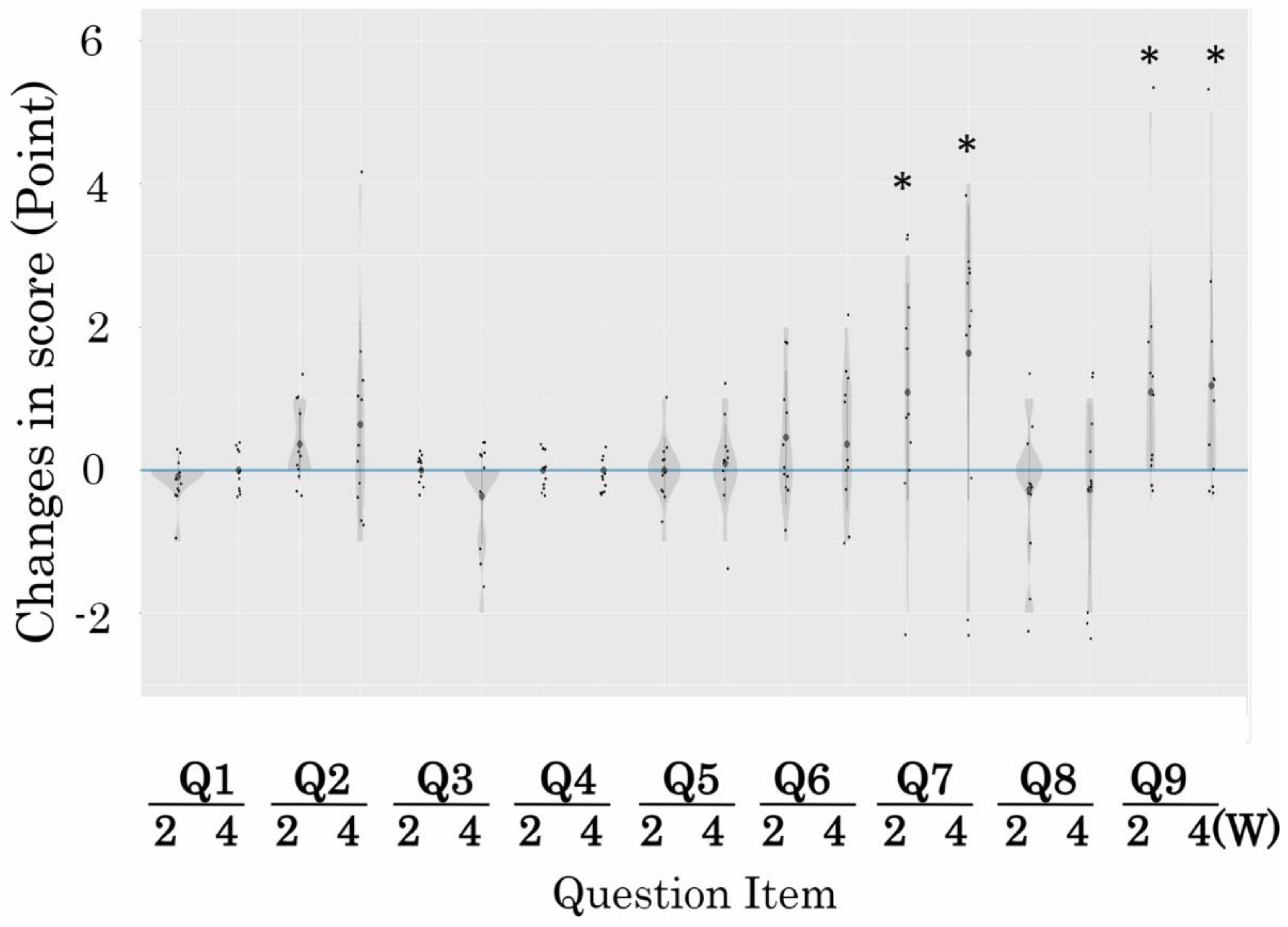
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matsuda, Y.; Haniu, H.; Tsukahara, T.; Inoue, T.; Sako, K.; Sugita, K.; Mabuchi, T.; Emizu, T.; Sato, K. Effects of Porcine Liver Decomposition Product on the cognitive function in non-dementia patients. Jpn. J. Med. Pharm. Sci. 2016, 73, 1057–1066. (In Japanese) [Google Scholar]
- Matsuda, Y.; Haniu, H.; Tsukahara, T.; Uemura, T.; Inoue, T.; Sako, K.I.; Kojima, J.; Mori, T.; Sato, K. Oral administration of porcine liver decomposition product for 4 weeks enhances visual memory and delayed recall in healthy adults over 40 years of age: A randomized, double-blind, placebo-controlled study. Exp. Gerontol. 2020, 141, 111064. [Google Scholar] [CrossRef]
- Cohen, E.L.; Wurtman, R.J. Brain acetylcholine: Control by dietary choline. Science 1976, 191, 561–562. [Google Scholar] [CrossRef]
- Tsukahara, T.; Haniu, H.; Uemura, T.; Matsuda, Y. Porcine liver decomposition product-derived lysophospholipids promote microglial activation in vitro. Sci. Rep. 2020, 10, 3748. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, T.; Haniu, H.; Uemura, T.; Matsuda, Y. Therapeutic potential of porcine liver decomposition product: New insights and perspectives for microglia-mediated neuroinflammation in neurodegenerative diseases. Biomedicines 2020, 8, 446. [Google Scholar] [CrossRef]
- Kato, T. Neuro-immunological hypothesis of psychiatric diseases via microglia. Jpn. J. Biol. Psychiatry 2010, 21, 229–236. (In Japanese) [Google Scholar]
- Otsubo, T.; Tanaka, K.; Koda, R.; Shinoda, J.; Sano, N.; Tanaka, S.; Aoyama, H.; Mimura, M.; Kamijima, K. Reliability and validity of Japanese version of the Mini-International Neuropsychiatric Interview. Psychiatry Clin. Neurosci. 2005, 59, 517–526. [Google Scholar] [CrossRef]
- Nagai, C. Neuropsychological Assessment Methods for General Clinician. Neurol. Ther. 2022, 39, 241–245. (In Japanese) [Google Scholar] [CrossRef]
- Toda, T.; Nagami, S.; Fukunaga, S. Efficacy of verbal fluency task in patients with Alzheimer’s disease. JAHS 2018, 9, 142–148. [Google Scholar] [CrossRef]
- Audenaert, K.; Brans, B.; Van Laere, K.; Lahorte, P.; Versijpt, J.; Van Heeringen, K.; Dierckx, R. Verbal fluency as a prefrontal activation probe: A validation study using 99mTc-ECD brain SPET. Eur. J. Nucl. Med. 2000, 27, 1800–1808. [Google Scholar] [CrossRef] [PubMed]
