Socioeconomic Inequalities Affect Brain Responses of Infants Growing Up in Germany
Abstract
1. Introduction
1.1. Impact of Socioeconomic Inequalities on Family Dynamics
1.2. Impact of Socioeconomic Inequalities on Cognitive and Neural Development
1.3. Infant N2–P3a Complex following Unexpected Deviants
1.4. Aim of the Study
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Demographic Group Variables
2.4. Experimental Procedure
2.5. EEG Measurement
2.6. EEG Pre-Processing
2.7. Analysis of the Stimulus-Locked N2 and P3a in the Time Domain
2.8. Statistical Analysis
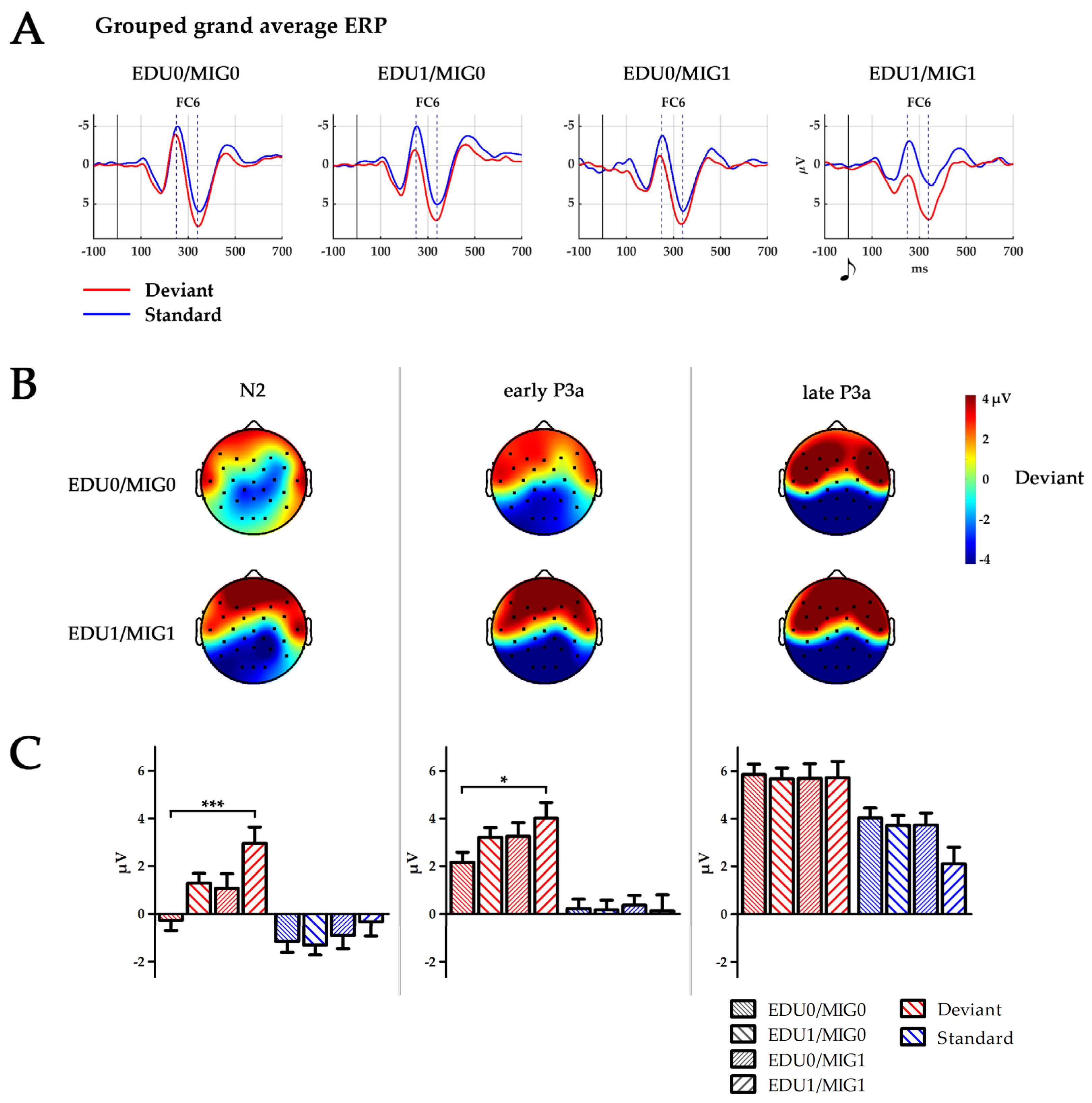
3. Results
3.1. Characterisation of the Infant N2–P3a Complex
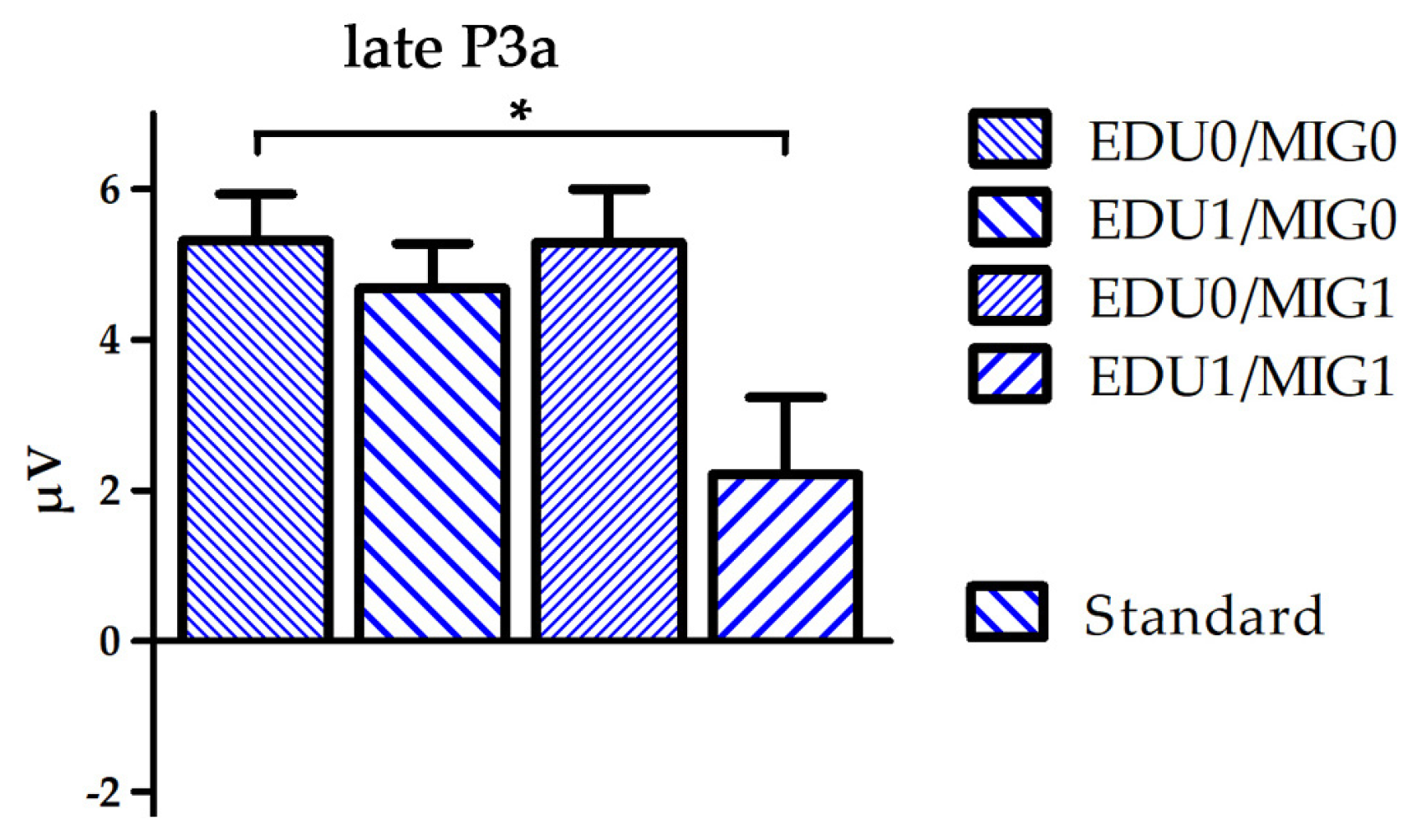
3.2. Group Differences
4. Discussion
4.1. Immature Processing of Stimulus Changes—The Infant N2–P3a Complex
4.2. Changes of the Infant N2–P3a Complex due to Social and Cultural Challenges
4.3. Embedding Results into the BRISE Longitudinal Study
4.4. Classification of Socioeconomic Backgrounds
4.5. Limitations and Directions for Future Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berger, L.M.; Paxson, C.; Waldfogel, J. Income and Child Development. Child. Youth Serv. Rev. 2009, 31, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Coll, C.G.; Lamberty, G.; Jenkins, R.; McAdoo, H.P.; Crnic, K.; Wasik, B.H.; García, H.V. An Integrative Model for the Study of Developmental Competencies in Minority Children. Child. Dev. 1996, 67, 1891–1914. [Google Scholar] [CrossRef]
- Letourneau, N.L.; Linda, D.L.; Leah, L.; Barry, W.; Catherine, Y.M. Socioeconomic Status and Child Development: A Meta-Analysis. J. Emot. Behav. Disord. 2013, 21, 211–224. [Google Scholar] [CrossRef]
- McLoyd, V.C. Socioeconomic disadvantage and child development. Am. Psychol. 1998, 53, 185–204. [Google Scholar] [CrossRef]
- Conger, R.D.; Conger, K.J.; Martin, M.J. Socioeconomic Status, Family Processes, and Individual Development. J. Marriage Fam. 2010, 72, 685–704. [Google Scholar] [CrossRef] [PubMed]
- Bradley, R.H.; Corwyn, R.F. Socioeconomic status and child development. Annu. Rev. Psychol. 2002, 53, 371–399. [Google Scholar] [CrossRef] [PubMed]
- Duncan, G.J.; Magnuson, K. Investing in Preschool Programs. J. Econ. Perspect. 2013, 27, 109–132. [Google Scholar] [CrossRef] [PubMed]
- Conger, R.D.; Donnellan, M.B. An interactionist perspective on the socioeconomic context of human development. Annu. Rev. Psychol. 2007, 58, 175–199. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Orozco, C.; Motti-Stefanidi, F.; Marks, A.; Katsiaficas, D.A.-O. An integrative risk and resilience model for understanding the adaptation of immigrant-origin children and youth. Am. Psychol. 2018, 73, 781–796. [Google Scholar] [CrossRef]
- Schneider, J.M.; Behboudi, M.H.; Maguire, M.J. The Necessity of Taking Culture and Context into Account When Studying the Relationship between Socioeconomic Status and Brain Development. Brain Sci. 2024, 14, 392. [Google Scholar] [CrossRef]
- Sirin, S.R. Socioeconomic Status and Academic Achievement: A Meta-Analytic Review of Research. Rev. Educ. Res. 2005, 75, 417–453. [Google Scholar] [CrossRef]
- Heineck, G.; Riphahn, R.T. Intergenerational Transmission of Educational Attainment in Germany—The Last Five Decades. Jahrbücher Natl. Stat. 2009, 229, 36–60. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Holloway, S.D. Parental Expectations and Children’s Academic Performance in Sociocultural Context. Educ. Psychol. Rev. 2010, 22, 189–214. [Google Scholar] [CrossRef]
- Berry, J.W. Acculturation: Living successfully in two cultures. Int. J. Intercult. Relat. 2005, 29, 697–712. [Google Scholar] [CrossRef]
- Portes, A. Legacies: The Story of the Immigrant Second Generation; Rumbaut, R.G., Ed.; University of California Press: Berkeley, CA, USA, 2001. [Google Scholar]
- Johnson, S.B.; Riis, J.L.; Noble, K.G. State of the Art Review: Poverty and the Developing Brain. Pediatrics 2016, 137, e20153075. [Google Scholar] [CrossRef] [PubMed]