- Gourovitch, M.L.; Kirkby, B.S.; Goldberg, T.E.; Weinberger, D.R.; Gold, J.M.; Esposito, G.; Van Horn, J.D.; Berman, K.F. A comparison of rCBF patterns during letter and semantic fluency. Neuropsychology 2000, 14, 353–360. [Google Scholar] [CrossRef]
- Anand, A.; Li, Y.; Wang, Y.; Wu, J.; Gao, S.; Bukhari, L.; Mathews, V.P.; Kalnin, A.; Lowe, M.J. Activity and connectivity of brain mood regulating circuit in depression: A functional magnetic resonance study. Biol. Psychiatry 2005, 57, 1079–1088. [Google Scholar] [CrossRef]
- Drevets, W.C.; Videen, T.O.; Price, J.L.; Preskorn, S.H.; Carmichael, S.T.; Raichle, M.E. A functional anatomical study of unipolar depression. J. Neurosci. 1992, 12, 3628–3641. [Google Scholar] [CrossRef]
- Surguladze, S.S.; Brammer, M.J.; Keedwell, P.; Giampietro, V.; Young, A.W.; Travis, M.J.; Williams, S.C.R.; Phillips, M.L. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol. Psychiatry 2005, 57, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, T.; Hara, H.; Haniu, H.; Matsuda, Y. The combined effects of lysophospholipids against lipopolysaccharide-induced inflammation and oxidative stress in microglial cells. J. Oleo Sci. 2021, 70, 947–954. [Google Scholar] [CrossRef]
- Badimon, A.; Strasburger, H.J.; Ayata, P.; Chen, X.; Nair, A.; Ikegami, A.; Hwang, P.; Chan, A.T.; Graves, S.M.; Uweru, J.O.; et al. Negative feedback control of neuronal activity by microglia. Nature 2020, 586, 417–423. [Google Scholar] [CrossRef]
- Ransohoff, R.M. A polarizing question: Do M1 and M2 microglia exist? Nat. Neurosci. 2016, 19, 987–991. [Google Scholar] [CrossRef]
- Setiawan, E.; Wilson, A.A.; Mizrahi, R.; Rusjan, P.M.; Miler, L.; Rajkowska, G.; Suridjan, I.; Kennedy, J.L.; Rekkas, P.V.; Houle, S.; et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 2015, 72, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Holmes, S.E.; Hinz, R.; Conen, S.; Gregory, C.J.; Matthews, J.C.; Anton-Rodriguez, J.M.; Gerhard, A.; Talbot, P.S. Elevated translocator protein in anterior cingulate in major depression and a role for inflammation in suicidal thinking: A positron emission tomography study. Biol. Psychiatry 2018, 83, 61–69. [Google Scholar] [CrossRef]
- McKim, D.B.; Niraula, A.; Tarr, A.J.; Wohleb, E.S.; Sheridan, J.F.; Godbout, J.P. Neuroinflammatory dynamics underlie memory impairments after repeated social defeat. J. Neurosci. 2016, 36, 2590–2604. [Google Scholar] [CrossRef]