- Farah, M.J. The Neuroscience of Socioeconomic Status: Correlates, Causes, and Consequences. Neuron 2017, 96, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Perkins, S.C.; Finegood, E.D.; Swain, J.E. Poverty and language development: Roles of parenting and stress. Innov. Clin. Neurosci. 2013, 10, 10–19. [Google Scholar] [PubMed]
- Hirsh-Pasek, K.; Adamson, L.B.; Bakeman, R.; Owen, M.T.; Golinkoff, R.M.; Pace, A.; Yust, P.K.; Suma, K. The Contribution of Early Communication Quality to Low-Income Children’s Language Success. Psychol. Sci. 2015, 26, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Evans, G.W. Child Development and the Physical Environment. Annu. Rev. Psychol. 2006, 57, 423–451. [Google Scholar] [CrossRef]
- Farah, M.J.; Shera, D.M.; Savage, J.H.; Betancourt, L.; Giannetta, J.M.; Brodsky, N.L.; Malmud, E.K.; Hurt, H. Childhood poverty: Specific associations with neurocognitive development. Brain Res. 2006, 1110, 166–174. [Google Scholar] [CrossRef]
- Duncan, G.J.; Yeung, W.J.; Brooks-Gunn, J.; Smith, J.R. How Much Does Childhood Poverty Affect the Life Chances of Children? Am. Sociol. Rev. 1998, 63, 406–423. [Google Scholar] [CrossRef]
- Noble, K.G.; McCandliss, B.D.; Farah, M.J. Socioeconomic gradients predict individual differences in neurocognitive abilities. Dev. Sci. 2007, 10, 464–480. [Google Scholar] [CrossRef] [PubMed]
- Lawson, G.M.; Hook, C.J.; Farah, M.J. A meta-analysis of the relationship between socioeconomic status and executive function performance among children. Dev. Sci. 2018, 21, e12529. [Google Scholar] [CrossRef] [PubMed]
- Stevens, C.; Bavelier, D. The role of selective attention on academic foundations: A cognitive neuroscience perspective. Dev. Cogn. Neurosci. 2012, 2 (Suppl. S1), S30–S48. [Google Scholar] [CrossRef] [PubMed]
- Halle, T.; Forry, N.D.; Hair, E.C.; Perper, K.; Wandner, L.D.; Wessel, J.S.; Vick, J. Disparities in Early Learning and Development: Lessons from the Early Childhood Longitudinal Study—Birth Cohort (ECLS-B): (571822009-001); Child Trends: Washington, DC, USA, 2009. [Google Scholar]
- Blossfeld, H.P.; Roßbach, H.G. Education as a Lifelong Process: The German National Educational Panel Study (NEPS); Springer: Wiesbaden, Germany, 2019. [Google Scholar] [CrossRef]
- Linberg, A.; Attig, M.; Weinert, S. Social disparities in the vocabulary of 2-year-old children and the mediating effect of language-stimulating interaction behavior. J. Educ. Res. Online 2020, 12, 12–35. [Google Scholar] [CrossRef] [PubMed]
- Lupien, S.J.; King, S.; Meaney, M.J.; McEwen, B.S. Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Dev. Psychopathol. 2001, 13, 653–676. [Google Scholar] [CrossRef] [PubMed]
- D’Angiulli, A.; Herdman, A.; Stapells, D.; Hertzman, C. Children’s Event-Related Potentials of Auditory Selective Attention Vary With Their Socioeconomic Status. Neuropsychology 2008, 22, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Stevens, C.; Lauinger, B.; Neville, H. Differences in the neural mechanisms of selective attention in children from different socioeconomic backgrounds: An event-related brain potential study. Dev. Sci. 2009, 12, 634–646. [Google Scholar] [CrossRef]
- Lipina, S.J.; Segretin, M.S. Strengths and weakness of neuroscientific investigations of childhood poverty: Future directions. Front. Hum. Neurosci. 2015, 9, 53. [Google Scholar] [CrossRef]
- Ursache, A.; Noble, K.G.; Pediatric Imaging, N.; Genetics, S. Socioeconomic status, white matter, and executive function in children. Brain Behav. 2016, 6, e00531. [Google Scholar] [CrossRef]
- Ursache, A.; Noble, K.G. Neurocognitive development in socioeconomic context: Multiple mechanisms and implications for measuring socioeconomic status. Psychophysiology 2016, 53, 71–82. [Google Scholar] [CrossRef]