- Ahmmed, M.K.; Hachem, M.; Ahmmed, F.; Rashidinejad, A.; Oz, F.; Bekhit, A.A.; Carne, A.; Bekhit, A.E.A. Marine Fish-derived lysophosphatidylcholine: Properties, extraction, quantification, and brain health application. Molecules 2023, 28, 3088. [Google Scholar] [CrossRef]
- Wen, J.; Satyanarayanan, S.K.; Li, A.; Yan, L.; Zhao, Z.; Yuan, Q.; Su, K.P.; Su, H. Unraveling the impact of Omega-3 polyunsaturated fatty acids on blood-brain barrier (BBB) integrity and glymphatic function. Brain Behav. Immun. 2024, 115, 335–355. [Google Scholar] [CrossRef] [PubMed]
- Murota, K. Digestion and absorption of dietary glycerophospholipids in the small intestine: Their significance as carrier molecules of choline and n-3 polyunsaturated fatty acids. Biocatal. Agric. Biotechnol. 2020, 26, 101633. [Google Scholar] [CrossRef]
- Inoue, T.; Matsuda, Y.; Sato, T.; Sakurada, C.; Haniu, H.; Tsukahara, T.; Sugita, K.; Mabuchi, T.; Emizu, T.; Sato, K. The impact of repeated administration of choline chloride on spatial cognitive memory in rats. Jpn. J. Med. Pharm. Sci. 2016, 73, 1009–1016. (In Japanese) [Google Scholar]
- Rose, C.R.; Blum, R.; Kafitz, K.W.; Kovalchuk, Y.; Konnerth, A. From modulator to mediator: Rapid effects of BDNF on ion channels. BioEssays 2004, 26, 1185–1194. [Google Scholar] [CrossRef]
- Tong, L.; Prieto, G.A.; Kramár, E.A.; Smith, E.D.; Cribbs, D.H.; Lynch, G.; Cotman, C.W. Brain-derived neurotrophic factor-dependent synaptic plasticity is suppressed by interleukin-1β via p38 mitogen-activated protein kinase. J. Neurosci. 2012, 32, 17714–17724. [Google Scholar] [CrossRef]
| Moisture (normal pressure heating drying method 105 °C, 3 h) | 72.90% | |
| Crude fat (ether extraction method) | 3.09% | |
| Phospholipid (LC/MS/MS method) | Total Phosphatidylcholine (PC) | 21.716 mg |
| Total Phosphatidylethanolamine (PE) | 2.569 mg | |
| Total Phosphatidylinositol (PI) | 1.076 mg | |
| Total Phosphatidylserine (PS) | 0.085 mg | |
| Total Phosphatidic acid (PA) | 0.648 mg | |
| Total Lysophosphatidylcholine (LPC) | 3.390 mg | |
| Total Sphingomyelin (SM) | 0.764 mg | |
| Question Items | |
|---|---|
| Q1 | Age |
| Q2 | Orientation to dates |
| Q3 | Orientation to place |
| Q4 | Verbal memory |
| Q5 | Attention |
| Q6 | Recent memory |
| Q7 | Delayed recall of verbal memory |
| Q8 | Visual memory |
| Q9 | Word recall/verbal fluency tasks |
| Study Indicator | Study Design | Participant | Result |
|---|---|---|---|
| HDS-R [1] | Study 1 Dose confirmation test (open test) (Participants: HDS-R > 20) | Low dose (N = 5) Age: 81 ± 4 High dose (N = 8) Age: 75 ± 4 | No effect at 2 cap/day, significant increase at (4 cap/day) |