- Lawson, G.M.; Duda, J.T.; Avants, B.B.; Wu, J.; Farah, M.J. Associations between children’s socioeconomic status and prefrontal cortical thickness. Dev. Sci. 2013, 16, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Pietto, M.L.; Kamienkowski, J.E.; Lipina, S.J. Electrophysiological Approaches in the Study of the Influence of Childhood Poverty on Cognition. In Neuroscience and Social Science: The Missing Link; Ibáñez, A., Sedeño, L., García, A.M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 349–381. [Google Scholar] [CrossRef]
- Noble, K.G.; Engelhardt, L.E.; Brito, N.H.; Mack, L.J.; Nail, E.J.; Angal, J.; Barr, R.; Fifer, W.P.; Elliott, A.J. Socioeconomic disparities in neurocognitive development in the first two years of life. Dev. Psychobiol. 2015, 57, 535–551. [Google Scholar] [CrossRef] [PubMed]
- Hackman, D.A.; Gallop, R.; Evans, G.W.; Farah, M.J. Socioeconomic status and executive function: Developmental trajectories and mediation. Dev. Sci. 2015, 18, 686–702. [Google Scholar] [CrossRef] [PubMed]
- Moyano, S.; Rico-Pico, J.; Conejero, A.; Hoyo, A.; Ballesteros-Duperon, M.L.A.; Rueda, M.R. Influence of the environment on the early development of attentional control. Infant. Behav. Dev. 2023, 71, 101842. [Google Scholar] [CrossRef] [PubMed]
- Blair, C.; Granger, D.A.; Willoughby, M.; Mills-Koonce, R.; Cox, M.; Greenberg, M.T.; Kivlighan, K.T.; Fortunato, C.K.; Investigators, t.F. Salivary Cortisol Mediates Effects of Poverty and Parenting on Executive Functions in Early Childhood. Child. Dev. 2011, 82, 1970–1984. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.L.; Hair, N.; Shen, D.G.; Shi, F.; Gilmore, J.H.; Wolfe, B.L.; Pollak, S.D. Family poverty affects the rate of human infant brain growth. PLoS ONE 2013, 8, e80954. [Google Scholar] [CrossRef] [PubMed]
- Conejero, A.; Guerra, S.; Abundis-Gutiérrez, A.; Rueda, M.R. Frontal theta activation associated with error detection in toddlers: Influence of familial socioeconomic status. Dev. Sci. 2018, 21. [Google Scholar] [CrossRef] [PubMed]
- Cantiani, C.; Ortiz-Mantilla, S.; Riva, V.; Piazza, C.; Bettoni, R.; Musacchia, G.; Molteni, M.; Marino, C.; Benasich, A.A. Reduced left-lateralized pattern of event-related EEG oscillations in infants at familial risk for language and learning impairment. Neuroimage-Clin. 2019, 22, 101778. [Google Scholar] [CrossRef] [PubMed]
- Kushnerenko, E.V.; van den Bergh, B.R.H.; Winkler, I. Separating acoustic deviance from novelty during the first year of life: A review of event-related potential evidence. Front. Psychol. 2013, 4, 595. [Google Scholar] [CrossRef]
- Kushnerenko, E.; Ceponiene, R.; Balan, P.; Fellman, V.; Näätänen, R. Maturation of the auditory change detection response in infants: A longitudinal ERP study. Neuroreport 2002, 13, 1843–1848. [Google Scholar] [CrossRef]
- Mathes, B.; Kemmerich, R.; Wienke, A.S. Experimentelles Arbeiten mit Kindern aus verschiedenen (Sprach-)Kulturen und mit familiären Herausforderungen. Frühe Bild. 2024, 13, 116–118. [Google Scholar] [CrossRef]
- Frischkorn, G.T.; Hilger, K.; Kretzschmar, A.; Schubert, A.-L. Intelligenzdiagnostik der Zukunft. Psychol. Rundsch. 2022, 73, 173–189. [Google Scholar] [CrossRef]
- Bryant, G.A. Vocal communication across cultures: Theoretical and methodological issues. Philos. Trans. R. Soc. B Biol. Sci. 2022, 377, 20200387. [Google Scholar] [CrossRef]
- Chandrasekaran, B.; Krishnan, A.; Fau-Gandour, J.T.; Gandour, J.T. Sensory processing of linguistic pitch as reflected by the mismatch negativity. Ear Hear. 2009, 30, 552–558. [Google Scholar] [CrossRef]
- van Dinteren, R.; Arns, M.; Jongsma, M.L.A.; Kessels, R.P.C. P300 Development across the Lifespan: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e87347. [Google Scholar] [CrossRef]
- Başar-Eroglu, C.; Schmiedt-Fehr, C.; Marbach, S.; Brand, A.; Mathes, B. Altered oscillatory alpha and theta networks in schizophrenia. Brain Res. 2008, 1235, 143–152. [Google Scholar] [CrossRef]
- Justo-Guillén, E.; Ricardo-Garcell, J.; Rodríguez-Camacho, M.; Rodríguez-Agudelo, Y.; Lelo de Larrea-Mancera, E.S.; Solís-Vivanco, R. Auditory mismatch detection, distraction, and attentional reorientation (MMN-P3a-RON) in neurological and psychiatric disorders: A review. Int. J. Psychophysiol. 2019, 146, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Müller, V.; Jirsa, V.; Perdikis, D.; Sleimen-Malkoun, R.; von Oertzen, T.; Lindenberger, U. Lifespan Changes in Network Structure and Network Topology Dynamics During Rest and Auditory Oddball Performance. Front. Aging Neurosci. 2019, 11, 138. [Google Scholar] [CrossRef] [PubMed]
- Basar, E.; Schmiedt-Fehr, C.; Mathes, B.; Femir, B.; Emek-Savas, D.D.; Tulay, E.; Tan, D.; Duzgun, A.; Güntekin, B.; Ozerdem, A.; et al. What does the broken brain say to the neuroscientist? Oscillations and connectivity in schizophrenia, Alzheimer’s disease, and bipolar disorder. Int. J. Psychophysiol. 2016, 103, 135–148. [Google Scholar] [CrossRef]
- Fellman, V.; Kushnerenko, E.; Mikkola, K.; Ceponiene, R.; Leipala, J.; Näätänen, R. Atypical auditory event-related potentials in preterm infants during the first year of life: A possible sign of cognitive dysfunction? Pediatr. Res. 2004, 56, 291–297. [Google Scholar] [CrossRef]
- Kujala, T.; Tervaniemi, M.; Schröger, E. The mismatch negativity in cognitive and clinical neuroscience: Theoretical and methodological considerations. Biol. Psychol. 2007, 74, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Huotilainen, M.; Kujala, A.; Hotakainen, M.; Parkkonen, L.; Taulu, S.; Simola, J.; Nenonen, J.; Karjalainen, M.; Näätänen, R. Short-term memory functions of the human fetus recorded with magnetoencephalography. Neuroreport 2005, 16, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Piazza, C.; Cantiani, C.; Akalin-Acar, Z.; Miyakoshi, M.; Benasich, A.A.; Reni, G.; Bianchi, A.M.; Makeig, S. ICA-derived cortical responses indexing rapid multi-feature auditory processing in six-month-old infants. Neuroimage 2016, 133, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Tomé, D.; Barbosa, F.; Nowak, K.; Marques-Teixeira, J. The development of the N1 and N2 components in auditory oddball paradigms: A systematic review with narrative analysis and suggested normative values. J. Neural. Trans. 2015, 122, 375–391. [Google Scholar] [CrossRef] [PubMed]
- Näätänen, R.; Kujala, T.; Winkler, I. Auditory processing that leads to conscious perception: A unique window to central auditory processing opened by the mismatch negativity and related responses. Psychophysiology 2011, 48, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef] [PubMed]
- Folstein, J.R.; van Petten, C. Influence of cognitive control and mismatch on the N2 component of the ERP: A review. Psychophysiology 2008, 45, 152–170. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, N.; Benasich, A.A. Maturation of auditory evoked potentials from 6 to 48 months: Prediction to 3 and 4 year language and cognitive abilities. Clin. Neurophysiol. 2011, 122, 320–338. [Google Scholar] [CrossRef] [PubMed]
- Wetzel, N.; Schröger, E. On the development of auditory distraction: A review. PsyCh J. 2014, 3, 72–91. [Google Scholar] [CrossRef]
- Hämäläinen, J.A.; Ortiz-Mantilla, S.; Benasich, A.A. Source localization of event-related potentials to pitch change mapped onto age-appropriate MRIs at 6 months of age. Neuroimage 2011, 54, 1910–1918. [Google Scholar] [CrossRef]
- He, C.; Hotson, L.; Trainor, L.J. Mismatch responses to pitch changes in early infancy. J. Cogn. Neurosci. 2007, 19, 878–892. [Google Scholar] [CrossRef] [PubMed]
- Hackman, D.A.; Farah, M.J. Socioeconomic status and the developing brain. Trends Cogn. Sci. 2009, 13, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Schütte, K.; Hasselhorn, M.; Köller, O. Bildungsungleichheit besser verstehen und vermindern. Frühe Bild. 2024, 13, 65–70. [Google Scholar] [CrossRef]