| Study 2 Placebo-controlled double-blind study and safety confirmation study (Participants: HDS-R > 20) | Placebo (N = 25) Age: 58 ± 4 PLDP (N = 25) Age: 60 ± 4 | Placebo (4 cap/day): no significant change PLDP (4 cap/day): Significant increase No change in safety | |
| WMS-R [2] | Study 3 Placebo-controlled double-blind study | Under 40 years Placebo (N = 15) Age: 24 ± 2 PLDP (N = 15) Age: 25 ± 3 Over 40 years Placebo (N = 15) Age: 58 ± 8 PLDP (N = 13) Age: 63 ± 15 | PLDP (4 cap/day): Significant score increases compared to placebo in visual memory and delayed recall (aged over 40 years) |
| Patient No. | Age | HDS-R | Gender | Purpose of Visit |
|---|---|---|---|---|
| No. 1 | 80 | 23 | Male | Hypertension |
| No. 2 | 75 | 21 | Male | Prostatic hyperplasia |
| No. 3 | 53 | 23 | Male | Hypertension |
| No. 4 | 84 | 22 | Male | Edema |
| No. 5 | 86 | 23 | Female | Osteoporosis |
| No. 6 | 84 | 19 | Male | Dyslipidemia |
| No. 7 | 79 | 28 | Female | Hypertension |
| No. 8 | 80 | 23 | Female | Hypertension |
| No. 9 | 65 | 23 | Female | Hypertension |
| No. 10 | 79 | 23 | Female | Diabetes |
| No. 11 | 84 | 17 | Female | Hypertension |
| Mean ± SD | 77 ± 3 | 21.4 ± 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, M.; Sato, I.; Sato, M.; Iwasaki, H.; Saito, T.; Kimura, M.; Sako, K.; Maeda, T.; Haniu, H.; Tsukahara, T.; et al. Pork Liver Decomposition Product May Improve Frontal Lobe Function in Humans—Open Trial. Brain Sci. 2024, 14, 586. https://doi.org/10.3390/brainsci14060586
Suzuki M, Sato I, Sato M, Iwasaki H, Saito T, Kimura M, Sako K, Maeda T, Haniu H, Tsukahara T, et al. Pork Liver Decomposition Product May Improve Frontal Lobe Function in Humans—Open Trial. Brain Sciences. 2024; 14(6):586. https://doi.org/10.3390/brainsci14060586
Chicago/Turabian StyleSuzuki, Miiru, Ikuya Sato, Masatsugu Sato, Hideki Iwasaki, Takahiro Saito, Masahiko Kimura, Kenichi Sako, Tomoji Maeda, Hisao Haniu, Tamotsu Tsukahara, and et al. 2024. "Pork Liver Decomposition Product May Improve Frontal Lobe Function in Humans—Open Trial" Brain Sciences 14, no. 6: 586. https://doi.org/10.3390/brainsci14060586
APA StyleSuzuki, M., Sato, I., Sato, M., Iwasaki, H., Saito, T., Kimura, M., Sako, K., Maeda, T., Haniu, H., Tsukahara, T., & Matsuda, Y. (2024). Pork Liver Decomposition Product May Improve Frontal Lobe Function in Humans—Open Trial. Brain Sciences, 14(6), 586. https://doi.org/10.3390/brainsci14060586