- Katus, L.; Mason, L.; Milosavljevic, B.; McCann, S.; Rozhko, M.; Moore, S.E.; Elwell, C.E.; Lloyd-Fox, S.; de Haan, M.; Drammeh, S.; et al. ERP markers are associated with neurodevelopmental outcomes in 1–5 month old infants in rural Africa and the UK. NeuroImage 2020, 210, 116591. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Hotson, L.; Trainor, L.J. Maturation of cortical mismatch responses to occasional pitch change in early infancy: Effects of presentation rate and magnitude of change. Neuropsychologia 2009, 47, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Marie, C.; Trainor, L.J. Development of Simultaneous Pitch Encoding: Infants Show a High Voice Superiority Effect. Cereb. Cortex 2013, 23, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, T.; Mourad, N.; Trainor, L.J. Development of auditory-specific brain rhythm in infants. Eur. J. Neurosci. 2011, 33, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Chatrian, G.E.; Lettich, E.; Nelson, P.L. Modified nomenclature for the “10%” electrode system. J. Clin. Neurophysiol. 1988, 5, 183–186. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- McIsaac, H.; Polich, J. Comparison of infant and adult P300 from auditory stimuli. J. Exp. Child. Psychol. 1992, 53, 115–128. [Google Scholar] [CrossRef]
- Posner, M.I.; Rothbart, M.K. Research on attention networks as a model for the integration of psychological science. Annu. Rev. Psychol. 2007, 58, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Marie, C.; Trainor, L.J. Early development of polyphonic sound encoding and the high voice superiority effect. Neuropsychologia 2014, 57, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Butler, B.E.; Trainor, L.J. Brief pitch-priming facilitates infants’ discrimination of pitch-evoking noise: Evidence from event-related potentials. Brain Cogn. 2013, 83, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Ceponiene, R.; Rinne, T.; Näätänen, R. Maturation of cortical sound processing as indexed by event-related potentials. Clin. Neurophysiol. 2002, 113, 870–882. [Google Scholar] [CrossRef]
- Cheour, M.; Shestakova, A.; Alku, P.; Ceponiene, R.; Näätänen, R. Mismatch negativity shows that 3-6-year-old children can learn to discriminate non-native speech sounds within two months. Neurosci. Lett. 2002, 325, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Morr, M.L.; Shafer, V.L.; Kreuzer, J.A.; Kurtzberg, D. Maturation of Mismatch Negativity in Typically Developing Infants and Preschool Children. Ear Hear. 2002, 23, 118–136. [Google Scholar] [CrossRef] [PubMed]
- Alho, K.; Sainio, K.; Sajaniemi, N.; Reinikainen, K.; Näätänen, R. Event-related brain potential of human newborns to pitch change of an acoustic stimulus. Electroencephalogr. Clin. Neurophysiol. 1990, 77, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Trainor, L.J.; Lee, K.; Bosnyak, D.J. Cortical Plasticity in 4-Month-Old Infants: Specific Effects of Experience with Musical Timbres. Brain Topogr. 2011, 24, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Leppänen, P.H.; Lyytinen, H. Auditory event-related potentials in the study of developmental language-related disorders. Audiol. Neurootol. 1997, 2, 308–340. [Google Scholar] [CrossRef]
- Benasich, A.A.; Choudhury, N.A.; Realpe-Bonilla, T.; Roesler, C.P. Plasticity in developing brain: Active auditory exposure impacts prelinguistic acoustic mapping. J. Neurosci. 2014, 34, 13349–13363. [Google Scholar] [CrossRef]
- Sambeth, A.; Pakarinen, S.; Ruohio, K.; Fellman, V.; van Zuijen, T.L.; Huotilainen, M. Change detection in newborns using a multiple deviant paradigm: A study using magnetoencephalography. Clin. Neurophysiol. 2009, 120, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Kushnerenko, E.; Ceponiene, R.; Balan, P.; Fellman, V.; Huotilaine, M.; Näätänen, R. Maturation of the auditory event-related potentials during the first year of life. Neuroreport 2002, 13, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Demiralp, T.; Ademoglu, A.; Istefanopulos, Y.; Başar-Eroglu, C.; Başar, E. Wavelet analysis of oddball P300. Int. J. Psychophysiol. 2001, 39, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Başar, E.; Başar-Eroglu, C.; Karakas, S.; Schürmann, M. Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int. J. Psychophysiol. 2001, 39, 241–248. [Google Scholar] [CrossRef]
- Başar, E.; Schürmann, M.; Demiralp, T.; Başar-Eroglu, C.; Ademoglu, A. Event-related oscillations are ‘real brain responses’—Wavelet analysis and new strategies. Int. J. Psychophysiol. 2001, 39, 91–127. [Google Scholar] [CrossRef] [PubMed]
- Basar, E. The theory of the whole-brain-work. Int. J. Psychophysiol. 2006, 60, 133–138. [Google Scholar] [CrossRef]
- Braver, T.S.; Barch, D.M.; Gray, J.R.; Molfese, D.L.; Snyder, A. Anterior cingulate cortex and response conflict: Effects of frequency, inhibition and errors. Cereb. Cortex 2001, 11, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Coderre, E.; Conklin, K.; van Heuven, W.J. Electrophysiological measures of conflict detection and resolution in the Stroop task. Brain Res. 2011, 1413, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Niendam, T.A.; Laird, A.R.; Ray, K.L.; Dean, Y.M.; Glahn, D.C.; Carter, C.S. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn. Affect. Behav. Neurosci. 2012, 12, 241–268. [Google Scholar] [CrossRef]
- Isler, J.R.; Grieve, P.G.; Czernochowski, D.; Stark, R.I.; Friedman, D. Cross-frequency phase coupling of brain rhythms during the orienting response. Brain Res. 2008, 1232, 163–172. [Google Scholar] [CrossRef]
- Prada, L.; Barceló, F.; Herrmann, C.S.; Escera, C. EEG delta oscillations index inhibitory control of contextual novelty to both irrelevant distracters and relevant task-switch cues. Psychophysiology 2014, 51, 658–672. [Google Scholar] [CrossRef] [PubMed]
- Kolev, V.; Demiralp, T.; Yordanova, J.; Ademoglu, A.; Isoglu-Alkac, U. Time-frequency analysis reveals multiple functional components during oddball P300. Neuroreport 1997, 8, 2061–2065. [Google Scholar] [CrossRef] [PubMed]
- Marshall, P.J.; Bar-Haim, Y.; Fox, N.A. Development of the EEG from 5 months to 4 years of age. Clin. Neurophysiol. 2002, 113, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Rico-Pico, J.; Moyano, S.; Conejero, A.; Hoyo, A.; Ballesteros-Duperon, M.A.; Rueda, M.R. Early development of electrophysiological activity: Contribution of periodic and aperiodic components of the EEG signal. Psychophysiology 2023, 60, e14360. [Google Scholar] [CrossRef] [PubMed]
- Saby, J.N.; Marshall, P.J. The utility of EEG band power analysis in the study of infancy and early childhood. Dev. Neuropsychol. 2012, 37, 253–273. [Google Scholar] [CrossRef] [PubMed]
- Kolev, V.; Başar-Eroglu, C.; Aksu, F.; Başar, E. EEG rhythmicities evoked by visual stimuli in three-year-old children. Int. J. Neurosci. 1994, 75, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Başar-Eroglu, C.; Kolev, V.; Ritter, B.; Aksu, F.; Başar, E. EEG, auditory evoked potentials and evoked rhythmicities in three-year-old children. Int. J. Neurosci. 1994, 75, 239–255. [Google Scholar] [CrossRef]
- Mathes, B.; Khalaidovski, K.; Wienke, A.S.; Schmiedt-Fehr, C.; Başar-Eroglu, C. Maturation of the P3 and concurrent oscillatory processes during early and late adolescence. Clin. Neurophysiol. 2016, 127, 2599–2609. [Google Scholar] [CrossRef] [PubMed]
- Wienke, A.S.; Başar-Eroglu, C.; Schmiedt-Fehr, C.; Mathes, B. Novelty N2-P3a Complex and Theta Oscillations Reflect Improving Neural Coordination Within Frontal Brain Networks During Adolescence. Front. Behav. Neurosci. 2018, 12. [Google Scholar] [CrossRef]
- Giovanis, E.; Akdede, S. Cultural Integration of First-Generation Immigrants: Evidence from European Union Countries. Review of Economic Analysis 2023, 15, 97–125. [Google Scholar] [CrossRef]
- Hauge, L.J.; Torgersen, L.; Vollrath, M. Associations between maternal stress and smoking: Findings from a population-based prospective cohort study. Addiction 2012, 107, 1168–1173. [Google Scholar] [CrossRef] [PubMed]
- Luby, J.; Belden, A.; Botteron, K.; Marrus, N.; Harms, M.P.; Babb, C.; Nishino, T.; Barch, D. The Effects of Poverty on Childhood Brain Development: The Mediating Effect of Caregiving and Stressful Life Events. JAMA Pediatr. 2013, 167, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Farah, M.J. Socioeconomic status and the brain: Prospects for neuroscience-informed policy. Nat. Rev. Neurosci. 2018, 19, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Brito, N.H.; Fifer, W.P.; Myers, M.M.; Elliott, A.J.; Noble, K.G. Associations among family socioeconomic status, EEG power at birth, and cognitive skills during infancy. Dev. Cogn. Neurosci. 2016, 19, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Tomalski, P.; Moore, D.G.; Ribeiro, H.; Axelsson, E.L.; Murphy, E.; Karmiloff-Smith, A.; Johnson, M.H.; Kushnerenko, E. Socioeconomic status and functional brain development—associations in early infancy. Dev. Sci. 2013, 16, 676–687. [Google Scholar] [CrossRef]
- Otero, G.A. EEG spectral analysis in children with sociocultural handicaps. Int. J. Neurosci. 1994, 79, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Tarullo, A.R.; Obradovic, J.; Keehn, B.; Rasheed, M.A.; Siyal, S.; Nelson, C.A.; Yousafzai, A.K. Gamma power in rural Pakistani children: Links to executive function and verbal ability. Dev. Cogn. Neurosci. 2017, 26, 1–8. [Google Scholar] [CrossRef]
- Pierce, L.J.; Thompson, B.L.; Gharib, A.; Schlueter, L.; Reilly, E.; Valdes, V.; Roberts, S.; Conroy, K.; Levitt, P.; Nelson, C.A. Association of Perceived Maternal Stress During the Perinatal Period With Electroencephalography Patterns in 2-Month-Old Infants. Jama Pediatr. 2019, 173, 561–570. [Google Scholar] [CrossRef]
- Troller-Renfree, S.V.; Sperber, J.F.; Hart, E.R.; Costanzo, M.A.; Gennetian, L.A.; Meyer, J.S.; Fox, N.A.; Noble, K.G. Associations between maternal stress and infant resting brain activity among families residing in poverty in the U.S. Biol. Psychol. 2023, 184, 108683. [Google Scholar] [CrossRef]
- Möwisch, D.; Wienke, A.S.; Meier-Faust, E.; Mathes, B.; Attig, M. Cumulative socioeconomic risk factors and child temperament. Z. Für Erzieh. 2024. under review. [Google Scholar]
- Pedrós Barnils, N.; Eurenius, E.; Gustafsson, P.A.-O. Self-rated health inequalities in the intersection of gender, social class and regional development in Spain: Exploring contributions of material and psychosocial factors. Int. J. Equity Health 2020, 19, 85. [Google Scholar] [CrossRef]
- Römer, P.; Kemmerich, R.; Petermann, F.; Mathes, B.; Zierul, C. Alcohol and Nicotine Consumption during Pregnancy. SUCHT 2023, 69, 99–111. [Google Scholar] [CrossRef]
- Kiel, N.; Samdan, G.; Wienke, A.S.; Reinelt, T.; Pauen, S.; Mathes, B.; Herzmann, C. From co-regulation to self-regulation: Maternal soothing strategies and self-efficacy in relation to maternal reports of infant regulation at 3 and 7 months. Infant. Ment. Health J. 2024, 45, 135–152. [Google Scholar] [CrossRef] [PubMed]
- Samdan, G.; Reinelt, T.; Kiel, N.; Mathes, B.; Pauen, S. Maternal self-efficacy development from pregnancy to 3 months after birth. Infant. Ment. Health J. 2022, 43, 864–877. [Google Scholar] [CrossRef] [PubMed]
- Möwisch, D.; Attig, M.; Weinert, S. Einflussfaktoren auf die frühe Mutter-Kind-Interaktion. Frühe Bild. 2024, 13, 84–92. [Google Scholar] [CrossRef]
- Norton, E.S.; MacNeill, L.A.; Harriott, E.M.; Allen, N.; Krogh-Jespersen, S.; Smyser, C.D.; Rogers, C.E.; Smyser, T.A.; Luby, J.; Wakschlag, L. EEG/ERP as a pragmatic method to expand the reach of infant-toddler neuroimaging in HBCD: Promises and challenges. Dev. Cogn. Neurosci. 2021, 51, 100988. [Google Scholar] [CrossRef] [PubMed]
- Dotson, V.M.; Duarte, A. The importance of diversity in cognitive neuroscience. Ann. N. Y. Acad. Sci. 2020, 1464, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Blair, C.; Raver, C.C. Poverty, Stress, and Brain Development: New Directions for Prevention and Intervention. Acad. Pediatr. 2016, 16, S30–S36. [Google Scholar] [CrossRef] [PubMed]
- Hurt, H.; Betancourt, L.M. Effect of socioeconomic status disparity on child language and neural outcome: How early is early? Pediatr. Res. 2016, 79, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Tarullo, A.R.; Leppänen, J.M.; Evans, D.; Coetzee, L.; Lopera-Perez, D.C.; Brady, S.P.; Gabard-Durnam, L.J.; Fink, G.; Hamer, D.H.; Yousafzai, A.K.; et al. Neonatal Physical Growth Predicts Electroencephalography Power in Rural South African Children. Brain Sci. 2024, 14, 552. [Google Scholar] [CrossRef]
- Draper, C.E.; Barnett, L.M.; Cook, C.J.; Cuartas, J.A.; Howard, S.J.; McCoy, D.C.; Merkley, R.; Molano, A.; Maldonado-Carreño, C.; Obradović, J.; et al. Publishing child development research from around the world: An unfair playing field resulting in most of the world’s child population under-represented in research. Infant. Child. Dev. 2022, 32, e2375. [Google Scholar] [CrossRef]
- Bell, M.A.; Cuevas, K. Using EEG to Study Cognitive Development: Issues and Practices. J. Cogn. Dev. 2012, 13, 281–294. [Google Scholar] [CrossRef]
- Evans, G.W.; Kim, P. Childhood Poverty, Chronic Stress, Self-Regulation, and Coping. Child. Dev. Perspect. 2013, 7, 43–48. [Google Scholar] [CrossRef]
- González, L.; Cortés-Sancho, R.; Murcia, M.; Ballester, F.; Rebagliato, M.; Rodríguez-Bernal, C.L. The role of parental social class, education and unemployment on child cognitive development. Gac. Sanit. 2020, 34, 51–60. [Google Scholar] [CrossRef]
- D’Angiulli, A.; Lipina, S.J.; Olesinska, A. Explicit and implicit issues in the developmental cognitive neuroscience of social inequality. Front. Human. Neurosci. 2012, 6, 00254. [Google Scholar] [CrossRef] [PubMed]
- Conejero, A.; Rueda, M.R. Infant temperament and family socio-economic status in relation to the emergence of attention regulation. Sci. Rep. 2018, 8, 11232. [Google Scholar] [CrossRef] [PubMed]
- van Zuijen, T.L.; Plakas, A.; Maassen BA, M.; Been, P.; Maurits, N.M.; Krikhaar, E.; van Driel, J.; van der Leij, A. Temporal auditory processing at 17 months of age is associated with preliterate language comprehension and later word reading fluency: An ERP study. Neurosci. Lett. 2012, 528, 31–35. [Google Scholar] [CrossRef]
- Adams, E.J.; Scott, M.E.; Amarante, M.; Ramirez, C.A.; Rowley, S.J.; Noble, K.G.; Troller-Renfree, S.V. Fostering inclusion in EEG measures of pediatric brain activity. NPJ Sci. Learn. 2024, 9, 27. [Google Scholar] [CrossRef]

| N | 255 | |
|---|---|---|
| Mean age, months (SD) | 8.3 (1.2) | |
| Gender (% female) | 45.9 | |
| Parental Education | ||
| Higher (university entrance degree of at least one parent) | Lower (neither parent with university entrance degree) | |
| N | 177 | 78 |
| Infant Migration Background | ||
| None or 2nd grade | 1st grade | |
| N | 145 | 110 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wienke, A.S.; Mathes, B. Socioeconomic Inequalities Affect Brain Responses of Infants Growing Up in Germany. Brain Sci. 2024, 14, 560. https://doi.org/10.3390/brainsci14060560
Wienke AS, Mathes B. Socioeconomic Inequalities Affect Brain Responses of Infants Growing Up in Germany. Brain Sciences. 2024; 14(6):560. https://doi.org/10.3390/brainsci14060560
Chicago/Turabian StyleWienke, Annika Susann, and Birgit Mathes. 2024. "Socioeconomic Inequalities Affect Brain Responses of Infants Growing Up in Germany" Brain Sciences 14, no. 6: 560. https://doi.org/10.3390/brainsci14060560
APA StyleWienke, A. S., & Mathes, B. (2024). Socioeconomic Inequalities Affect Brain Responses of Infants Growing Up in Germany. Brain Sciences, 14(6), 560. https://doi.org/10.3390/brainsci14060560






